Abstract
Purpose
PD-L1 is the main ligand for the immune inhibitory receptor PD-1. This ligand is frequently expressed by melanoma cells. In this study we investigated whether PD-L1 expression is controlled by melanoma driver mutations and modified by oncogenic signaling inhibition.
Experimental Design
Expression of PD-L1 was investigated in a panel of 51 melanoma cell lines containing different oncogenic mutations, including cell lines with innate and acquired resistance to BRAF inhibitors. The effects of targeted therapy drugs on expression of PD-L1 by melanoma cells were investigated.
Results
No association was found between the level of PD-L1 expression and mutations in BRAF, NRAS, PTEN or amplification of AKT. Resistance to vemurafenib due to the activation of alternative signaling pathways was accompanied with the induction of PD-L1 expression, while the resistance due to the reactivation of the MAPK pathway had no effect on PD-L1 expression. In melanoma cell lines the effects of BRAF, MEK and PI3K inhibitors on expression of PD-L1 were variable from reduction to induction, particularly in the presence of INFγ. In PD-L1-exposed lymphocytes, vemurafenib paradoxically restored activity of the MAPK pathway and increased the secretion of cytokines.
Conclusions
In melanoma cell lines, including BRAF inhibitor-resistant cells, PD-L1 expression is variably regulated by oncogenic signaling pathways. PD-L1-exposed lymphocytes decrease MAPK signaling, which is corrected by exposure to vemurafenib, providing potential benefits of combining this drug with immunotherapies.
Keywords: PD-1, PD-L1, BRAF inhibitor, melanoma, targeted therapy, acquired resistance
Background
Recent developments in the field of targeted therapy and immunotherapy have increased the life expectancy of patients with advanced melanoma. These therapies include BRAF inhibitors (BRAFi) such as vemurafenib and dabrafenib (1, 2), the MEK inhibitor (MEKi) trametinib (3), and the anti-cytotoxic T lymphocyte-4 (CTLA4) antibody ipilimumab (4, 5). Further advances are being made with other therapies including anti-programmed cell death 1 (PD-1) and programmed death ligand-1 (PD-L1, B7-H1 or CD274) antibodies (6, 7, 8), and tumor infiltrating lymphocytes (TIL) or T cell receptor (TCR)-engineered adoptive cell transfer (ACT) therapy (9).
BRAF mutation at position V600 is detected in approximately half of melanomas. This mutation causes over-activity of downstream signaling in the MAPK pathway (10). Growth of melanoma cells with the BRAFV600 mutation can be effectively blocked by BRAFi (11, 12). Interestingly, contrary to BRAF mutant cancer cells, in other cells with wild type BRAF gene, BRAFi paradoxically activates the MAPK pathway (13), which is also evident in activated lymphocytes (14). Despite the initial response of BRAF mutant tumors to the BRAF inhibitors, acquired resistance develops frequently and most patients will relapse within months (15). To prevent the emergence of resistance through the reactivation of MAPK pathway, treatment regiments with the combinations of BRAF and MEK inhibitors have been investigated in clinical trials indicating higher beneficiary effects and lower side effects (3). Moreover, to block the resistance through alternative signaling pathways (16), clinical trials with the combinations of BRAF and PI3K/AKT inhibitors have been initiated (US cooperative group clinical trial S1221, NCT 01902173).
Melanoma has been considered an immunogenic malignancy. However, the endogenous anti-tumor immune response is not sufficient to control the cancer in the great majority of cases. Therefore, different strategies have been devised to augment the immune reaction against the melanoma cells (17, 18, 19). Ipilimumab is an anti-CTLA4 antibody that blocks the CTLA4-induced T cell inhibition in the activation phase of an antitumor immune response. In two randomized clinical trials, this anti-CTLA4 antibody has shown improvement in overall survival of patients with metastatic melanoma (4, 20).
The co-inhibitory receptor/ligand pair, PD-1/PD-L1, is one of the main peripheral regulatory mechanisms for induction of anergy in immune cells and maintenance of peripheral tolerance (21). PD-1 expression is induced on the cell surface upon T cell activation (22). The cytoplasmic domain of PD-1 contains a tyrosine-based inhibitory motif (ITIM) and an immunoreceptor tyrosine-based switch motif (ITSM). Interaction with PD-L1 causes phosphorylation of a tyrosine in the ITSM motif of PD-1 receptor. Consequently SH2-domain containing tyrosine phosphatase 2 (SHP-2), and possibly SHP-1, are recruited to this motif of PD-1 resulting in down-regulation of the PI3K/AKT and MAPK signaling pathways downstream of the TCR and blockade of cell cycle progression in the immune cells (23, 24).
Constitutive PD-L1 expression has been detected in different tumors including melanoma (21). Moreover, on tumor cells, expression of PD-L1 can be induced by interferon gamma (INFγ) and other cytokines that are produced by the activated lymphocytes (25). In other words, tumor-reacting immune cells inadvertently trigger an inhibitory mechanism, which has been termed adaptive immune resistance (26). Therefore, therapeutic attempts to block PD-L1 and PD-1 interaction should shift the balance toward higher activity of anti-tumor immune cells. Indeed, in a recent clinical trial with the anti–PD-1 antibody nivolumab objective responses in 18–28% of patients with different cancers was observed (7), and another anti-PD-1 antibody MK-3475 (lambrolizumab), had a response rate of 38% in patients with advanced melanoma (6).
Both targeted therapy and immunotherapy have shown to be effective in melanoma, while they function through different mechanisms. Therefore, the idea of combining these two types of therapies has been very appealing. However, the effects of targeted therapy drugs on the effectors and targets of immune system have not been clearly understood (27). Moreover, the associations or the cross talk between the oncogenic driver pathways in cancer cells and the immunoregulatory pathways, such as PD-L1, have not been fully elucidated. In this study, we investigated the expression level of PD-L1 in a panel of 51 melanoma cell lines, including vemurafenib sensitive and resistant BRAF mutants, NRAS mutants and NRAS/BRAF wild types. Some of the cell lines also contained additional mutations in signaling molecules involved in the PI3K/AKT pathway. Moreover, in the presence or absence of INFγ, or in a co-culture of melanoma cells and lymphocytes, we studied the effects of blocking the main oncogenic driver pathways on expression of PD-L1. The paradoxical MAPK activating effect of vemurafenib on signaling and cytokine production of lymphocytes that are exposed to the inhibitory effects of PD-1/PD-L1 interaction was studied as well.
Materials & Methods
Reagents and Cell Lines
Vemurafenib and trametinib were purchased from Selleck chemicals (Houston, TX) and the pan-PI3K inhibitor (PI3Ki) GSK2126458 was obtained from GlaxoSmithKline (GSK, PA) under a material transfer agreement. Interferon γ (INFγ) was obtained from Sigma Aldrich (St Louis, MO). Human melanoma cell lines (M series) were established from patient's biopsies under UCLA IRB 11-003254 as previously described (16). WM1366 and SBCL2 were obtained from the American Type Culture Collection (ATCC, Rockville, MD). Growing media, maintenance and mycoplasma testing of the cell lines were as described before (16). BRAF mutant cell lines with the in vitro acquired vemurafenib resistance are indicated by the AR suffixes. Except M249AR4 that contains a secondary NRAS mutation, the rest of acquired resistant cell lines were maintained in the growing media containing 1µM of vemurafenib.
Mutation Identification of the Cell Lines
Mutations in the cell lines were determined as it has been described before (12). In brief, the genomic DNA isolated from each cell line was subjected to analysis by OncoMap 3 or Iontrone, and the mutations were confirmed by direct Sanger sequencing.
Cell Proliferation and Viability Assays
The growth and viability of the cells treated with serial dilutions of vemurafenib were determined by the bioluminescence assay (Promega, Fitchburg, WI) as it was described previously (16). Growth inhibition rates were used to calculate the IC50 for each cell line. Each assay was performed at least twice in duplicates.
siRNA Transfection
Cell lines were transfected with the PD-L1 specific or no target control siRNAs (Dharmacon, Lafayette, CO) as it has been described before (16). Transfected cells were harvested after 72 hours for detection of PD-L1 levels by flow cytometry assays.
Lymphocyte Preparation
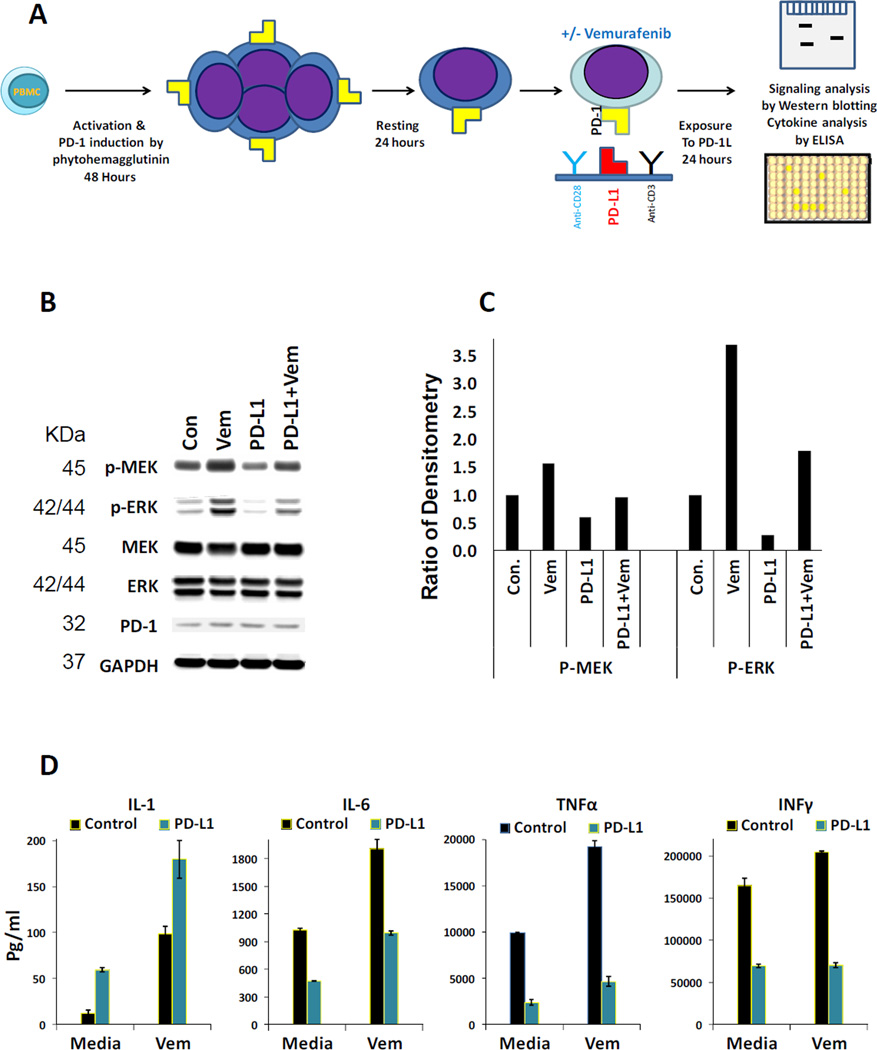
To induce production of PD-1, frozen PBMCs (obtained under UCLA IRB 10-001598) were defrosted and after treatment with 227U/ml of DNase (Sigma, St Louis, MO) for 30 minutes, transferred to RPMI1640 containing 5µg/ml phytohemagglutinin (Sigma, St Louis, MO), 5% heat inactivated human AB serum, 1% penicillin, streptomycin and amphotericin (Omega Scientific, Tarzana, CA) and incubated for 48 hours (24). This PBMC culturing method was used to induce proliferation of activated T lymphocytes by mitogen activation and precondition them to express PD-1. Then cells were rested overnight in the same growth condition minus the phytohemagglutinin (Figure 4 A). These cells then were used for the co-culture with the melanoma cells or for the exposure to the recombinant PD-L1 (SinoBio Biotech, Shanghai, China).
Figure 4. Restoration of the MAPK pathway activity and enhancement of cytokine production by vemurafenib in lymphocytes exposed to PD-L1.
A) PBMCs were primed by mitogen activation to express PD-1. This priming by mitogen activation leads to proliferation of activated T lymphocytes. Then in the presence and absence of vemurafenib, cells were exposed to anti-CD3/antiCD28 or anti-CD3/antiCD28 plus recombinant PD-L1 coated plates for 24 hours. B) Western blot analysis of the MAPK pathway activity in the lymphocytes exposed to the mentioned conditions described in the section A. C) Quantitation of the same Western blot analysis by densitometry. D) ELISA assays were performed in duplicates to detect the concentration of cytokines in the supernatants of lymphocytes primed and treated according to the same experimental settings described in the section A. Error bars are two standard deviations.
Drug Treatment of Melanoma Cells and Co-culture with Lymphocytes
Melanoma cells were plated in two sets in 12 well plates and the following day, they were treated either with DMSO, BRAFi (vemurafenib), MEKi (trametinib) or the PI3Ki. After another 24 hours, one set was treated with media and the other set with INFγ. The final concentrations of the drugs were 1.5µM of vemurafenib, 25nM of trametinib, 100nM of PI3Ki and 200U/ml of INFγ. In the case of co-cultures, all the steps and conditions were the same except at the start of drug treatment the media was switched to the media containing 5% of human AB serum instead of FBS and the next day a suspension of lymphocytes (primed as described in the above) was added to each well. The final concentration of lymphocytes was 550,000 cells/ml. Each assay was repeated at least twice.
Exposure of Lymphocytes to PD-L1 and Cytokine Analysis by ELISA
Non-tissue culture 24 well plates were coated overnight with a mixture of 60µg/ml of Fc-PD-L1 recombinant protein (SinoBio Biotech, Shanghai, China), 15.4µg/ml of anti-CD3 and 19.4 µg/ml of anti-CD28 (both from BD biosciences San Jose, CA) in PBS or the same mixture containing Fc instead of Fc-PD-L1 as the control. The next day, plates were blocked with 2.5% human serum albumin (Octapharma, Stockholm, Sweden) in PBS for one hour. After removing the blocking solution, at a concentration of 700,000 cells/ml, lymphocytes (prepared as described in the above) were added to the wells in the presence of DMSO or 1.5µM of vemurafenib in RPMI1640 + 5% of human AB serum and 1% of antibiotics. After 24 hours, each supernatant was collected for performing cytokine ELISA assays and the cells were harvested for the Western blot analysis. Concentrations of IL-1, IL-6, TNFα and INFγ were determined by eBioscience (San Diego, CA) ELISA kits. The assays were performed in duplicates according to the instruction of the manufacturer after diluting the samples 1:100 for TNFα and INFγ assays and 1:10 for IL-1 and IL-6 assays.
Western Blotting
Lymphocytes were primed and treated as it was mentioned in the above and after harvest were lysed and subjected to Western blotting. Primary antibodies included p-ERK Thr204/205, ERK, p-MEK Ser217/221, MEK, GAPDH (all from Cell Signaling Technology, Danvers, MA) and PD-1 from Biolegend (San Diego, CA). Western blotting was performed as previously described (16). Densitometry of bands were performed by using ImageJ program.
Flow Cytometry
Melanoma cells were probed with anti-PD-L1 (CD274) in APC (catalog # 329708, Biolegend). In the co-culture experiments to distinguish melanoma cells from the immune cells, samples were stained with both anti-CD274 and anti-CD45 in Brilliant Violet 605 (catalog # 304042, Biolegend). All Live/Dead discrimination was performed with 7AAD (A07704, Beckman Coulter, Brea, CA). Lymphocytes were stained with CD45, CD8a in Brilliant Violet 650(301041, Biolegend, San Diego, CA), CD4 in Brilliant Violet 510 (317444, Biolegend, San Diego, CA). All samples were run on a Beckton Dickinson LSRII flow cytometer. The optimal staining amounts of antibodies were determined by internal lab experiments, and applied to the cells at fifteen minutes at room temperature, while protected from light. Cells were gated according to the following schema: Morphology was determined by using the area of the forward scatter emission peak (FSC-A) versus the area of the side scatter emission peak (SSC-A). Segregation of single cells was determined using SSC-A versus the width of the side scatter emission (SSC-W). Comparing the 7AAD to the APC emission peak allowed analysis of PD-L1 for the melanoma cells. For co-culture assays, Live/Dead and lymphocyte discrimination was determined by comparing area of the 7AAD emission peak versus the area of the CD45 emission peak and then 7AAD versus APC for melanoma cell. Median Fluorescent Intensity (MFI) of PD-L1 was taken from plots of PD-L1.
Statistical Analysis
Analyses were performed in the R environment (R Core Team (2013)), and MS Excel.
Results
Distribution of PD-L1 expression among melanoma cell lines with different oncogenic driver mutations
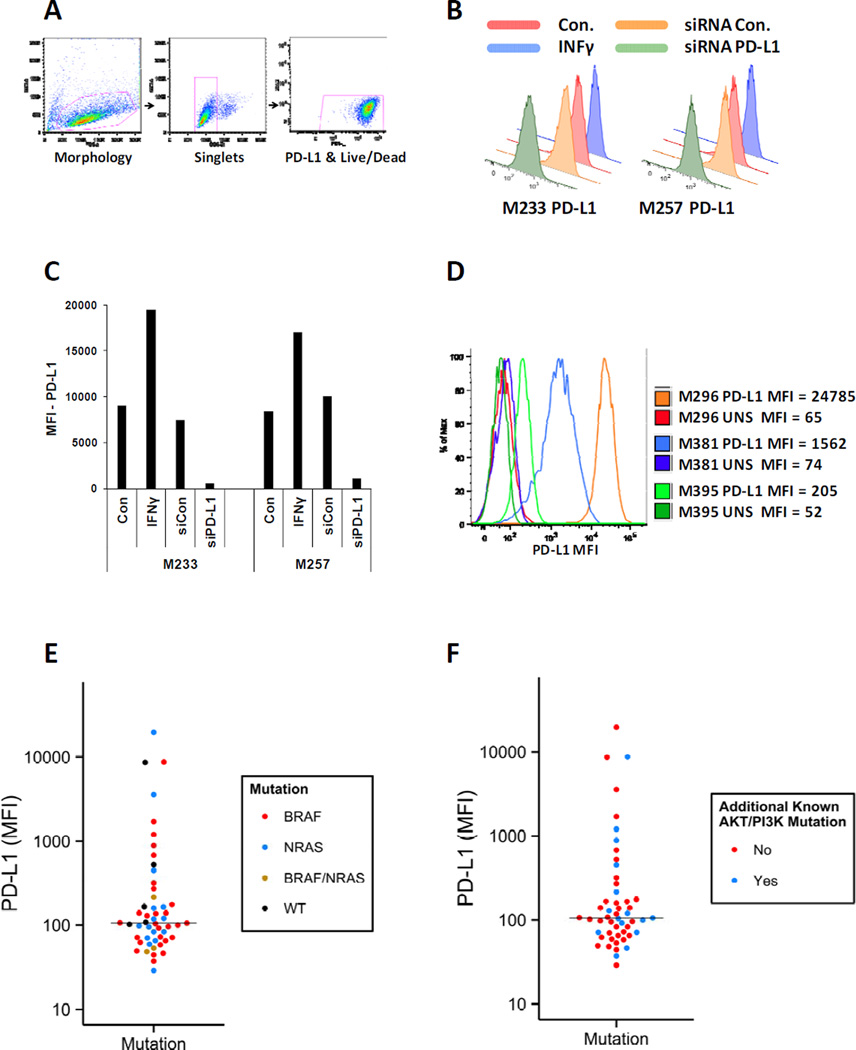
To investigate the levels and prevalence of PD-L1 expression, a panel of 51 melanoma cell lines was analyzed by flow cytometry. For this purpose, at first, the validity of the anti-PD-L1 antibody was evaluated by its ability to detect the increase in expression of PD-L1 upon treatment of two melanoma cell lines (M233 and M257) with INFγ and the decrease of PD-L1 level after siRNA knockdown of this ligand. By using the optimal titration of the antibody, flow cytometry assay indicated around two fold induction of MFI by INFγ and around 90% reduction of MFI by the PD-L1 siRNA pool in comparison with the non-targeted control siRNA pool (Figure 1 A, B and C).
Figure 1. PD-L1 expression by melanoma cell lines according to their mutations in the MAPK or PI3K/AKT pathways.
A) Gating strategy for detection of PD-L1 levels in melanoma cell lines stained with APC anti-PD-L1 antibody (catalog # 329708, Biolegend). For differentiation of live/dead, 7AAD exclusion staining was used. B) Validation of the anti-PD-L1 antibody by investigating its ability to detect induction of this ligand in the presence of 200u/ml of INFγ and reduction of this ligand after siRNA knockdown. Histograms show PD-L1 levels on the surface of two melanoma cell lines (M233 and M257) in the mentioned conditions. C) Bar graph of PD-L1 MFIs for the same cell lines in the mentioned conditions. D) Examples of flow cytometry histograms of PD-L1 levels in three melanoma cells lines with almost two log fold differences in expressions of this ligand. The PD-L1 levels were determined in the absence of INFγ. E) Expression levels of PD-L1 in a panel of melanoma cell lines containing 27 BRAF mutants, 15 RAS mutants, 3 BRAF/NRAS double mutant and 6 BRAF/NRAS wild types were measured by flow cytometry and graphed by using the MFI of this ligand for each cell line. The assays on each single sample were repeated at least twice. Each dot represents one cell line and is color coded according to the mutation status of BRAF and RAS genes. The median of PD-L1 expression for all the cell lines is shown by the horizontal line around MFI of 100. The MFI distributions of the different mutants are not significantly different (Kolmogorov–Smirnov test). F) The same cell lines in the panel are color coded according to the absence (red dots) or presence (blue dots) of mutations or amplification of genes involved in the PI3K/AKT pathway. Both MFI distributions are not significantly different (Kolmogorov–Smirnov test). The PD-L1 levels in figure E and F were determined in the absence of INFγ.
The panel of melanoma cell lines used in this study contained 27 BRAF mutants, 15 RAS mutants, 3 BRAF/NRAS double mutants and 6 BRAF/RAS wild types (Table 1). Expression level of PD-L1 was variable among melanoma cell lines showing a range of more than 2 log difference (Figure 1 D). Of note, in this analysis we evaluated the un-stimulated (constitutive) expression of PD-L1 without exposure to interferons. In this panel of cell lines, the measured MFI levels of PD-L1 showed random distributions (Kolmogorov–Smirnov test) independent of the driver mutation in the melanoma cells (Figure 1 E). The majority of cell lines showed very low levels of PD-L1 expression (MFI<100). The highest PD-L1 expression was detected in four of the cell lines (M296, M233, M257 and WM1366), which were 4 to 22 fold higher than the average expression of PD-L1 on all the cell lines in the panel. M296 and WM1366 are NRAS mutants, M233 is a BRAF mutant, and M257 is a NRAS/BRAF wild type. These results indicate that very high expression of PD-L1 is not specific to any particular group of driver oncogene mutated melanoma cell lines.
Table 1.
Characterization of the cell lines.
| Cell line | Mutation/alteration in MAPK pathway |
Sensitivity to Vemurafenib |
AKT/PI3K Mutation or Amplification |
|---|---|---|---|
| M229 | BRAF | S | AKT1 Amplification, Heterozygous PTEN |
| M229AR | BRAF | R | AKT1 Amplification, Heterozygous PTEN |
| M233 | BRAF | R | AKT1 Amplification, PTEN null |
| M238 | BRAF | S | Heterozygous PTEN |
| M238AR | BRAF | R | Heterozygous PTEN |
| M249 | BRAF | S | AKT2 Amplification, PTEN null |
| M255 | BRAF | R | AKT2 Amplification |
| M262 | BRAF | S | AKT1 Mutation |
| M263 | BRAF | S | |
| M297 | BRAF | S | |
| M299 | BRAF | R | |
| M308 | BRAF | R | AKT2 Amplification |
| M370 | BRAF | R | |
| M381 | BRAF | R | |
| M383 | BRAF | R | |
| M395 | BRAF | S | |
| M397 | BRAF | S | PTEN Null |
| M399 | BRAF | R | PTEN Null |
| M406 | BRAF | S | |
| M407 | BRAF | S | |
| M409 | BRAF | R | |
| M409AR | BRAF | R | |
| M410 | BRAF | R | |
| M411 | BRAF | S | PTEN Null |
| M414 | BRAF | R | |
| M417 | BRAF | R | |
| M397AR | BRAF/(Truncated BRAF) | R | PTEN Null |
| M249AR | BRAF/NRAS | R | AKT2 Amplification, PTEN null |
| M376 | BRAF/NRAS | R | |
| M398 | BRAF/NRAS | R | |
| M418 | KRAS | NA | |
| M202 | NRAS | NA | |
| M207 | NRAS | NA | Heterozygous PTEN |
| M243 | NRAS | NA | |
| M244 | NRAS | NA | |
| M245 | NRAS | NA | |
| M296 | NRAS | NA | |
| M311 | NRAS | NA | |
| M318 | NRAS | NA | PI3KCA mutation |
| M408 | NRAS | NA | |
| M412A | NRAS | NA | |
| M412B | NRAS | NA | |
| SBCL2 | NRAS | NA | |
| SKMEL 173 | NRAS | NA | |
| WM1366 | NRAS | NA | |
| M230 | WT | NA | |
| M257 | WT | NA | |
| M285 | WT | NA | |
| M368 | WT | NA | |
| M375 | WT | NA | |
| PB | WT | NA |
AR: Acquired Resistance, WT: BRAF and NRAS Wild Type. S: Sensitive, R: Resistant, NA: Not Applicable
It had been previously proposed that PTEN deletion and increased PI3K/AKT signaling resulted in increased PD-L1 expression in glioblastoma (28). To investigate a possible association between over activity of PI3K/AKT pathway and the expression of PD-L1, the same panel of melanoma cell lines was analyzed according to the absence or presence of known mutations, deletions or amplification of genes involved in activity of the PI3K/AKT pathway (Table 1). Cell lines with PI3KCA and heterozygous PTEN mutations, PTEN null expression, and amplification or mutation in any of the AKT isoforms showed a distribution pattern of PD-L1 expression similar to the other cell lines (Kolmogorov–Smirnov test) ranging from very low to very high levels (Figure 1 F). Therefore, PD-L1 expression in this large panel of melanoma cell lines with defined oncogenic alterations was independent of the oncogenic events in the MAPK and PI3K/AKT pathways.
Expression of PD-L1 in vemurafenib sensitive and resistant BRAF mutant cell lines
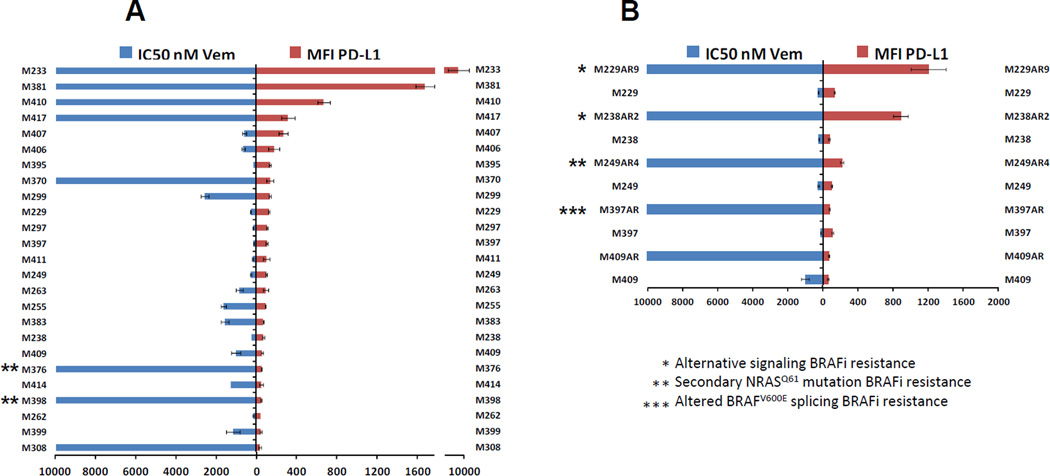
It has been reported that vemurafenib-resistant melanoma cell lines exhibit induction of PD-L1 expression (29). In the current study, after performing growth inhibition assays, any cell line with an IC50>1000nM of vemurafenib was considered to be resistant to this drug. Expression of PD-L1 on BRAF mutated cell lines and their levels of sensitivity to vemurafenib were graphed side by side (Figure 2 A). Cell lines were arranged from the highest to the lowest expression of PD1-L. As compared with the expression levels of PD-L1, cell lines with different IC50s were randomly distributed. Although no association between the level of PD-L1 and the response to the BRAF inhibitor could be seen, all the sensitive cell lines (IC50<1000nM) showed relatively low levels of PD-L1 expression (MFI<275) with the median MFIs of 106. Cell lines with an average resistance to vemurafenib (1000<IC50<3000nM) also had low levels of PD-L1 expression with the median of MFIs equal to 65. However, very resistant cell lines (IC50>10000nM) showed a bimodal distribution of PD-L1 expression with an overall median of MFIs equal to 229. Out of eight very resistant cell lines, four exhibited low levels of PD-L1 (MFI<139) and four showed high levels between 320 to 9000 PD-L1 MFI. It should be mentioned that the BRAF mutant resistant cell lines, M376 and M398, contain secondary NRAS mutations (16, 30) which is the cause of resistance to vemurafenib. Both of these cell lines showed very low levels of PD-L1 expression.
Figure 2. Comparison of PD-L1 expression in BRAF mutated melanoma cell lines with their responses to the BRAF inhibitor vemurafenib.
A) Expressions of PD-L1 in BRAF mutated cell lines and their levels of sensitivities to the BRAF inhibitor vemurafenib were graphed side by side. Each assay was performed twice and resistance to vemurafenib was defined as an IC50 of higher than 1000nM. B) Side by side expression of PD-L1 and the IC50 levels of five pairs of sensitive parental cell lines and their in vitro developed vemurafenib resistant sublines indicated by the AR suffixes after the cell lines names. Mechanism of resistance due to the activation of alternative pathways, secondary NRAS mutation and truncated BRAF has been indicated by one, two or 3 asterisks, respectively. In both graph A and B, a bimodal pattern of PD-L1 expression among the highly resistant cell lines can be seen.
The same analysis was performed with 5 pairs of sensitive parental and their in vitro acquired vemurafenib resistant sub-cell lines. Similar to the innately resistant cell lines, in vitro acquired vemurafenib resistant sub-cell lines also showed a bimodal pattern of PD-L1 expression. As shown in figure 2 B, two of the resistant cell lines (M229AR9 and M238AR2) exhibited more than 9 fold induction of the ligand in comparison to their parental sensitive cell lines. Expression of PD-L1 in M249AR4 was still at low levels (MFI=216), although it was twice of PD-L1 expression in the M249 parental cell line. Two of these resistant lines (M397AR and M409AR) showed the same or slightly lower levels of PD-L1 expression compared to their parental lines. M229AR9 and M238AR2, the two resistant cell lines with induction of PD-L1, also show induction of PDGFRβ expression and their mechanism of resistance to vemurafenib is through the activation of alternative signaling pathways other than the MAPK (30). M249AR4 is a PTEN null cell line with a secondary NRAS mutation that causes reactivation of the MAPK pathway and resistance to vemurafenib (16, 30). Mechanism of resistance in M397AR is alternative splicing of BRAF mRNA and expression of truncated BRAF, which renders vemurafenib ineffective (31). The mechanism of resistance in M409AR is still under investigation. Therefore, it seems that the resistance due to the activation of alternative signaling pathways is accompanied with a high induction of PD-L1, while the resistance due to the reactivation of MAPK has no or slight inducing effect on the expression of this ligand.
Effect of major signaling pathway blockade by targeted therapy agents on expression of PD-L1
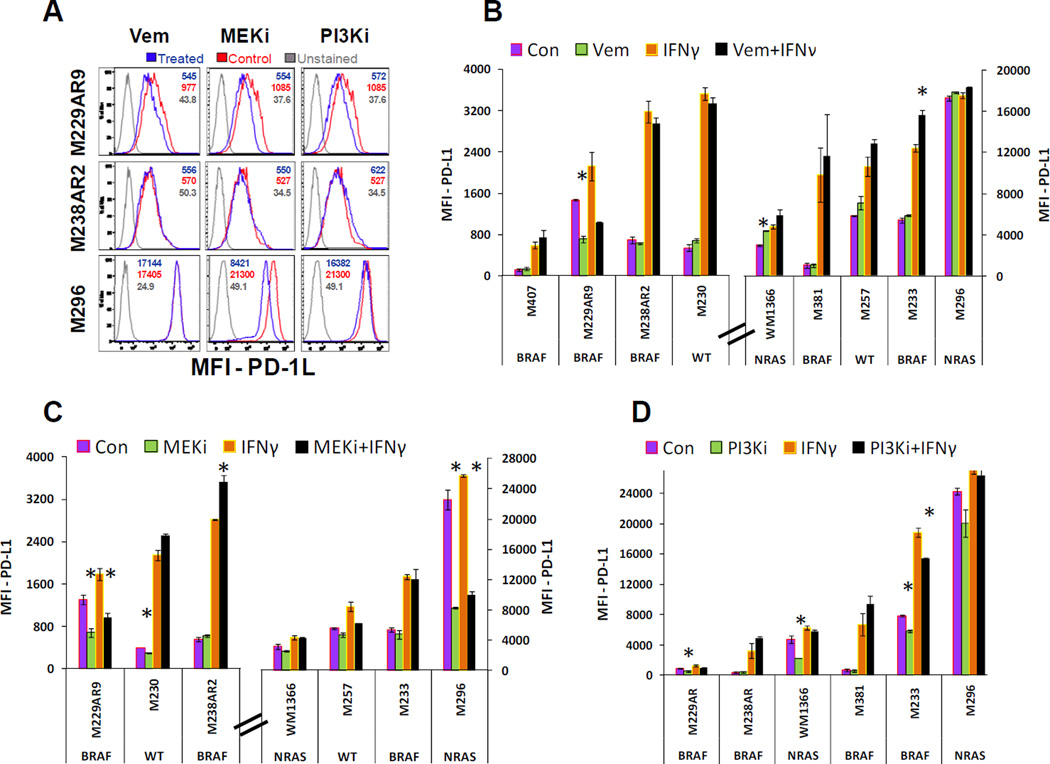
The expression of PD-L1 is mainly controlled by INFγ through the activation of the JAK/STAT pathway (25), whereas over-activity of the MAPK pathway is the main oncogenic driver in the majority of melanomas. In addition, the role of the PI3K/AKT pathway and its cross talk with the MAPK pathway in survival and resistance to inhibitors of the MAPK pathway has been described (16). To investigate the potential effects of these pathways on expression of PD-L1, in the presence and absence of INFγ, cell lines representative of BRAF mutant, NRAS mutant and BRAF/NRAS wild type melanoma cell lines were treated with solvent, vemurafenib, the MEK inhibitor trametinib or a PI3Ki and analyzed for the MFI of PD-L1 surface expression (Figure 3 A).
Figure 3. Effects of targeted therapy drugs in combination with INFγ on expression of PD-L1 in melanoma cell lines.
A) Three examples of flow cytometry assays performed on melanoma cell lines treated with either the solvent (control), vemurafenib (1.5µM), MEKi (25nM), or PI3Ki (100nM) shown by histogram plots. In M229AR9 and M296 cell lines, MFI as the indication of PD-L1 expression level can be decreased significantly by some of the treatments, while in comparison with the unstained sample, the cell population is still in the positive range for expression of this ligand. B) The effect of vemurafenib (1.5µM) on expression of PD-L1 in melanoma cell lines in the presence and absence of INFγ (200u/ml). C) The effect of the MEK inhibitor, trametinib, at 25nM on expression of PD-L1 in melanoma cell lines in the presence and absence of INFγ (200u/ml). D) The effect of the PI3Ki at 100nM concentration on expression of PD-L1 in the presence and absence of INFγ (200u/ml). Graphs in panels B and C are separated between cell lines with low or high PD-L1 MFI to allow adequate evaluation of the different conditions. In panels B, C and D, the mutations in BRAF or NRAS genes of cell lines have been indicated under the name of each cell line. The assays on each single sample were performed at least twice and error bars are the standard errors. Significant effects of the signaling pathway inhibitors (Vem, MEKi, and PI3Ki) in the presence or absence of IFNγ are indicated (asterisk, t-test p-value<0.05). The effects of these targeted therapy drugs on expression of PD-L1 seem to be variable among the different cell lines causing decreases in some cases and induction of expression in some others particularly in the presence of INFγ.
In the majority of cell lines, vemurafenib at 1.5µM level had no effect on the expression of PD-L1. However, in one NRAS mutant cell line (WM1366), vemurafenib caused a slight induction of the ligand (Figure 3 B), which may be related to paradoxical MAPK activation. Also, in the presence of INFγ, vemurafenib caused induction of PD-L1 in one of the vemurafenib resistant cell lines (M233). On the other hand, M229AR9, despite being resistant to the growth inhibitory effect of vemurafenib, was the only BRAF mutant cell line that showed a reduction of PD-L1 upon the treatment with vemurafenib or combination of vemurafenib and INFγ (Figure 3 B). For these studies, the control cultures of this resistant cell lines were off the drug for 24 hours, and treated sample had the reintroduction of the drug. The difference between the expression of PD-L1 in the control and treated samples can be interpreted in two ways: a) the induction of PD-L1 in the control in the absence of vemurafenib, or b) the reduction of PD-L1 upon reintroduction of vemurafenib. Either way it seems that vemurafenib causes the decrease in PD-L1 because either removing it from the control is accompanied with the induction of this protein in the control cells or vemurafenib causing the decrease in expression of this protein upon addition of the drug to the treated cells.
In two of the tested cell lines (M229AR9 and M296), the MEK inhibitor trametinib, at 25nM concentration, caused a significant reduction in the expression of PD-L1 (Figure 3 C). Note that M229AR9 showed a similar pattern in the presence of vemurafenib, whereas M296, a NRAS mutant cell line, responded only to MEKi. These results indicate that at least in some cases expression of PD-L1 can be modulated by inhibitors of the MAPK pathway. On the contrary, in some of the tested cell lines combination of the MEKi with INFγ caused a slight (M230) or significant induction (M238AR2) of PD-L1.
The effect of blocking PI3K/AKT pathway on the expression of PD-L1 was also variable from cell line to cell line. The PI3Ki at 100nM concentration decreased expression of PD-L1 significantly in three cell lines. However, this effect was eliminated in the presence of INFγ, except for one cell line (Figure 3 D). These results indicate that, similar to the MAPK pathway, inhibition of PI3K/AKT pathway can, in principle, also affect the expression of PD-L1. However, these effects are variable among different cell lines and perhaps are regulated by the specific signaling context of each cell line.
Restoration of the MAPK pathway in PD-L1 engaged-lymphocytes upon vemurafenib exposure
Interaction of PD-L1 with its receptor PD-1 on the surface of lymphocytes triggers the inhibitory signaling and decreases the activity of signaling pathways such as PI3K/AKT and MAPK (23, 24). On the contrary, vemurafenib can paradoxically activate the MAPK pathway in lymphocytes, which do not carry BRAF gene mutations. We investigated the possibility of restoring the MAPK pathway activity and enhancement of cytokine production by vemurafenib in PBMC cultures in conditions that expand T lymphocytes. These PBMC cultures were preconditioned to express PD-1 by mitogen activation leading to proliferation of activated T lymphocytes. The analysis of these primed cells indicated that out of all the live CD45+ T cells, 43.9% were CD4+ and 38.6% were CD8+ T lymphocytes (a total of 82.5%). The primed cells were then cultured in plates coated with a mixture of anti-CD3 and anti-CD28 and either Fc (control) or recombinant Fc-PD-L1, in the presence or absence of vemurafenib (Figure 4 A).
In the presence of vemurafenib alone, p-MEK and p-ERK were induced due to paradoxical activation of the MAPK pathway in lymphocytes. Vemurafenib alone also induced higher levels of four tested cytokines (IL-1, IL-6, TNFα, and INFγ) as compared to the control (Figure 4 B, C and D). On the contrary, exposure of lymphocytes to PD-L1 for 24 hours caused a reduction in the activity of the MAPK pathway as indicated by the decreases in p-MEK and p-ERK (Figure 4 B and C). PD-L1 also caused lower levels of cytokines IL-6, TNFα, and INFγ but higher levels of IL-1 in the supernatant of lymphocytes. Meanwhile, in the PD-L1-exposed lymphocytes, vemurafenib restored the phosphorylation of p-MEK and p-ERK to levels similar to the untreated control sample (Figure 4 B). Except for INFγ, levels of all other tested cytokines were induced in the presence of vemurafenib and PD-L1 compared to PD1-L alone. However, induction of IL-6 and TNFα was not sufficient to restore these cytokines to the control levels (Figure 4 D). In unprimed resting PBMCs the MAPK pathway is not active even in the presence of vemurafenib (supplementary figure 1). Moreover, in unprimed resting PBMCs expression of cytokines was at the background level even in the presence of vemurafenib (data not shown)
Effect of MAPK inhibitors on PD-L1 expression in co-cultures of melanoma cells and lymphocytes
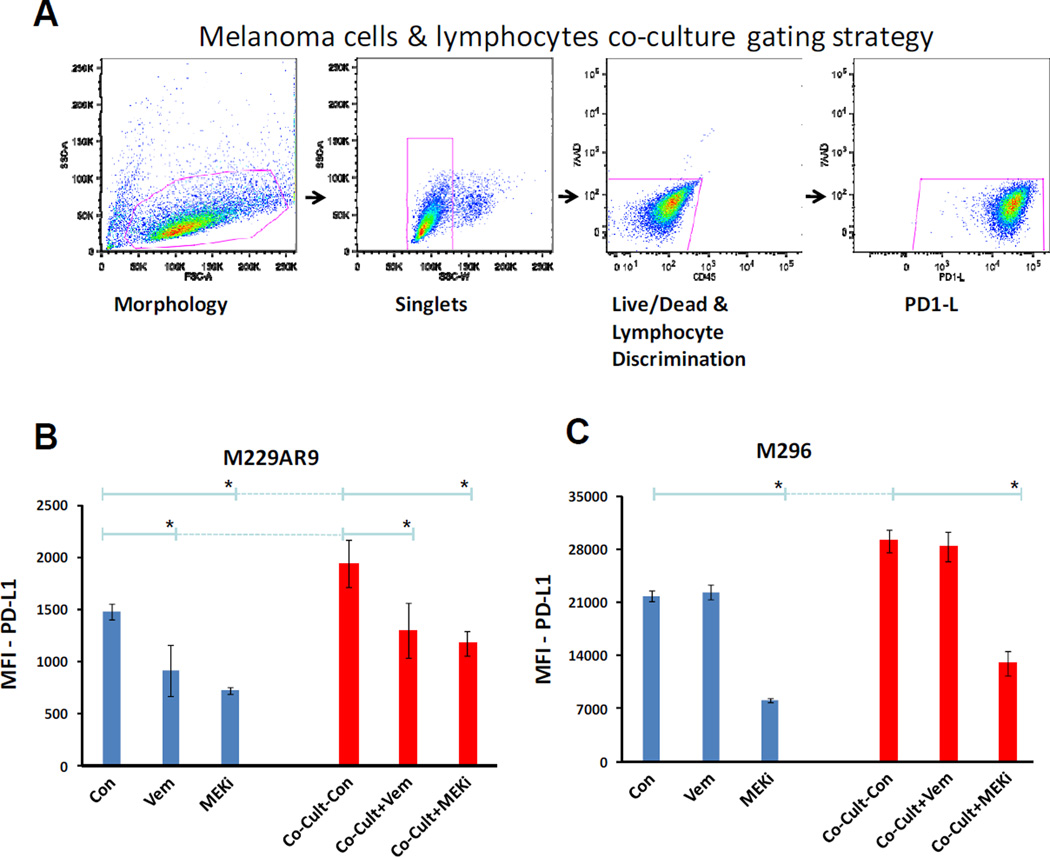
In patients, it is common that lymphocytes infiltrate tumors and therefore tumor cells are directly exposed to the cytokines produced by the tumor infiltrating lymphocytes. Our results indicated that expression of PD-L1 was reduced in the BRAF mutant M229AR9 melanoma cell line by vemurafenib and MEKi. The NRAS mutated cell line M296, showed decrease in PD-L1 expression by MEKi but not vemurafenib (Figure 3 B). Meanwhile, vemurafenib induced the production of some cytokines by lymphocytes (Figure 4 C), which potentially can induce higher levels of PD-L1 expression in the adjacent tumor cells. However, MEKi does not cause induction of cytokines in lymphocytes (data not shown). To investigate the net effects of vemurafenib and MEKi on PD-L1 expression in a mixture of melanoma cells and lymphocytes, an in vitro co-culture experiment was set up where these cells were treated with either one of these drugs. Similar to what was observed and interpreted before in figure 3 B, vemurafenib treatment resulted in a significant reduction of PD-L1 expression in the M229AR9 cell line (Anova p-value<0.05), but not in the M296 cell line (Figure 5 B, C). M229AR9 alone or in co-culture showed a 35% PD-L1 reduction upon vemurafenib treatment. MEKi treatment led to a significant PD-L1 reduction in both cell lines (Anova p-value<0.05).
Figure 5. Inducing effect of lymphocytes and the reducing effect of MAPK pathway inhibitors on the expression of PD-L1 by melanoma cell lines.
A) Gating strategy of the co-cultures. Melanoma cells were gated according to morphology (FSC-A vs SSC-A) to single cell discrimination (SSC-W vs SSC-A). The melanoma cells were then gated to perform live/dead and lymphocyte discrimination (CD45 vs 7AAD). These cells were then checked for PD-L1 positivity (PD-L1 vs 7AAD). The assays on each single sample were repeated at least twice. B) Evaluation of the expression of PD-L1 in the BRAF mutant melanoma cell line M229AR9 alone or co-cultured with lymphocytes in the absence and presence of the BRAF inhibitor, vemurafenib (1.5µM), or the MEKi, trametinib (25nM). M229AR9 control was off vemurafenib for 3 days before the harvest for flow cytometry (method and material section). Asterisks indicate significant statistical differences in PD-L1 expression upon treatment with vemurafenib and MEKi (Anova p-value<0.05). C) PD-L1 expression levels in the NRAS mutant melanoma cell line M296 alone or co-cultured with lymphocytes and the same treatments described in the section B. Asterisks indicate significant statistical differences in PD-L1 expression upon treatment with MEKi (Anova p-value<0.05).
Discussion
Prior studies have reported several mechanisms by which PD-L1 can be expressed by cancer cells, including the well documented inducible PD-L1 expression in response to interferons, and reports on expression of PD-L1 downstream of oncogenic signaling through the MAPK or the PI3K/AKT pathways. In a study analyzing anaplastic large cell lymphoma and Hodgkin lymphoma, PD-L1 expression was shown to be controlled by MAPK pathway signaling (32). Furthermore, in a study using glioblastoma cell lines, Parsa et al. found that higher activity of AKT pathway, particularly upon the mutation and loss of PTEN, was associated with the increased PD-L1 expression (28). However, in our study we did not identify an association between the level of PD-L1 expression and alterations in MAPK signaling, mutations in PTEN or other activating mutations or gene amplifications in the PI3K/AKT pathway. The disagreement in the findings of these studies with our series may be due to the differences in signaling context of melanoma and other cancers. However, similar to what has been shown in another study in melanoma cell lines (29), we also found that, in the presence of a PI3K inhibitor, expression of PD-L1 was decreased moderately only in some cell lines. Interestingly, in some cell lines the reducing effect of PI3Ki on expression of PD-L1 was abrogated in the presence of INFγ.
In a recent study using melanoma cell lines, Jiang et al. found that the development of resistance to vemurafenib was associated with higher levels of c-Jun transcription factor which in turn caused higher PD-L1 expression (29). The apparent discrepancies with our results are related to the fact that resistance to vemurafenib can occur through a variety of mechanisms. These resistant mechanisms include activation of alternative signaling pathways such as PI3K/AKT (16) or reactivation of the MAPK pathway through a secondary mutation in NRAS (30), amplification of BRAF gene (33), activating mutations in MEK (34), over expression of MAP3K8 (COT/Tp12) and continuous dimerization of truncated BRAF isoforms (35, 31). Resistance due to the activation of alternative signaling pathways is usually accompanied with high expression of receptor tyrosine kinases such as PDGFRβ, higher expression and dependency on c-jun and phenotypic switch of melanoma cells toward a more mesenchymal phenotype (30 and Titz et al. sumbitted). In our panel of cell lines, resistant sub-lines that were categorized under the activation of alternative signaling pathway resistant mechanism also showed significantly higher expression of PD-L1 in comparison to their parental sensitive lines (M229AR9 and M238AR2). This is in agreement with the findings of Jiang et al. regarding the higher expression of c-Jun and PD-L1 in the resistant cell lines (29). However, this effect does not occur in all the mechanisms of acquired resistance to BRAFi, since no or only a slight increase in expression of PD-L1 could be detected in the resistant cell lines with reactivation of the MAPK pathway.
The effects of targeted therapy drugs on the signaling and function of immune cells and the possibility of combining targeted therapy with different immunotherapy strategies have been the focal points of several studies (36, 37, 14). Our findings in the current study indicate that the effects of MAPK targeted therapy agents on expression of PD-L1 are complex and variable among the cell lines. The dual effects of BRAF inhibitors such as vemurafenib on melanoma and immune cells are of particular interest (27). Aside from their inhibitory effect on mutated BRAF in cancer cells, these drugs can paradoxically activate MAPK pathway in the cells with normal BRAF. This paradoxical effect can be particularly important in the context of PD-L1/PD-1 inhibitory therapy. In this study we showed that the exposure of lymphocytes to PD-L1 caused a decrease in the activity of MAPK pathway and reduced production of some of the cytokines. However, in such conditions vemurafenib restored the MAPK activity and induced positive effects on cytokine secretion.
In conclusion, our studies analyzing PD-L1 expression in a large panel of melanoma cell lines with or without exposure to MAPK inhibitors show that there is no straightforward cell-intrinsic explanation of PD-L1 expression as it had been previously hypothesized. The functional significance of constitutive and targeted therapy-modulated PD-L1 expression in vivo needs to be evaluated in comparison with the expression of PD-L1 induced by T cell infiltration. It is possible that only when PD-L1 is expressed upon T cell infiltration, in response to interferons and other immune stimulating cytokines, will it be important as a biomarker for response to PD-1 or PD-L1 blocking antibodies. Constitutive PD-L1 expression, or PD-L1 expression upon alterations of signaling pathways, in the absence of a T cell infiltration, may not serve as a biomarker. Encouragingly, the MAPK paradoxical activation effects of BRAF inhibitors on T cells can serve to offset the inhibition of TCR signaling through the MAPK pathway induced by PD-L1 expression, providing hope for synergistic activity of BRAF inhibition and PD-1/PD-L1 blockade.
Supplementary Material
Statement of Translational Relevance.
The idea of combining targeted therapies and cancer immunotherapy has been attractive in the melanoma field. One of the main clinical concerns is the development of resistance to BRAF inhibitors, which in turn may alter the expression of the immunoregulatory protein PD-L1 and therefore affect the activity of immunotherapy. In this study we provide evidence of how changes in oncogenic signaling impact on PD-L1 expression, and the combined effects of BRAF inhibitors on melanoma and immune cells. Our findings provide preclinical supporting evidence for clinical trials with MAPK targeted therapies and immunotherapies for melanoma.
Acknowledgements
The authors thank Jennifer Tsoi for her help in revising some of graphs in this paper.
Financial support: This work was funded by NIH grant P01 CA168585, The Seaver Institute, the Dr. Robert Vigen memorial fund, the Ressler Family Foundation, the Wesley Coyle Memorial Fund, the Louise Belley and Richard Schnarr Fund, the Garcia-Corsini Family Fund, the Bila Alon Hacker Memorial Fund, the Fred L. Hartley Family Foundation, the Ruby Family Foundation, the Jonsson Cancer Center Foundation, and the Caltech-UCLA Joint Center for Translational Medicine (to A.R.). MA was partially supported by P50 CA086306 In Vivo Imaging in Cancer Biology (ICMIC) career development award. L.R. was supported by the V Foundation-Gil Nickel Family Endowed Fellowship in Melanoma Research and a grant from the Spanish Society of Medical Oncology (SEOM) for Translational Research in Reference Centers. T.G.G. is the recipient of a Research Scholar Award from the American Cancer Society (RSG-12-257-01-TBE) and an Established Investigator Award from the Melanoma Research Alliance (20120279).
Footnotes
No potential conflicts of interest were disclosed.
References
- 1.Alcalá AM, Flaherty KT. BRAF inhibitors for the treatment of metastatic melanoma: clinical trials and mechanisms of resistance. Clin Cancer Res. 2012 Jan 1;18(1):33–39. doi: 10.1158/1078-0432.CCR-11-0997. [DOI] [PubMed] [Google Scholar]
- 2.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010 Aug 26;363(9):809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012 Nov;367(18):1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 Aug 19;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Thomas L, Bondarenko I, O'Day S, MD JW, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011 Jun 30;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 6.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013 Jul 11;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012 Jun 28;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013 Jul 11;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma C, Cheung AF, Chodon T, Koya RC, Wu Z, Ng C, et al. Multifunctional T-cell analyses to study response and progression in adoptive cell transfer immunotherapy. Cancer Discov. 2013 Apr;3(4):418–429. doi: 10.1158/2159-8290.CD-12-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002 Jun 27;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 11.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010 Sep 30;467(7315):596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Søndergaard JN, Nazarian R, Wang Q, Guo D, Hsueh T, Mok S, et al. Differential sensitivity of melanoma cell lines with BRAFV600E mutation to the specific Raf inhibitor PLX4032. J Transl Med. 2010 Apr 20;8:39. doi: 10.1186/1479-5876-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012 Jan 19;366(3):207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koya RC, Mok S, Otte N, Blacketor KJ, Comin-Anduix B, Tumeh PC, et al. BRAF inhibitor vemurafenib improves the antitumor activity of adoptive cell immunotherapy. Cancer Res. 2012 Aug 15;72(16):3928–3937. doi: 10.1158/0008-5472.CAN-11-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012 Feb 23;366(8):707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atefi M, von Euw E, Attar N, Ng C, Chu C, Guo D, et al. Reversing melanoma cross-resistance to BRAF and MEK inhibitors by co-targeting the AKT/mTOR pathway. PLoS One. 2011;6(12):e28973. doi: 10.1371/journal.pone.0028973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butterfield LH, Comin-Anduix B, Vujanovic L, Lee Y, Dissette VB, Yang JQ, et al. Adenovirus MART-1-engineered autologous dendritic cell vaccine for metastatic melanoma. J Immunother. 2008 Apr;31(3):294–309. doi: 10.1097/CJI.0b013e31816a8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011 Jun 2;364(22):2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011 Dec 20;29(36):4828–4836. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McArthur GA, Ribas A. Targeting oncogenic drivers and the immune system in melanoma. J Clin Oncol. 2013 Feb 1;31(4):499–506. doi: 10.1200/JCO.2012.45.5568. [DOI] [PubMed] [Google Scholar]
- 21.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012 Mar 28;4(127):127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005 Nov;25(21):9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012 Jun 26;5(230):ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274) FEBS Lett. 2006 Feb 6;580(3):755–762. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 26.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012 Mar 22;12(4):252–264. doi: 10.1038/nrc3239. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribas A, Wolchok JD. Combining cancer immunotherapy and targeted therapy. Curr Opin Immunol. 2013 Apr;25(2):291–296. doi: 10.1016/j.coi.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007 Jan;13(1):84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 29.Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, Hodi FS. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res. 2013 Feb 1;19(3):598–609. doi: 10.1158/1078-0432.CCR-12-2731. [DOI] [PubMed] [Google Scholar]
- 30.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010 Dec 16;468(7326):973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011 Nov 23;480(7377):387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto R, Nishikori M, Tashima M, Sakai T, Ichinohe T, Takaori-Kondo A, et al. B7-H1 expression is regulated by MEK/ERK signaling pathway in anaplastic large cell lymphoma and Hodgkin lymphoma. Cancer Sci. 2009 Nov;100(11):2093–2100. doi: 10.1111/j.1349-7006.2009.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi H, Moriceau G, Kong X, Lee MK, Lee H, Koya RC, et al. Melanoma whole-exome sequencing identifies (V600E) B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat Commun. 2012 Mar 6;3:724. doi: 10.1038/ncomms1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emery CM, Vijayendran KG, Zipser MC, Sawyer AM, Niu L, Kim JJ, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A. 2009 Dec 1;106(48):20411–20416. doi: 10.1073/pnas.0905833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010 Dec 16;468(7326):968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Comin-Anduix B, Chodon T, Sazegar H, Matsunaga D, Mock S, Jalil J, et al. The oncogenic BRAF kinase inhibitor PLX4032/RG7204 does not affect the viability or function of human lymphocytes across a wide range of concentrations. Clin Cancer Res. 2010 Dec 15;16(24):6040–6048. doi: 10.1158/1078-0432.CCR-10-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012 Mar 1;18(5):1386–1394. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.