Abstract
Chemotherapy is one of the most widely used approaches in combating advanced prostate cancer, but its therapeutic efficacy is usually insufficient due to lack of specificity and associated toxicity. Lack of targeted delivery to prostate cancer cells is also the primary obstacles in achieving feasible therapeutic effect of other promising agents including peptide, protein, and nucleic acid. Consequently, there remains a critical need for strategies to increase the selectivity of anti-prostate cancer agents. This review will focus on various prostate cancer-specific antigens and enzymes that could be exploited for prostate cancer targeted drug delivery. Among various targeting strategies, active targeting is the most advanced approach to specifically deliver drugs to their designated cancer cells. In this approach, drug carriers are modified with targeting ligands that can specifically bind to prostate cancer-specific antigens. Moreover, there are several specific enzymes in the tumor microenvironment of prostate cancer that can be exploited for stimulus-responsive drug delivery systems. These systems can specifically release the active drug in the tumor microenvironment of prostate cancer, leading to enhanced tumor penetration efficiency.
Keywords: prostate cancer, tumor microenvironment, PSMA, PSA, PSCA, HER2, MUC1, MMP
1. Introduction
Prostate cancer is currently the most common male malignancy and remains the leading cause of death in American men, in spite of extensive efforts and recent advances in early diagnosis and surgical intervention.1 According to the classification by the U.S. National Cancer Institute, prostate cancer can be divided into four different stages after diagnosis. In stage I, the cancer is small and confined to the prostate gland. In stage II, the cancer is larger but still limited to the prostate gland. In stage III, the cancer spreads out of the prostate gland and reaches the tissues near the prostate. The cancer may reach the seminal vesicles. In stage IV, the cancer spreads to distant organs and tissues, such as rectum, lymph nodes, bones, lung, etc. When prostate cancer spreads out of the prostate gland and metastasizes to distant parts of the body, it is called advanced prostate cancer.2 Patients with high risk of prostate cancer progression and/or death are also considered as advanced prostate cancer.3
The current standard therapies include surgery, radiation, and adjuvant hormonal therapy. Although these therapies are relatively effective in the early stages of disease, the majority of patients initially diagnosed with localized prostate cancer ultimately relapse. As a result, the major risk faced by prostate cancer patients is the development of advanced prostate cancer.1
Although chemotherapy is one of the most widely used approaches in combating advanced prostate cancer, its therapeutic efficacy is usually insufficient due to lack of specificity and associated toxicity. Lack of targeted delivery to prostate cancer cells is one of the primary obstacles in achieving feasible therapeutic effect of other promising agents including small molecules, peptides, proteins, and nucleic acids. Consequently, there remains a critical need for strategies to increase the selectivity of anti-prostate cancer agents.
Among various targeting strategies, active targeting is the most advanced approach to specifically deliver drugs to their designated cancer cells. In this approach, drug carriers are modified with targeting ligands that can specifically bind to prostate cancer-specific antigens, leading to accumulation of drugs in cancer cells. Extensive efforts have been devoted to identifying potential prostate cancer-specific antigens and corresponding ligands, such as monoclonal antibodies/fragments, peptides, aptamers, or small molecules.
On the other hand, the tumor microenvironment in prostate cancer contains several overexpressed enzymes that can be used to achieve selective drug release in the interstitial spaces surrounding prostate cancer cells.
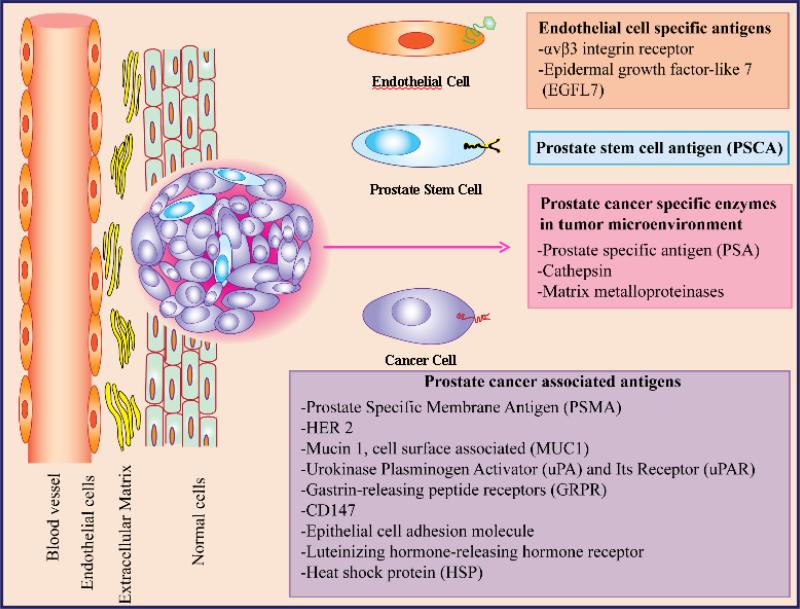
The aim of this review is to critically evaluate various prostate cancer-specific antigens and enzymes (Figure 1) that have been exploited for prostate cancer targeted drug delivery. We will also introduce some of the antigens that have not been explored but shown great promise as prostate cancer-specific marker.
Figure 1.
Prostate cancer-specific antigens and enzymes in the tumor microenvironment.
2. Prostate Cancer Associated Antigen
2.1 Prostate Specific Membrane Antigen (PSMA)
PSMA, also known as glutamate carboxypeptidase II, N-acetyl-α-linked acidic dipeptidase I, or folate hydrolase, is a 100 KDa type II transmembrane glycosylated protein. PSMA consists of an extensively glycosylated extracellular domain of 707 amino acids, a transmembrane domain of 24 amino acids and an intracellular domain of 19 amino acids.4-7 The overall crystal structure of PSMA is composed of a symmetric dimer, in which each polypeptide contains three distinct structural and functional domains: a protease domain (amino acids 56-116), an apical domain (amino acids 117- 351), and a C-terminal/helical domain (amino acids 592-750).5, 8 PSMA is a member of the family of zinc-dependent exopeptidases with a bi nuclear zinc active site and it can work as a glutamate carboxypeptidase. Normally, PSMA is expressed on membranes of prostate epithelial cells and its expression level is increased in prostate cancer cells. Many studies have reported that PSMA overexpresses in nearly all prostate cancers and notably in almost all tumor stages and its expression level increases with cancer progression.9-11,11-13
Although PSMA is expressed in some normal tissues, such as small intestine, proximal renal tubules and salivary glands, 14 but its expression level is 100 to 1000 fold higher in prostate cancer cells compare to normal tissues. 15,16 In addition the site of expression of normal tissue is not exposed to direct blood circulation so that the interaction with PSMA-specific antibodies or other ligands can be ignored. 17,14 Moreover, PSMA is also expressed on the neovasculature of the most of the solid malignant tumors, but not in normal vasculature.18 The over expression of PSMA is a very primitive characteristic of prostate cancer cells and the expression level enhances with the aggressiveness and recurrence of tumor. The expression level of higher-grade and androgen-independent tumors is highest in the metastatic state. 19 Comparing to prostate-specific antigen (PSA) and prostatic acid phosphatase (PAP), PSMA is not a secretory protein. Instead, PSMA has internalization function and this transport effect could be increased three fold when PSMA is bound by anti-PSMA antibodies. Therefore, PSMA is emerged as one of the most potential and important antigen target and diagnostic biomarker in prostate cancer. 15, 20
Since the expression level of PSMA is exceptionally high in prostate cancer cells, PSMA has been extensively used as a target in targeted drug delivery strategies. Many PSMA targeting aptamers, mAb,21 or peptides 22 have been developed and employed in prodrug or nanoparticles to improve their targeting efficiency to prostate cancer cells. In starting of last decade 2002, by employing in vitro selection, Shawn E Lupold et al found two RNA aptamers which were named A9 and A10 aptamers, and these two aptamers have high binding affinity to PSMA and can inhibit its NAALADase/ Glutamate carboxypeptidase II activity. Similarly, researchers have been used phage display to screen and identify peptide sequences such as KYLAYPDSVHIW 23 and WQPDTAHHWATL 22 which can also specific bind to PSMA and inhibit its enzymatic activity. These targeting ligands have been widely used for targeted drug delivery. In most of these approaches the drug or the delivery system is conjugated with a PSMA-targeting ligand which leads to the binding of PSMA positive cells. Among them, A10 aptamer is one of the widely used ligand. Recently, A10 aptamer is conjugated on the surface of micelles, 24 and results demonstrated significantly higher drug uptake in PSMA positive CWR22Rv1 cancer cell both in vitro and in vivo studies.24 Many groups have studied and reported the PSMA targeted delivery of diagnostic agents and therapeutics. Some of the representative approaches are summarized in Table 1 and 2.Various PSMA based diagnostic and therapeutic agents are under phase1, 2 and 3 trials.25
Table 1.
PSMA-specific imaging agents
| Targeting Agents | Reference |
|---|---|
| 111Indium (111In) labeled anti-PSMA monoclonal antibody 7E11 | 30, 31 |
| 90Yttrium-(90Y) labeled 7E11 | 32, 33 |
| 64Copper (64Cu) labeled anti-PSMA monoclonal antibody 3/A12, 3/E7, and 3/F11 | 34, 35 |
| 177Lutetium (177Lu; β particle emitter) labeled Antibody 3/F11 | 36 |
| PSMA binding (extracellularly) antibodies J415, J533, and J591 | |
| 213Bismuth (213Bi; alpha particle emitter) labeled J591 | 37 |
| 131Iod (131I) labeled antibodies J591, J415, and 7E11 | 25 |
| 131I and 90Y labeled antibody (humanized) J591 (huJ591) | 38 |
| DUPA-Tc99m | 39 |
Table 2.
PSMA-specific therapeutic agents
| Targeting moiety | Drug/cytotoxic agent | Delivery system | Reference |
|---|---|---|---|
| De-immunized mAb J591 | Maytansinoid 1 | Antibody-Drug Conjugate | 40 |
| Humanized antibody huJ591** | Plant toxin saporin | Fusion protein | 41 |
| scFv A5 (generated from the mAb 3/A12) | Toxin domain, the shortened form of Pseudomonas exotoxin A (PE40) | Fusion protein | 42, 43,35 |
| scFv D7 (generated from the anti-PSMA antibody 3/F11) | Toxin domain, the shortened form of Pseudomonas exotoxin A (PE40) | Fusion protein | 43 |
| 2-[3-(1,3dicarboxypropyl)ureido]pentanedioic acid (DUPA) | Chemotherapeutic drugs | Conjugate | 44 |
| A10 Aptamer | Doxorubicin | Polyplex | 45 |
| A10 Aptamer | Doxorubicin | Micelle | 24 |
| Monoclonal antibody (J415, J533, and J591) | Bismuth-213 | Radiolabled antibody | 25, 37, 38 |
| Anti-PSMA Monoclonal antibody | Monomethylauristatin E | Antibody-drug conjugate | 46 |
| Prostate-specific membrane aptamers (PSMAa10) | TGX-221 | Prodrug | 47 |
| A10 Aptamer | androgen receptor silencing constructs | Biodegradable nanoparticles | 45 |
| Pseudomimetic dipeptideN-[N-[(S)-1,3-dicarboxypropyl]carbamoyl]-(S)-lysine (DCL)] | (-)-epigallocatechin 3-gallate (EGCG | Nanoparticles | 48 |
| A10 aptamer | Cisplatin | A10 aptamer functionalized PLGA-PEG nanoparticles) | 49, 50 |
*= Phase 1
phase 2
In addition, PSMA is utilized successfully in some other approaches too, such as, in radiotherapy, radiolabeled anti-PSMA mAbs are used to target PSMA-positive prostate tumor cells. ProstaScint® scan (Cytogen Corporation, Princeton, NJ) is one of the examples of this approach. It is an FDA-approved radiographic test that uses the anti-PSMA antibody (mAb 7E11) by linking it to 111indium to form the radiodiagnostic marker, 111indium-capromab pendetide.26 Immunotherapy several Anti-PSMA mAbs, scFv or RNA oligonucleotides are conjugated to the surface of immunotoxins or nanoparticles to achieve high targeting specificity to prostate cancer. In addition, T cells are employed against the prostate cancer cells by stimulating them with anti-PSMA/anti-CD3 diabodies or anti-PSMA scFv-based chimeric antigen receptors. 27, 28 Vaccines are another very interesting area which utilized PSMA as target to potent cellular and humoral immune responses againts cancer cells.29
PSMA is a widely used marker for prostate cancer cells. Its overexpression is associated with cancer. Currently it is most appropriate prognostic marker. A lot of promising clinical applications employing PSMA have been done and also many other are being developed. On basis of current scenario in the future, PSMA would play an important impact on prostate cancer diagnosis and treatment.
2.2 Prostate stem cell antigen (PSCA)
Cancer stem cells are cancer cells that possess the properties of normal stem cells, such as self-renewal and differentiation into heterogeneous cell types.51 These cancer stem cells are rare but highly tumorigenic and play key role in tumor homeostasis and metastasis.49, 52
PSCA is a glycosylphosphatidylinositol (GPI)-anchored cell membrane protein in the Thy-1/Ly-6 family of cell surface antigens, consisting of 123 amino acids.51 It shows 30% identity to stem cell antigen type 2 (SCA-2), which is a cell surface marker of immature thymic lymphocytes. 53, 54
Similar to PSMA, PSCA is also expressed in some normal tissues, such as the bladder, colon, kidney, and stomach, but its expression in prostate cancer tissue is much higher compared to normal tissues. It is overexpressed in local as well as metastatic prostate cancer cells.55, 56 Moreover, the PSCA expression level in high-grade prostatic intraepithelial neoplasm is fourfold higher than that in benign prostatic hyperplasia.57,58,58 An in situ analysis of 126 prostate cancer specimens, including high-grade prostatic intraepithelial neoplasia and androgen-dependent and androgen-independent tumors, showed moderate to strong PSCA expression in more than 85% of the samples.56 The higher level of PSCA expression is directly associated with the advanced stages, higher degree and androgen independency of the disease. In another study of 112 samples, 94% specimens of primary prostate tumors and 100% of bone metastases specimens showed PSCA expression.59 Its expression level increases in cases of higher Gleason score, advanced tumor stage and progression of disease to androgen-independent state.57, 59 Moreover, the limited expression in normal prostate tissues, direct connection with tumor grade or stage, and expression on the surface of tumor cells suggest that PSCA could become a prominent target in drug delivery to prostate cancer.58
The use of PSCA in targeted drug delivery has emerged as a prominent area of research. In one interesting approach, dual-functional nanoparticles modified with PSCA-specific ScFv were developed for targeted delivery to prostate cancer cells. The nanoparticles demonstrated prostate cancer-specific accumulation of docetaxel and imaging agent in vitro and in vivo. Moreover, the nanoparticles reversed tumor growth in nude mice bearing prostate cancer xenografts without significant systemic toxicity. 60 In a similar approach, dual docetaxel and super paramagnetic iron oxide-loaded nanoparticles were prepared and delivered specifically to prostate cancer PC3 cells by using the single chain PSCA antibody scAb-PSCA61. In addition, the PSCA-targeted monoclonal antibody was linked to a copolymer complex containing super paramagnetic iron oxide. The functionalized nanoprobe can specifically target prostate cancer cells and work as a novel MRI nanoprobe for early diagnosis of prostate cancer.62 In addition, anti-PSCA mAbs such as 1G8 and 3C5 59 have been tested for their inherent cytotoxic activity using subcutaneous and orthotopic CaP xenograft models. 63 A dendritic cell-based immunotherapy and PSCA based vaccine have been developed and tested for hormone-refractory prostate cancer. 64, 65
The overexpression of PSCA in prostate cancer cells and its successful preliminary investigations support the candidature of PSCA for targeted drug delivery and diagnosis in prostate cancer.
2.3 HER-2
HER-2, or ErbB-2, is a transmembrane glycoprotein that belongs to the ErbB protein family. It consists of three regions: an intracellular tyrosine kinase domain, a single α-helix transmembrane domain (TM), and an N-terminal extracellular domain (ECD).66 Among these, the N-terminal ECD is the largest region containing about 630 amino acids. It consists of four domains: I/L1, II/CR1, III/L2 and IV/CR2. These extracellular domains can dimerize after ligand binding, and their specific tyrosine residues can be auto phosphorylated by the activated cytoplasmic kinase and then initiate downstream cell proliferation. HER2 activates pathways that promote cell division and suppress apoptosis, resulting in enhanced cell motility.67, 68 In addition, even in the absence of androgens HER2 is able to activate the androgen receptor (AR) pathway. 67, 69-71 This provides help to HER-2-expressing cancer cells in their survival and growth and also accelerates the progression of the tumor towards androgen independence. HER-2 activation of AR also shows an association with aggressive behavior and makes the cells more resistant to therapy.67, 72-74
Although HER-2 is a well-known membrane receptor in breast cancer, it is also overexpressed in prostate cancers.75 It has been reported that 25% of untreated primary prostate tumors, 78% of castrate metastatic tumors, and 59% of localized tumors after hormone treatment overexpress the HER-2 protein.75 Although the monoclonal anti-HER2 antibody has not shown a significant therapeutic effect in prostate cancer patients, 76 the overexpressed HER-2 on prostate cancers is a promising molecular target for targeted drug delivery systems. Previous reports showed that Herceptin (a monoclonal anti-HER-2 antibody) can significantly inhibit the growth of androgen-dependent prostate tumors in animal studies.77 However, little efficacy of Herceptin was observed in combating Hormone Refractory Prostate Cancer (HRPC) in a clinical study.76
Tai et al. employed an HER2-specific peptide KCCYSL as a targeting moiety for delivery of a TGX221 analogue. This peptide-drug conjugate revealed a significantly higher prostate cancer cell uptake compared to the parent drug.78 Other HER-2 specific ligands, such as the peptide LTVSPWY, 79 the aptamer HB5, 80 and the HER-2 specific affibody, 81, 82 have been investigated for targeted delivery of various agents to HER-2 positive breast and prostate cancer cells. All these evidence support the potential role of using HER-2 specific ligands for prostate cancer targeted drug delivery.
2.4 Mucin 1
Mucins are a family of high molecular weight glycoproteins that are found exclusively on the apical surface of various glandular epithelia, including in the reproductive, urinary, respiratory, and gastrointestinal tracts.83 Twenty-one mucin genes have been identified in the human genome, and their proteins are named MUC1 to MUC 21. These proteins are classified into two major categories: transmembrane proteins (MUC1, MUC3A, MUC3B, MUC4, MUC12, MUC13, MUC15, MUC16, MUC17, MUC20, and MUC21) and secreted proteins (MUC2, MUC5AC, MUC5B, MUC6, MUC7, MUC8 and MUC19).84-86 Mucins are produced by epithelial tissues, and their main function is to protect and lubricate epithelial surfaces.83 They also play roles in cell signaling by interacting with other signal transducing molecules, such as beta-catenin, glycogen synthase kinase 3beta, Grb2, and the Sos/Ras exchange protein.87-89 The molecular weight of MUC1 is 120–225 kDa, and it can increase to 250-500 kDa after glycosylation.90 MUC1 is a type I transmembrane protein and consists of a large extracellular domain that contains variable number of tandem repeats of 20 amino acids (GVTSAPDTRPAPGSTAPPAH), a transmembrane domain, and an intracellular domain of 69 amino acids. 84, 85
MUC1 is expressed in many normal cells but is overexpressed in a variety of adenocarcinomas, such as breast cancer, lung cancer, pancreatic cancer, and prostate cancer.91 In normal tissues, MUC1 is found at the apical side of epithelial cells and typically with high glycosylation level. However, in MUC1-overexpressing tumor tissues, it is expressed not only on the apical side but all around the cell surface, and it also shows a low glycosylation level.92 MUC1 is overexpressed in almost 60% of primary prostate cancers and 90% of lymph node prostate cancer metastases.93-95 The overexpression of MUC1 is prevalent in malignant prostate tumors, and its expression level correlates with tumor grade and stage.95, 96 Immunoblot analysis reveals that MUC1 is present in androgen-independent and AR-negative cell lines, such as PC-3 (low level) and DU-145 (high level), and is absent or scarce in androgen-dependent and AR-positive cell lines, such as LNCaP, CWR22Rv1 and MDA-PCa-2b.93 However, some other findings suggest that MUC1 may not have the same cancer-promoting role in prostate cancer cells as that in other epithelial cancers, such as colon, breast, and pancreatic cancer.93, 97
Various strategies have been adopted to target cancer therapeutic agents to MUC1. Numerous MUC1-specific mAb, 98 single-chain Fv,99 and aptamers 100 have been developed for targeted drug delivery to MUC1-overexpressing cancer cells.98, 99, 100, 101 For example, Paclitaxeland doxorubicin-containing nanoparticles modified with anti-MUC1 aptamers as a targeting moiety were formulated and targeted to breast cancer cells overexpressing MUC1.The aptamer modified nanoparticles exhibit higher cellular uptake and cytotoxicity in MUC1 overexpressing cells compared to unmodified nanoparticles.101, 102
Liposome-based BLP25 (L-BLP25) vaccine and TG4010 containing MVA-MUC1-IL2 vaccine immunotherapy are reported to identify and destroy prostate cancer cells overexpressing MUC1.103, 104 Moreover, a MUC1-inhibiting compound, GO-201, can interact with MUC1 receptors and inhibits the proliferation of DU145 and PC3 cells.93
Although not much work has been conducted in the use of MUC1 for targeted drug delivery to prostate cancer cells, the overexpression of MUC1 in prostate cancer cells and successful delivery of cytotoxic agents to other MUC1-overexpressing cancer cells strongly support the candidature of MUC1 in targeted drug delivery to prostate cancer.
2.5 Urokinase plasminogen activator receptor (uPAR)
The uPA system, which mainly comprises urokinase plasminogen activator (uPA) and its receptor urokinase plasminogen activator receptor (uPAR), attracts the attention of researchers because of its role in many important processes, such as cell differentiation, proliferation, adhesion, and signaling.105 uPAR is a membrane-bound receptor. uPA interacts with uPAR and forms a uPAR-uPA conjugate that enters cells by clathrin-coated, receptor-mediated endocytosis.106, 107 The uPAR-uPA conjugate is involved in activating various cellular activities, such as plasminogen activation,108 extracellular matrix invasion,106, 109, 110 cell adhesion, and metastasis.111, 112 uPA and uPAR also play important role in prostate cancer metastasis, and the knockdown of uPA and uPAR expression by shRNA in the PC-3 and DU145 cell lines leads to apoptosis and significant inhibition of metastasis in orthotopic mouse prostate cancer model.113
uPAR is overexpressed on several cancer cells including prostate cancer cells.114, 115 Although uPA and uPAR are expressed in normal cells, the activity and expression of uPAR are much higher in malignant tumors, including prostate cancer.114 Immunohistochemical examination showed that overexpression of uPAR is expressed in 64% of primary CaP tissues and in more than 90% of lymph node metastases.116 The overexpression of uPAR and uPAR mRNA is also reported in more than 80% samples from the patients of high-grade prostate cancer with a Gleason score greater than 7. 115, 117
Because uPAR expression is commonly observed in prostate cancer, especially in late stage disease, it is therefore a potential target for prostate cancer therapy. A number of approaches have been investigated to target uPA/uPAR for diagnosis118, 119 as well as for the targeted delivery of drug to prostate cancer cells.117 Several uPAR-specific peptides 106, 120, 121 and a monoclonal anti-uPAR antibody 122 have been identified and used for targeting uPAR-overexpressing cancer cells 123,124 including prostate cancer cells.106 These peptides work as a targeting moieties and are used in the preparation of conjugates that specifically target and deliver the drug or radionuclide to uPAR-expressing cancer cells. The targeted delivery of Noscapine 125 and plasmid DNA in uPAR-targeted nanoparticles106 to prostate cancer cells has been reported.
The overexpression of uPAR in prostate cancer cells, especially in advanced forms of disease, and their successful utilization for targeted delivery of therapeutic or diagnostic agents to prostate cancer cells suggest that these receptors have a prominent future in targeted drug delivery to prostate cancer cells. It is an attractive target and several molecules that target uPAR directly or operate through the uPA system to deliver therapeutic payloads have been designed, investigated and are heading toward the clinic, but further investigations are required to validate their present status.
2.6 Gastrin-releasing peptide receptor (GRPR)
Gastrin-releasing peptide receptor (GRPR), also known as BB2, is a glycosylated 7-transmembrane G protein-coupled receptor that is a member of a family of four bombesin (BN) receptor subtypes. The others are neuromedin B (NMB) receptor or BB1, the BRS-3 or BB3, and BB4. 126 GRP mediates many physiological and pathophysiological processes by interacting with GRP receptors, 127 but from an oncologic point of view, GRP plays a role in growth and/or differentiation by inducing activation of number of enzymes and pathways in various human tumors including prostate cancers. 127
GRPR is overexpressed in various malignancies, including prostate, breast, pancreatic, gastric, colorectal, and esophageal cancers, GI carcinoids, renal cell cancer, lung cancer, head and neck cancer, neuroblastomas and brain cancer. 128, 129 There is compelling evidence that prostate tumors overexpress GRPR. 126, 128, 130-132 More importantly, overexpression of these receptors is limited to the malignant cells, and most normal and hyperplastic prostate tissues are GRPR-negative.126 GRPR overexpression is found in 77% to 100% of prostate tumor samples.126, 130 GRPR expression is androgen-dependent, and androgen ablation results in down-regulation of GRPR. The higher expression of GRPR in hormone independent prostate tumors and lower expression in hormone-dependent tumors also support this hypothesis. 133
Moreover, GRPR possesses high binding affinity to GRP peptides. Bombesin is a linear tetra decapeptide with the sequence EQRLGNQWAVGHLM, which possesses homology to GRP at the amidated C terminal sequence in the final 7 amino acids, and therefore, bombesin is widely used to target GRPR.134
Many studies have reported the targeted delivery of radionuclides or other cytotoxic agents by conjugating them with bombesin analogs. For instance, an enhanced delivery of a photosensitizer (Sulfonated aluminum phthalocyanines) was achieved by coupling it with bombesin. Bombesin acts as a targeting moiety and delivers the drug specifically to prostate cancer cells expressing GRPR.135 Also, many attempts have been made to combine the targeted radiotherapy with protein kinase inhibitor 136 or with antineoplastic agents such as rapamycin137 to achieve targeted delivery of these agents to prostate cancer cells. Moreover, the targeted delivery of liposomes containing chemotherapeutic agents using bombesin peptide antagonists to prostate cancer cells has also been sought in recent years. 138 Several groups followed similar strategies for site specific delivery of a number of radio-labeled bombesin analogs, 139-144 such as 18F-labeled, 144, 145 64Cu-labeled,143, 144 99mTc-N2S2-Tat(49–57)-labeled bombesin142 for diagnostic and therapeutic purposes in prostate cancer. However, it is worth pointing out that the suboptimal biodistribution profiles of some peptide ligands could be a potential hurdle for the application of them in in vivo studies.
The above-mentioned prostate cancer-specific expression pattern and successful use in targeted drug delivery to prostate cancer cells suggests that GRPR possesses most of the merits to become a prominent target molecule in prostate cancer diagnosis and therapy.
2.7 CD147
Cluster of differentiation 147 (CD147), also known as extracellular matrix metalloproteinase inducer (EMMPRIN) or Basigin, is a highly glycosylated type 1 integral transmembrane protein of the immunoglobulin superfamily.146 It consists of a 185-amino-acid extracellular domain with multiple glycosylation sites, a 24-amino-acid transmembrane domain containing a glutamic acid residue that can associate with other proteins, and a 39-amino-acid intracellular domain.147 CD147 is a multifunctional protein important in wound healing, embryo implantation, neuronal signaling, cell differentiation, angiogenesis, cell adhesion, migration, drug resistance and apoptosis. 148-150 In tumor cells, CD147 plays key role in cancer metastasis and angiogenesis. 147 For example, CD147 regulates the expression of matrix metalloproteinases (MMP), which degrades extracellular matrix present around primary tumors, leading to invasion and metastasis of epithelial tumor cells.151, 152
CD147 is weakly expressed in normal tissues but increased remarkably in various malignant tumor tissues,153 including prostate tumors, and facilitates tumor metastasis. 151, 154-156 It is highly expressed on the surface of cancer cells and induces synthesis of various matrix metalloproteinases (MMP-1, MMP-2, MMP-3) 157 by inducing neighboring fibroblasts and tumor cells. 158, 159
In prostate cancer patients, the overexpression of CD147 is directly correlated with the progression of cancer, metastasis and poor prognosis.160, 161 CD147 is overexpressed in 47% to 80% of prostate cancer patients, which is almost 7 times more than in benign prostate hyperplasia (BPH) patients and 9 times higher than normal prostate tissues. 161, 162
CD147 has attracted attention because of its specific overexpression in cancer cells and the role in cancer progression. As a result, anti-CD147 antibodies have been developed. Anti-CD147 antibody-labeled liposomal delivery of a glutathione-doxorubicin conjugate to prostate cancer PC3 cells has been reported. The results showed specific accumulation of the aCD147abliposome in target prostate cancer cells but not in CD147-deficient cells.163 Moreover, the use of monoclonal antibody targeting CD147 for targeted delivery of small molecule drugs to CD147-overexpressing HNSCC (head and neck squamous cell carcinoma) cells showed significantly decreased cellular proliferation and cell viability. 164
The above examples indicate that CD147 may become a good target for targeted drug delivery to prostate cancer cells. However, more studies are required to establish CD147 as a prominent and reliable target for prostate cancer-specific drug delivery systems.
2.8 Epithelial cell-adhesion molecule (EpCAM)
EpCAM is a calcium-independent type I transmembrane protein with a molecular weight of 39-42 kDa. Although the function of EpCAM is still basically unidentified, recent research suggests that EpCAM plays important roles not only in cell adhesion but also in cellular signaling, cell migration, proliferation and differentiation.165, 166 EpCAM shows tumor initiation, proliferation, invasion, metastasis 167 and chemo-/radio sensitivity through PI3K/Akt/mTOR signaling pathway activation in prostate cancer cells.168
EpCAM is expressed abundantly in several solid tumors, such as prostate cancer, breast cancer, liver cancer, lung cancer, pancreatic cancer, and melanoma, and it shows limited expression in normal epithelial tissues. 169, 170 Numerous immunohistochemical studies have shown that the expression of EpCAM is much higher in prostate cancer cells compared to normal cells.169, 171, 172 This overexpression of EpCAM is found in 71-98% of cases,170, 173, 174 and the expression level is many time higher than that of benign 172 or normal prostate tissues.171 Moreover, EpCAM is expressed 76-fold higher in the tumor-associated stroma and 170-fold higher in tumor stroma with Gleason score 4 or 5 compared to normal stroma.175 In tumor cells EpCAM is highly expressed at the apical surface, whereas in normal cells the expression is basolateral.176 EpCAM has also been found on prostate cancer stem cells.177, 178 Taken together, these data provide the evidence that EpCAM is a prominent candidate not only for the detection of circulating and metastasizing prostate cancer cells but also for targeted drug delivery to prostate cancer cells.
A number of research groups have explored the role of EpCAM in targeted drug delivery to cancer cells. An EpCAM-targeting aptamer 179 and an antibody 180 were developed and employed for targeted drug delivery to retinoblastoma.179, 180 In some other approaches, anti-EpCAM antibody-drug conjugate 181 to target pancreatic carcinoma, EpCAM-targeted delivery of nanocomplexed siRNA to target MCF-7 breast cancer cells, and EpCAM scFv-based delivery of siRNA to colon cancer cells were also studied. In all these examples, significant EpCAM-mediated targeted drug delivery to various EpCAM-overexpressing cancer cells was achieved. Cancer immunotherapy strategies employing EpCAM as a target are also well known; adecatumumab, edrecolomab and some other prototype recombinant anti-EpCAM mAbs were developed. Adecatumumab has been tested on prostate cancer patients and reached Phase II trials.182
Although not much research has been done on using EpCAM as a target for drug delivery to prostate cancer, its expression pattern in prostate tumor cells and successful use of EpCAM-specific antibodies, aptamers and scFv for targeted drug delivery to various other EpCAM overexpressing tumors suggests EpCAM as a prominent target for prostate cancer targeted drug delivery.
2.9 Luteinizing hormone-releasing hormone receptor
The luteinizing hormone-releasing hormone receptor (LHRHR), or gonadotropin-releasing hormone receptor (GNRHR), belongs to the seven-transmembrane G protein-coupled receptor (GPCR) family. These receptors are responsible for stimulating the actions of LHRH after its release from the hypothalamus. These receptors are mainly expressed on the surface of pituitary gonadotrope cells but also on some extra-pituitary organs.183 LHRH receptor is expressed in tissues of the reproductive tract, such as ovary, endometrium, prostate and breast, and also the tumors derived from these organs.183
Apart from the pituitary, LHRH receptor is expressed on the plasma membranes of several human cancer cells, including prostate cancer cells,184, 185 and its expression level is much higher compared to normal tissues. 186, 187 Studies using ligand-binding assays and reverse transcriptase-PCR (RT-PCR) showed that 86% of human prostate cancer specimens expressed LHRH receptor.188 In another immunohistochemistry (IHC) study, 95.7% of surgical specimens showed expression of LHRH receptor, with almost 70% samples showing moderate to strong expression.189 In hormone-refractory prostate carcinoma, 100% of specimens showed the expression of mRNA for LHRH receptors. 190, 191 The selective and persistent expression pattern of LHRH receptors in prostate cancer cells provides a rational to use these receptors for targeted drug delivery to prostate cancers.
The use of LHRH agonists and antagonists in prostate cancer therapy is well established. 189 Many research groups have used these molecules for targeted delivery of drugs into cells expressing LHRH receptor. For example, cytotoxic compounds, such as chlorambucil (Chl), melphalan (Mel), and metal complex related to the cytotoxic complexes cisplatin were coupled with an LHRH analogue to increase their cytotoxic activity against LHRH overexpressing cancer cells.192 Others used the similar strategy to conjugate cytotoxic drugs such as anthraquinone and methotrexate with LHRH agonist [D-Lys6] and demonstrated enhanced anti-tumor effect compared to the cytotoxic drugs alone.193 These agents successfully inhibited prostate tumor growth. AN-152 (now called AEZS-108) is one of the best examples of such a strategy. This analogue contains doxorubicin coupled with the LHRH agonist [D-Lys(6)]LHRH. 194 It has shown some promising results, and phase II studies are in progress for their use in castration-resistant prostate cancer.195 Because of promising results in earlier phases, targeted chemotherapy using LHRH-linked analogues, like AEZS-108, is scheduled to enter phase III studies in advanced endometrial tumors that are positive for LHRH receptor.195 In addition, deslorelin-docetaxel analogues were also developed and showed 15-fold higher potency than docetaxel alone at 72 h in LNCaP and androgen-independent PC-3 cell lines. 196
The results on LHRH receptor-targeted agents and their encouraging preclinical data in prostate cancer therapy suggest that it has potential as a viable target, and agents targeting this receptor may provide great benefit to patients with prostate cancer or other LHRH receptor-expressing cancers.
2.10 Heat shock proteins (HSPs)
HSPs are a group of proteins present in almost all living organisms including humans. They were first identified in Drosophila melanogaster in 1962. These proteins are abundant in most cells: they make up about 1-2% of total protein, which increases to 4-6% in stressed conditions such as high temperature, inflammation, change in pH, change in cell environment, the presence of toxins and hypoxia. 197-199 In normal conditions, HSPs are bound with inactive monomers of heat shock transcription factors (HSF) in the cytosol. In stressed conditions, HSPs are stimulated rapidly by dissociating with HSFs.200-202 In humans, these HSPs are divided broadly into two groups depending upon their size: the higher molecular weight HSPs, such as HSP90, HSP70 and HSP60, and small molecular weight HSPs, such as HSP27. The numbers represent their molecular weights.203 In cancer cells, these HSPs are up regulated and show cytoprotective actions through various mechanisms,204-206 helping cancer cells survive.207
HSPs are overexpressed in many cancers, including prostate cancer. The overexpression of these proteins reflects a poor prognosis in terms of survival and response to cancer therapy. Among many other HSPs, HSP70, HSP78 (GRP78) 208 and HSP27 are overexpressed in various cancer cells including breast and prostate cancer cells.203 Higher expression of HSP70 is observed in aggressively malignant prostate cancer cell lines, 209, 210 whereas the expression of HSP27 increases shortly after androgen ablation, and its level and uniformity increase in treatment-resistant prostate cancer. 209, 211-213 One of the studies conducted on prostate cancer patients revealed that 73% of 164 cases showed high Grp78 expression in localized prostate cancer, whereas in castration-resistant prostate cancer 100% of cases showed high Grp78 expression. 214
On the basis of the expression and roles of HSPs in prostate cancer progression, various compounds were found exhibiting significant antitumor activity against prostate cancer through anti-HSP therapy. Some of them are in phase I, II and III trials.209 Anti-GRP78 scFv 215 was also identified and used for delivery and internalization of Quantum dot-conjugate.215 HSP-targeted drug delivery is also an exciting and useful area to explore. A few HSP-70 specific peptide sequences, such as WIFPWIQL 216 and WDLAWMFRLPVG,216, 217 were also identified and used for targeted delivery of cytotoxic agents to cells overexpressing HSPs, including prostate cancer cells. 217-219,216 These peptides were successfully employed as ligand for HPMA copolymer–drug conjugate and efficiently delivered to prostate tumor cells. 217-219
The expression of HSPs in tumor cells and their successful use in targeted delivery of drugs /drug delivery systems suggests that HSPs could become a prominent tool in targeting prostate cancer cells and could improve the efficiency of current drug regimens. But some further studies and validation of their use in targeted drug delivery to prostate cancer cells is still needed.
3 Prostate Cancer Specific Enzymes
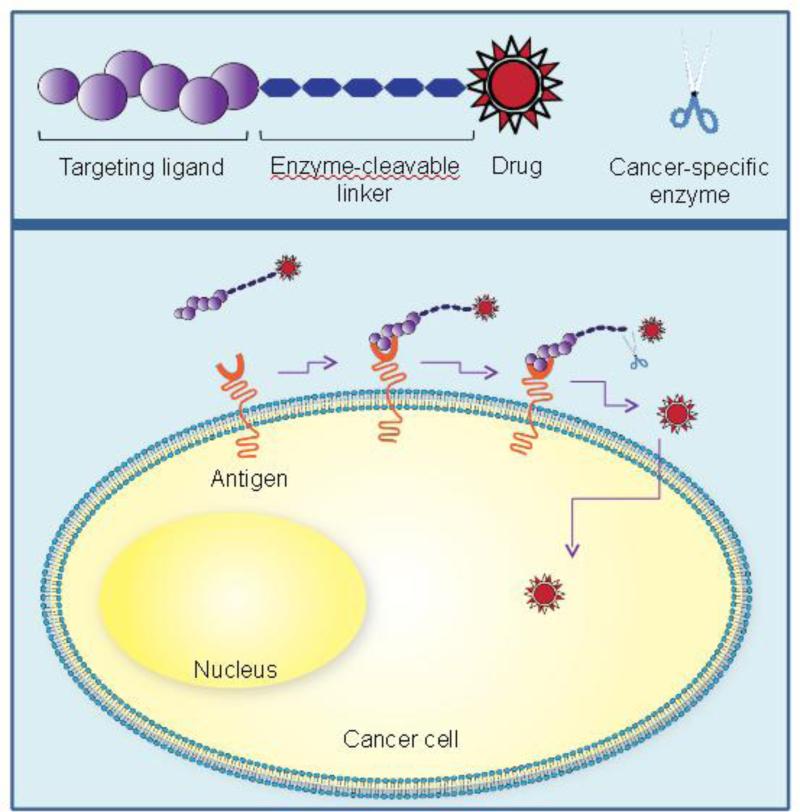
One strategy to achieve tumor-specific accumulation of a drug is to design a stimulus-responsive system that can specifically release the active drug in the tumor microenvironment. Prostate cancer specific enzymes can therefore be utilized for this approach.220 The drug can be linked to its carrier using the substrate of a tumor-specific enzyme (Figure 2). Alternatively, the drug can be encapsulated in a carrier which can be specifically degraded in tumor microenvironment by these enzymes. The advantage of this strategy is that released drug molecules have better penetration efficacy in tumor tissues compared to intact drug delivery systems.
Figure 2.
Prostate cancer-specific enzyme mediated drug release in tumor microenvironment. The prostate cancer-specific ligand delivers the drug conjugate to prostate cancer cells. The prostate cancer-specific enzyme cleaves the substrate and release the parent drug in the tumor microenvironment. The released drug molecules have better penetration efficacy in tumor microenvironment compared to intact drug conjugate.
3.1 Prostate specific antigen (PSA)
Prostate-specific antigen (PSA), a 33k Da single chain glycoprotein, is an androgen-regulated protease that belongs to the glandular Kallikrein family, which is a group of serine proteases.221 PSA is secreted by the normal human prostate epithelium and enters the lumen as a zymogen. In the lumen, seven amino acids from the N-terminus of PSA are cleaved by protease such as human kallikrein 2, leading to activation of PSA.222 PSA is one of the three most abundant proteins in semen and its major function is proteolytic fragmentation of semenogelin I and II, which is responsible for mediating gel formation of semen.223
PSA, either in inactive or active form, can enter the blood stream through basal cells and the basement membrane. In the blood stream, active PSA is bound by protease inhibitors rapidly, while inactive PSA stays in the unbound state. In prostate cancer patients, the total PSA level in the blood stream is significantly increased due to the disruption of the normal prostate gland.224 The higher a patient's serum PSA level, the likelier he is to have prostate cancer. In 1986, FDA approved a PSA test as an evaluation for prostate cancer progression, and in 1994, FDA defined 4.0 ng/ml of PSA in the blood stream to be the upper limit of normal prostate tissue.225 In the past few years, as a consequence, PSA testing has been the most widely employed prostate cancer diagnosis method and biomarker for evaluation of future risk of prostate cancer progression despite its several disadvantages, such as lack of specificity and potential over diagnosis.225
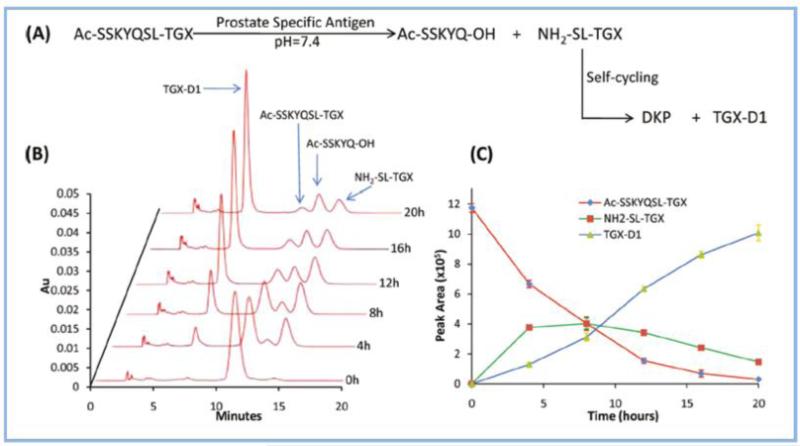
Since active PSA rapidly loses its enzymatic activity after it enters the blood stream by binding to protease inhibitors, the concentration of active PSA in the tumor microenvironment is much higher than that in the circulation. Accordingly, it is possible to design a PSA substrate-drug conjugate as a novel target drug delivery strategy for prostate cancer therapy. Denmeade et al. identified a 7-mer peptide sequence (His-Ser-Ser-Lys-Leu-Gln-Leu) that can be specifically cleaved by PSA.226 Later, the same group designed a prodrug by conjugating this peptide to doxorubicin. They found this doxorubicin-peptide prodrug had much higher cytotoxic effect on PSA-producing prostate cancer cells than PSA-nonproducing prostate cancer cells in vitro.227 This result indicates that the peptide can be cleaved by PSA and the parent drug is released to induce prostate cancer cell apoptosis. Tai et al. employed the same peptide as a PSA substrate and linked this peptide to a HER-2-targeting peptide and a TGX-221 derivative. The peptide-drug conjugate was cleaved by PSA in prostate cancer cells to release the parent drug, inducing prostate cancer cells apoptosis in vitro.78 In this approach, PSA enzymes in prostate cancers recognize a particular peptide sequence (SSKYQ) and cleave it between the residues Gln (Q) and Ser (S) to form the dipeptide drug conjugate (NH2-SL-TGX) which further undergoes a self-cyclization reaction to release the parent drug TGX-D1 in a physiological pH. The in vitro cleavage of the peptide-drug conjugate in the presence of PSA is demonstrated in Figure 3.Similarly, Chandran et al. developed a macromolecular carrier, in which a HPMA-based copolymer was covalently couple to a PSA-activated peptide drug conjugate (HSSKLQL12ADT). The parent drug L12ADT can be cleaved from the copolymer in the presence of PSA. The polymer-drug conjugate not only induce apoptosis of prostate cancer cells in vitro but also releases and accumulates L12ADT in the tumor tissues in animal studies. 228
Figure 3.
PSA-mediated drug release from the peptide drug conjugate (Ac-SSKYQSL-TGX). Ac-SSKYQSL-TGX is cleaved by PSA to release the intermediate NH2-SL-TGX, which underwent self-cyclization to release the parent drug TGX-D1. (A) Illustration of PSA activation of the peptide drug conjugate. (B) HPLC chromatogram monitored at 268 nm. (C) Release profile of TGX-D1 from Ac-SSKYQSL-TGX. (Adapted from reference 78)
Similarly, Defeo-Jones et al. designed a peptide-doxorubicin prodrug by covalently linking of doxorubicin and another PSA-specific peptide Nglutaryl-(4-hydroxyprolyl)Ala-Sercyclohexaglycyl-Gln-Ser-Leu-CO2H. Compared to free doxorubicin, the prodrug significantly reduced cytotoxicity in the cells that do not secrete PSA. Additionly, the peptide-doxorubicin prodrug exhibited much higher antitumor efficacy and less toxicity compared to doxorubicin alone in animal studies.229
In addition to the prodrug approach, a prostate-specific replication-competent adenovirus (CV787) was developed for targeted prostate cancer therapy. The adenovirus vector contains the prostate-specific rat probasin promoter and the human prostate-specific enhancer/promoter. CV787 replicated about 104-105 times more efficiently in PSA-positive cells than PSA-negative cells. Accordingly, CV787 kills PSA-positive prostate cancer cells 10000-fold efficiently than PSA-negative cells, indicating a very high specificity against prostate cancer cells. A single injection of the adenovirus can eliminate xenografted PSA-positive tumors in several weeks.230
3.2 Cathepsin
Cathepsins are overexpressed in various human cancers231. They are a family of endopeptidases that contains more than a dozen members: cathepsins A, B, C, D, E, F, G, H, L, K, O, S, V, and W. Among them, cathepsin B, C, F, H, L, K, O, S, V, W and X are cysteine proteases, while cathepsin A and G are serine carboxy peptidases and cathepsins D and E are aspartic proteases.232, 233 Each member has a different structure, protein substrates, and mechanism of catalysis and therefore plays a different role in proliferation, angiogenesis, and metastasis of tumors.233 All cathepsins are produced as an inactive form, and most of these members can be activated by the low pH condition that is found in lysosomes.234
Cathepsins are expressed on the cell surface and released to the extracellular space. Cathepsins are overexpressed on various cancers, such as breast, lung, colon, liver, gastric, ovarian, and prostate cancer233. Fernandez et al. reported that cathepsin B and cathepsin S are often expressed together in prostate cancer cells.235 Brubaker et al. found that the expression level of cathepsin K, a cysteine protease, in bone metastases is significantly higher than primary prostate cancer. By contrast, there is no expression of cathepsin K in normal prostate tissues.236 Moreover, cathepsin H exhibits higher expression in prostate tumors.237 Because cathepsins are overexpressed in prostate cancers, it is therefore possible to employ some cathepsins as potential targets for prostate cancer-specific drug delivery.
Currently, there are a few prodrug strategies that employ cathepsins as a tumor-specific enzyme. For instance, a cathepsin B-specific tetrapeptide (Gly-Phe-Leu-Gly) was used as a linker to conjugate doxorubicin to a synthetic N-(2-hydroxypropyl)methacrylamide copolymer . The polymer-drug conjugate showed 15-fold longer half-life in the blood stream than free doxorubicin. In animal studies, the polymer-drug conjugate showed significantly higher efficiency than free doxorubicin in inhibiting the growth of MAC15A tumors, which overexpress cathepsin B. On the contrary, the enhanced activity of the polymer-drug conjugate was not observed in MAC26 tumors. This is in accordance with the fact that the expression level and enzyme activity of cathepsin B in MAC15A is higher than MAC26, and the released doxorubicin in MAC15A tumors is 7-fold greater than MAC26 tumors.238 This study clearly demonstrates the importance of releasing parent drug in tumors.
Although there is no report on similar drug delivery systems targeting cathepsins for prostate cancer therapy, cathepsins are believed to be overexpressed on prostate cancer cells, so targeting cathepsins could be considered a potential strategy for prostate cancer treatment in the future.
3.3 Matrix metalloproteinases
Matrix metalloproteinases (MMPs) are a family of zinc-dependent proteases with over twenty distinct members in humans239. Each member shares a similar catalytic domain and contains different substrate domains240. MMPs can degrade the components of extracellular matrix and play important roles in cell growth, proliferation and apoptosis.241 There are four subgroups of MMPs: the collagenases include MMP-1, -8 and -13, and their major function is to degrade interstitial collagens; the gelatinases include MMP-2 and -9, and they can degrade collagens that are located in basal membranes; the stromelysins include MMP-3, -10, and -11, and they can degrade proteoglycans; and the matrilysins include MMP-14, -15, -16, -17, -23, -24, and -25, and they can degrade proteins in ECM.241, 242 Endogenous tissue inhibitors of metalloproteinases (TIMPs) regulate the proteolytic activities of MMPs.243 Because some MMPs, such as MMP-1, 2, 7 and 9, play important roles in the development of prostate cancer,239 TIMPs have been employed as agents for inhibition of prostate tumor progression and metastases. Moreover, some synthetic inhibitors of MMPs are under research for prostate cancer therapy.
MMPs are overexpressed in prostate cancers cells, and their expression levels are correlated with the progression of tumors.244, 245 On the other hand, the expression level of TIMPs is complex; both decreasing and increasing of TIMP expression have been reported in prostate cancer. 246, 247 Brehmer et al. reported that palpable tumors expressed a significantly higher level of MMP-2 but less MMP-9 than nonpalpable tumors. TIMP-1 is expressed significantly less in malignant epithelium. This change of expression level leads to imbalance of the ratio of MMPs and TIMPs, which is frequently found in prostate cancer tissues.244 Wood et al. also found that the expression levels of MMP-2 and MMP-9 are relatively low in normal and low-Gleason-score tissues, whereas significantly increase in higher Gleason sum tissues.245
Because MMPs are overexpressed on various tumors, it is possible to employ MMPs as a tumor-specific enzyme to trigger the release of active agents in prostate tumor tissues. One strategy is to design a prodrug or a target delivery system in which a MMP substrate peptide is used as the cleavable linker between the active agent and its cargo. This novel delivery system only allows the release of parent drug at tumor sites so that it can achieve targeted therapy without inducing toxicity in other tissues. In the past decade, several novel delivery systems have been developed by employing MMP-cleavable peptides for treatment of various cancers that overexpress MMPs. For example, Terada et al. developed galactosylated liposomes containing the Gly-Pro-Leu-Gly-Ile-Ala-Gly-Gln peptide, which is a MMP-2 substrate, for hepatocellular carcinoma therapy.248 Similarly, Gu et al. employed MMP-2/9 cleavable small molecular weight protamine (ALMWP) to modify PEG-co-PCL nanoparticles and developed a novel delivery system for targeted glioblastoma therapy.249 Although currently there is no similar research related to prostate cancer therapy, MMP substrate could be used in prostate cancer-specific drug delivery because MMPs are overexpressed in prostate tumors.
4. Prostate Cancer Endothelium-associated Antigens
4.1 αvβ3 integrin receptor
The integrins are heterodimers that consist of two types I transmembrane subunits: α and β. There are 18 α and 8 β subunits that make up 24 different integrins250. The integrins can recognize and bind to major adhesive components, which are located in extracellular matrix. Although different integrins consists of different α and β subunits, but most integrins bind to the same or overlapping ligands. 250 The major function of integrins is to regulate the adhesion between cells or the attachment between cells and their environments. Besides, integrins play important roles in the regulation of cell differentiation, progression, proliferation and apoptosis251. The integrins also regulate the activity of cancer cells, including prostate cancer. They play important roles in tumor invasion and metastasis.252
Prostate cancer cells express abnormal amount of integrins and are surrounded by aberrant extracellular matrix (ECM).253 Some integrins are downregulated, while others are upregulated. In prostate cancer, among α subunits, α3, α4, α5, α7, and αv are downregulated, while αIIb is upregulated. Among β subunits, β1C and β4 are downregulated, while β1, β3, and β6 are upregulated.253 Integrins αvβ3 is widely expressed on tumor-associated new blood vessels but not on vessels in normal tissues. Integrins αvβ3 is also overexpressed on the surface of various cancer cells, including breast, pancreatic, and prostate cancer.254 Zheng et al. reported that αvβ3 is overexpressed on PC-3 cells, which are highly invasive prostate cancer cells. On the contrary, it is not expressed on noninvasive LNCaP cells.255 The integrin αvβ3 promotes prostate cancer metastasis to the bone.
Cyclic Arg-Gly-Asp Peptides (RGD) are naturally present in ECM and can specifically bind to eight integrins, including αvβ3.254 Therefore, it is widely used as a αvβ3-targeting ligand in various delivery systems for prostate cancer therapy and imaging. Nora et al. employed the cyclic pentapeptide c(RGDfK) as a ligand for their PLGA-PEG nanoparticles. This novel delivery system successfully delivered the therapeutic agent cisplatin to prostate tumors and enhanced its antitumor activity in animal studies.256 Similarly, Danhier et al. used RGD-grafted PLGA-nanoparticles as a novel system to deliver paclitaxel to prostate cancer tissues. They reported that this novel delivery system showed better therapeutic effect in vivo compared to paclitaxel with non-targeted nanoparticles.257 In another study, a peptide heterodimer containing RGD and bombesin analog for dual-receptor targeting was conjugated to (18)F as an imaging agent. The dual integrin αvβ3 and GRPR targeting agent exhibited higher tumor-targeting efficacy compared to (18)F-labeled RGD or (18)F-labeled bembesin analog.258
4.2 Epidermal growth factor-like 7 (Egfl7)
Epidermal growth factor-like 7 (Egfl7), also known as vascular endothelial statin (VE-statin), is a protein secreted by endothelial cells, and its expression is restricted to actively remodeling vascular endothelium.259-261 The expression of Egfl7 in tumors is deregulated and promotes tumor progression by inhibiting the expression of endothelial molecules that mediate immune cell infiltration. Egfl7 also plays a key role in the process of blood vessel formation, but the exact mechanism is still not clear.262, 263 Analysis of 211 human breast cancer specimens shows that Egfl7 is overexpressed in breast cancer cells. Particularly, the expression of Egfl7 is dramatically higher in invasive ductal carcinoma.264 A recent study investigated the Egfl7 expression in normal human tissues and ten different tumors including prostate cancer. The results shows significant higher expression of Egfl7 in prostate cancer cells compared to normal prostate tissues.265 Moreover, as a non-endothelial tissue, prostate is naturally deficient in Egfl7 expression.259 As a result, Egfl7 may be a potential marker for diagnosis and targeted therapeutics in prostate cancer.
5. Conclusions
This review summarizes various prostate cancer-specific antigens and enzymes (Figure 1) that could be exploited for prostate cancer targeted drug delivery. To be eligible for prostate cancer-specific delivery, these antigens and enzymes should have either unique or higher expression level in the tumor compared to other organs. Ideally, the expression level of the antigens or enzymes is correlated with tumor progression, thus leading to more specific delivery to advanced prostate cancer cells. Some of the antigens and enzymes, such as PSMA, PSA, PSCA have been extensively used for prostate cancer diagnosis, imaging, and therapeutics (Table 1, 2, 3, and 4). Other markers, such as HER-2, MUCIN1, uPAR, GRPR, CD147, EpCAM, LHRH, and HSP (Table 3 and 4) have been widely used for targeted delivery to a variety of cancers, but not extensively exploited in prostate cancer drug delivery. However, these markers may also become prominent targets for prostate cancer therapeutics because of their overexpression in prostate cancer cells.
Table 3.
Imaging agents targeting prostate cancer-relevant antigens
| Antigen/Receptor | Targeting Agents | Reference |
|---|---|---|
| PSCA | Single chain mono clonal antibofy(scab PSCA) | 138 |
| uPAR | (18)F-AlF-NOTA-AE105 | 119 |
| GRPR | 64Cu-labeled NOTA-Bn-SCN-Aoc-bombesin analogue | 139 |
| 64Cu-labeled SarAr-bombesin analogs | 140 | |
| (99m)Tc-N2S2-Tat(49-57)-bombesin | 142 | |
| (18)F-labeled bombesin analog, (18)F-BAY 86-4367 | 141 |
Table 4.
Therapeutic agents targeting prostate cancer-relevant antigens
| Antigen/Receptor | Targeting moiety | Drug/cytotoxic agent | Delivery system | Reference |
|---|---|---|---|---|
| PSCA | Single chain PSCA antibody scAb-PSCA | Dual docetaxel and super paramagnetic iron oxide-loaded nanoparticles | Nanoparticles | 266 |
| GRPR | Bombesin | Methotrexate | Conjugate | 267 |
| Bombesin | Photosensitizer (Sulfonated aluminum phthalocyanines) | Conjugate | 135 | |
| Bombesin peptide antagonists | Chemotherapeutic agents | Liposome | 268 | |
| HER2 | HER2 targeting peptide | TGX221 | Prodrug | 78 |
| Mucin 1 | ** MUC1 specific lipopeptide | Immunotherapeutic | Peptide vaccine | 103, 269 |
| PSA | PSA-cleavable peptide | Doxorubicin | Prodrug | 227 |
| PSA-cleavable peptide | TGX-221D1 | Prodrug | 78 | |
| PSA-cleavable peptide | L12ADT | Polymer-drug conjugate | 228 |
*= Phase 1
phase 2
Imaging agents can be linked directly to the ligands of these antigens for a better sensitivity and accuracy. Therapeutic agents can either be linked to these ligands or encapsulated in carriers that are modified with these ligands to improve their efficacy and minimize toxicity in other normal tissues. There are several prostate cancer specific enzymes, such as PSA, Cathepsin, and MMP can be utilized to design enzyme-cleavable drug conjugates (Figure 2) or carriers as a stimulus-responsive system.
Taken together, tremendous progress has been made in the past two decades to exploit cancer specific antigens and enzymes for targeted delivery to various cancers including prostate cancer. Successful prostate cancer drug targeting is, however, very complicated. Researchers need to select the best targeting strategy based on the property and pharmacological mechanism of each individual drug. Moreover, dual-receptor targeting may provide better specificity than mono-targeting. Another major hurdle in the successful application of these targeting ligands is the transition from exciting in vitro studies to successful in vivo studies. While many of the targeting ligands exhibit specific and high affinity to their antigens in vitro, the in vivo conditions are more complicated and the presence of a great variety of cells, proteins and other molecules in the circulation may comprise the binding affinity of the targeting ligands. The targeting ligands may need to be modified to achieve the optimal biodistribution profile and targeting efficacy in vivo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Mabjeesh NJ, Zhong H, Simons JW. Gene therapy of prostate cancer: current and future directions. Endocr Relat Cancer. 2002;9(2):115–39. doi: 10.1677/erc.0.0090115. [DOI] [PubMed] [Google Scholar]
- 2.Moul JW, Wu H, Sun L, McLeod DG, Amling C, Donahue T, Kusuda L, Sexton W, O'Reilly K, Hernandez J, Chung A, Soderdahl D. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol. 2004;171(3):1141–7. doi: 10.1097/01.ju.0000113794.34810.d0. [DOI] [PubMed] [Google Scholar]
- 3.Moul JW. The evolving definition of advanced prostate cancer. Rev Urol. 2004;6(Suppl 8):S10–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Ananias HJ, van den Heuvel MC, Helfrich W, de Jong IJ. Expression of the gastrin-releasing peptide receptor, the prostate stem cell antigen and the prostate-specific membrane antigen in lymph node and bone metastases of prostate cancer. Prostate. 2009;69(10):1101–8. doi: 10.1002/pros.20957. [DOI] [PubMed] [Google Scholar]
- 5.Davis MI, Bennett MJ, Thomas LM, Bjorkman PJ. Crystal structure of prostate-specific membrane antigen, a tumor marker and peptidase. Proc Natl Acad Sci U S A. 2005;102(17):5981–6. doi: 10.1073/pnas.0502101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesters JR, Barinka C, Li W, Tsukamoto T, Majer P, Slusher BS, Konvalinka J, Hilgenfeld R. Structure of glutamate carboxypeptidase II, a drug target in neuronal damage and prostate cancer. EMBO J. 2006;25(6):1375–84. doi: 10.1038/sj.emboj.7600969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang QL, Yao MY. [Updated application of prostate-specific membrane antigen to the diagnosis and treatment of prostate cancer]. Zhonghua Nan Ke Xue. 2008;14(1):79–82. [PubMed] [Google Scholar]
- 8.Bukrinsky JT, Bjerrum MJ, Kadziola A. Native carboxypeptidase A in a new crystal environment reveals a different conformation of the important tyrosine 248. Biochemistry. 1998;37(47):16555–64. doi: 10.1021/bi981678i. [DOI] [PubMed] [Google Scholar]
- 9.Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82(11):2256–61. doi: 10.1002/(sici)1097-0142(19980601)82:11<2256::aid-cncr22>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Wright GL, Jr., Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. 1995;1(1):18–28. doi: 10.1016/1078-1439(95)00002-y. [DOI] [PubMed] [Google Scholar]
- 11.Israeli RS, Powell CT, Corr JG, Fair WR, Heston WD. Expression of the prostate-specific membrane antigen. Cancer Res. 1994;54(7):1807–11. [PubMed] [Google Scholar]
- 12.Wright GL, Jr., Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, Troyer J, Konchuba A, Schellhammer PF, Moriarty R. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48(2):326–34. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 13.Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52(4):637–40. doi: 10.1016/s0090-4295(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 14.Troyer JK, Beckett ML, Wright GL., Jr. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int J Cancer. 1995;62(5):552–8. doi: 10.1002/ijc.2910620511. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91(3):528–39. doi: 10.1002/jcb.10661. [DOI] [PubMed] [Google Scholar]
- 16.Sokoloff RL, Norton KC, Gasior CL, Marker KM, Grauer LS. A dual-monoclonal sandwich assay for prostate-specific membrane antigen: levels in tissues, seminal fluid and urine. Prostate. 2000;43(2):150–7. doi: 10.1002/(sici)1097-0045(20000501)43:2<150::aid-pros10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Nanus DM, Milowsky MI, Kostakoglu L, Smith-Jones PM, Vallabahajosula S, Goldsmith SJ, Bander NH. Clinical use of monoclonal antibody HuJ591 therapy: targeting prostate specific membrane antigen. J Urol. 2003;170(6 Pt 2):S84–8. doi: 10.1097/01.ju.0000095151.97404.7c. discussion S88-9. [DOI] [PubMed] [Google Scholar]
- 18.Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59(13):3192–8. [PubMed] [Google Scholar]
- 19.Ross JS, Sheehan CE, Fisher HA, Kaufman RP, Jr., Kaur P, Gray K, Webb I, Gray GS, Mosher R, Kallakury BV. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9(17):6357–62. [PubMed] [Google Scholar]
- 20.Elsasser-Beile U, Buhler P, Wolf P. Targeted therapies for prostate cancer against the prostate specific membrane antigen. Curr Drug Targets. 2009;10(2):118–25. doi: 10.2174/138945009787354601. [DOI] [PubMed] [Google Scholar]
- 21.Thomas TP, Patri AK, Myc A, Myaing MT, Ye JY, Norris TB, Baker JR., Jr. In vitro targeting of synthesized antibody-conjugated dendrimer nanoparticles. Biomacromolecules. 2004;5(6):2269–74. doi: 10.1021/bm049704h. [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal S, Singh P, Topaloglu O, Isaacs JT, Denmeade SR. A dimeric peptide that binds selectively to prostate-specific membrane antigen and inhibits its enzymatic activity. Cancer Res. 2006;66(18):9171–7. doi: 10.1158/0008-5472.CAN-06-1520. [DOI] [PubMed] [Google Scholar]
- 23.Qin B, Tai W, Shukla RS, Cheng K. Identification of a LNCaP-specific binding peptide using phage display. Pharm Res. 2011;28(10):2422–34. doi: 10.1007/s11095-011-0469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu W, Siddiqui IA, Nihal M, Pilla S, Rosenthal K, Mukhtar H, Gong S. Aptamer-conjugated and doxorubicin-loaded unimolecular micelles for targeted therapy of prostate cancer. Biomaterials. 2013;34(21):5244–53. doi: 10.1016/j.biomaterials.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith-Jones PM, Vallabhajosula S, Navarro V, Bastidas D, Goldsmith SJ, Bander NH. Radiolabeled monoclonal antibodies specific to the extracellular domain of prostate-specific membrane antigen: preclinical studies in nude mice bearing LNCaP human prostate tumor. J Nucl Med. 2003;44(4):610–7. [PubMed] [Google Scholar]
- 26.Chang SS. Overview of prostate-specific membrane antigen. Rev Urol. 2004;6(Suppl 10):S13–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Baum V, Buhler P, Gierschner D, Herchenbach D, Fiala GJ, Schamel WW, Wolf P, Elsasser-Beile U. Antitumor activities of PSMAxCD3 diabodies by redirected T-cell lysis of prostate cancer cells. Immunotherapy. 2013;5(1):27–38. doi: 10.2217/imt.12.136. [DOI] [PubMed] [Google Scholar]
- 28.Buhler P, Wolf P, Gierschner D, Schaber I, Katzenwadel A, Schultze-Seemann W, Wetterauer U, Tacke M, Swamy M, Schamel WW, Elsasser-Beile U. A bispecific diabody directed against prostate-specific membrane antigen and CD3 induces T-cell mediated lysis of prostate cancer cells. Cancer Immunol Immunother. 2008;57(1):43–52. doi: 10.1007/s00262-007-0348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durso RJ, Andjelic S, Gardner JP, Margitich DJ, Donovan GP, Arrigale RR, Wang X, Maughan MF, Talarico TL, Olmsted RA, Heston WD, Maddon PJ, Olson WC. A novel alphavirus vaccine encoding prostate-specific membrane antigen elicits potent cellular and humoral immune responses. Clin Cancer Res. 2007;13(13):3999–4008. doi: 10.1158/1078-0432.CCR-06-2202. [DOI] [PubMed] [Google Scholar]
- 30.Kahn D, Williams RD, Manyak MJ, Haseman MK, Seldin DW, Libertino JA, Maguire RT. 111Indium-capromab pendetide in the evaluation of patients with residual or recurrent prostate cancer after radical prostatectomy. The ProstaScint Study Group. J Urol. 1998;159(6):2041–6. doi: 10.1016/S0022-5347(01)63239-7. [DOI] [PubMed] [Google Scholar]
- 31.Kahn D, Williams RD, Seldin DW, Libertino JA, Hirschhorn M, Dreicer R, Weiner GJ, Bushnell D, Gulfo J. Radioimmunoscintigraphy with 111indium labeled CYT-356 for the detection of occult prostate cancer recurrence. J Urol. 1994;152(5 Pt 1):1490–5. doi: 10.1016/s0022-5347(17)32453-9. [DOI] [PubMed] [Google Scholar]
- 32.Deb N, Goris M, Trisler K, Fowler S, Saal J, Ning S, Becker M, Marquez C, Knox S. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin Cancer Res. 1996;2(8):1289–97. [PubMed] [Google Scholar]
- 33.Kahn D, Austin JC, Maguire RT, Miller SJ, Gerstbrein J, Williams RD. A phase II study of [90Y] yttrium-capromab pendetide in the treatment of men with prostate cancer recurrence following radical prostatectomy. Cancer Biother Radiopharm. 1999;14(2):99–111. doi: 10.1089/cbr.1999.14.99. [DOI] [PubMed] [Google Scholar]
- 34.Elsasser-Beile U, Reischl G, Wiehr S, Buhler P, Wolf P, Alt K, Shively J, Judenhofer MS, Machulla HJ, Pichler BJ. PET imaging of prostate cancer xenografts with a highly specific antibody against the prostate-specific membrane antigen. J Nucl Med. 2009;50(4):606–11. doi: 10.2967/jnumed.108.058487. [DOI] [PubMed] [Google Scholar]
- 35.Wolf P, Alt K, Buhler P, Katzenwadel A, Wetterauer U, Tacke M, Elsasser-Beile U. Anti-PSMA immunotoxin as novel treatment for prostate cancer? High and specific antitumor activity on human prostate xenograft tumors in SCID mice. Prostate. 2008;68(2):129–38. doi: 10.1002/pros.20684. [DOI] [PubMed] [Google Scholar]
- 36.Behe M, Alt K, Deininger F, Buhler P, Wetterauer U, Weber WA, Elsasser-Beile U, Wolf P. In vivo testing of 177Lu-labelled anti-PSMA antibody as a new radioimmunotherapeutic agent against prostate cancer. In Vivo. 25(1):55–9. [PubMed] [Google Scholar]
- 37.McDevitt MR, Barendswaard E, Ma D, Lai L, Curcio MJ, Sgouros G, Ballangrud AM, Yang WH, Finn RD, Pellegrini V, Geerlings MW, Jr., Lee M, Brechbiel MW, Bander NH, Cordon-Cardo C, Scheinberg DA. An alpha-particle emitting antibody ([213Bi]J591) for radioimmunotherapy of prostate cancer. Cancer Res. 2000;60(21):6095–100. [PubMed] [Google Scholar]
- 38.Vallabhajosula S, Smith-Jones PM, Navarro V, Goldsmith SJ, Bander NH. Radioimmunotherapy of prostate cancer in human xenografts using monoclonal antibodies specific to prostate specific membrane antigen (PSMA): studies in nude mice. Prostate. 2004;58(2):145–55. doi: 10.1002/pros.10281. [DOI] [PubMed] [Google Scholar]
- 39.Kularatne SA, Wang K, Santhapuram HK, Low PS. Prostate-specific membrane antigen targeted imaging and therapy of prostate cancer using a PSMA inhibitor as a homing ligand. Mol Pharm. 2009;6(3):780–9. doi: 10.1021/mp900069d. [DOI] [PubMed] [Google Scholar]
- 40.Henry MD, Wen S, Silva MD, Chandra S, Milton M, Worland PJ. A prostate-specific membrane antigen-targeted monoclonal antibody-chemotherapeutic conjugate designed for the treatment of prostate cancer. Cancer Res. 2004;64(21):7995–8001. doi: 10.1158/0008-5472.CAN-04-1722. [DOI] [PubMed] [Google Scholar]
- 41.Kuroda K, Liu H, Kim S, Guo M, Navarro V, Bander NH. Saporin toxin-conjugated monoclonal antibody targeting prostate-specific membrane antigen has potent anticancer activity. Prostate. 70(12):1286–94. doi: 10.1002/pros.21164. [DOI] [PubMed] [Google Scholar]
- 42.Wolf P, Elsasser-Beile U. Pseudomonas exotoxin A: from virulence factor to anti-cancer agent. Int J Med Microbiol. 2009;299(3):161–76. doi: 10.1016/j.ijmm.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Wolf P, Gierschner D, Buhler P, Wetterauer U, Elsasser-Beile U. A recombinant PSMA-specific single-chain immunotoxin has potent and selective toxicity against prostate cancer cells. Cancer Immunol Immunother. 2006;55(11):1367–73. doi: 10.1007/s00262-006-0131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kularatne SA, Venkatesh C, Santhapuram HK, Wang K, Vaitilingam B, Henne WA, Low PS. Synthesis and biological analysis of prostate-specific membrane antigen-targeted anticancer prodrugs. J Med Chem. 53(21):7767–77. doi: 10.1021/jm100729b. [DOI] [PubMed] [Google Scholar]
- 45.Kim E, Jung Y, Choi H, Yang J, Suh JS, Huh YM, Kim K, Haam S. Prostate cancer cell death produced by the co-delivery of Bcl-xL shRNA and doxorubicin using an aptamerconjugated polyplex. Biomaterials. 31(16):4592–9. doi: 10.1016/j.biomaterials.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 46.Ma D, Hopf CE, Malewicz AD, Donovan GP, Senter PD, Goeckeler WF, Maddon PJ, Olson WC. Potent antitumor activity of an auristatin-conjugated, fully human monoclonal antibody to prostate-specific membrane antigen. Clin Cancer Res. 2006;12(8):2591–6. doi: 10.1158/1078-0432.CCR-05-2107. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y, Duan S, Zeng X, Liu C, Davies NM, Li B, Forrest ML. Prodrug strategy for PSMA-targeted delivery of TGX-221 to prostate cancer cells. Mol Pharm. 9(6):1705–16. doi: 10.1021/mp3000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanna V, Pintus G, Roggio AM, Punzoni S, Posadino AM, Arca A, Marceddu S, Bandiera P, Uzzau S, Sechi M. Targeted biocompatible nanoparticles for the delivery of (−)-epigallocatechin 3-gallate to prostate cancer cells. J Med Chem. 2011;54(5):1321–32. doi: 10.1021/jm1013715. [DOI] [PubMed] [Google Scholar]
- 49.Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc Natl Acad Sci U S A. 2011;108(5):1850–5. doi: 10.1073/pnas.1011379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc Natl Acad Sci U S A. 2008;105(45):17356–61. doi: 10.1073/pnas.0809154105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lam JS, Yamashiro J, Shintaku IP, Vessella RL, Jenkins RB, Horvath S, Said JW, Reiter RE. Prostate stem cell antigen is overexpressed in prostate cancer metastases. Clin Cancer Res. 2005;11(7):2591–6. doi: 10.1158/1078-0432.CCR-04-1842. [DOI] [PubMed] [Google Scholar]
- 52.Tang DG, Patrawala L, Calhoun T, Bhatia B, Choy G, Schneider-Broussard R, Jeter C. Prostate cancer stem/progenitor cells: identification, characterization, and implications. Mol Carcinog. 2007;46(1):1–14. doi: 10.1002/mc.20255. [DOI] [PubMed] [Google Scholar]
- 53.Antica M, Wu L, Scollay R. Stem cell antigen 2 expression in adult and developing mice. Immunol Lett. 1997;55(1):47–51. doi: 10.1016/s0165-2478(96)02682-x. [DOI] [PubMed] [Google Scholar]
- 54.Classon BJ, Coverdale L. Mouse stem cell antigen Sca-2 is a member of the Ly-6 family of cell surface proteins. Proc Natl Acad Sci U S A. 1994;91(12):5296–300. doi: 10.1073/pnas.91.12.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raff AB, Gray A, Kast WM. Prostate stem cell antigen: a prospective therapeutic and diagnostic target. Cancer Lett. 2009;277(2):126–32. doi: 10.1016/j.canlet.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reiter RE, Gu Z, Watabe T, Thomas G, Szigeti K, Davis E, Wahl M, Nisitani S, Yamashiro J, Le Beau MM, Loda M, Witte ON. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci U S A. 1998;95(4):1735–40. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taeb J, Asgari M, Abolhasani M, Farajollahi MM, Madjd Z. Expression of prostate stem cell antigen (PSCA) in prostate cancer: a tissue microarray study of Iranian patients. Pathol Res Pract. 2014;210(1):18–23. doi: 10.1016/j.prp.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 58.Zhigang Z, Wenlv S. Prostate stem cell antigen (PSCA) expression in human prostate cancer tissues: implications for prostate carcinogenesis and progression of prostate cancer. Jpn J Clin Oncol. 2004;34(7):414–9. doi: 10.1093/jjco/hyh073. [DOI] [PubMed] [Google Scholar]
- 59.Gu Z, Thomas G, Yamashiro J, Shintaku IP, Dorey F, Raitano A, Witte ON, Said JW, Loda M, Reiter RE. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene. 2000;19(10):1288–96. doi: 10.1038/sj.onc.1203426. [DOI] [PubMed] [Google Scholar]
- 60.Gao X, Luo Y, Wang Y, Pang J, Liao C, Lu H, Fang Y. Prostate stem cell antigen-targeted nanoparticles with dual functional properties: in vivo imaging and cancer chemotherapy. Int J Nanomedicine. 2012;7:4037–51. doi: 10.2147/IJN.S32804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ling Y, Wei K, Luo Y, Gao X, Zhong S. Dual docetaxel/superparamagnetic iron oxide loaded nanoparticles for both targeting magnetic resonance imaging and cancer therapy. Biomaterials. 32(29):7139–50. doi: 10.1016/j.biomaterials.2011.05.089. [DOI] [PubMed] [Google Scholar]
- 62.Zhou J, Huang L, Wang W, Pang J, Zou Y, Shuai X, Gao X. Prostate cancer targeted MRI nanoprobe based on superparamagnetic iron oxide and copolymer of poly(ethylene glycol) and polyethyleneimin. Chinese Science Bulletin. 2009;54(18):3137–3146. [Google Scholar]
- 63.Li Y, Cozzi PJ, Russell PJ. Promising tumor-associated antigens for future prostate cancer therapy. Med Res Rev. 30(1):67–101. doi: 10.1002/med.20165. [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Hernandez Mde L, Gray A, Hubby B, Klinger OJ, Kast WM. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res. 2008;68(3):861–9. doi: 10.1158/0008-5472.CAN-07-0445. [DOI] [PubMed] [Google Scholar]
- 65.Thomas-Kaskel AK, Zeiser R, Jochim R, Robbel C, Schultze-Seemann W, Waller CF, Veelken H. Vaccination of advanced prostate cancer patients with PSCA and PSA peptide-loaded dendritic cells induces DTH responses that correlate with superior overall survival. Int J Cancer. 2006;119(10):2428–34. doi: 10.1002/ijc.22097. [DOI] [PubMed] [Google Scholar]
- 66.Tai W, Mahato R, Cheng K. The role of HER2 in cancer therapy and targeted drug delivery. J Control Release. 2010;146(3):264–75. doi: 10.1016/j.jconrel.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malmberg J, Perols A, Varasteh Z, Altai M, Braun A, Sandstrom M, Garske U, Tolmachev V, Orlova A, Karlstrom AE. Comparative evaluation of synthetic anti-HER2 Affibody molecules site-specifically labelled with 111In using N-terminal DOTA, NOTA and NODAGA chelators in mice bearing prostate cancer xenografts. Eur J Nucl Med Mol Imaging. 2012;39(3):481–92. doi: 10.1007/s00259-011-1992-9. [DOI] [PubMed] [Google Scholar]
- 68.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 69.Gioeli D, Ficarro SB, Kwiek JJ, Aaronson D, Hancock M, Catling AD, White FM, Christian RE, Settlage RE, Shabanowitz J, Hunt DF, Weber MJ. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J Biol Chem. 2002;277(32):29304–14. doi: 10.1074/jbc.M204131200. [DOI] [PubMed] [Google Scholar]
- 70.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5(3):280–5. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 71.Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54(20):5474–8. [PubMed] [Google Scholar]
- 72.Minner S, Jessen B, Stiedenroth L, Burandt E, Kollermann J, Mirlacher M, Erbersdobler A, Eichelberg C, Fisch M, Brummendorf TH, Bokemeyer C, Simon R, Steuber T, Graefen M, Huland H, Sauter G, Schlomm T. Low level HER2 overexpression is associated with rapid tumor cell proliferation and poor prognosis in prostate cancer. Clin Cancer Res. 2010;16(5):1553–60. doi: 10.1158/1078-0432.CCR-09-2546. [DOI] [PubMed] [Google Scholar]
- 73.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23(32):8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 74.So A, Gleave M, Hurtado-Col A, Nelson C. Mechanisms of the development of androgen independence in prostate cancer. World J Urol. 2005;23(1):1–9. doi: 10.1007/s00345-004-0473-1. [DOI] [PubMed] [Google Scholar]
- 75.Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, Balk S, Thomas G, Kaplan I, Hlatky L, Hahnfeldt P, Kantoff P, Loda M. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst. 2000;92(23):1918–25. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 76.Ziada A, Barqawi A, Glode LM, Varella-Garcia M, Crighton F, Majeski S, Rosenblum M, Kane M, Chen L, Crawford ED. The use of trastuzumab in the treatment of hormone refractory prostate cancer; phase II trial. Prostate. 2004;60(4):332–7. doi: 10.1002/pros.20065. [DOI] [PubMed] [Google Scholar]
- 77.Agus DB, Scher HI, Higgins B, Fox WD, Heller G, Fazzari M, Cordon-Cardo C, Golde DW. Response of prostate cancer to anti-Her-2/neu antibody in androgen-dependent and -independent human xenograft models. Cancer Res. 1999;59(19):4761–4. [PubMed] [Google Scholar]
- 78.Tai W, Shukla RS, Qin B, Li B, Cheng K. Development of a peptide-drug conjugate for prostate cancer therapy. Mol Pharm. 2011;8(3):901–12. doi: 10.1021/mp200007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shadidi M, Sioud M. Identification of novel carrier peptides for the specific delivery of therapeutics into cancer cells. FASEB J. 2003;17(2):256–8. doi: 10.1096/fj.02-0280fje. [DOI] [PubMed] [Google Scholar]
- 80.Liu Z, Duan JH, Song YM, Ma J, Wang FD, Lu X, Yang XD. Novel HER2 aptamer selectively delivers cytotoxic drug to HER2-positive breast cancer cells in vitro. J Transl Med. 2012;10:148. doi: 10.1186/1479-5876-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao J, Chen K, Miao Z, Ren G, Chen X, Gambhir SS, Cheng Z. Affibody-based nanoprobes for HER2-expressing cell and tumor imaging. Biomaterials. 2011;32(8):2141–8. doi: 10.1016/j.biomaterials.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alexis F, Basto P, Levy-Nissenbaum E, Radovic-Moreno AF, Zhang L, Pridgen E, Wang AZ, Marein SL, Westerhof K, Molnar LK, Farokhzad OC. HER-2-targeted nanoparticle-affibody bioconjugates for cancer therapy. ChemMedChem. 2008;3(12):1839–43. doi: 10.1002/cmdc.200800122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia. 2001;6(3):339–53. doi: 10.1023/a:1011379725811. [DOI] [PubMed] [Google Scholar]
- 84.Yonezawa S, Higashi M, Yamada N, Yokoyama S, Kitamoto S, Kitajima S, Goto M. Mucins in human neoplasms: clinical pathology, gene expression and diagnostic application. Pathol Int. 61(12):697–716. doi: 10.1111/j.1440-1827.2011.02734.x. [DOI] [PubMed] [Google Scholar]
- 85.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9(12):874–85. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4(1):45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 87.Li Y, Bharti A, Chen D, Gong J, Kufe D. Interaction of glycogen synthase kinase 3beta with the DF3/MUC1 carcinoma-associated antigen and beta-catenin. Mol Cell Biol. 1998;18(12):7216–24. doi: 10.1128/mcb.18.12.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamamoto M, Bharti A, Li Y, Kufe D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. J Biol Chem. 1997;272(19):12492–4. doi: 10.1074/jbc.272.19.12492. [DOI] [PubMed] [Google Scholar]
- 89.Pandey P, Kharbanda S, Kufe D. Association of the DF3/MUC1 breast cancer antigen with Grb2 and the Sos/Ras exchange protein. Cancer Res. 1995;55(18):4000–3. [PubMed] [Google Scholar]
- 90.Brayman M, Thathiah A, Carson DD. MUC1: a multifunctional cell surface component of reproductive tissue epithelia. Reprod Biol Endocrinol. 2004;2:4. doi: 10.1186/1477-7827-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995;57:607–34. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- 92.Singh R, Bandyopadhyay D. MUC1: a target molecule for cancer therapy. Cancer Biol Ther. 2007;6(4):481–6. doi: 10.4161/cbt.6.4.4201. [DOI] [PubMed] [Google Scholar]
- 93.Joshi MD, Ahmad R, Yin L, Raina D, Rajabi H, Bubley G, Kharbanda S, Kufe D. MUC1 oncoprotein is a druggable target in human prostate cancer cells. Mol Cancer Ther. 2009;8(11):3056–65. doi: 10.1158/1535-7163.MCT-09-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cozzi PJ, Wang J, Delprado W, Perkins AC, Allen BJ, Russell PJ, Li Y. MUC1, MUC2, MUC4, MUC5AC and MUC6 expression in the progression of prostate cancer. Clin Exp Metastasis. 2005;22(7):565–73. doi: 10.1007/s10585-005-5376-z. [DOI] [PubMed] [Google Scholar]
- 95.Kirschenbaum A, Itzkowitz SH, Wang JP, Yao S, Eliashvili M, Levine AC. MUC1 Expression in Prostate Carcinoma: Correlation with Grade and Stage. Mol Urol. 1999;3(3):163–168. [PubMed] [Google Scholar]
- 96.Arai T, Fujita K, Fujime M, Irimura T. Expression of sialylated MUC1 in prostate cancer: relationship to clinical stage and prognosis. Int J Urol. 2005;12(7):654–61. doi: 10.1111/j.1442-2042.2005.01112.x. [DOI] [PubMed] [Google Scholar]
- 97.Singh AP, Chauhan SC, Bafna S, Johansson SL, Smith LM, Moniaux N, Lin MF, Batra SK. Aberrant expression of transmembrane mucins, MUC1 and MUC4, in human prostate carcinomas. Prostate. 2006;66(4):421–9. doi: 10.1002/pros.20372. [DOI] [PubMed] [Google Scholar]
- 98.van Bracht E, Stolle S, Hafmans TG, Boerman OC, Oosterwijk E, van Kuppevelt TH, Daamen WF. Specific targeting of tumor cells by lyophilisomes functionalized with antibodies. Eur J Pharm Biopharm. 2014 doi: 10.1016/j.ejpb.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 99.Henderikx P, Kandilogiannaki M, Petrarca C, von Mensdorff-Pouilly S, Hilgers JH, Krambovitis E, Arends JW, Hoogenboom HR. Human single-chain Fv antibodies to MUC1 core peptide selected from phage display libraries recognize unique epitopes and predominantly bind adenocarcinoma. Cancer Res. 1998;58(19):4324–32. [PubMed] [Google Scholar]
- 100.Hu Y, Duan J, Zhan Q, Wang F, Lu X, Yang XD. Novel MUC1 aptamer selectively delivers cytotoxic agent to cancer cells in vitro. PLoS One. 2012;7(2):e31970. doi: 10.1371/journal.pone.0031970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu C, Hu Y, Duan J, Yuan W, Wang C, Xu H, Yang XD. Novel aptamer-nanoparticle bioconjugates enhances delivery of anticancer drug to MUC1-positive cancer cells in vitro. PLoS One. 2011;6(9):e24077. doi: 10.1371/journal.pone.0024077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tan L, Neoh KG, Kang ET, Choe WS, Su X. PEGylated anti-MUC1 aptamerdoxorubicin complex for targeted drug delivery to MCF7 breast cancer cells. Macromol Biosci. 11(10):1331–5. doi: 10.1002/mabi.201100173. [DOI] [PubMed] [Google Scholar]
- 103.Sangha R, North S. L-BLP25: a MUC1-targeted peptide vaccine therapy in prostate cancer. Expert Opin Biol Ther. 2007;7(11):1723–30. doi: 10.1517/14712598.7.11.1723. [DOI] [PubMed] [Google Scholar]
- 104.North S, Butts C. Vaccination with BLP25 liposome vaccine to treat non-small cell lung and prostate cancers. Expert Rev Vaccines. 2005;4(3):249–57. doi: 10.1586/14760584.4.3.249. [DOI] [PubMed] [Google Scholar]
- 105.Nalla AK, Gorantla B, Gondi CS, Lakka SS, Rao JS. Targeting MMP-9, uPAR, and cathepsin B inhibits invasion, migration and activates apoptosis in prostate cancer cells. Cancer Gene Ther. 2010;17(9):599–613. doi: 10.1038/cgt.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang M, Lowik DW, Miller AD, Thanou M. Targeting the urokinase plasminogen activator receptor with synthetic self-assembly nanoparticles. Bioconjug Chem. 2009;20(1):32–40. doi: 10.1021/bc8001908. [DOI] [PubMed] [Google Scholar]
- 107.Andreasen PA, Sottrup-Jensen L, Kjoller L, Nykjaer A, Moestrup SK, Petersen CM, Gliemann J. Receptor-mediated endocytosis of plasminogen activators and activator/inhibitor complexes. FEBS Lett. 1994;338(3):239–45. doi: 10.1016/0014-5793(94)80276-9. [DOI] [PubMed] [Google Scholar]
- 108.Myohanen H, Vaheri A. Regulation and interactions in the activation of cell-associated plasminogen. Cell Mol Life Sci. 2004;61(22):2840–58. doi: 10.1007/s00018-004-4230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dano K, Behrendt N, Hoyer-Hansen G, Johnsen M, Lund LR, Ploug M, Romer J. Plasminogen activation and cancer. Thromb Haemost. 2005;93(4):676–81. doi: 10.1160/TH05-01-0054. [DOI] [PubMed] [Google Scholar]
- 110.Behrendt N. The urokinase receptor (uPAR) and the uPAR-associated protein (uPARAP/Endo180): membrane proteins engaged in matrix turnover during tissue remodeling. Biol Chem. 2004;385(2):103–36. doi: 10.1515/BC.2004.031. [DOI] [PubMed] [Google Scholar]
- 111.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3(12):932–43. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 112.Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci. 2000;57(1):25–40. doi: 10.1007/s000180050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pulukuri SM, Gondi CS, Lakka SS, Jutla A, Estes N, Gujrati M, Rao JS. RNA interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival, and tumorigenicity in vivo. J Biol Chem. 2005;280(43):36529–40. doi: 10.1074/jbc.M503111200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH. Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev. 2008;34(2):122–36. doi: 10.1016/j.ctrv.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 115.Gavrilov D, Kenzior O, Evans M, Calaluce R, Folk WR. Expression of urokinase plasminogen activator and receptor in conjunction with the ets family and AP-1 complex transcription factors in high grade prostate cancers. Eur J Cancer. 2001;37(8):1033–40. doi: 10.1016/s0959-8049(01)00077-6. [DOI] [PubMed] [Google Scholar]
- 116.Cozzi PJ, Wang J, Delprado W, Madigan MC, Fairy S, Russell PJ, Li Y. Evaluation of urokinase plasminogen activator and its receptor in different grades of human prostate cancer. Hum Pathol. 2006;37(11):1442–51. doi: 10.1016/j.humpath.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 117.Li Y, Cozzi PJ. Targeting uPA/uPAR in prostate cancer. Cancer Treat Rev. 2007;33(6):521–7. doi: 10.1016/j.ctrv.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 118.Yang L, Sajja HK, Cao Z, Qian W, Bender L, Marcus AI, Lipowska M, Wood WC, Wang YA. uPAR-targeted optical imaging contrasts as theranostic agents for tumor margin detection. Theranostics. 2013;4(1):106–18. doi: 10.7150/thno.7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Persson M, Liu H, Madsen J, Cheng Z, Kjaer A. First (18)F-labeled ligand for PET imaging of uPAR: in vivo studies in human prostate cancer xenografts. Nucl Med Biol. 2013;40(5):618–24. doi: 10.1016/j.nucmedbio.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Persson M, Madsen J, Ostergaard S, Jensen MM, Jorgensen JT, Juhl K, Lehmann C, Ploug M, Kjaer A. Quantitative PET of human urokinase-type plasminogen activator receptor with 64Cu-DOTA-AE105: implications for visualizing cancer invasion. J Nucl Med. 2012;53(1):138–45. doi: 10.2967/jnumed.110.083386. [DOI] [PubMed] [Google Scholar]
- 121.Ploug M, Ostergaard S, Gardsvoll H, Kovalski K, Holst-Hansen C, Holm A, Ossowski L, Dano K. Peptide-derived antagonists of the urokinase receptor. affinity maturation by combinatorial chemistry, identification of functional epitopes, and inhibitory effect on cancer cell intravasation. Biochemistry. 2001;40(40):12157–68. doi: 10.1021/bi010662g. [DOI] [PubMed] [Google Scholar]
- 122.Rabbani SA, Ateeq B, Arakelian A, Valentino ML, Shaw DE, Dauffenbach LM, Kerfoot CA, Mazar AP. An anti-urokinase plasminogen activator receptor antibody (ATN-658) blocks prostate cancer invasion, migration, growth, and experimental skeletal metastasis in vitro and in vivo. Neoplasia. 2010;12(10):778–88. doi: 10.1593/neo.10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Van Buren G, 2nd, Gray MJ, Dallas NA, Xia L, Lim SJ, Fan F, Mazar AP, Ellis LM. Targeting the urokinase plasminogen activator receptor with a monoclonal antibody impairs the growth of human colorectal cancer in the liver. Cancer. 2009;115(14):3360–8. doi: 10.1002/cncr.24371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Persson M, Rasmussen P, Madsen J, Ploug M, Kjaer A. New peptide receptor radionuclide therapy of invasive cancer cells: in vivo studies using 177Lu-DOTA-AE105 targeting uPAR in human colorectal cancer xenografts. Nucl Med Biol. 2012;39(7):962–9. doi: 10.1016/j.nucmedbio.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 125.Abdalla MO, Karna P, Sajja HK, Mao H, Yates C, Turner T, Aneja R. Enhanced noscapine delivery using uPAR-targeted optical-MR imaging trackable nanoparticles for prostate cancer therapy. J Control Release. 149(3):314–22. doi: 10.1016/j.jconrel.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res. 1999;59(5):1152–9. [PubMed] [Google Scholar]
- 127.Hohla F, Schally AV. Targeting gastrin releasing peptide receptors: New options for the therapy and diagnosis of cancer. Cell Cycle. 2010;9(9):1738–41. doi: 10.4161/cc.9.9.11347. [DOI] [PubMed] [Google Scholar]
- 128.Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev. 2008;60(1):1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cornelio DB, Roesler R, Schwartsmann G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann Oncol. 2007;18(9):1457–66. doi: 10.1093/annonc/mdm058. [DOI] [PubMed] [Google Scholar]
- 130.Korner M, Waser B, Rehmann R, Reubi JC. Early over-expression of GRP receptors in prostatic carcinogenesis. Prostate. 2014;74(2):217–24. doi: 10.1002/pros.22743. [DOI] [PubMed] [Google Scholar]
- 131.Reubi JC, Wenger S, Schmuckli-Maurer J, Schaer JC, Gugger M. Bombesin receptor subtypes in human cancers: detection with the universal radioligand (125)I-[D-TYR(6), beta-ALA(11), PHE(13), NLE(14)] bombesin(6-14). Clin Cancer Res. 2002;8(4):1139–46. [PubMed] [Google Scholar]
- 132.Sun B, Halmos G, Schally AV, Wang X, Martinez M. Presence of receptors for bombesin/gastrin-releasing peptide and mRNA for three receptor subtypes in human prostate cancers. Prostate. 2000;42(4):295–303. doi: 10.1002/(sici)1097-0045(20000301)42:4<295::aid-pros7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 133.de Visser M, van Weerden WM, de Ridder CM, Reneman S, Melis M, Krenning EP, de Jong M. Androgen-dependent expression of the gastrin-releasing peptide receptor in human prostate tumor xenografts. J Nucl Med. 2007;48(1):88–93. [PubMed] [Google Scholar]
- 134.Schroeder RP, van Weerden WM, Bangma C, Krenning EP, de Jong M. Peptide receptor imaging of prostate cancer with radiolabelled bombesin analogues. Methods. 2009;48(2):200–4. doi: 10.1016/j.ymeth.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 135.Dubuc C, Langlois R, Benard F, Cauchon N, Klarskov K, Tone P, van Lier JE. Targeting gastrin-releasing peptide receptors of prostate cancer cells for photodynamic therapy with a phthalocyanine-bombesin conjugate. Bioorg Med Chem Lett. 2008;18(7):2424–7. doi: 10.1016/j.bmcl.2008.02.051. [DOI] [PubMed] [Google Scholar]
- 136.Dumont RA, Tamma M, Braun F, Borkowski S, Reubi JC, Maecke H, Weber WA, Mansi R. Targeted radiotherapy of prostate cancer with a gastrin-releasing Peptide receptor antagonist is effective as monotherapy and in combination with rapamycin. J Nucl Med. 54(5):762–9. doi: 10.2967/jnumed.112.112169. [DOI] [PubMed] [Google Scholar]
- 137.Okarvi SM, Al Jammaz I. Synthesis and evaluation of a technetium-99m labeled cytotoxic bombesin peptide conjugate for targeting bombesin receptor-expressing tumors. Nucl Med Biol. 37(3):277–88. doi: 10.1016/j.nucmedbio.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 138.Accardo A, Salsano G, Morisco A, Aurilio M, Parisi A, Maione F, Cicala C, Tesauro D, Aloj L, De Rosa G, Morelli G. Peptide-modified liposomes for selective targeting of bombesin receptors overexpressed by cancer cells: a potential theranostic agent. Int J Nanomedicine. 7:2007–17. doi: 10.2147/IJN.S29242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Craft JM, De Silva RA, Lears KA, Andrews R, Liang K, Achilefu S, Rogers BE. In vitro and in vivo evaluation of a 64Cu-labeled NOTA-Bn-SCN-Aoc-bombesin analogue in gastrin-releasing peptide receptor expressing prostate cancer. Nucl Med Biol. 2012;39(5):609–16. doi: 10.1016/j.nucmedbio.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lears KA, Ferdani R, Liang K, Zheleznyak A, Andrews R, Sherman CD, Achilefu S, Anderson CJ, Rogers BE. In vitro and in vivo evaluation of 64Cu-labeled SarAr-bombesin analogs in gastrin-releasing peptide receptor-expressing prostate cancer. J Nucl Med. 2011;52(3):470–7. doi: 10.2967/jnumed.110.082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Honer M, Mu L, Stellfeld T, Graham K, Martic M, Fischer CR, Lehmann L, Schubiger PA, Ametamey SM, Dinkelborg L, Srinivasan A, Borkowski S. 18F-labeled bombesin analog for specific and effective targeting of prostate tumors expressing gastrin-releasing peptide receptors. J Nucl Med. 2011;52(2):270–8. doi: 10.2967/jnumed.110.081620. [DOI] [PubMed] [Google Scholar]
- 142.Santos-Cuevas CL, Ferro-Flores G, Arteaga de Murphy C, Ramirez Fde M, Luna-Gutierrez MA, Pedraza-Lopez M, Garcia-Becerra R, Ordaz-Rosado D. Design, preparation, in vitro and in vivo evaluation of (99m)Tc-N2S2-Tat(49-57)-bombesin: a target-specific hybrid radiopharmaceutical. Int J Pharm. 2009;375(1-2):75–83. doi: 10.1016/j.ijpharm.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 143.Garrison JC, Rold TL, Sieckman GL, Figueroa SD, Volkert WA, Jurisson SS, Hoffman TJ. In vivo evaluation and small-animal PET/CT of a prostate cancer mouse model using 64Cu bombesin analogs: side-by-side comparison of the CB-TE2A and DOTA chelation systems. J Nucl Med. 2007;48(8):1327–37. doi: 10.2967/jnumed.107.039487. [DOI] [PubMed] [Google Scholar]
- 144.Yang YS, Zhang X, Xiong Z, Chen X. Comparative in vitro and in vivo evaluation of two 64Cu-labeled bombesin analogs in a mouse model of human prostate adenocarcinoma. Nucl Med Biol. 2006;33(3):371–80. doi: 10.1016/j.nucmedbio.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 145.Honer M, Mu L, Stellfeld T, Graham K, Martic M, Fischer CR, Lehmann L, Schubiger PA, Ametamey SM, Dinkelborg L, Srinivasan A, Borkowski S. 18F-labeled bombesin analog for specific and effective targeting of prostate tumors expressing gastrin-releasing peptide receptors. J Nucl Med. 52(2):270–8. doi: 10.2967/jnumed.110.081620. [DOI] [PubMed] [Google Scholar]
- 146.Weidle UH, Scheuer W, Eggle D, Klostermann S, Stockinger H. Cancer-related issues of CD147. Cancer Genomics Proteomics. 2010;7(3):157–69. [PubMed] [Google Scholar]
- 147.Nabeshima K, Iwasaki H, Koga K, Hojo H, Suzumiya J, Kikuchi M. Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol Int. 2006;56(7):359–67. doi: 10.1111/j.1440-1827.2006.01972.x. [DOI] [PubMed] [Google Scholar]
- 148.Hao J, Madigan MC, Khatri A, Power CA, Hung TT, Beretov J, Chang L, Xiao W, Cozzi PJ, Graham PH, Kearsley JH, Li Y. In vitro and in vivo prostate cancer metastasis and chemoresistance can be modulated by expression of either CD44 or CD147. PLoS One. 2012;7(8):e40716. doi: 10.1371/journal.pone.0040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Naor D, Wallach-Dayan SB, Zahalka MA, Sionov RV. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol. 2008;18(4):260–7. doi: 10.1016/j.semcancer.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 150.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4(1):33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 151.Zhong WD, Han ZD, He HC, Bi XC, Dai QS, Zhu G, Ye YK, Liang YX, Qin WJ, Zhang Z, Zeng GH, Chen ZN. CD147, MMP-1, MMP-2 and MMP-9 protein expression as significant prognostic factors in human prostate cancer. Oncology. 2008;75(3-4):230–6. doi: 10.1159/000163852. [DOI] [PubMed] [Google Scholar]
- 152.Sun J, Hemler ME. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 2001;61(5):2276–81. [PubMed] [Google Scholar]
- 153.Riethdorf S, Reimers N, Assmann V, Kornfeld JW, Terracciano L, Sauter G, Pantel K. High incidence of EMMPRIN expression in human tumors. Int J Cancer. 2006;119(8):1800–10. doi: 10.1002/ijc.22062. [DOI] [PubMed] [Google Scholar]
- 154.Tsuchiya N, Narita S, Kumazawa T, Inoue T, Ma Z, Tsuruta H, Saito M, Horikawa Y, Yuasa T, Satoh S, Ogawa O, Habuchi T. Clinical significance of a single nucleotide polymorphism and allelic imbalance of matrix metalloproteinase-1 promoter region in prostate cancer. Oncol Rep. 2009;22(3):493–9. doi: 10.3892/or_00000462. [DOI] [PubMed] [Google Scholar]
- 155.Millimaggi D, Mari M, D'Ascenzo S, Carosa E, Jannini EA, Zucker S, Carta G, Pavan A, Dolo V. Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia. 2007;9(4):349–57. doi: 10.1593/neo.07133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sidhu SS, Mengistab AT, Tauscher AN, LaVail J, Basbaum C. The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene. 2004;23(4):956–63. doi: 10.1038/sj.onc.1207070. [DOI] [PubMed] [Google Scholar]
- 157.Gabison EE, Hoang-Xuan T, Mauviel A, Menashi S. EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie. 2005;87(3-4):361–8. doi: 10.1016/j.biochi.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 158.Zhu H, Zhao J, Zhu B, Collazo J, Gal J, Shi P, Liu L, Strom AL, Lu X, McCann RO, Toborek M, Kyprianou N. EMMPRIN regulates cytoskeleton reorganization and cell adhesion in prostate cancer. Prostate. 72(1):72–81. doi: 10.1002/pros.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Muramatsu T, Miyauchi T. Basigin (CD147): a multifunctional transmembrane protein involved in reproduction, neural function, inflammation and tumor invasion. Histol Histopathol. 2003;18(3):981–7. doi: 10.14670/HH-18.981. [DOI] [PubMed] [Google Scholar]
- 160.Hao JL, Cozzi PJ, Khatri A, Power CA, Li Y. CD147/EMMPRIN and CD44 are potential therapeutic targets for metastatic prostate cancer. Curr Cancer Drug Targets. 10(3):287–306. doi: 10.2174/156800910791190193. [DOI] [PubMed] [Google Scholar]
- 161.Han ZD, Bi XC, Qin WJ, He HC, Dai QS, Zou J, Ye YK, Liang YX, Zeng GH, Chen ZN, Zhong WD. CD147 expression indicates unfavourable prognosis in prostate cancer. Pathol Oncol Res. 2009;15(3):369–74. doi: 10.1007/s12253-008-9131-z. [DOI] [PubMed] [Google Scholar]
- 162.Zhong WD, Liang YX, Lin SX, Li L, He HC, Bi XC, Han ZD, Dai QS, Ye YK, Chen QB, Wang YS, Zeng GH, Zhu G, Zhang Z, Chen ZN, Wu CL. Expression of CD147 is associated with prostate cancer progression. Int J Cancer. 2012;130(2):300–8. doi: 10.1002/ijc.25982. [DOI] [PubMed] [Google Scholar]
- 163.Matsudaira H, Asakura T, Aoki K, Searashi Y, Matsuura T, Nakajima H, Tajiri H, Ohkawa K. Target chemotherapy of anti-CD147 antibody-labeled liposome encapsulated GSHDXR conjugate on CD147 highly expressed carcinoma cells. Int J Oncol. 36(1):77–83. [PubMed] [Google Scholar]
- 164.Sweeny L, Hartman YE, Zinn KR, Prudent JR, Marshall DJ, Shekhani MS, Rosenthal EL. A novel extracellular drug conjugate significantly inhibits head and neck squamous cell carcinoma. Oral Oncol. 2013;49(10):991–7. doi: 10.1016/j.oraloncology.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, Kieu C, Papior P, Baeuerle PA, Munz M, Gires O. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol. 2009;11(2):162–71. doi: 10.1038/ncb1824. [DOI] [PubMed] [Google Scholar]
- 166.Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171(2):386–95. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Patriarca C, Macchi RM, Marschner AK, Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: a short review. Cancer Treat Rev. 2012;38(1):68–75. doi: 10.1016/j.ctrv.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 168.Ni J, Cozzi P, Hao J, Beretov J, Chang L, Duan W, Shigdar S, Delprado W, Graham P, Bucci J, Kearsley J, Li Y. Epithelial cell adhesion molecule (EpCAM) is associated with prostate cancer metastasis and chemo/radioresistance via the PI3K/Akt/mTOR signaling pathway. Int J Biochem Cell Biol. 2013;45(12):2736–48. doi: 10.1016/j.biocel.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 169.Went P, Vasei M, Bubendorf L, Terracciano L, Tornillo L, Riede U, Kononen J, Simon R, Sauter G, Baeuerle PA. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br J Cancer. 2006;94(1):128–35. doi: 10.1038/sj.bjc.6602924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, Dirnhofer S. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35(1):122–8. doi: 10.1016/j.humpath.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 171.Ni J, Cozzi PJ, Duan W, Shigdar S, Graham PH, John KH, Li Y. Role of the EpCAM (CD326) in prostate cancer metastasis and progression. Cancer Metastasis Rev. 31(3-4):779–91. doi: 10.1007/s10555-012-9389-1. [DOI] [PubMed] [Google Scholar]
- 172.Benko G, Spajic B, Kruslin B, Tomas D. Impact of the EpCAM expression on biochemical recurrence-free survival in clinically localized prostate cancer. Urol Oncol. 31(4):468–74. doi: 10.1016/j.urolonc.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 173.Datta-Mannan A, Witcher DR, Tang Y, Watkins J, Wroblewski VJ. Monoclonal antibody clearance. Impact of modulating the interaction of IgG with the neonatal Fc receptor. J Biol Chem. 2007;282(3):1709–17. doi: 10.1074/jbc.M607161200. [DOI] [PubMed] [Google Scholar]
- 174.Rao CG, Chianese D, Doyle GV, Miller MC, Russell T, Sanders RA, Jr., Terstappen LW. Expression of epithelial cell adhesion molecule in carcinoma cells present in blood and primary and metastatic tumors. Int J Oncol. 2005;27(1):49–57. [PubMed] [Google Scholar]
- 175.Mukherjee S, Richardson AM, Rodriguez-Canales J, Ylaya K, Erickson HS, Player A, Kawasaki ES, Pinto PA, Choyke PL, Merino MJ, Albert PS, Chuaqui RF, Emmert-Buck MR. Identification of EpCAM as a molecular target of prostate cancer stroma. Am J Pathol. 2009;175(6):2277–87. doi: 10.2353/ajpath.2009.090013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mitra M, Kandalam M, Rangasamy J, Shankar B, Maheswari UK, Swaminathan S, Krishnakumar S. Novel epithelial cell adhesion molecule antibody conjugated polyethyleneimine-capped gold nanoparticles for enhanced and targeted small interfering RNA delivery to retinoblastoma cells. Mol Vis. 2013;19:1029–38. [PMC free article] [PubMed] [Google Scholar]
- 177.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L, Tang DG. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25(12):1696–708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 178.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 179.Subramanian N, Raghunathan V, Kanwar JR, Kanwar RK, Elchuri SV, Khetan V, Krishnakumar S. Target-specific delivery of doxorubicin to retinoblastoma using epithelial cell adhesion molecule aptamer. Mol Vis. 2012;18:2783–95. [PMC free article] [PubMed] [Google Scholar]
- 180.Mitra M, Misra R, Harilal A, Sahoo SK, Krishnakumar S. Enhanced in vitro antiproliferative effects of EpCAM antibody-functionalized paclitaxel-loaded PLGA nanoparticles in retinoblastoma cells. Mol Vis. 2011;17:2724–37. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 181.Moldenhauer G, Salnikov AV, Luttgau S, Herr I, Anderl J, Faulstich H. Therapeutic potential of amanitin-conjugated anti-epithelial cell adhesion molecule monoclonal antibody against pancreatic carcinoma. J Natl Cancer Inst. 2012;104(8):622–34. doi: 10.1093/jnci/djs140. [DOI] [PubMed] [Google Scholar]
- 182.Marschner N, Ruttinger D, Zugmaier G, Nemere G, Lehmann J, Obrist P, Baeuerle PA, Wolf A, Schmidt M, Abrahamsson PA, Reinhardt C, Heidenreich A. Phase II study of the human anti-epithelial cell adhesion molecule antibody adecatumumab in prostate cancer patients with increasing serum levels of prostate-specific antigen after radical prostatectomy. Urol Int. 2010;85(4):386–95. doi: 10.1159/000318055. [DOI] [PubMed] [Google Scholar]
- 183.Aguilar-Rojas A, Huerta-Reyes M. Human gonadotropin-releasing hormone receptor-activated cellular functions and signaling pathways in extra-pituitary tissues and cancer cells (Review). Oncol Rep. 2009;22(5):981–90. doi: 10.3892/or_00000525. [DOI] [PubMed] [Google Scholar]
- 184.Schally AV, Nagy A. New approaches to treatment of various cancers based on cytotoxic analogs of LHRH, somatostatin and bombesin. Life Sci. 2003;72(21):2305–20. doi: 10.1016/s0024-3205(03)00113-9. [DOI] [PubMed] [Google Scholar]
- 185.Limonta P, Moretti RM, Marelli MM, Dondi D, Parenti M, Motta M. The luteinizing hormone-releasing hormone receptor in human prostate cancer cells: messenger ribonucleic acid expression, molecular size, and signal transduction pathway. Endocrinology. 1999;140(11):5250–6. doi: 10.1210/endo.140.11.7087. [DOI] [PubMed] [Google Scholar]
- 186.Tolkach Y, Joniau S, Van Poppel H. Luteinizing hormone-releasing hormone (LHRH) receptor agonists vs antagonists: a matter of the receptors? BJU Int. 2013;111(7):1021–30. doi: 10.1111/j.1464-410X.2013.11796.x. [DOI] [PubMed] [Google Scholar]
- 187.Tieva A, Stattin P, Wikstrom P, Bergh A, Damber JE. Gonadotropin-releasing hormone receptor expression in the human prostate. Prostate. 2001;47(4):276–84. doi: 10.1002/pros.1072. [DOI] [PubMed] [Google Scholar]
- 188.Halmos G, Arencibia JM, Schally AV, Davis R, Bostwick DG. High incidence of receptors for luteinizing hormone-releasing hormone (LHRH) and LHRH receptor gene expression in human prostate cancers. J Urol. 2000;163(2):623–9. [PubMed] [Google Scholar]
- 189.Liu SV, Liu S, Pinski J. Luteinizing hormone-releasing hormone receptor targeted agents for prostate cancer. Expert Opin Investig Drugs. 2011;20(6):769–78. doi: 10.1517/13543784.2011.574611. [DOI] [PubMed] [Google Scholar]
- 190.Nagy A, Schally AV. Targeting of cytotoxic luteinizing hormone-releasing hormone analogs to breast, ovarian, endometrial, and prostate cancers. Biol Reprod. 2005;73(5):851–9. doi: 10.1095/biolreprod.105.043489. [DOI] [PubMed] [Google Scholar]
- 191.Straub B, Muller M, Krause H, Schrader M, Goessl C, Heicappell R, Miller K. Increased incidence of luteinizing hormone-releasing hormone receptor gene messenger RNA expression in hormone-refractory human prostate cancers. Clin Cancer Res. 2001;7(8):2340–3. [PubMed] [Google Scholar]
- 192.Bajusz S, Janaky T, Csernus VJ, Bokser L, Fekete M, Srkalovic G, Redding TW, Schally AV. Highly potent analogues of luteinizing hormone-releasing hormone containing D-phenylalanine nitrogen mustard in position 6. Proc Natl Acad Sci U S A. 1989;86(16):6318–22. doi: 10.1073/pnas.86.16.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pinski J, Schally AV, Yano T, Szepeshazi K, Halmos G, Groot K, Comaru-Schally AM, Radulovic S, Nagy A. Inhibition of growth of experimental prostate cancer in rats by LH-RH analogs linked to cytotoxic radicals. Prostate. 1993;23(2):165–78. doi: 10.1002/pros.2990230209. [DOI] [PubMed] [Google Scholar]
- 194.Letsch M, Schally AV, Szepeshazi K, Halmos G, Nagy A. Preclinical evaluation of targeted cytotoxic luteinizing hormone-releasing hormone analogue AN-152 in androgen-sensitive and insensitive prostate cancers. Clin Cancer Res. 2003;9(12):4505–13. [PubMed] [Google Scholar]
- 195.Engel J, Emons G, Pinski J, Schally AV. AEZS-108 : a targeted cytotoxic analog of LHRH for the treatment of cancers positive for LHRH receptors. Expert Opin Investig Drugs. 2012;21(6):891–9. doi: 10.1517/13543784.2012.685128. [DOI] [PubMed] [Google Scholar]
- 196.Sundaram S, Durairaj C, Kadam R, Kompella UB. Luteinizing hormone-releasing hormone receptor-targeted deslorelin-docetaxel conjugate enhances efficacy of docetaxel in prostate cancer therapy. Mol Cancer Ther. 2009;8(6):1655–65. doi: 10.1158/1535-7163.MCT-08-0988. [DOI] [PubMed] [Google Scholar]
- 197.Nahleh Z, Tfayli A, Najm A, El Sayed A, Nahle Z. Heat shock proteins in cancer: targeting the ‘chaperones’. Future Med Chem. 2012;4(7):927–35. doi: 10.4155/fmc.12.50. [DOI] [PubMed] [Google Scholar]
- 198.Kim LS, Kim JH. Heat shock protein as molecular targets for breast cancer therapeutics. J Breast Cancer. 2011;14(3):167–74. doi: 10.4048/jbc.2011.14.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5(10):761–72. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 200.Tutar L, Tutar Y. Heat shock proteins; an overview. Curr Pharm Biotechnol. 11(2):216–22. doi: 10.2174/138920110790909632. [DOI] [PubMed] [Google Scholar]
- 201.Walter S, Buchner J. Molecular chaperones--cellular machines for protein folding. Angew Chem Int Ed Engl. 2002;41(7):1098–113. doi: 10.1002/1521-3773(20020402)41:7<1098::aid-anie1098>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 202.Mathew A, Morimoto RI. Role of the heat-shock response in the life and death of proteins. Ann N Y Acad Sci. 1998;851:99–111. doi: 10.1111/j.1749-6632.1998.tb08982.x. [DOI] [PubMed] [Google Scholar]
- 203.Hessenkemper W, Baniahmad A. Targeting heat shock proteins in prostate cancer. Curr Med Chem. 2013;20(22):2731–40. doi: 10.2174/0929867311320220001. [DOI] [PubMed] [Google Scholar]
- 204.Parcellier A, Gurbuxani S, Schmitt E, Solary E, Garrido C. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem Biophys Res Commun. 2003;304(3):505–12. doi: 10.1016/s0006-291x(03)00623-5. [DOI] [PubMed] [Google Scholar]
- 205.Concannon CG, Orrenius S, Samali A. Hsp27 inhibits cytochrome c-mediated caspase activation by sequestering both pro-caspase-3 and cytochrome c. Gene Expr. 2001;9(4-5):195–201. doi: 10.3727/000000001783992605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schneider GB, Hamano H, Cooper LF. In vivo evaluation of hsp27 as an inhibitor of actin polymerization: hsp27 limits actin stress fiber and focal adhesion formation after heat shock. J Cell Physiol. 1998;177(4):575–84. doi: 10.1002/(SICI)1097-4652(199812)177:4<575::AID-JCP8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 207.Jego G, Hazoume A, Seigneuric R, Garrido C. Targeting heat shock proteins in cancer. Cancer Lett. 2013;332(2):275–85. doi: 10.1016/j.canlet.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 208.Hendershot LM, Valentine VA, Lee AS, Morris SW, Shapiro DN. Localization of the gene encoding human BiP/GRP78, the endoplasmic reticulum cognate of the HSP70 family, to chromosome 9q34. Genomics. 1994;20(2):281–4. doi: 10.1006/geno.1994.1166. [DOI] [PubMed] [Google Scholar]
- 209.Sarkars R, Mukherjee S, Roy M. Targeting heat shock proteins by phenethyl isothiocyanate results in cell-cycle arrest and apoptosis of human breast cancer cells. Nutr Cancer. 2013;65(3):480–93. doi: 10.1080/01635581.2013.767366. [DOI] [PubMed] [Google Scholar]
- 210.Tang D, Khaleque MA, Jones EL, Theriault JR, Li C, Wong WH, Stevenson MA, Calderwood SK. Expression of heat shock proteins and heat shock protein messenger ribonucleic acid in human prostate carcinoma in vitro and in tumors in vivo. Cell Stress Chaperones. 2005;10(1):46–58. doi: 10.1379/CSC-44R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rocchi P, Beraldi E, Ettinger S, Fazli L, Vessella RL, Nelson C, Gleave M. Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis. Cancer Res. 2005;65(23):11083–93. doi: 10.1158/0008-5472.CAN-05-1840. [DOI] [PubMed] [Google Scholar]
- 212.Rocchi P, So A, Kojima S, Signaevsky M, Beraldi E, Fazli L, Hurtado-Coll A, Yamanaka K, Gleave M. Heat shock protein 27 increases after androgen ablation and plays a cytoprotective role in hormone-refractory prostate cancer. Cancer Res. 2004;64(18):6595–602. doi: 10.1158/0008-5472.CAN-03-3998. [DOI] [PubMed] [Google Scholar]
- 213.Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, Ke Y, Foster CS. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60(24):7099–105. [PubMed] [Google Scholar]
- 214.Pootrakul L, Datar RH, Shi SR, Cai J, Hawes D, Groshen SG, Lee AS, Cote RJ. Expression of stress response protein Grp78 is associated with the development of castration-resistant prostate cancer. Clin Cancer Res. 2006;12(20 Pt 1):5987–93. doi: 10.1158/1078-0432.CCR-06-0133. [DOI] [PubMed] [Google Scholar]
- 215.Xu W, Liu L, Brown NJ, Christian S, Hornby D. Quantum dot-conjugated anti-GRP78 scFv inhibits cancer growth in mice. Molecules. 17(1):796–808. doi: 10.3390/molecules17010796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Arap MA, Lahdenranta J, Mintz PJ, Hajitou A, Sarkis AS, Arap W, Pasqualini R. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6(3):275–84. doi: 10.1016/j.ccr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 217.Larson N, Gormley A, Frazier N, Ghandehari H. Synergistic enhancement of cancer therapy using a combination of heat shock protein targeted HPMA copolymer-drug conjugates and gold nanorod induced hyperthermia. J Control Release. 2013;170(1):41–50. doi: 10.1016/j.jconrel.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gormley AJ, Larson N, Sadekar S, Robinson R, Ray A, Ghandehari H. Guided Delivery of Polymer Therapeutics Using Plasmonic Photothermal Therapy. Nano Today. 2012;7(3):158–167. doi: 10.1016/j.nantod.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Larson N, Ray A, Malugin A, Pike DB, Ghandehari H. HPMA copolymer-aminohexylgeldanamycin conjugates targeting cell surface expressed GRP78 in prostate cancer. Pharm Res. 2010;27(12):2683–93. doi: 10.1007/s11095-010-0267-7. [DOI] [PubMed] [Google Scholar]
- 220.Mahato R, Tai W, Cheng K. Prodrugs for improving tumor targetability and efficiency. Adv Drug Deliv Rev. 2011;63(8):659–70. doi: 10.1016/j.addr.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yousef GM, Diamandis EP. The new human tissue kallikrein gene family: structure, function, and association to disease. Endocr Rev. 2001;22(2):184–204. doi: 10.1210/edrv.22.2.0424. [DOI] [PubMed] [Google Scholar]
- 222.Lovgren J, Rajakoski K, Karp M, Lundwall a., Lilja H. Activation of the zymogen form of prostate-specific antigen by human glandular kallikrein 2. Biochem Biophys Res Commun. 1997;238(2):549–55. doi: 10.1006/bbrc.1997.7333. [DOI] [PubMed] [Google Scholar]
- 223.Christensson A, Laurell CB, Lilja H. Enzymatic activity of prostate-specific antigen and its reactions with extracellular serine proteinase inhibitors. Eur J Biochem. 1990;194(3):755–63. doi: 10.1111/j.1432-1033.1990.tb19466.x. [DOI] [PubMed] [Google Scholar]
- 224.Balk SP, Ko YJ, Bubley GJ. Biology of prostate-specific antigen. J Clin Oncol. 2003;21(2):383–91. doi: 10.1200/JCO.2003.02.083. [DOI] [PubMed] [Google Scholar]
- 225.Cary KC, Cooperberg MR. Biomarkers in prostate cancer surveillance and screening: past, present, and future. Ther Adv Urol. 2013;5(6):318–29. doi: 10.1177/1756287213495915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Denmeade SR, Lou W, Lovgren J, Malm J, Lilja H, Isaacs JT. Specific and efficient peptide substrates for assaying the proteolytic activity of prostate-specific antigen. Cancer Res. 1997;57(21):4924–30. [PMC free article] [PubMed] [Google Scholar]
- 227.Denmeade SR, Nagy A, Gao J, Lilja H, Schally AV, Isaacs JT. Enzymatic activation of a doxorubicin-peptide prodrug by prostate-specific antigen. Cancer Res. 1998;58(12):2537–40. [PubMed] [Google Scholar]
- 228.Chandran SS, Nan A, Rosen DM, Ghandehari H, Denmeade SR. A prostate-specific antigen activated N-(2-hydroxypropyl) methacrylamide copolymer prodrug as dual-targeted therapy for prostate cancer. Mol Cancer Ther. 2007;6(11):2928–37. doi: 10.1158/1535-7163.MCT-07-0392. [DOI] [PubMed] [Google Scholar]
- 229.DeFeo-Jones D, Garsky VM, Wong BK, Feng DM, Bolyar T, Haskell K, Kiefer DM, Leander K, McAvoy E, Lumma P, Wai J, Senderak ET, Motzel SL, Keenan K, Van Zwieten M, Lin JH, Freidinger R, Huff J, Oliff A, Jones RE. A peptide-doxorubicin ‘prodrug’ activated by prostate-specific antigen selectively kills prostate tumor cells positive for prostate-specific antigen in vivo. Nat Med. 2000;6(11):1248–52. doi: 10.1038/81351. [DOI] [PubMed] [Google Scholar]
- 230.Yu DC, Chen Y, Seng M, Dilley J, Henderson DR. The addition of adenovirus type 5 region E3 enables calydon virus 787 to eliminate distant prostate tumor xenografts. Cancer Res. 1999;59(17):4200–3. [PubMed] [Google Scholar]
- 231.Choi KY, Saravanakumar G, Park JH, Park K. Hyaluronic acid-based nanocarriers for intracellular targeting: interfacial interactions with proteins in cancer. Colloids Surf B Biointerfaces. 2012;99:82–94. doi: 10.1016/j.colsurfb.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6(10):764–75. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 233.Tan GJ, Peng ZK, Lu JP, Tang FQ. Cathepsins mediate tumor metastasis. World J Biol Chem. 2013;4(4):91–101. doi: 10.4331/wjbc.v4.i4.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 2001;20(17):4629–33. doi: 10.1093/emboj/20.17.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Fernandez PL, Farre X, Nadal A, Fernandez E, Peiro N, Sloane BF, Shi GP, Chapman HA, Campo E, Cardesa A. Expression of cathepsins B and S in the progression of prostate carcinoma. Int J Cancer. 2001;95(1):51–5. doi: 10.1002/1097-0215(20010120)95:1<51::aid-ijc1009>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 236.Brubaker KD, Vessella RL, True LD, Thomas R, Corey E. Cathepsin K mRNA and protein expression in prostate cancer progression. J Bone Miner Res. 2003;18(2):222–30. doi: 10.1359/jbmr.2003.18.2.222. [DOI] [PubMed] [Google Scholar]
- 237.Waghray A, Keppler D, Sloane BF, Schuger L, Chen YQ. Analysis of a truncated form of cathepsin H in human prostate tumor cells. J Biol Chem. 2002;277(13):11533–8. doi: 10.1074/jbc.M109557200. [DOI] [PubMed] [Google Scholar]
- 238.Loadman PM, Bibby MC, Double JA, Al-Shakhaa WM, Duncan R. Pharmacokinetics of PK1 and doxorubicin in experimental colon tumor models with differing responses to PK1. Clin Cancer Res. 1999;5(11):3682–8. [PubMed] [Google Scholar]
- 239.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: structures, evolution, and diversification. FASEB J. 1998;12(12):1075–95. [PubMed] [Google Scholar]
- 241.Ozden F, Saygin C, Uzunaslan D, Onal B, Durak H, Aki H. Expression of MMP-1, MMP-9 and TIMP-2 in prostate carcinoma and their influence on prognosis and survival. J Cancer Res Clin Oncol. 2013;139(8):1373–82. doi: 10.1007/s00432-013-1453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Escaff S, Fernandez JM, Gonzalez LO, Suarez A, Gonzalez-Reyes S, Gonzalez JM, Vizoso FJ. Study of matrix metalloproteinases and their inhibitors in prostate cancer. Br J Cancer. 2010;102(5):922–9. doi: 10.1038/sj.bjc.6605569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 244.Brehmer B, Biesterfeld S, Jakse G. Expression of matrix metalloproteinases (MMP-2 and - 9) and their inhibitors (TIMP-1 and -2) in prostate cancer tissue. Prostate Cancer Prostatic Dis. 2003;6(3):217–22. doi: 10.1038/sj.pcan.4500657. [DOI] [PubMed] [Google Scholar]
- 245.Wood M, Fudge K, Mohler JL, Frost AR, Garcia F, Wang M, Stearns ME. In situ hybridization studies of metalloproteinases 2 and 9 and TIMP-1 and TIMP-2 expression in human prostate cancer. Clin Exp Metastasis. 1997;15(3):246–58. doi: 10.1023/a:1018421431388. [DOI] [PubMed] [Google Scholar]
- 246.Stearns ME, Fudge K, Garcia F, Wang M. IL-10 inhibition of human prostate PC-3 ML cell metastases in SCID mice: IL-10 stimulation of TIMP-1 and inhibition of MMP-2/MMP-9 expression. Invasion Metastasis. 1997;17(2):62–74. [PubMed] [Google Scholar]
- 247.Jung K, Nowak L, Lein M, Priem F, Schnorr D, Loening SA. Matrix metalloproteinases 1 and 3, tissue inhibitor of metalloproteinase-1 and the complex of metalloproteinase-1/tissue inhibitor in plasma of patients with prostate cancer. Int J Cancer. 1997;74(2):220–3. doi: 10.1002/(sici)1097-0215(19970422)74:2<220::aid-ijc14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 248.Terada T, Iwai M, Kawakami S, Yamashita F, Hashida M. Novel PEG-matrix metalloproteinase-2 cleavable peptide-lipid containing galactosylated liposomes for hepatocellular carcinoma-selective targeting. J Control Release. 2006;111(3):333–42. doi: 10.1016/j.jconrel.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 249.Gu G, Xia H, Hu Q, Liu Z, Jiang M, Kang T, Miao D, Tu Y, Pang Z, Song Q, Yao L, Chen H, Gao X, Chen J. PEG-co-PCL nanoparticles modified with MMP-2/9 activatable low molecular weight protamine for enhanced targeted glioblastoma therapy. Biomaterials. 2013;34(1):196–208. doi: 10.1016/j.biomaterials.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 250.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- 251.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 252.Jin H, Varner J. Integrins: roles in cancer development and as treatment targets. Br J Cancer. 2004;90(3):561–5. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Goel HL, Li J, Kogan S, Languino LR. Integrins in prostate cancer progression. Endocr Relat Cancer. 2008;15(3):657–64. doi: 10.1677/ERC-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Sutherland M, Gordon A, Shnyder SD, Patterson LH, Sheldrake HM. RGD-Binding Integrins in Prostate Cancer: Expression Patterns and Therapeutic Prospects against Bone Metastasis. Cancers (Basel) 2012;4(4):1106–45. doi: 10.3390/cancers4041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59(7):1655–64. [PubMed] [Google Scholar]
- 256.Graf N, Bielenberg DR, Kolishetti N, Muus C, Banyard J, Farokhzad OC, Lippard SJ. alpha(V)beta(3) integrin-targeted PLGA-PEG nanoparticles for enhanced anti-tumor efficacy of a Pt(IV) prodrug. ACS Nano. 2012;6(5):4530–9. doi: 10.1021/nn301148e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Danhier F, Vroman B, Lecouturier N, Crokart N, Pourcelle V, Freichels H, Jerome C, Marchand-Brynaert J, Feron O, Preat V. Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with paclitaxel. J Control Release. 2009;140(2):166–73. doi: 10.1016/j.jconrel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 258.Li ZB, Wu Z, Chen K, Ryu EK, Chen X. 18F-labeled BBN-RGD heterodimer for prostate cancer imaging. J Nucl Med. 2008;49(3):453–61. doi: 10.2967/jnumed.107.048009. [DOI] [PubMed] [Google Scholar]
- 259.Musiyenko A, Bitko V, Barik S. Ectopic expression of miR-126*, an intronic product of the vascular endothelial EGF-like 7 gene, regulates prostein translation and invasiveness of prostate cancer LNCaP cells. J Mol Med (Berl) 2008;86(3):313–22. doi: 10.1007/s00109-007-0296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Campagnolo L, Leahy A, Chitnis S, Koschnick S, Fitch MJ, Fallon JT, Loskutoff D, Taubman MB, Stuhlmann H. EGFL7 is a chemoattractant for endothelial cells and is up-regulated in angiogenesis and arterial injury. Am J Pathol. 2005;167(1):275–84. doi: 10.1016/S0002-9440(10)62972-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fitch MJ, Campagnolo L, Kuhnert F, Stuhlmann H. Egfl7, a novel epidermal growth factor-domain gene expressed in endothelial cells. Dev Dyn. 2004;230(2):316–24. doi: 10.1002/dvdy.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY, De Sauvage FJ, Ye W. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428(6984):754–8. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- 263.Hogan BL, Kolodziej PA. Organogenesis: molecular mechanisms of tubulogenesis. Nat Rev Genet. 2002;3(7):513–23. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- 264.Philippin-Lauridant G, Baranzelli MC, Samson C, Fournier C, Pinte S, Mattot V, Bonneterre J, Soncin F. Expression of Egfl7 correlates with low-grade invasive lesions in human breast cancer. Int J Oncol. 2013;42(4):1367–75. doi: 10.3892/ijo.2013.1820. [DOI] [PubMed] [Google Scholar]
- 265.Fan C, Yang LY, Wu F, Tao YM, Liu LS, Zhang JF, He YN, Tang LL, Chen GD, Guo L. The expression of Egfl7 in human normal tissues and epithelial tumors. Int J Biol Markers. 2013;28(1):71–83. doi: 10.5301/JBM.2013.10568. [DOI] [PubMed] [Google Scholar]
- 266.Ling Y, Wei K, Luo Y, Gao X, Zhong S. Dual docetaxel/superparamagnetic iron oxide loaded nanoparticles for both targeting magnetic resonance imaging and cancer therapy. Biomaterials. 2011;32(29):7139–50. doi: 10.1016/j.biomaterials.2011.05.089. [DOI] [PubMed] [Google Scholar]
- 267.Okarvi SM, Al Jammaz I. Synthesis and evaluation of a technetium-99m labeled cytotoxic bombesin peptide conjugate for targeting bombesin receptor-expressing tumors. Nucl Med Biol. 2010;37(3):277–88. doi: 10.1016/j.nucmedbio.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 268.Accardo A, Salsano G, Morisco A, Aurilio M, Parisi A, Maione F, Cicala C, Tesauro D, Aloj L, De Rosa G, Morelli G. Peptide-modified liposomes for selective targeting of bombesin receptors overexpressed by cancer cells: a potential theranostic agent. Int J Nanomedicine. 2012;7:2007–17. doi: 10.2147/IJN.S29242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sangha R, Butts C. L-BLP25: a peptide vaccine strategy in non small cell lung cancer. Clin Cancer Res. 2007;13(15 Pt 2):s4652–4. doi: 10.1158/1078-0432.CCR-07-0213. [DOI] [PubMed] [Google Scholar]