Abstract
Despite the best available treatments for primary tumors, cancer can return, even after a long disease-free interval. During this period, cancer cells are believed to lie dormant in either primary, metastatic sites, or independent sites like bone marrow, effectively escaping adjuvant cytotoxic treatments. To date, little is known about how these cells transition to dormancy, or how they are reactivated if cancer recurs. Recent studies have revealed the effects of tumor microenvironment, or niche, on the regulation of tumor dormancy via the signaling pathways of growth arrest-specific 6 (GAS6), bone morphogenetic protein 7 (BMP7), and transforming growth factor beta-1 (TGF-β1), and that the balance between activation of p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK) MAPK plays a pivotal role in tumor dormancy. In this review, we will discuss tumor dormancy from the perspective of the niche, and consider potential therapeutic targets. Greater understanding of the mechanisms involved will help guide innovation in the care of advanced cancer patients.
Background
Even with the successful removal of a primary tumor, cancer can recur after years, or even decades, of a disease-free interval. When disseminated tumor cells (DTCs) from a primary tumor reach a metastatic site, they begin communicating with the microenvironment. Whether these DTCs form a metastasis or enter dormancy may in part depend on the signals from this metastatic microenvironment. During the dormant period, cancer cells can undergo G1/G0 growth arrest and become clinically undetectable, which serves to protect them from cytotoxic drug exposure, since these drugs are designed to target cells in mitotic division. Once latent cancer cells are reactivated, the disease frequently becomes impossible to cure. Prevention of cancer recurrence or eradication of these dormant cells, therefore, remains a major challenge.
Accumulating evidence suggests that the microenvironment plays a pivotal role in the regulation of tumor dormancy (1–3). Similar findings suggest that the microenvironment plays a major role in regulating quiescence of normal adult stem cells. Indeed, the microenvironment surrounding stem cells exists in a dormant state to keep the stem cells dormant (4), but stem cells leave this state as their microenvironment matures (5). These findings suggest that the immaturity of the microenvironment plays a crucial role in maintaining dormancy of stem cells.
In the hematopoietic field, Schofield hypothesized that the microenvironment, which he named the “niche”, actively participates in the regulation of hematopoietic stem cell (HSC) fate (6). This HSC microenvironment has been particularly well studied (7–10). In humans, bone marrow is the major location for hematopoiesis (6), and within their niche, HSCs are likely to stay dormant owing to cell intrinsic and extrinsic factors. Osteoblasts and sinusoidal endothelium are thought to be two major components of the bone marrow HSC niche, while adipocytes, mesenchymal stem cells, and CXCL12 expressing reticular cells also participate in maintenance of HSC functions (10). In the marrow, several signaling pathways, including Wnt, Notch, Hedgehog, and the BMPs have been known to control HSC quiescence (10). The interactions between HSCs and osteoblasts through Tie2/Ang-1 and/or Mpl/THPO signaling pathway also play pivotal roles in HSC quiescence (10). Additionally, HSCs can enter quiescence when presented with hypoxic conditions (10). The region where osteoblasts reside has been shown to be more hypoxic than the area of sinusoidal endothelium (10). Therefore, the osteoblastic niche facilitates quiescence and self-renewal of HSCs, while the vascular niche is associated with the proliferation and differentiation of HSCs.
In considering the metastatic fate of DTCs, the hematopoietic niche becomes important, since cancers with origins in prostate and breast preferentially metastasize to bone, and are known to take advantage of the bone marrow. Bone metastatic prostate cancer cells, for example, chiefly parasitize the HSC niche to survive within the marrow environment (11). In fact, prostate cancer cells compete for the bone marrow niche with HSCs by using a process equivalent to that used by HSCs to access the niche (11). Since the major role of the niche is to keep HSCs dormant, it is possible that the bone marrow niche may also be essential for tumor dormancy.
Recent studies have revealed that cancers target their own particular metastatic niche(s) during dissemination (11–13). Likewise, DTCs from other forms of cancer may have similar “niche”-induced dormancy. Though the effects of the bone marrow niche on tumor dormancy, progression, and metastasis have been investigated, the cell types composing the niches for cancer cells and how they may directly interact with these cells still remains to be elucidated.
Although growing evidence has provided clues to possible mechanisms whereby the microenvironment regulates tumor dormancy, our knowledge remains incomplete. Moreover, little is known as to how cancer cells reactivate after long periods of dormancy. Since dormant tumor cells may be seeds for recurrence or metastasis, there is an urgent need to uncover the interaction between the microenvironment and tumor dormancy in order to develop therapies that insure that cancer does not return. In this review, we will discuss tumor dormancy from the perspective of the microenvironment, and explore potential therapeutic targets.
Tumor cell dormancy in the “Niche”
In examining the metastatic niche and its effect on tumor dormancy, the corollary seen in the HSC niche is instructive. Interplay between a niche and its associated cells can regulate the state of dormancy or proliferative activity. Direct cell-to-cell contact between an HSC and its niche is important for maintaining the cellular functions of HSCs (7–10). Recent work by our group revealed that adhesion molecule Annexin II (Anxa2) expressed by the osteoblastic HSC niche directly supports the survival of HSCs (14). In our cancer studies, we found that prostate cancer cells which have disseminated to bone sites also bind to the osteoblastic niche through Anxa2, and that when the prostate cancer cells reach this niche, the tyrosine kinase receptor Axl is upregulated (15).
Axl is one of the TAM (Tyro3 (SKY), Axl, MerTK) family of receptor tyrosine kinases, which have extracellular and cytoplasmic domains consisting of 2 immunoglobin-like domains and 2 fibronectin type III repeats. Growth arrest-specific 6 (GAS6) is ligand for the TAM receptors and GAS6 mainly activates downstream signaling, including phosphoinositide 3-kinase (PI3K)/Akt and extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) pathways, via the TAM receptors (16). In addition, it has been demonstrated that GAS6 secreted by fibroblasts in the marrow converts HSCs into a dormant state (17).
As with HSCs, GAS6 produced by the bone marrow osteoblastic niche initiates dormancy in disseminated prostate cancer cells (18) and acute lymphoblastic leukemia (19). GAS6 inhibits cell proliferation, while it prevents apoptosis induced by cytotoxic agents (18, 19). These effects may be due to the activation of the ERK MAPK pathway (18). Additionally, GAS6 increases the number of cells in the G0 phase (18). More recent studies have demonstrated that the ratio of receptors for GAS6 also plays a crucial role in the regulation of tumor dormancy (20). When Axl expression is higher than that of Tyro3 (subfamily of Axl), prostate cancer cells remain dormant (20). On the other hand, when prostate cancer cells have relatively higher Tyro3 expression, they are likely to be more proliferative (20). Moreover, this receptor switch seems to be tissue dependent: high levels of Tyro3 are observed in the primary tumor site, whereas DTCs in the marrow express high levels of Axl (20).
In a similar way, bone morphogenetic protein 7 (BMP7) produced by bone marrow stromal cells triggers dormancy of prostate cancer cells. When prostate cancer cells were co-cultured with bone marrow stromal cells, prostate cancer cells entered dormancy through the activation of the cell cycle inhibitor p21, the metastasis suppressor gene N-myc downstream-regulated gene 1 (NDRG1), and p38 MAPK phosphorylation (21). Additionally, BMP7 dramatically suppresses the ERK MAPK pathway (21). These observations are consistent with previous studies by Aguirre-Ghiso’s group, proposing that the ratio of ERK and p38 MAPK pathways plays a pivotal role in the determination of tumor dormancy (22, 23). Dormancy is prevented when BMP7 secreted by bone marrow stromal cells is blocked by shRNA or pan-BMP inhibitor Noggin (21). Moreover, the effects of BMP7 on dormancy of prostate cancer cells both in vitro and in vivo are attenuated when BMP receptor 2 (BMPR2) on prostate cancer cells is down-regulated (21).
Tumor dormancy is not only observed in the bone marrow microenvironment, but also in other metastatic sites. When metastatic breast cancer 4T07 cells in the lung over-express Coco, an antagonist of transforming growth factor beta (TGF-β) ligands, cells escape dormancy by blocking the binding of microenvironmental BMP4 ligands to the BMPR on the cancer cells (24). In contrast, Coco knock-down breast cancer 4T1 cells have a dormant phenotype (24). Moreover, when Coco was knocked-down in 4T07 cells, phosphorylation of Smad proteins, downstream of the BMP4 signaling pathway, is prevented (24). Importantly, this event occurs in lung, but not in bone or brain, suggesting that the lung microenvironment is a unique venue for Coco-influenced tumor dormancy (24), providing a niche for metastatic cells.
Cancer cells also maintain dormancy during the dissemination process itself. An in vivo orthopedic model reveals that Ki67 negative dormant breast cancer MCF-7 and MDA-MB-231 cells are found near the thrombospondin-1 (TSP-1) expressing perivascular niche in lung and bone marrow (25). This effect is inhibited by anti-TSP-1 antibody in an in vitro co-culture system (25). Additionally, breast cancer cells enter dormancy when they contact the dormant microvasculature, which expresses low levels of TGF-β1 and periostin (POSTN). However, dormancy is reversed when the microvasculature becomes active, where both TGF-β1 and POSTN are upregulated (25). These findings suggest that microvasculature may serve as another potential niche for a dormancy switch.
The interaction between extracellular matrix (ECM) and integrins expressed by cancer cells also induces a variety of intracellular signals and is another regulator of tumor dormancy (26–30). Integrin-mediated adhesion to ECM induces the proliferation of cancer cells, whereas the disruption of these interactions promotes tumor dormancy. When the levels of urokinase receptor (uPAR) are decreased in human carcinoma HEp3 cells, α5β1 integrin is likewise downregulated. Consequently, the cancer cells fail to bind to fibronectin (31), resulting in a reduction of the ERK MAPK pathway and activation of the p38 MAPK pathway, which leads to cell dormancy (31). As with BMP7 (21), tumor dormancy is induced when the p38 MAPK pathway is relatively higher than the ERK MAPK pathway.
Tumor dormancy in primary sites can also be regulated by a niche microenvironment. In prostate cancer, the proximal region of prostatic ducts serves as a niche to keep cancer cells dormant in the primary tumor (32). The proximal region expresses higher levels of TGF-β1 than the distal region (32), and this TGF-β1 expression induces dormancy of both the region and the prostate cancer cells associated with it by activating phosphorylation of Smad 2/3 (32). In in vitro conditions, TGF-β1 inhibits the proliferation of cells isolated from both the proximal and distal regions of the prostate, however cells from the distal region differentiate with this treatment, while proximal region cells do not (32). Moreover, TGF-β1 induces apoptosis of distal cells, while proximal cells retain resistance to apoptotic effects of TGF-β1 since they express higher levels of the apoptosis suppressor gene bcl-2 (32). There is some controversy about the role of TGF-β1 in cancer progression. Although TGF-β1 promotes cancer progression within microvasculature, it acts as a tumor dormancy inducer in the primary site, suggesting that its effects may therefore differ between microenvironments. Clearly, further studies in this area are warranted.
Clinical-translational advances
It is postulated that dormant cancer cells survive the cytotoxic treatments used to combat actively dividing cells, affording the opportunity for these cells to reactivate in the future. Although there are some preclinical models to test this hypothesis (33), the effect of cytotoxic agents on dormant cancer cells still remains unknown.
As discussed above, the GAS6/Axl axis appears to be a factor in the mechanisms of tumor dormancy (18, 20). In addition, Axl overexpression is well known to promote invasion and metastasis (34–38), and is correlated with increased tumor grade and poor clinical outcome in many tumor types (39–41), suggesting that this receptor tyrosine kinase may be a new therapeutic target. Several small-molecule inhibitors of Axl are now being evaluated as candidate drugs for cancer treatment (16, 42). One of these Axl inhibitors, BGB324 (formally R428), prevents breast cancer (human MDA-MB-231 cells: approximately 70% tumor reduction, p<0.05; and mouse 4T1 cells: approximately 50% tumor reduction, p<0.05) metastasis and extends the survival of mice inoculated with mouse 4T1 cells (median survival >80 days vs. 52 days, p<0.05) by affecting both tumor cells and their microenvironment (43). Additionally, BGB 324 synergistically enhances the suppressive effects of cisplatin on liver micrometastasis (43). More importantly, BergenBio AZ (Bergen, Norway) has initiated phase I clinical trials of BGB324 (44). According to their website, a phase I a clinical trial in healthy volunteers has been recently completed and phase I b clinical trials are planned in acute myeloid leukemia and non-small cell lung cancer in 2014 (45).
Another treatment, a monoclonal antibody against both human and murine Axl, YW327.6S2, reduces the growth and metastasis of A549 non-small-cell lung cancer (NSCLC) (approximately 25% tumor reduction) and MDA-MB-231 breast cancer (approximately 25% tumor reduction), and these effects are enhanced by anti-VEGF treatment, epidermal growth factor receptor (EGFR) inhibitor (erlotinib), and chemotherapy in xenograft mouse models (46). Additionally, YW327.6S2 also negatively affects the secretion of inflammatory cytokines and chemokines from tumor-associated macrophages (46). Consistent with these findings, increased activation of Axl in human NSCLCs during EMT induces resistance to erlotinib (47). Although blocking Axl signaling seems very promising for cancer treatment, whether the GAS6/Axl axis is a potential therapeutic target for tumor dormancy remains unclear, however the fact that these treatments also affect the metastatic niche cells is intriguing. Further study in this area is clearly needed.
As discussed, BMP7 is another molecule that appears to induce tumor dormancy in prostate cancer (21). BMP7 also upregulates human telomerase reverse transcriptase (hTERT) gene repression, which results in the growth arrest of cervical cancer cells (48). Since BMP7 is already FDA-approved for bone fractures, it may be worth considering its use for maintaining tumor dormancy to prevent metastatic growth. On the other hand, some caution should be used, since the effect of BMP7 can be dependent on cell types and/or microenvironment (49). As previously described (21), BMP7 inhibits the growth of prostate cancer within the bone marrow microenvironment (the tissue volume of tibia bearing BMP7-overexpressing vs. control prostate cancer cells: 0.56 ± 7.3 vs. 0.78 ± 4.8 mm2, p = 0.031) (49). However, in the primary site, while BMP7 reduces tumor burden of androgen-dependent prostate cancer cells, it does not affect the proliferation of castration-resistant prostate cancer cells (49). Further careful research in this area is therefore essential, and may reveal that BMP7 is suitable for some, but not all, cancer therapy.
In addition, liver and lung metastasis of 4T1 cells and MDA-MB-231T cells are suppressed when implanted animals are treated with lysophosphatidic acid receptor 1 (LPA1) inhibitor Debio-0719 (4T1 cells: liver metastasis 73.0% tumor reduction, p<0.001 and lung metastasis 88.5% tumor reduction, p<0.001; and MDA-MB-231T cells: lung metastasis 77.1% tumor reduction, p<0.001) or when LPA1 in the tumors is knock-downed using an shRNA technique (4T1 cells: liver metastasis 43.7% tumor reduction, p=0.01 and lung metastasis 50.0% tumor reduction, p=0.004) (50). However these treatments fail to affect primary tumor growth (50). Interestingly, Debio-0719 induces dormancy of metastatic 4T1 cells by reducing proliferative markers Ki67 and the phosphorylation of ERK MAPK, but activating the phosphorylation of the p38 MAPK (50), which reflects a change in the balance of ERK MAPK/p38 MAPK as previously described (21, 31). Furthermore, when angiogenesis is completely inhibited with a vascular endothelial growth factor A (VEGF-A) inhibitor, bevacizumab, micrometastases of lung carcinoma are kept in a chronic dormancy state (51), although these cells revive when bevacizumab is withdrawn (51). These findings suggest that as well as eradicating dormant cancer cells, maintaining the tumor-dormant state or switching proliferating cancer cells to dormant cancer cells indefinitely may be a new therapeutic approach.
Conclusions
Once cancer recurs, survival rates of cancer patients drastically decline. Although significant progress has been made in early diagnosis and treatment, we are still losing the battle against cancer. Therefore, a better understanding of the mechanisms underlying cancer recurrence, leading to new therapeutic approaches, is crucial.
In this review, we discussed the molecular mechanisms whereby the microenvironment, or “the niche”, controls tumor dormancy (Figure 1). We also outlined several therapeutic targets that may help eradicate these challenging dormant cancer cells. Waking up dormant cancer cells by targeting treatment prior to chemotherapy might be worth considering in future trials. Although the outcome seems promising, this work is still in the development and testing stage. Alternatively, an approach which works to sustain tumor dormancy ad infinitum may also offer avenues for cancer treatment. In this strategy, although cancer cells would still be present, they would no longer divide, and therefore would not lead to metastatic disease. More discoveries in this area are obviously essential prior to initiating clinical studies. In the long run, our research efforts towards better and more effective treatments hold the promise of changing the future of cancer therapy.
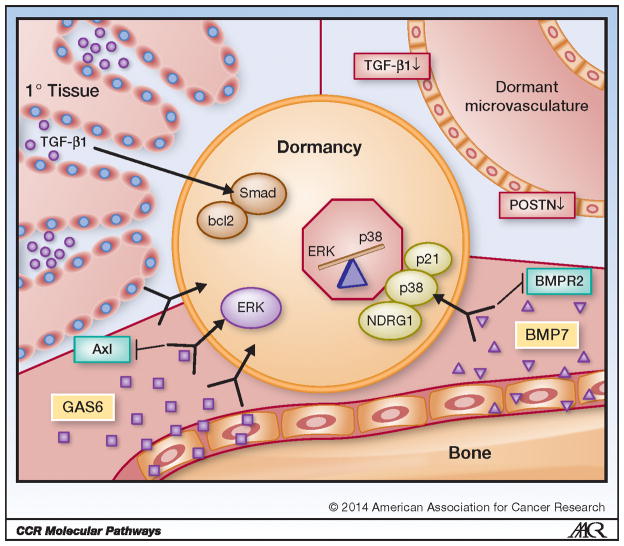
Figure 1. Niche Mediated Tumor Cell Dormancy.
Tumor cells become and stay dormant via signaling from the surrounding tissue (niche). High levels of transforming growth factor beta-1 (TGF-β1) in the proximal region of primary tissue cause activation of Smad and upregulation of the apoptosis suppressor gene bcl2, which in turn induces dormancy in resident tumor cells. Tumor cells have also been shown to enter dormancy within dormant microvasculature, which is believed to occur because of characteristically low levels of TGF-β1 and periostin (POSTN). Osteoblast derived growth arrest specific 6 (GAS6), and stromal cell derived bone morphogenetic protein 7 (BMP7) in the bone marrow also induces tumor cell dormancy. GAS6 is recognized by tyrosine kinase receptor Axl, which activates the extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) pathway. BMP7 is received by BMP receptor 2 (BMPR2), which causes activation of cell cycle inhibitor p21, upregulation of N-myc downstream-regulated gene 1 (NDRG1) and p38 MAPK phosphorylation. Strong evidence suggests tumor cells respond to external stimuli by shifting the balance of the ERK MAPK and p38 MAPK pathways: in dormant tumor cells the p38 MAPK pathway is generally more activated than ERK MAPK.
Acknowledgments
This work is supported by the National Cancer Institute (CA093900; to R.S. Taichman, and CA163124 and CA166307; to Y. Shiozawa and R.S. Taichman), the Department of Defense (W81XWH-11-1-0636 and PC130359; to Y. Shiozawa and R.S. Taichman), and the Prostate Cancer Foundation (Y. Shiozawa and R.S. Taichman).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Pantel K, Alix-Panabieres C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009;6:339–51. doi: 10.1038/nrclinonc.2009.44. [DOI] [PubMed] [Google Scholar]
- 2.Morgan TM, Lange PH, Porter MP, Lin DW, Ellis WJ, Gallaher IS, et al. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res. 2009;15:677–83. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nash KT, Phadke PA, Navenot JM, Hurst DR, Accavitti-Loper MA, Sztul E, et al. Requirement of KISS1 secretion for multiple organ metastasis suppression and maintenance of tumor dormancy. J Natl Cancer Inst. 2007;99:309–21. doi: 10.1093/jnci/djk053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci U S A. 2007;104:10063–8. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–60. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 7.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 8.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–9. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 10.Shiozawa Y, Taichman RS. Getting blood from bone: an emerging understanding of the role that osteoblasts play in regulating hematopoietic stem cells within their niche. Exp Hematol. 2012;40:685–94. doi: 10.1016/j.exphem.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sleeman JP. The metastatic niche and stromal progression. Cancer Metastasis Rev. 2012;31:429–40. doi: 10.1007/s10555-012-9373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Descot A, Oskarsson T. The molecular composition of the metastatic niche. Exp Cell Res. 2013 doi: 10.1016/j.yexcr.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Jung Y, Wang J, Song J, Shiozawa Y, Wang J, Havens A, et al. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood. 2007;110:82–90. doi: 10.1182/blood-2006-05-021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J, et al. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008;105:370–80. doi: 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korshunov VA. Axl-dependent signalling: a clinical update. Clin Sci (Lond) 2012;122:361–8. doi: 10.1042/CS20110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dormady SP, Zhang XM, Basch RS. Hematopoietic progenitor cells grow on 3T3 fibroblast monolayers that overexpress growth arrest-specific gene-6 (GAS6) Proc Natl Acad Sci U S A. 2000;97:12260–5. doi: 10.1073/pnas.97.22.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, Jung Y, et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12:116–27. doi: 10.1593/neo.91384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiozawa Y, Pedersen EA, Taichman RS. GAS6/Mer axis regulates the homing and survival of the E2A/PBX1-positive B-cell precursor acute lymphoblastic leukemia in the bone marrow niche. Exp Hematol. 2010;38:132–40. doi: 10.1016/j.exphem.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taichman RS, Patel LR, Bedenis R, Wang J, Weidner S, Schumann T, et al. GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS One. 2013;8:e61873. doi: 10.1371/journal.pone.0061873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011;208:2641–55. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L. Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell. 2001;12:863–79. doi: 10.1091/mbc.12.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK) Cancer Res. 2003;63:1684–95. [PubMed] [Google Scholar]
- 24.Gao H, Chakraborty G, Lee-Lim AP, Mo Q, Decker M, Vonica A, et al. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell. 2012;150:764–79. doi: 10.1016/j.cell.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807–17. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barkan D, Green JE, Chambers AF. Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur J Cancer. 2010;46:1181–8. doi: 10.1016/j.ejca.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70:5706–16. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barkan D, Kleinman H, Simmons JL, Asmussen H, Kamaraju AK, Hoenorhoff MJ, et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68:6241–50. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henriet P, Zhong ZD, Brooks PC, Weinberg KI, DeClerck YA. Contact with fibrillar collagen inhibits melanoma cell proliferation by up-regulating p27KIP1. Proc Natl Acad Sci U S A. 2000;97:10026–31. doi: 10.1073/pnas.170290997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roth JM, Akalu A, Zelmanovich A, Policarpio D, Ng B, MacDonald S, et al. Recombinant alpha2(IV)NC1 domain inhibits tumor cell-extracellular matrix interactions, induces cellular senescence, and inhibits tumor growth in vivo. Am J Pathol. 2005;166:901–11. doi: 10.1016/s0002-9440(10)62310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol. 1999;147:89–104. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salm SN, Burger PE, Coetzee S, Goto K, Moscatelli D, Wilson EL. TGF-{beta} maintains dormancy of prostatic stem cells in the proximal region of ducts. J Cell Biol. 2005;170:81–90. doi: 10.1083/jcb.200412015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naumov GN, Townson JL, MacDonald IC, Wilson SM, Bramwell VH, Groom AC, et al. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast Cancer Res Treat. 2003;82:199–206. doi: 10.1023/B:BREA.0000004377.12288.3c. [DOI] [PubMed] [Google Scholar]
- 34.Song X, Wang H, Logsdon CD, Rashid A, Fleming JB, Abbruzzese JL, et al. Overexpression of receptor tyrosine kinase Axl promotes tumor cell invasion and survival in pancreatic ductal adenocarcinoma. Cancer. 2011;117:734–43. doi: 10.1002/cncr.25483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C, et al. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 2011;30:1436–48. doi: 10.1038/onc.2010.509. [DOI] [PubMed] [Google Scholar]
- 36.Mudduluru G, Leupold JH, Stroebel P, Allgayer H. PMA up-regulates the transcription of Axl by AP-1 transcription factor binding to TRE sequences via the MAPK cascade in leukaemia cells. Biol Cell. 2010;103:21–33. doi: 10.1042/BC20100094. [DOI] [PubMed] [Google Scholar]
- 37.Gustafsson A, Martuszewska D, Johansson M, Ekman C, Hafizi S, Ljungberg B, et al. Differential expression of Axl and Gas6 in renal cell carcinoma reflecting tumor advancement and survival. Clin Cancer Res. 2009;15:4742–9. doi: 10.1158/1078-0432.CCR-08-2514. [DOI] [PubMed] [Google Scholar]
- 38.Vajkoczy P, Knyazev P, Kunkel A, Capelle HH, Behrndt S, von Tengg-Kobligk H, et al. Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proc Natl Acad Sci U S A. 2006;103:5799–804. doi: 10.1073/pnas.0510923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee CH, Yen CY, Liu SY, Chen CK, Chiang CF, Shiah SG, et al. Axl is a prognostic marker in oral squamous cell carcinoma. Ann Surg Oncol. 2012;19 (Suppl 3):S500–8. doi: 10.1245/s10434-011-1985-8. [DOI] [PubMed] [Google Scholar]
- 40.Hector A, Montgomery EA, Karikari C, Canto M, Dunbar KB, Wang JS, et al. The Axl receptor tyrosine kinase is an adverse prognostic factor and a therapeutic target in esophageal adenocarcinoma. Cancer Biol Ther. 2010;10:1009–18. doi: 10.4161/cbt.10.10.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koorstra JB, Karikari CA, Feldmann G, Bisht S, Rojas PL, Offerhaus GJ, et al. The Axl receptor tyrosine kinase confers an adverse prognostic influence in pancreatic cancer and represents a new therapeutic target. Cancer Biol Ther. 2009;8:618–26. doi: 10.4161/cbt.8.7.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting Axl and Mer kinases in cancer. Mol Cancer Ther. 2011;10:1763–73. doi: 10.1158/1535-7163.MCT-11-0116. [DOI] [PubMed] [Google Scholar]
- 43.Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W, et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010;70:1544–54. doi: 10.1158/0008-5472.CAN-09-2997. [DOI] [PubMed] [Google Scholar]
- 44.Sheridan C. First Axl inhibitor enters clinical trials. Nat Biotechnol. 2013;31:775–6. doi: 10.1038/nbt0913-775a. [DOI] [PubMed] [Google Scholar]
- 45.http://www.bergenbio.com/BGB324
- 46.Ye X, Li Y, Stawicki S, Couto S, Eastham-Anderson J, Kallop D, et al. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene. 2010;29:5254–64. doi: 10.1038/onc.2010.268. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44:852–60. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassar L, Li H, Pinto AR, Nicholls C, Bayne S, Liu JP. Bone morphogenetic protein-7 inhibits telomerase activity, telomere maintenance, and cervical tumor growth. Cancer Res. 2008;68:9157–66. doi: 10.1158/0008-5472.CAN-08-1323. [DOI] [PubMed] [Google Scholar]
- 49.Morrissey C, Brown LG, Pitts TE, Vessella RL, Corey E. Bone morphogenetic protein 7 is expressed in prostate cancer metastases and its effects on prostate tumor cells depend on cell phenotype and the tumor microenvironment. Neoplasia. 2010;12:192–205. doi: 10.1593/neo.91836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marshall JC, Collins JW, Nakayama J, Horak CE, Liewehr DJ, Steinberg SM, et al. Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer. J Natl Cancer Inst. 2012;104:1306–19. doi: 10.1093/jnci/djs319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16:116–22. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]