Abstract
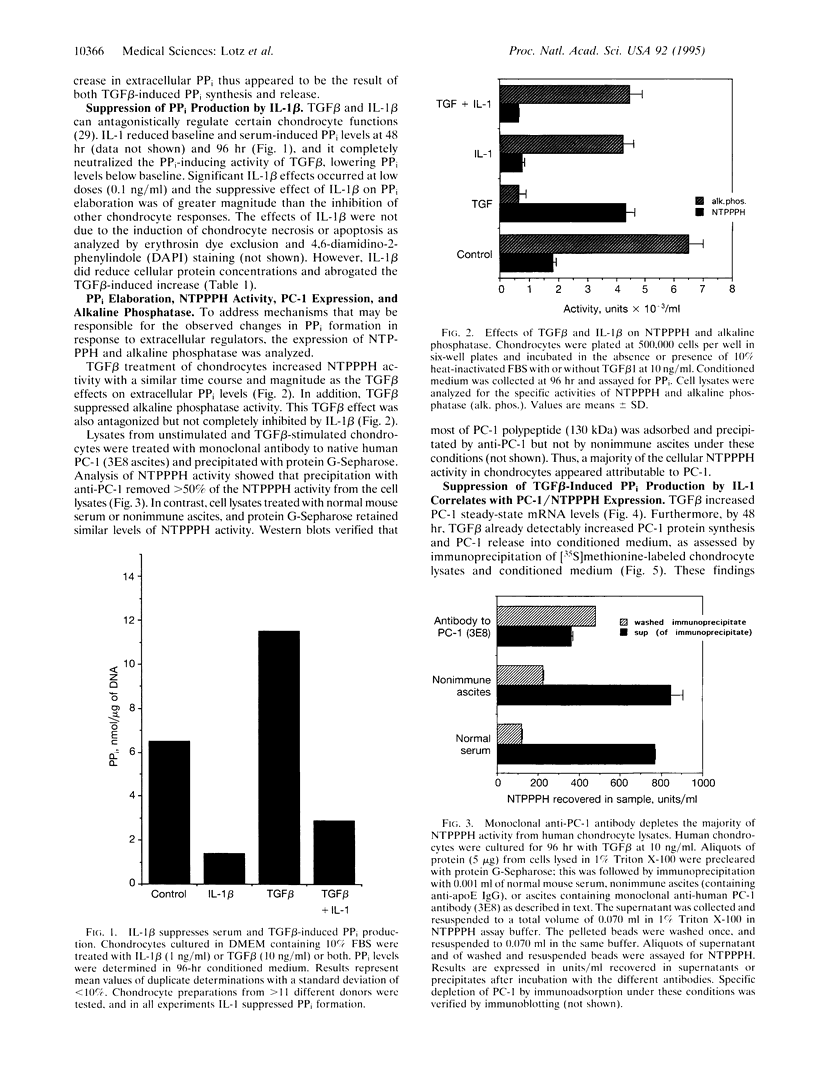
Articular cartilage chondrocytes have the unique ability to elaborate large amounts of extracellular pyrophosphate (PPi), and transforming growth factor beta (TGF beta) appears singular among cartilage regulatory factors in stimulating PPi production. TGF beta caused a time and dose-dependent increase in intracellular and extracellular PPi in human articular chondrocyte cultures. TGF beta and interleukin 1 beta (IL-1 beta) antagonistically regulate certain chondrocyte functions. IL-1 beta profoundly inhibited basal and TGF beta-induced PPi elaboration. To address mechanisms involved with the regulation of PPi synthesis by IL-1 beta and TGF beta, we analyzed the activity of the PPi-generating enzyme NTP pyrophosphohydrolase (NTPPPH) and the PPi-hydrolyzing enzyme alkaline phosphatase. Human chondrocyte NTPPPH activity was largely attributable to plasma cell membrane glycoprotein 1, PC-1. Furthermore, TGF beta induced comparable increases in the activity of extracellular PPi, intracellular PPi, and cellular NTPPPH and in the levels of PC-1 protein and mRNA in chondrocytes as well as a decrease in alkaline phosphatase. All of these TGF beta-induced responses were completely blocked by IL-1 beta. Thus, IL-1 beta may be an important regulator of mineralization in chondrocytes by inhibiting TGF beta-induced PPi production and PC-1 expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belli S. I., Goding J. W. Biochemical characterization of human PC-1, an enzyme possessing alkaline phosphodiesterase I and nucleotide pyrophosphatase activities. Eur J Biochem. 1994 Dec 1;226(2):433–443. doi: 10.1111/j.1432-1033.1994.tb20068.x. [DOI] [PubMed] [Google Scholar]
- Belli S. I., Sali A., Goding J. W. Divalent cations stabilize the conformation of plasma cell membrane glycoprotein PC-1 (alkaline phosphodiesterase I). Biochem J. 1994 Nov 15;304(Pt 1):75–80. doi: 10.1042/bj3040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belli S. I., van Driel I. R., Goding J. W. Identification and characterization of a soluble form of the plasma cell membrane glycoprotein PC-1 (5'-nucleotide phosphodiesterase). Eur J Biochem. 1993 Oct 1;217(1):421–428. doi: 10.1111/j.1432-1033.1993.tb18261.x. [DOI] [PubMed] [Google Scholar]
- Cheung C. P., Suhadolnik R. J. A rapid quantitative method for measuring UTP, UDP, and UMP in the picomole range. Anal Biochem. 1977 Nov;83(1):52–56. doi: 10.1016/0003-2697(77)90508-5. [DOI] [PubMed] [Google Scholar]
- Derfus B. A., Rachow J. W., Mandel N. S., Boskey A. L., Buday M., Kushnaryov V. M., Ryan L. M. Articular cartilage vesicles generate calcium pyrophosphate dihydrate-like crystals in vitro. Arthritis Rheum. 1992 Feb;35(2):231–240. doi: 10.1002/art.1780350218. [DOI] [PubMed] [Google Scholar]
- Eastgate J. A., Symons J. A., Wood N. C., Grinlinton F. M., di Giovine F. S., Duff G. W. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet. 1988 Sep 24;2(8613):706–709. doi: 10.1016/s0140-6736(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Guerne P. A., Carson D. A., Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol. 1990 Jan 15;144(2):499–505. [PubMed] [Google Scholar]
- Guerne P. A., Sublet A., Lotz M. Growth factor responsiveness of human articular chondrocytes: distinct profiles in primary chondrocytes, subcultured chondrocytes, and fibroblasts. J Cell Physiol. 1994 Mar;158(3):476–484. doi: 10.1002/jcp.1041580312. [DOI] [PubMed] [Google Scholar]
- Howell D. S., Martel-Pelletier J., Pelletier J. P., Morales S., Muniz O. NTP pyrophosphohydrolase in human chondrocalcinotic and osteoarthritic cartilage. II. Further studies on histologic and subcellular distribution. Arthritis Rheum. 1984 Feb;27(2):193–199. doi: 10.1002/art.1780270211. [DOI] [PubMed] [Google Scholar]
- Huang R., Rosenbach M., Vaughn R., Provvedini D., Rebbe N., Hickman S., Goding J., Terkeltaub R. Expression of the murine plasma cell nucleotide pyrophosphohydrolase PC-1 is shared by human liver, bone, and cartilage cells. Regulation of PC-1 expression in osteosarcoma cells by transforming growth factor-beta. J Clin Invest. 1994 Aug;94(2):560–567. doi: 10.1172/JCI117370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. C., Chuck A. J., Arie E. A., Green D. J., Doherty M. Diseases associated with calcium pyrophosphate deposition disease. Semin Arthritis Rheum. 1992 Dec;22(3):188–202. doi: 10.1016/0049-0172(92)90019-a. [DOI] [PubMed] [Google Scholar]
- Leyva A., Jr, Kelley W. N. Measurement of DNA in cultured human cells. Anal Biochem. 1974 Nov;62(1):173–179. doi: 10.1016/0003-2697(74)90378-9. [DOI] [PubMed] [Google Scholar]
- Lotz M., Blanco F. J., von Kempis J., Dudler J., Maier R., Villiger P. M., Geng Y. Cytokine regulation of chondrocyte functions. J Rheumatol Suppl. 1995 Feb;43:104–108. [PubMed] [Google Scholar]
- Lotz M., Terkeltaub R., Villiger P. M. Cartilage and joint inflammation. Regulation of IL-8 expression by human articular chondrocytes. J Immunol. 1992 Jan 15;148(2):466–473. [PubMed] [Google Scholar]
- Lust G., Faure G., Netter P., Seegmiller J. E. Increased pyrophosphate in fibroblasts and lymphoblasts from patients with hereditary diffuse articular chondrocalcinosis. Science. 1981 Nov 13;214(4522):809–810. doi: 10.1126/science.6270793. [DOI] [PubMed] [Google Scholar]
- Lust G., Nuki G., Seegmiller J. E. Inorganic pyrophosphate and proteoglycan metabolism in cultured human articular chondrocytes and fibroblasts. Arthritis Rheum. 1976 May-Jun;19 (Suppl 3):479–487. doi: 10.1002/1529-0131(197605/06)19:3+<479::aid-art1780190723>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Malemud C. J. The role of growth factors in cartilage metabolism. Rheum Dis Clin North Am. 1993 Aug;19(3):569–580. [PubMed] [Google Scholar]
- McGuire M. B., Colman C. H., Baghat N., Russell R. G. Radiometric measurement of pyrophosphate in cell cultures. Biochem Soc Trans. 1980 Oct;8(5):529–530. doi: 10.1042/bst0080529. [DOI] [PubMed] [Google Scholar]
- Oyajobi B. O., Russell R. G., Caswell A. M. Modulation of ecto-nucleoside triphosphate pyrophosphatase activity of human osteoblast-like bone cells by 1 alpha,25-dihydroxyvitamin D3, 24R,25-dihydroxyvitamin D3, parathyroid hormone, and dexamethasone. J Bone Miner Res. 1994 Aug;9(8):1259–1266. doi: 10.1002/jbmr.5650090816. [DOI] [PubMed] [Google Scholar]
- Pattrick M., Hamilton E., Hornby J., Doherty M. Synovial fluid pyrophosphate and nucleoside triphosphate pyrophosphatase: comparison between normal and diseased and between inflamed and non-inflamed joints. Ann Rheum Dis. 1991 Apr;50(4):214–218. doi: 10.1136/ard.50.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins A. P., Kiljan E., van de Stadt R. J., van der Korst J. K. Inorganic pyrophosphate release by rabbit articular chondrocytes in vitro. Arthritis Rheum. 1986 Dec;29(12):1485–1492. doi: 10.1002/art.1780291210. [DOI] [PubMed] [Google Scholar]
- Rachow J. W., Ryan L. M. Inorganic pyrophosphate metabolism in arthritis. Rheum Dis Clin North Am. 1988 Aug;14(2):289–302. [PubMed] [Google Scholar]
- Rebbe N. F., Tong B. D., Finley E. M., Hickman S. Identification of nucleotide pyrophosphatase/alkaline phosphodiesterase I activity associated with the mouse plasma cell differentiation antigen PC-1. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5192–5196. doi: 10.1073/pnas.88.12.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. K., Cheung H. S., Ryan L. M. Transforming growth factor beta 1 stimulates inorganic pyrophosphate elaboration by porcine cartilage. Arthritis Rheum. 1991 Jul;34(7):904–911. doi: 10.1002/art.1780340717. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. K., McCarty B. A., Cheung H. S., Ryan L. M. A comparison of the effect of transforming growth factor beta 1 on pyrophosphate elaboration from various articular tissues. Arthritis Rheum. 1993 Apr;36(4):539–542. doi: 10.1002/art.1780360415. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. K., Ryan L. M. Probenecid inhibits transforming growth factor-beta 1 induced pyrophosphate elaboration by chondrocytes. J Rheumatol. 1994 May;21(5):896–900. [PubMed] [Google Scholar]
- Russell R. G., Mühlbauer R. C., Bisaz S., Williams D. A., Fleisch H. The influence of pyrophosphate, condensed phosphates, phosphonates and other phosphate compounds on the dissolution of hydroxyapatite in vitro and on bone resorption induced by parathyroid hormone in tissue culture and in thyroparathyroidectomised rats. Calcif Tissue Res. 1970;6(3):183–196. doi: 10.1007/BF02196199. [DOI] [PubMed] [Google Scholar]
- Ryan L. M., Kurup I. V., Derfus B. A., Kushnaryov V. M. ATP-induced chondrocalcinosis. Arthritis Rheum. 1992 Dec;35(12):1520–1525. doi: 10.1002/art.1780351216. [DOI] [PubMed] [Google Scholar]
- Ryan L. M., Kurup I., Cheung H. S. Stimulation of cartilage inorganic pyrophosphate elaboration by ascorbate. Matrix. 1991 Aug;11(4):276–281. doi: 10.1016/s0934-8832(11)80235-0. [DOI] [PubMed] [Google Scholar]
- Ryan L. M., Kurup I., McCarty D. J., Cheung H. S. Cartilage inorganic pyrophosphate elaboration is independent of sulfated glycosaminoglycan synthesis. Arthritis Rheum. 1990 Feb;33(2):235–240. doi: 10.1002/art.1780330212. [DOI] [PubMed] [Google Scholar]
- Ryan L. M., Wortmann R. L., Karas B., Lynch M. P., McCarty D. J. Pyrophosphohydrolase activity and inorganic pyrophosphate content of cultured human skin fibroblasts. Elevated levels in some patients with calcium pyrophosphate dihydrate deposition disease. J Clin Invest. 1986 May;77(5):1689–1693. doi: 10.1172/JCI112487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. A., Hummel C. F., Carty R. P. The role of nucleoside triphosphate pyrophosphohydrolase in in vitro nucleoside triphosphate-dependent matrix vesicle calcification. J Biol Chem. 1983 Jul 25;258(14):8601–8607. [PubMed] [Google Scholar]
- Sokoloff L., Varma A. A. Chondrocalcinosis in surgically resected joints. Arthritis Rheum. 1988 Jun;31(6):750–756. doi: 10.1002/art.1780310608. [DOI] [PubMed] [Google Scholar]
- Tenenbaum J., Muniz O., Schumacher H. R., Good A. E., Howell D. S. Comparison of phosphohydrolase activities from articular cartilage in calcium pyrophosphate deposition disease and primary osteoarthritis. Arthritis Rheum. 1981 Mar;24(3):492–500. doi: 10.1002/art.1780240307. [DOI] [PubMed] [Google Scholar]
- Terkeltaub R., Rosenbach M., Fong F., Goding J. Causal link between nucleotide pyrophosphohydrolase overactivity and increased intracellular inorganic pyrophosphate generation demonstrated by transfection of cultured fibroblasts and osteoblasts with plasma cell membrane glycoprotein-1. Relevance to calcium pyrophosphate dihydrate deposition disease. Arthritis Rheum. 1994 Jun;37(6):934–941. doi: 10.1002/art.1780370624. [DOI] [PubMed] [Google Scholar]
- Villiger P. M., Geng Y., Lotz M. Induction of cytokine expression by leukemia inhibitory factor. J Clin Invest. 1993 Apr;91(4):1575–1581. doi: 10.1172/JCI116363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiger P. M., Lotz M. Expression of prepro-enkephalin in human articular chondrocytes is linked to cell proliferation. EMBO J. 1992 Jan;11(1):135–143. doi: 10.1002/j.1460-2075.1992.tb05036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]