Abstract
Purpose
Regorafenib, a multi-kinase inhibitor targeting the Ras/Raf/MEK/ERK pathway, has recently been approved for the treatment of metastatic colorectal cancer (CRC). However, the mechanisms of action of regorafenib in CRC cells have been unclear. We investigated how regorafenib suppresses CRC cell growth and potentiates effects of other chemotherapeutic drugs.
Experimental Design
We determined whether and how regorafenib induces the expression of PUMA, a p53 target and a critical mediator of apoptosis in CRC cells. We also investigated whether PUMA is necessary for the killing and chemosensitization effects of regorafenib in CRC cells. Furthermore, xenograft tumors were used to test if PUMA mediates the in vivo antitumor, antiangiogenic and chemosensitization effects of regorafenib.
Results
We found that regorafenib treatment induces PUMA in CRC cells irrespective of p53 status through the NF-κB pathway following ERK inhibition and glycogen synthase kinase 3β (GSK3β) activation. Upregulation of PUMA is correlated with apoptosis induction in different CRC cell lines. PUMA is necessary for regorafenib-induced apoptosis in CRC cells. Chemosensitization by regorafenib is mediated by enhanced PUMA induction through different pathways. Furthermore, deficiency in PUMA abrogates the in vivo antitumor, antiangiogenic and chemosensitization effects of regorafenib.
Conclusions
Our results demonstrate a key role of PUMA in mediating the anticancer effects of regorafenib in CRC cells. They suggest that PUMA induction can be used as an indicator of regorafenib sensitivity, and also provide a rationale for manipulating the apoptotic machinery to improve the therapeutic efficacy of regorafenib and other targeted drugs.
Keywords: regorafenib, PUMA, apoptosis, NF-κB, GSK3β, colon cancer
Introduction
Colorectal cancer (CRC) represents the third leading cause of cancer-related death in the US (1). Recurrent or metastatic colorectal tumors are largely incurable with median overall survival of ~2 years (2). Conventional chemotherapy for CRC treatment involves combinations of cytotoxic drugs such as 5-fluorouracil (5-FU), oxaliplatin and irinotecan, and has limited efficacy and substantial side effects due to lack of specificity (3). The development of targeted anticancer agents has significantly improved efficacy of chemotherapy against metastatic CRC (4). For example, cetuximab, an anti-epidermal growth factor receptor (EGFR) monoclonal antibody, is effective as a single agent or in combination with cytotoxic drugs for treatment of metastatic CRC (4). The inclusion of targeted therapy is expected to move oncology practice towards personalized treatment (5).
Regorafenib (Stivarga), a multi-kinase inhibitor that targets the Ras/Raf/MEK/ERK pathway, has recently been approved for the treatment of metastatic CRC and gastrointestinal stromal tumors (6, 7). A variety of clinical trials have also been initiated to use regorafenib to treat other malignancies, including those of lung, esophageal, and liver. Regorafenib inhibits several kinases that are aberrantly activated in tumor cells, including c-Raf, B-Raf, vascular endothelial growth factor receptors (VEGFRs), platelet-derived growth factor receptor (PDGFR), c-Kit, and Tie-2 (8). The antitumor activity of regorafenib has been demonstrated in a variety of preclinical models, and is associated with suppression of cell proliferation, induction of apoptosis, and inhibition of tumor angiogenesis (8, 9). However, it is unclear whether any of these effects is essential for the antitumor activity of regorafenib.
Induction of apoptosis in tumor cells has emerged as a key mechanism of targeted therapies (10). p53-upregulated modulator of apoptosis (PUMA), a BH3-only Bcl-2 family member, functions as a critical regulator of apoptosis in CRC cells (11). PUMA is transcriptionally activated by p53 and initiates apoptotic response to DNA damage (12). It can also be induced in a p53-independent manner by a variety of non-genotoxic stimuli, such as the pan-kinase inhibitor UCN-01 (13), the EGFR tyrosine kinase inhibitors (TKIs) gefitinib and erlotinib (14), and tumor necrosis factor-α (TNF-α) (15). The p53-independent induction of PUMA by these stimuli is mediated by the transcription factor Forkhead Box O3a (FoxO3a), p73, or nuclear factor κB (NF-κB), respectively (13–15). Upon its induction, PUMA potently promotes apoptosis in CRC cells by antagonizing antiapoptotic Bcl-2 family members such as Bcl-XL, activating proapoptotic members Bax and Bak, and resulting in mitochondrial dysfunction and caspase activation (16–18).
Our previous study revealed that sorafenib, a regorafenib analog approved for treating liver and kidney tumors, induces PUMA-dependent apoptosis (19). This prompted us to investigate the role of PUMA in mediating the effects of regorafenib against CRC cells. We found that PUMA is induced by regorafenib through the NF-κB pathway and plays a pivotal role in therapeutic response to regorafenib in CRC cells. Our results suggest that PUMA induction is indicative of the therapeutic efficacy of regorafenib, and likely other targeted agents as well.
Materials and Methods
Cell culture and drug treatment
Human CRC cell lines were purchased from American Type Culture Collection (Manassas, VA) or obtained from Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. Isogenic p53-knockout (KO) and PUMA-KO HCT116, and PUMA-KO DLD1 cell lines were previously described (18, 20). The cell lines were last tested and authenticated for genotypes, drug response, morphology, and absence of mycoplasma in October, 2012. All CRC cell lines were cultured in McCoy’s 5A modified media (Invitrogen, Carlsbad, CA). Immortalized wild-type (WT), p65-KO, and PUMA-KO mouse embryonic fibroblast (MEF) cells were previously described (19), and were cultured in DMEM media (Invitrogen). Cells were maintained in a 37°C incubator at 5% CO2. Cell culture media were supplemented with 10% defined FBS (HyClone, Logan, UT), 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen).
Cells were plated and treated by drugs at 40–50% density in 12-well plates. The anticancer agents and chemicals used include regorafenib (Active Biochem, Maplewood, NJ), BAY 11-7082, PD98059 (Merck Chemicals, Darmstadt, Germany), 5-fluorouracil (5-FU), UCN-01, cisplatin, oxaliplatin (Sigma, St Louis, MO), gefitinib (AstraZeneca, Wilmington, DE) and cetuximab (ImClone, New York, NY). All agents were diluted with DMSO (Sigma), except for cisplatin, which was dissolved in 0.9% NaCl. For NF-κB inhibition, cells were pre-treated with BAY 11-7082 for 1 hour before regorafenib treatment.
Real-time reverse transcriptase (RT) PCR
Total RNA was isolated from regorafenib-treated cells using Mini RNA Isolation II Kit (Zymo Research, Irvine, CA) according to the manufacturer’s protocol. One μg of total RNA was used to generate cDNA using SuperScript II reverse transcriptase (Invitrogen). Real-time PCR was performed for PUMA and β-actin using previously described primers and conditions (13).
Western blotting
Western blotting was performed as previously described (16), with antibodies for PUMA (18), Akt, phospho-Akt (S473), Bid, active caspase 3, caspase 8, caspase 9, ERK, phospho-ERK (T202/Y204), IκB, phospho-IκB (S22/23), p65, phospho-p65 (S536, S276, and S468), phospho-FoxO3a, STAT1, phospho-STAT1 (Y701), glycogen synthase kinase 3β (GSK3β), phospho-GSK3β (S9) (Cell Signaling Technology, Beverly, MA), Bak, FoxO3a (Millipore, Bellerica, MA), cytochrome oxidase subunit IV (Invitrogen), Mcl-1, IκB, cytochrome c, lamin A/C (Santa Cruz Biotechnology, Santa Cruz, CA), β-actin (Sigma), Bim, α-tubulin (EMD Biosciences, Philadelphia, PA), and Bcl-XL (BD Transduction, San Jose, CA).
Apoptosis assays
Nuclear staining with Hoechst 33258 (Invitrogen) was performed as previously described (13). Annexin V/propidium iodide (PI) staining was performed using annexin-Alexa 488 (Invitrogen) and PI as described (21). Cytochrome c release was analyzed by cytochrome c Western blotting of mitochondrial and cytosolic fractions isolated by differential centrifugation (17). Colony formation was assayed by plating the treated cells in 12-well plates at appropriate dilutions, and allowing for cell growth for 10–14 days, followed by crystal violet (Sigma) staining of cell colonies. Mitochondrial membrane potential changes were detected by flow cytometry of treated cells stained with MitoTracker Red CMXRos (Invitrogen) at room temperature for 15 minutes.
Transfection and siRNA knockdown
Cells were transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Knockdown experiments were performed 24 hours prior to regorafenib treatment using 200 pmole of siRNA. The control scrambled siRNA and siRNA for human p65 (15), c-Raf (AAGCACGCTTAGATTGGAATA-dTdT), PDGFRβ (GCAUCUUCAACAGCCUCUA-dTdT), B-Raf (ACAGAGACCUCAAGAGUAA-UU), and c-Kit (GGCCGACAAAAGGAGAUCU-dTdT) were from Dharmacon (La Fayette, CO). The siRNA for GSK3β (sc-35527) and VEGFR2 siRNA (sc-29318) were from Santa Cruz Biotechnology. A non-degradable IκBα super repressor mutant (S32/36A; IκBαM) was previously described (15).
Analysis of NF-κB nuclear translocation
HCT116 cells were pre-treated with BAY11-7082 or transfected with GSK3β siRNA, and then subjected to regorafenib treatment for 3 hours. Nuclear fractionation and immunofluorescence were used to analyze NF-κB nuclear translocation. For nuclear fractionation, nuclear extracts were isolated from cells treated in 75-cm2 flasks using the NE-PER nuclear/cytoplasmic extraction kit (Thermo Fisher, Waltham, MA) according to the manufacturer’s instructions, and analyzed by p65 Western blot. For immunofluoscence, cells treated in chamber slides were stained with anti-p65 (Cell Signaling) overnight at 4°C, following by secondary staining with the anti-rabbit AlexaFluor 488-conjugated secondary antibody (Invitrogen) for 1 hour at room temperature, as previously described (22). Images were acquired by an Olympus IX71 microscope (Olympus Imaging America, Inc., Center Valley, PA).
Luciferase assays
Luciferase reporter constructs containing WT or mutant PUMA promoter sequence in pBV-Luc vector were previously described (13, 23). To measure reporter activities, cells were transfected with the WT or mutant PUMA reporters along with the transfection control β-galactosidase reporter pCMVβ (Promega, Madison, WI). Luciferase activities were measured as previously described (22), and normalized to samples similarly transfected but without drug treatment. All reporter experiments were performed in triplicate and repeated three times.
Chromatin immunoprecipitation (ChIP)
ChIP with p65 antibody (Santa Cruz) was performed using the Chromatin Immunoprecipitation Assay Kit (Millipore) as previously described (20). The precipitates were analyzed by PCR using primers 5′-GTCGGTCTGTGTACGCATCG-3′ and 5′-CCCGCGTGACGCTACGGCCC-3′ to amplify a PUMA promoter fragment containing putative κB sites.
Animal tumor experiments
All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Female 5–6 week-old Nu/Nu mice (Charles River, Wilmington, MA) were housed in a sterile environment with micro isolator cages and allowed access to water and chow ad libitum. Mice were injected subcutaneously in both flanks with 4×106 WT or PUMA-KO HCT116 cells. After tumor growth for 7 days, mice were treated daily with regorafenib at 30 mg/kg by oral gavage for 10 consecutive days. For combination experiments, mice were treated with 15 mg/kg regorafenib daily by oral gavage, 25 mg/kg 5-FU (APP Pharmaceuticals, Schaumburg, IL) every other day by i.p. injection, or their combination for 10 consecutive days. Regorafenib was dissolved in Cremephor EL/95% ethanol (50:50) as a 4× stock solution (24), and 5-FU was supplied as a stock solution. Both drugs were diluted to the final concentration with sterile water before use. Tumor growth was monitored by calipers, and tumor volumes were calculated according to the formula ½ × length × width2. Mice were euthanized when tumors reached ~1.0 cm3 in size. Tumors were dissected and fixed in 10% formalin and embedded in paraffin. Terminal deoxynucleotidyl transferase mediated dUTP Nick End Labeling (TUNEL; Millipore), active caspase 3 (Cell Signaling), CD31 (Spring Bioscience, Pleasanton, CA), and Carbonic Anhydrase 9 (CA9; Santa Cruz) immunostaining was performed on 5 μM paraffin-embedded tumor sections as previously described (25), by using an AlexaFluor 488- or AlexaFluor 594-conjugated secondary antibody (Invitrogen) for detection.
Statistical Analysis
Statistical analyses were carried out using GraphPad Prism IV software (GraphPad Software, Inc., La Jolla, CA). P-values were calculated using the student’s t-test and were considered significant if P <0.05. The means ± one standard deviation (s.d.) were displayed in the figures.
Results
Upregulation of PUMA by regorafenib correlates with apoptosis induction in CRC cells
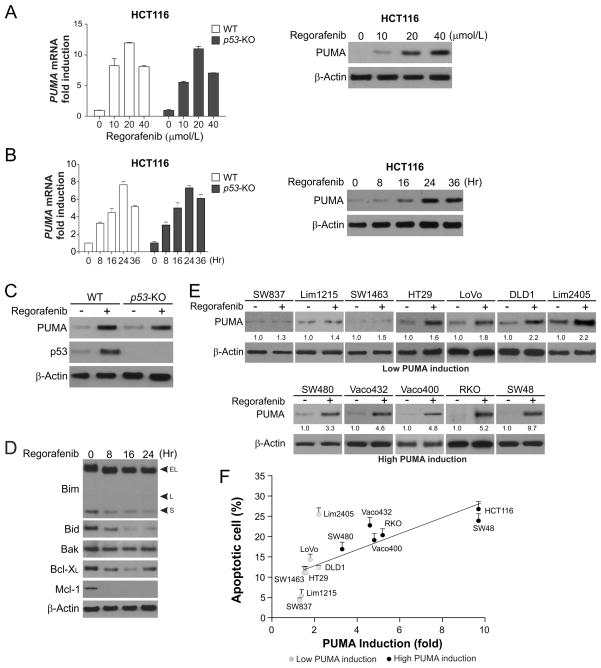
The recent approval of regorafenib for CRC treatment prompted us to investigate its mechanisms of action in CRC cells (7). Treating p53-wild type (WT) HCT116 colon cancer cells with regorafenib markedly induced PUMA protein and mRNA expression in a dose- and time-dependent manner (Fig. 1, A and B). The peaks of PUMA protein and mRNA induction were detected at 24 hours following 40 μmol/L regorafenib treatment (Fig. 1B). Regorafenib also induced PUMA protein and mRNA expression in p53-KO HCT116 cells (Fig. 1, A–C). In contrast, regorafenib treatment did not upregulate other proapoptotic Bcl-2 family members, including Bim, Bid and Bak, but reduced the expression of the antiapoptotic proteins Bcl-XL and Mcl-1 (Fig. 1E). PUMA was also induced by regorafenib in other CRC cell lines, including p53-WT Lim2405, LoVo, Lim1215, SW48, and RKO cells, and p53-mutant SW837, SW1463, SW480, Vaco432, Vaco400, DLD1, and HT29 cells (Fig. 1E). Analysis of 13 CRC cell lines revealed a correlation between the induction of PUMA and apoptosis by regorafenib (Fig. 1E and 1F). Following regorafenib treatment, cell lines with low endogenous expression but strong induction of PUMA had relatively high levels apoptosis, while those with low or no PUMA induction, such as SW837, Lim1215 and SW1463, had low or barely detectable levels of apoptosis, with the exception of Lim2045 (Fig. 1F). These results suggest that selective induction of PUMA by regorafenib contributes to apoptosis induction in CRC cells.
Figure 1. Upregulation of PUMA expression by regorafenib correlates with apoptosis induction in CRC cells.
(A) WT and p53-knockout (p53-KO) HCT116 colon cancer cells were treated with regorafenib at indicated concentrations for 24 hours. Left, PUMA mRNA induction by regorafenib was analyzed by real-time reverse transcriptase (RT) PCR, with β-actin as a control. Right, PUMA and β-actin expression was analyzed by Western blotting. (B) WT and p53-KO HCT116 cells were treated with 40 μmol/L regorafenib and analyzed at different time points after treatment. Left, time course of PUMA mRNA induction was determined by real-time RT PCR, with β-actin as a control. Right, time course of PUMA protein induction was analyzed by Western blotting. (C) WT and p53-KO HCT116 cells were treated with 40 μmol/L regorafenib for 24 hours. PUMA expression was analyzed by Western blotting. (D) The expression of indicated Bcl-2 family members was analyzed by Western blotting in HCT116 cells treated with 40 μmol/L regorafenib at indicated time points. (E) Western blot analysis of PUMA expression in indicated CRC cell lines treated with 40 μmol/L regorafenib for 24 hours. Relative PUMA expression, which was quantified by Image J program and normalized to that of β-actin, is indicated, with that in untreated cells arbitrarily set as 1.0. (F) Indicated CRC cell lines were treated with 40 μmol/L regorafenib for 48 hours. Apoptosis was quantified by counting condensed and fragmented nuclei after nuclear staining with Hoechst 33258, and plotted against PUMA induction from (E). The results represent means + SD of 3 independent experiments.
Regorafenib is dependent on PUMA to induce apoptosis in CRC cells
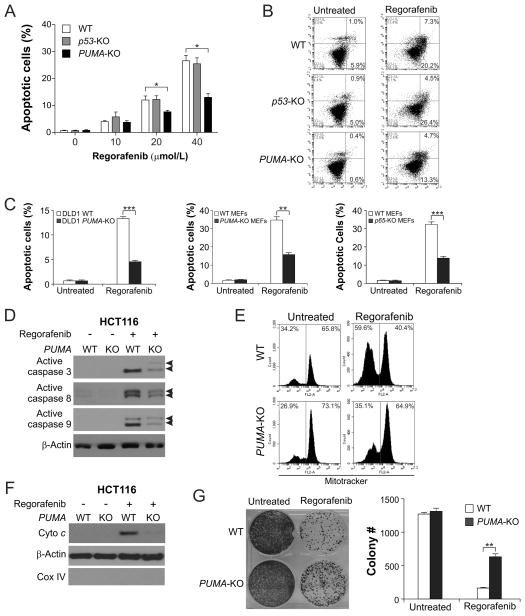
We then investigated the role of PUMA in regorafenib-induced apoptosis using isogenic HCT116 cell lines. Apoptosis induced by 10–40 μmol/L regorafenib was significantly reduced in PUMA-KO cells, but unaffected in p53-KO cells in comparison with WT HCT116 cells (Fig. 2A). Annexin V/PI staining confirmed the reduction of regorafenib-induced apoptosis in PUMA-KO cells (Fig. 2B). PUMA-dependent apoptotic response to regorafenib was also observed in DLD1 and mouse embryonic fibroblast (MEF) cells (Fig. 2C). The absence of PUMA in HCT116 cells abrogated regorafenib-induced mitochondrial events, including activation of caspases 3, 8, and 9 (Fig. 2D), mitochondrial membrane permeabilization (Fig. 2E), and cytochrome c release (Fig. 2F). Furthermore, PUMA-KO cells had improved long-term survival compared to WT HCT116 cells following regorafenib treatment (Fig. 2G). Therefore, PUMA is necessary for the apoptotic effect of regorafenib in CRC cells.
Figure 2. PUMA mediates the apoptotic and anticancer effects of regorafenib through the mitochondrial pathway.
(A) WT, p53-KO, and PUMA-KO HCT116 cells were treated with regorafenib at indicated concentrations for 48 hours. Apoptosis was analyzed by counting condensed and fragmented nuclei after nuclear staining with Hoechst 33258. (B) Apoptosis in cells treated with 40 μmol/L regorafenib for 48 hours was analyzed by annexin V/PI staining followed by flow cytometry. The percentages of annexin-positive apoptotic cells are indicated in the two right quadrants. (C) Comparison of apoptosis in WT and PUMA-KO DLD1 colon cancer cells (left), WT and PUMA-KO MEFs (middle), and WT and p65-KO MEFs (right) following treatment with 40 μmol/L regorafenib for 48 hours. Apoptosis was analyzed by nuclear staining as in (A). (D) Western blot analysis of active caspase 3, active caspase 8, and active caspase 9 (indicated by arrow heads) in WT and PUMA-KO HCT116 cells with or without regorafenib (40 μmol/L) treatment for 24 hours. (E) After treatment of WT and PUMA-KO HCT116 cells with 40 μmol/L regorafenib for 36 hours, mitochondrial membrane potential was analyzed by flow cytometry following MitoTracker Red CMXRos staining. (F) Cytosolic fractions isolated from WT and PUMA-KO HCT116 cells treated with 40 μmol/L regorafenib for 36 hours were probed for cytochrome c by Western blotting. α-Tubulin and cytochrome oxidase subunit IV (Cox IV), which are expressed in cytoplasm and mitochondria, respectively, were analyzed as the control for loading and fractionation. (G) Colony formation of WT and PUMA-KO HCT116 cells treated with 40 μmol/L regorafenib for 48 hours at 14 days following crystal violet staining of attached cells. Left, representative pictures of colonies; Right, quantification of colony numbers. Results in (A), (C), and (G) were expressed as means ± SD of 3 independent experiments. ***, P <0.001; **, P <0.01; *, P <0.05.
PUMA activation by regorafenib is mediated by NF-κB
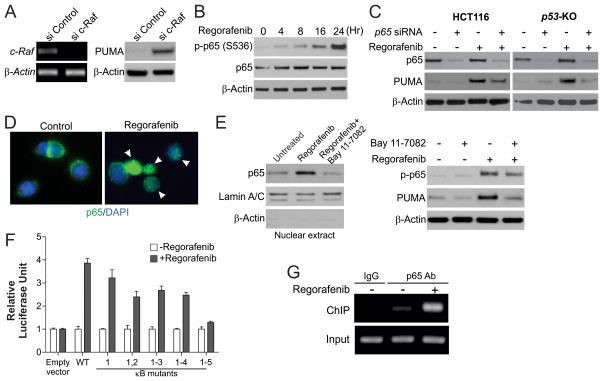
We then analyzed the mechanism of p53-independent PUMA induction by regorafenib in CRC cells. Knockdown of several known targets of regorafenib, including B-Raf, VEGFR2, PDGFR-β and c-Kit, by siRNA did not affect PUMA expression (Fig. S1, A and B). In contrast, depletion of c-Raf by siRNA led to increased levels of PUMA (Fig. 3A), suggesting that PUMA induction by regorafenib is due to c-Raf inhibition. Several transcription factors that can mediate PUMA upregulation in p53-deficient cells were examined (11). FoxO3a is not involved due to unchanged inhibitory phosphorylation following regorafenib treatment (Fig. S1C) (26), and lack of an effect of FoxO3a knockdown on PUMA induction (Fig. S1D). p73, a p53 family member (23), and STAT1, which mediates the effects of sorafenib in pancreatic cancer cells (27), were also ruled out due to lack of induction or a change in phosphorylation (Fig. S1C).
Figure 3. Activation of p65 mediates PUMA induction in response to regorafenib treatment.
(A) HCT116 cells were transfected with either a control scrambled siRNA or a c-Raf siRNA for 24 hours, and then treated with 40 μmol/L regorafenib for 24 hours. c-Raf mRNA and PUMA protein expression were analyzed by RT-PCR and Western blotting, respectively. (B) HCT116 cells were treated with 40 μmol/L regorafenib. Expression of p-p65 (S536) and β-actin at indicated time points was analyzed by Western blotting. (C) WT and p53-KO HCT116 cells were transfected with either a control scrambled siRNA or a p65 siRNA for 24 hours, and then treated with 40 μmol/L regorafenib for 24 hours. p65 and PUMA expression was analyzed by Western blotting. (D) HCT116 cells were treated with 40 μmol/L regorafenib for 3 hours and then fixed. Immunofluorescence was carried out as described in the Materials and Methods for p65 (green) and DAPI (blue). Representative pictures (400×) are shown. Arrows indicate cells with p65 nuclear translocation. (E) HCT116 cells were treated with 10 μmol/L BAY11-7082 for 1 hour, and then with 40 μmol/L regorafenib for 24 hours. Left, nuclear fractions were isolated from cells and analyzed for p65 expression by Western blotting. Lamin A/C and β-actin, which are expressed in nucleus and cytoplasm, respectively, were used as controls for loading and fractionation. Right, the levels of p-p65 (S536) and PUMA were analyzed by Western blotting. (F) p53-KO HCT116 cells were transfected overnight with a PUMA promoter luciferase reporter containing WT κB sites or indicated mutants, and then treated with 40 μmol/L regorafenib for 16 hours. Reporter activities were normalized to the untreated control samples and plotted. (G) Chromatin immunoprecipitation (ChIP) was performed using anti-p65 antibody on HCT116 cells following regorafenib (40 μmol/L) treatment for 8 hours. The IgG was used to control for antibody specificity. PCR was carried out using primers surrounding the p65 binding sites in the PUMA promoter.
The p65 subunit of NF-κB was recently identified as a transcriptional activator of PUMA in response to TNF-α or sorafenib treatment (15, 19). Activation of NF-κB signaling is characterized by phosphorylation of p65 on several residues and its subsequent translocation to the nucleus, where it activates transcription of target genes (28). We found that regorafenib treatment induced phosphorylation of S536, the major regulatory site of p65 (29), in a time- and dosage-dependent manner in HCT116 cells (Fig. 3B and S2A). Phosphorylation of S276, another site associated with p65 activation (29), was also increased after regorafenib treatment, while that of the controversial S468 site was not detected (Fig. S2B). Knock-down of p65 by siRNA abrogated PUMA induction by regorafenib in both WT and p53-KO HCT116 cells (Fig. 3C), as well as in DLD1 cells (Fig. S2C). The induction of PUMA and apoptosis by regorafenib was also suppressed in p65-KO MEFs (Fig. 2C and S2D). Furthermore, regorafenib treatment led to nuclear translocation of p65 as detected by immunofluorescence (Fig. 3D) and western blot (Fig. 3E, left).
p65 binds to the PUMA promoter to directly activate its transcription following regorafenib treatment
To investigate how NF-κB activates PUMA transcription in response to regorafenib treatment, cells were pre-treated with BAY 11-7082, an NF-κB inhibitor that suppresses p65 nuclear translocation (Fig. 3E, left). BAY 11-7082 treatment impeded PUMA expression and p65 phosphorylation induced by regorafenib (Fig. 3E, right), suggesting that PUMA induction by regorafenib is mediated by p65 nuclear translocation. To determine whether NF-κB can directly activate PUMA transcription, p53-KO HCT116 cells were transfected with luciferase reporter constructs containing different regions of the PUMA promoter (Fig. S2E) (23). Regorafenib treatment did not activate the previously identified NF-κB responsive element that is responsive to TNF-α treatment (15), in the distal PUMA promoter region (Frag D, Fig. S2E). In contrast, regorafenib treatment strongly activated the proximal 495-bp region of the PUMA promoter (Frag A, Fig. S2E). Analysis of the DNA sequence in this region identified at least 5 κB sites (Fig. S2F) (19). The effect of regorafenib treatment on the PUMA promoter was completely blocked only when all 5 κB sites were mutated (Fig. 3F and S2F), suggesting that all 5 κB sites contribute to PUMA induction by regorafenib. Furthermore, p65 was found to be recruited to the PUMA promoter containing the κB sites following regorafenib treatment by chromatin immunoprecipitation (ChIP) (Fig. 3G). These results indicate that p65 directly binds to multiple κB sites in the proximal PUMA promoter region to drive its transcriptional activation in response to regorafenib treatment.
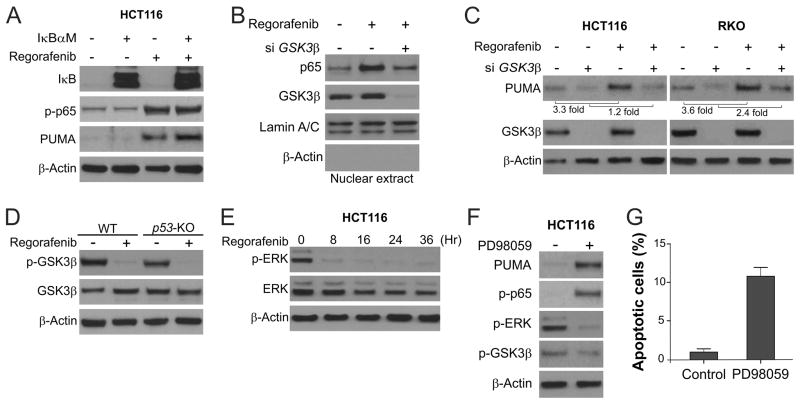
GSK3β-dependent, but IκB-independent p65 activation mediates PUMA induction by regorafenib
The canonical pathway of p65 activation is mediated by IκB phosphorylation and degradation (28). However, IκB phosphorylation or degradation was unaffected by regorafenib treatment (Fig. S2G). Transfecting cells with IκBαM, a non-degradable mutant of IκB (15), also did not affect regorafenib-induced PUMA expression (Fig. 4A), suggesting that the canonical NF-κB pathway is not involved in p65 activation or PUMA induction by regorafenib. Further analysis of other kinases known to activate NF-κB revealed that GSK3β is involved in regorafenib-induced p65 activation. Knockdown of GSK3β by siRNA suppressed regorafenib-induced p65 nuclear translocation (Fig. 4B), as well as PUMA expression in both HCT116 and RKO colon cancer cells (Fig. 4C). Furthermore, regorafenib treatment suppressed Ser9 phosphorylation of GSK3β, which inhibits its kinase activity (30), in both WT and p53-KO HCT116 cells (Fig. 4D). It has been shown that the ERK kinase can prime the inhibitory GSK3β Ser9 phosphorylation (31). We found that regorafenib treatment strongly suppressed phosphorylation of ERK1/2 (T202/Y204) (Fig. 4E). Treatment of the ERK inhibitor PD98059 phenocopied regorafenib treatment in blocking GSK3β Ser9 phosphorylation, and promoting p65 phosphorylation, PUMA expression and apoptosis induction (Fig. 4F and 4G), suggesting that ERK inhibition mediates the activation of GSK3β, p65 and PUMA by regorafenib. MEK inhibitors were also shown to suppress in vivo growth of CRC cells including HCT116 cells (32). Together, these results demonstrate that PUMA induction by regorafenib is mediated by ERK inhibition, relief of GSK3β inhibition, and subsequent p65 activation.
Figure 4. Regorafenib activates GSK3β and inhibits ERK to induce PUMA through p65.
(A) HCT116 cells were transfected with pCMV or IκBαM overnight, and then treated with 40 μmol/L regorafenib for 24 hours. PUMA, p-p65 (S536) and IκB were analyzed by Western blotting. (B) HCT116 cells were transfected with either a control scrambled siRNA or a GSK3β siRNA for 24 hours, and then treated with 40 μmol/L regorafenib for 3 hours. Nuclear fractions were isolated from cells treated with regorafenib and analyzed for p65 and GSK3β expression by Western blotting. (C) After GSK3β siRNA transfection as in (B), HCT116 and RKO cells were treated with 40 μmol/L regorafenib for 24 hours. GSK3β and PUMA were analyzed by Western blotting. PUMA induction was determined by quantifying PUMA expression using Image J program and normalizing to that of β-actin. (D) The levels of total GSK3β and p-GSK3β (S9) were analyzed by Western blotting in WT and p53-KO HCT116 cells treated with 40 μmol/L regorafenib for 24 hours. (E) The levels of total ERK and p-ERK (T202/Y204) were analyzed by Western blotting in HCT116 cells treated with 40 μmol/L regorafenib at indicated time points. (F) HCT116 cells were treated with 25 μmol/L of the ERK inhibitor PD98059 for 24 hours. The levels of PUMA, p-GSK3β (S9), p-ERK1/2 (T202/Y204) and p-p65 (S536) were analyzed by Western blotting. (G) Apoptosis in HCT116 cells treated with PD98059 as in (F) for 48 hours was determined by nuclear staining with Hoechst 33258.
PUMA is necessary for the in vivo antitumor and antiangiogenic activities of regorafenib
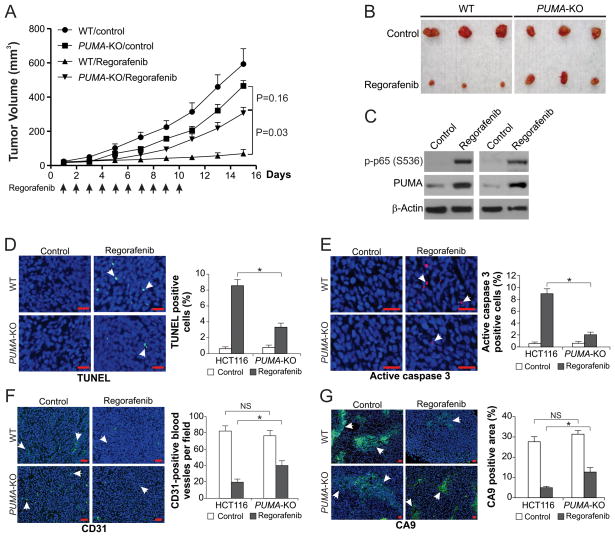
Regorafenib suppresses growth of CRC xenograft tumors effectively (8, 9). To determine if PUMA mediates tumor suppression by regorafenib, WT and PUMA-KO HCT116 cells were injected subcutaneously into nude mice to establish xenograft tumors. Mice were then treated with 30 mg/kg regorafenib or the control vehicle by oral gavage for 10 consecutive days, as previously described (8, 9). WT and PUMA-KO tumors were not significantly different in growth in the control group (Fig. 5, A and B). In line with previous studies (8, 9), regorafenib treatment suppressed the growth of WT tumors by 70–90% (Fig. 5A). In contrast, PUMA-KO tumors were significantly less sensitive to regorafenib treatment compared to WT tumors (Fig. 5, A and B), indicating that absence of PUMA abrogated the antitumor activity of regorafenib. Consistent with observations from cultured cells, p65 phosphorylation and PUMA expression were markedly increased in regorafenib-treated xenograft tumors (Fig. 5C). TUNEL staining revealed significant apoptosis induction in WT tumor tissues from the regorafenib-treated mice, but not the control mice. In contrast, apoptosis was largely reduced in the PUMA-KO tumors (Fig. 5D). Active caspase 3 staining confirmed regorafenib-induced and PUMA-dependent apoptosis in tumors (Fig. 5E). Analysis of tumor vasculature by CD31 staining showed that the antiangiogenic effect of regorafenib was reduced in the PUMA-KO tumors (Fig. 5F). Suppression of tumor hypoxia, analyzed by Carbonic Anhydrase 9 (CA9) staining, by regorafenib was also decreased in the PUMA-KO tumors compared to WT tumors (Fig. 5G). Therefore, the antitumor, apoptotic and antiangiogenic activities of regorafenib in vivo are largely dependent on PUMA.
Figure 5. The antitumor effects of regorafenib in vivo are PUMA-dependent.
(A) Nude mice were injected s.c. with 4 × 106 WT or PUMA-KO HCT116 cells. After 1 week, mice were oral gavaged with 30 mg/kg regorafenib or the vehicle control cremephor EL/ethanol for 10 consecutive days. Tumor volume at indicated time points after treatment was calculated and plotted with p values, n=5 in each group. Arrows indicate regorafenib injection. (B) Representative tumors at the end of the experiment in (A). (C) Mice with WT HCT116 xenograft tumors were treated with 30 mg/kg regorafenib or the vehicle as in (A) for 4 consecutive days. The levels of p-p65 (S536) and PUMA in randomly selected tumors were analyzed by Western blotting. (D) Paraffin-embedded sections of WT or PUMA-KO tumor tissues from mice treated as in (C) were analyzed by TUNEL staining. Left, representative TUNEL staining pictures; Right, TUNEL-positive cells were counted and plotted. (E) Tissue sections from (D) were analyzed by active caspase 3 staining. Left, representative staining pictures; Right, active caspase 3-positive cells were counted and plotted. (F) Tissue sections from (B) were analyzed for blood vessel formation by CD31 staining. Left, representative staining pictures; Right, CD31-positive cells were counted and plotted. (G) Tissue sections from (B) were analyzed for hypoxia by CA9 staining. Left, representative staining pictures; Right, CA9-positive areas were quantified by Image J program and plotted. In (D)–(G), results were expressed as means ± SD of 3 independent experiments. Arrows, example cells with positive staining; scale bars, 25 μm; *, P <0.05; NS, P > 0.05.
PUMA mediates the chemosensitization effects of regorafenib
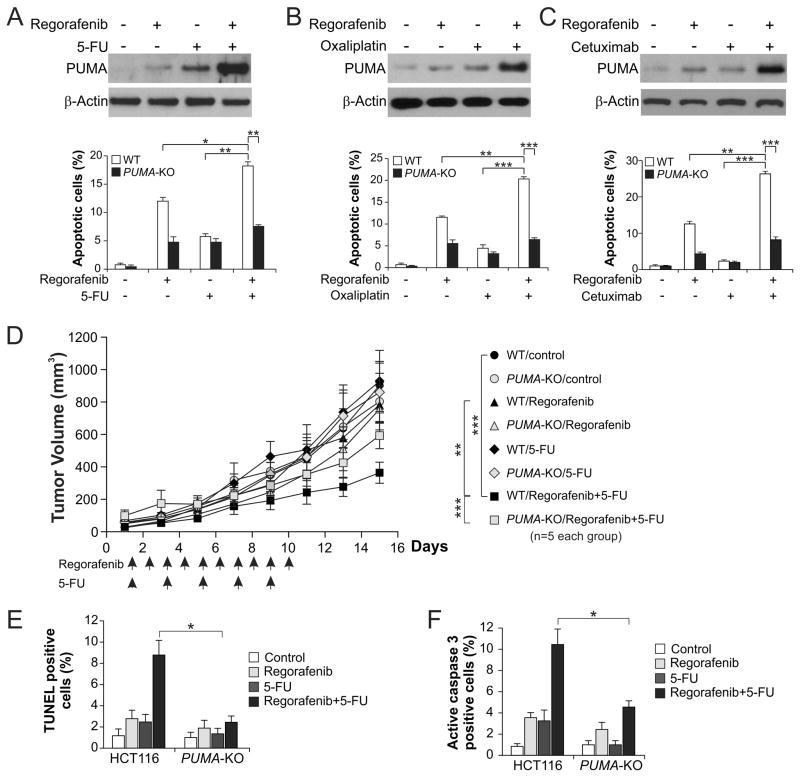
Regorafenib has been used in combination with other chemotherapeutic agents for CRC treatment (33). It is possible that the chemosensitization effects of regorafenib are mediated by simultaneous PUMA induction by regorafenib and other agents through different pathways. We found that regorafenib combined with 5-FU, oxaliplatin, or cisplatin induced higher levels of PUMA, compared to single agent alone (Fig. 6A, 6B and S3A). This is consistent with concurrent PUMA induction through both p53-dependent and -independent mechanism by DNA damage and regorafenib, respectively. Accordingly, the level of apoptosis was also significantly enhanced in WT HCT116 cells, but not in PUMA-KO cells following the combination treatment (Fig. 6A, 6B and S3B). Similarly, regorafenib combined with cetuximab, which induces PUMA through p73 via AKT inhibition (14), enhanced PUMA and apoptosis induction relative to regorafenib or cetuximab alone, and the enhanced apoptosis was PUMA-dependent (Fig. 6C). The PUMA-dependent sensitization effect was also observed in cells treated with regorafenib combined with the EGFR TKI gefitinib (Fig. S3C), or the broad-range kinase inhibitor UCN01 (Fig. S3D).
Figure 6. PUMA mediates the chemosensitization effects of regorafenib in vitro and in vivo.
(A) WT and PUMA-KO HCT116 cells were treated with 20 μmol/L regorafenib, 20 mg/L 5-fluorouracil (5-FU), or their combination. Upper, western blot analysis of PUMA expression in WT cells treated for 24 hours; lower, apoptosis in WT and PUMA-KO cells treated for 48 hours analyzed by nuclear staining with Hoechst 33258. (B) WT and PUMA-KO HCT116 cells were treated with 20 μmol/L regorafenib, 25 μmol/L oxaliplatin, or their combination. Upper, western blot analysis of PUMA expression in WT cells treated for 24 hours; lower, apoptosis in WT and PUMA-KO cells treated for 48 hours analyzed as in (A). (C) WT and PUMA-KO HCT116 cells were treated with 20 μmol/L regorafenib, 6 μg/mL of cetuximab, or their combination. Upper, western blot analysis of PUMA expression in WT cells treated for 24 hours; lower, apoptosis in WT and PUMA-KO cells treated for 48 hours analyzed as in (A). (D) Nude mice were injected s.c. with 4 × 106 WT or PUMA-KO HCT116 cells. After 1 week, mice were treated with 15 mg/kg regorafenib daily by oral gavage, 25 mg/kg 5-FU every other day by i.p. injection, or their combination for 10 consecutive days. Tumor volume at indicated time points after treatment was calculated and plotted with p values for indicated comparisons, n=5 in each group. Arrows indicate regorafenib or 5-FU injection. (E) Mice with WT or PUMA-KO HCT116 xenograft tumors were treated with 15 mg/kg regorafenib daily, 25 mg/kg 5-FU every other day, or their combination as in (D) for 4 consecutive days. Paraffin-embedded sections were analyzed by TUNEL staining. TUNEL-positive cells were counted and plotted. (F) Tissue sections from (E) were analyzed by active caspase 3 staining. Active caspase 3-positive cells were counted and plotted. Results in (E) and (F) were expressed as means ± SD of 3 independent experiments. ***, P <0.001; **, P <0.01; *, P <0.05.
To determine if PUMA mediates chemosensitization by regorafenib in vivo, nude mice with WT and PUMA-KO HCT116 xenograft tumors were treated with 15 mg/kg regorafenib, 25 mg/kg 5-FU, or their combination. The combination treatment more effectively suppressed the growth of WT tumors, compared to either regorafenib or 5-FU alone (Fig. 6D). However, the enhanced tumor suppression by the combination treatment was largely abolished in the PUMA-KO tumors (Fig. 6D and S4A), which was also associated with decreased apoptosis detected by TUNEL staining (Fig. 6E and S4B), and active caspase 3 staining (Fig. 6F and S4C). These findings suggest that PUMA mediates the chemosensitization effects of regorafenib in vitro and in vivo, and manipulating PUMA-mediated apoptosis can improve the therapeutic efficacy of regorafenib.
Discussion
The Ras/Raf/MEK/ERK pathway is aberrantly activated in a large fraction of colorectal tumors due to Ras and Raf mutations (34). Regorafenib is the first small molecule inhibitor of this pathway that has been approved by the Food and Drug Administration (FDA) for the treatment of metastatic CRC. Regorafenib differs from its analog sorafenib by single fluorine, which leads to a similar, yet distinct biochemical profile compared with sorafenib (24, 35). Although sorafenib has been extensively characterized, little is known about how regorafenib inhibits growth of CRC cells. The antiangiogenic activity of regorafenib is thought to largely contribute to its antitumor effects (35). Our results demonstrate for the first time that tumor suppression by regorafenib is dependent on the cell autonomous process of apoptosis induction, progressing from ERK inhibition, GSK3β activation, and p65 nuclear translocation, leading to PUMA induction and onset of mitochondria-mediated apoptosis. In initial clinical studies, regorafenib appears to be primarily cytostatic, which is unlike many chemotherapeutic drugs or targeted agents that are cytotoxic. Long-lasting tumor stabilization was observed in the majority of treated CRC patients (7). However, retrospective analysis revealed that a subset of treated patients had decreased radiological tumor density as well as cavitation of lung metastases during the course of treatment (36), which may be related to the pro-apoptotic activity of regorafenib. Changes in tumor microenvironment have recently emerged as an important mechanism of differential response and innate resistance to targeted therapies (37). Our findings suggest that both cell autonomous and microenvironmental effects of regorafenib contribute to tumor suppression, and they can affect each other through PUMA-mediated apoptosis. A systematic dissection of interactions between tumors and their microenvironment is necessary for understanding how regorafenib and other targeted agents inhibit tumor growth.
We performed most in vitro experiments using 40 μmol/L regorafenib to substantially induce PUMA and apoptosis in CRC cells. Importantly, the results of these experiments were confirmed by those from xenograft tumor experiments (Fig. 5 and 6). Phase 1 studies showed that the plasma concentrations of regorafenib can reach 5–10 μmol/L in patients (38, 39). The biologically active concentrations of regorafenib are likely to be higher than those in plasma due to binding to plasma proteins (35). Colorectal cells may also be topically exposed to orally administrated regorafenib at higher concentrations than those in circulation. Furthermore, regorafenib at a lower dose of 10 μmol/L was sufficient to induce PUMA expression (Fig. 1A). These observations suggest that induction of PUMA is likely to be involved in the effects of regorafenib at clinically relevant doses.
PUMA induction plays a role in apoptosis induced by a variety of chemotherapeutic agents, and may be a useful indicator of chemo sensitivity. We previously showed that PUMA induction correlates with differential sensitivity to EGFR TKIs in head and neck cancer cells, and lack of PUMA induction is associated with resistance to EGFR TKIs (14). Increased PUMA expression was associated with better prognosis in patients receiving 5-FU-based therapy in stage II and III CRC (40). Furthermore, a recent study demonstrated that response of isolated mitochondria from tumor cells to PUMA Bcl-2 homology 3 (BH3) peptide correlates with chemotherapy response in cancer patients (41). The results in this study suggest that PUMA induction may be useful as a surrogate biomarker for response of CRC to regorafenib. Although it is often difficult to obtain biopsies from colorectal tumors treated with chemotherapy after surgery, recent studies showed that biomarkers of therapeutic response can be analyzed using circulating tumor DNA (42, 43), or circulating tumor cells (44). It might be possible to determine PUMA induction by using such non-invasive approaches.
A number of clinical trials have been initiated to test combinations of regorafenib with conventional cytotoxic agents in CRC patients, such as 5-FU, oxaliplatin and irinotecan (33, 45). Our data indicate that enhanced PUMA induction mediates the in vitro and in vivo chemosensitization effects of regorafenib (Fig. 6). Regorafenib has also been combined with other targeted agents, such as the anti-VEGF antibody bevacizumab and the anti-EGFR antibodies cetuximab and panitumumab in ongoing clinical trials (33, 45). Incorporation of regorafenib is expected to sensitize CRC containing the G12 mutations in K-Ras or the V600E mutation in B-Raf, which compromise the effects of EGFR-targeted therapies (46). While regorafenib inhibits ERK and activates p65 to induce PUMA, EGFR inhibitors suppress AKT and induce p73 to activate PUMA (14). Concurrent PUMA induction through two distinct mechanisms can account for improved therapeutic efficacy of combining regorafenib and EGFR antibodies. In addition, p53-independent and/or -dependent PUMA induction also mediates apoptotic response to other targeted agents, such as the c-MET/ALK inhibitor crizotinib (47), the multi-kinase inhibitor drug sunitinib (48), and the HSP90 inhibitors (49). PUMA induction may serve as a useful indicator of effective drug combinations for developing more efficacious combination regimens with reduced doses and non-overlapping toxicities.
Drug resistance represents a major limitation of chemotherapy, and even more so for targeted therapies. Exploring PUMA-mediated apoptosis can be used to discover more effective anticancer agents to overcome chemo resistance. For this purpose, we have recently developed a high-throughput assay for detecting PUMA induction (unpublished data), which can be used for screening compound libraries to identify novel PUMA-inducing small molecules. A number of apoptosis-targeting agents have recently been developed and are evaluated in various clinical trials (50). These apoptosis-targeting agents may potentiate chemotherapeutic drugs such as regorafenib. Despite the encouraging results, the clinical relevance of our study remains to be further established using other preclinical models and human patient specimens from clinical trials, which will be the focus of our future investigations.
Supplementary Material
Statement of Translational Relevance.
Regorafenib, a multi-kinase inhibitor targeting the Ras/Raf/MEK/ERK pathway, has recently been approved for the treatment of metastatic colorectal cancer (CRC) and gastrointestinal stromal tumors (GIST). It is currently in many clinical trials in combination with other chemotherapeutic agents, such as the cytotoxic drugs 5-fluorouracil (5-FU), oxaliplatin and irinotecan, and the anti-epidermal growth factor receptor (EGFR) antibodies cetuximab and panitumumab, for CRC treatment. However, the anticancer mechanism of regorafenib in CRC cells has remained unclear. No effective biomarker has been described for predicting response of CRC to regorafenib. We demonstrate that the proapoptotic Bcl-2 family protein PUMA is critical for the antitumor, antiangiogenic and chemosensitization effects of regorafenib against CRC. Our results suggest that PUMA induction can be used as an indicator of responsiveness to regorafenib, and for developing more effective combination therapies involving regorafenib.
Acknowledgments
We thank Dr. Xin Huang for help with analysis of tumor hypoxia and our lab members for critical reading. This work is supported by NIH grants CA106348, CA121105, CA172136 (L.Z.), and CA129829 (J.Y.), and American Cancer Society grant RSG-10-124-01-CCE (J.Y.). This project used the UPCI shared glassware, cell imaging and animal facilities that were supported in part by award P30CA047904.
Abbreviations
- CA9
Carbonic Anhydrase 9
- ChIP
chromatin immunoprecipitation
- Cox IV
cytochrome oxidase subunit IV
- EGFR
epidermal growth factor receptor
- CRC
colorectal cancer
- 5-FU
5-fluorouracil
- FoxO3a
Forkhead Box O3a
- GIST
gastrointestinal stromal tumor
- GSK3β
glycogen synthase kinase 3β
- KO
knockout
- MEFs
mouse embryo fibroblasts
- NF-κB
nuclear factor κB
- PDGFR
platelet-derived growth factor receptor
- PI
propidium iodide
- PUMA
p53 upregulated modulator of apoptosis
- RT-PCR
reverse transcriptase-PCR
- siRNA
small interfering RNA
- TKI
tyrosine kinase inhibitor
- TNF-α
tumor necrosis factor-α
- TUNEL
Terminal deoxynucleotidyl transferase mediated dUTP Nick End Labeling
- VEGFR
vascular endothelial growth factor receptor
- WT
wild type
Footnotes
Disclosures: The authors declare no conflict of interest and all authors have agreed on the submission.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Poston GJ, Figueras J, Giuliante F, Nuzzo G, Sobrero AF, Gigot JF, et al. Urgent need for a new staging system in advanced colorectal cancer. J Clin Oncol. 2008;26:4828–33. doi: 10.1200/JCO.2008.17.6453. [DOI] [PubMed] [Google Scholar]
- 3.Segal NH, Saltz LB. Evolving treatment of advanced colon cancer. Annual review of medicine. 2009;60:207–19. doi: 10.1146/annurev.med.60.041807.132435. [DOI] [PubMed] [Google Scholar]
- 4.Chu E. An update on the current and emerging targeted agents in metastatic colorectal cancer. Clinical colorectal cancer. 2012;11:1–13. doi: 10.1016/j.clcc.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Martini M, Vecchione L, Siena S, Tejpar S, Bardelli A. Targeted therapies: how personal should we go? Nat Rev Clin Oncol. 2012;9:87–97. doi: 10.1038/nrclinonc.2011.164. [DOI] [PubMed] [Google Scholar]
- 6.Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–55. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 9.Abou-Elkacem L, Arns S, Brix G, Gremse F, Zopf D, Kiessling F, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther. 2013;12:1322–31. doi: 10.1158/1535-7163.MCT-12-1162. [DOI] [PubMed] [Google Scholar]
- 10.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–7. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 11.Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27 (Suppl 1):S71–83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu J, Yue W, Wu B, Zhang L. PUMA sensitizes lung cancer cells to chemotherapeutic agents and irradiation. Clin Cancer Res. 2006;12:2928–36. doi: 10.1158/1078-0432.CCR-05-2429. [DOI] [PubMed] [Google Scholar]
- 13.Dudgeon C, Wang P, Sun X, Peng R, Sun Q, Yu J, et al. PUMA induction by FoxO3a mediates the anticancer activities of the broad-range kinase inhibitor UCN-01. Mol Cancer Ther. 2010;9:2893–902. doi: 10.1158/1535-7163.MCT-10-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Q, Ming L, Thomas SM, Wang Y, Chen ZG, Ferris RL, et al. PUMA mediates EGFR tyrosine kinase inhibitor-induced apoptosis in head and neck cancer cells. Oncogene. 2009;18:2348–57. doi: 10.1038/onc.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang P, Qiu W, Dudgeon C, Liu H, Huang C, Zambetti GP, et al. PUMA is directly activated by NF-kappaB and contributes to TNF-alpha-induced apoptosis. Cell Death Differ. 2009;16:1192–202. doi: 10.1038/cdd.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ming L, Wang P, Bank A, Yu J, Zhang L. PUMA dissociates Bax and BCL-XL to induce apoptosis in colon cancer cells. J Biol Chem. 2006;281:16034–42. doi: 10.1074/jbc.M513587200. [DOI] [PubMed] [Google Scholar]
- 17.Yu J, Wang P, Ming L, Wood MA, Zhang L. SMAC/Diablo mediates the proapoptotic function of PUMA by regulating PUMA-induced mitochondrial events. Oncogene. 2007;26:4189–98. doi: 10.1038/sj.onc.1210196. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100:1931–6. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudgeon C, Peng R, Wang P, Sebastiani A, Yu J, Zhang L. Inhibiting oncogenic signaling by sorafenib activates PUMA via GSK3beta and NF-kappaB to suppress tumor cell growth. Oncogene. 2012;31:4848–58. doi: 10.1038/onc.2011.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Yu J, Zhang L. The nuclear function of p53 is required for PUMA-mediated apoptosis induced by DNA damage. Proc Natl Acad Sci U S A. 2007;104:4054–9. doi: 10.1073/pnas.0700020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Wang P, Sun Q, Ding WX, Yin XM, Sobol RW, et al. Following cytochrome c release, autophagy is inhibited during chemotherapy-induced apoptosis by caspase 8-mediated cleavage of Beclin 1. Cancer Res. 2011;71:3625–34. doi: 10.1158/0008-5472.CAN-10-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yue W, Sun Q, Dacic S, Landreneau RJ, Siegfried JM, Yu J, et al. Downregulation of Dkk3 activates beta-catenin/TCF-4 signaling in lung cancer. Carcinogenesis. 2008;29:84–92. doi: 10.1093/carcin/bgm267. [DOI] [PubMed] [Google Scholar]
- 23.Ming L, Sakaida T, Yue W, Jha A, Zhang L, Yu J. Sp1 and p73 Activate PUMA Following Serum Starvation. Carcinogenesis. 2008;29:1878–84. doi: 10.1093/carcin/bgn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 25.Qiu W, Wang X, Leibowitz B, Liu H, Barker N, Okada H, et al. Chemoprevention by nonsteroidal anti-inflammatory drugs eliminates oncogenic intestinal stem cells via SMAC-dependent apoptosis. Proc Natl Acad Sci U S A. 2010;107:20027–32. doi: 10.1073/pnas.1010430107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–63. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang S, Sinicrope FA. Sorafenib inhibits STAT3 activation to enhance TRAIL-mediated apoptosis in human pancreatic cancer cells. Mol Cancer Ther. 2010;9:742–50. doi: 10.1158/1535-7163.MCT-09-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutta J, Fan Y, Gupta N, Fan G, Gelinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;25:6800–16. doi: 10.1038/sj.onc.1209938. [DOI] [PubMed] [Google Scholar]
- 29.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 31.Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, et al. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–70. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Kim DJ, Lee MH, Reddy K, Li Y, Lim do Y, Xie H, et al. CInQ-03, a novel allosteric MEK inhibitor, suppresses cancer growth in vitro and in vivo. Carcinogenesis. 2013;34:1134–43. doi: 10.1093/carcin/bgt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strumberg D, Schultheis B. Regorafenib for cancer. Expert opinion on investigational drugs. 2012;21:879–89. doi: 10.1517/13543784.2012.684752. [DOI] [PubMed] [Google Scholar]
- 34.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 35.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–40. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricotta R, Sartore-Bianchi A, Verrioli A, Vanzulli A, Siena S. Regorafenib for metastatic colorectal cancer. Lancet. 2013;381:1537. doi: 10.1016/S0140-6736(13)60976-9. [DOI] [PubMed] [Google Scholar]
- 37.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–4. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mross K, Frost A, Steinbild S, Hedbom S, Buchert M, Fasol U, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:2658–67. doi: 10.1158/1078-0432.CCR-11-1900. [DOI] [PubMed] [Google Scholar]
- 39.Strumberg D, Scheulen ME, Schultheis B, Richly H, Frost A, Buchert M, et al. Regorafenib (BAY 73-4506) in advanced colorectal cancer: a phase I study. Br J Cancer. 2012;106:1722–7. doi: 10.1038/bjc.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinicrope FA, Rego RL, Okumura K, Foster NR, O’Connell MJ, Sargent DJ, et al. Prognostic impact of bim, puma, and noxa expression in human colon carcinomas. Clin Cancer Res. 2008;14:5810–8. doi: 10.1158/1078-0432.CCR-07-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–33. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–6. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diaz LA, Jr, Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537–40. doi: 10.1038/nature11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krebs MG, Metcalf RL, Carter L, Brady G, Blackhall FH, Dive C. Molecular analysis of circulating tumour cells-biology and biomarkers. Nat Rev Clin Oncol. 2014;11:129–44. doi: 10.1038/nrclinonc.2013.253. [DOI] [PubMed] [Google Scholar]
- 45.Festino L, Fabozzi A, Manzo A, Gambardella V, Martinelli E, Troiani T, et al. Critical appraisal of the use of regorafenib in the management of colorectal cancer. Cancer management and research. 2013;5:49–55. doi: 10.2147/CMAR.S34281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linardou H, Dahabreh IJ, Kanaloupiti D, Siannis F, Bafaloukos D, Kosmidis P, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. The lancet oncology. 2008;9:962–72. doi: 10.1016/S1470-2045(08)70206-7. [DOI] [PubMed] [Google Scholar]
- 47.Zheng X, He K, Zhang L, Yu J. Crizotinib induces PUMA-dependent apoptosis in colon cancer cells. Mol Cancer Ther. 2013;12:777–86. doi: 10.1158/1535-7163.MCT-12-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun J, Sun Q, Brown MF, Dudgeon C, Chandler J, Xu X, et al. The Multi-Targeted Kinase Inhibitor Sunitinib Induces Apoptosis in Colon Cancer Cells via PUMA. PLoS One. 2012;7:e43158. doi: 10.1371/journal.pone.0043158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He K, Zheng X, Zhang L, Yu J. Hsp90 inhibitors promote p53-dependent apoptosis through PUMA and Bax. Mol Cancer Ther. 2013 Aug 21; doi: 10.1158/1535-7163.MCT-13-0284. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang L, Ming L, Yu J. BH3 mimetics to improve cancer therapy; mechanisms and examples. Drug Resist Updat. 2007;10:207–17. doi: 10.1016/j.drup.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.