Abstract
The procurement and consumption of palatable, calorie-dense foods is influenced by the nutritional and hedonic value of foods. Although many factors can influence the control over behavior by foods rich in sugar and fat, emerging evidence indicates that biological sex may play a particularly crucial role in the types of foods individuals seek out, as well as the level of motivation individuals will exert to obtain those foods. However, a systematic investigation of food-seeking and consumption that disentangles the effects of the major sex-biasing factors, including sex chromosome complement and organizational and activational effects of sex hormones, has yet to be conducted. Using the four core genotypes mouse model system, we separated and quantified the effects of sex chromosome complement and gonadal sex on consumption of and motivation to obtain a highly-palatable solution (sweetened condensed milk – SCM). Gonadectomized mice with an XY sex chromosome complement, compared to those with two X chromosomes, independent of gonadal sex, appeared to be more sensitive to the reward value of the SCM solution and were more motivated to expend effort to obtain it, as evidenced by their dramatically greater expended effort in an instrumental task with progressively larger response-to-reward ratios. Gonadal sex independently affected free consumption of the solution but not motivation to obtain it. These data indicate that gonadal and chromosomal sex effects independently influence reward-related behaviors, contributing to sexually dimorphic patterns of behavior related to the pursuit and consumption of rewards.
Organisms seek out foods rich in sugars or fats both for their nutritional value, as well as for the associated experience of reward (Kelley, 2004, Kelley & Berridge, 2002, Richard et al., 2012). In humans and laboratory rodents, there are individual differences in the rewarding value of high sugar and/or high fat foods (Beaver et al., 2006, Carroll et al., 2008, Davis, 2009, Davis et al., 2004, Davis & Woodside, 2002, Small, 2009). Moreover, people with low sensitivity to rewards are more susceptible to consuming sugar/fat-rich foods in larger amounts, leading to greater risk for developing morbid obesity or other cardiometabolic disorders (Beaver et al., 2006, Davis et al., 2007, Davis et al., 2004, Stice et al., 2009).
For many species, goal-directed, effortful attempts to procure and then consume the food are usually required. In other words, instrumental actions, in which behavior triggers the availability of the reward, are necessary. A number of factors influence the control over behavior by high sugar/fat foods and the associated health consequences, particularly biological sex. Obese men and women differ in their preferred food items, with women preferring foods high in fat and sugar and men preferring foods high in protein and salt (Drewnowski et al., 1992). Similarly, female rodents exhibit greater preferences for and/or intake of sweet flavors compared to male rodents (Carroll et al., 2008, Dudley et al., 1979, Gentry & Wade, 1976a, Gentry & Wade, 1976b, Pankevich & Bale, 2008), suggesting that sexually dimorphic food preference may be a consequence of the underlying biological sex differences.
Motivational processes that lead to the initiation of food-seeking, culminating in food consumption, may also be susceptible to sex differences. Sex differences in reward sensitivity may, for example, explain differential susceptibility of female rats to instrumentally self-administer drugs of abuse (Carroll et al., 2004, Lynch et al., 2002). While some sex differences in reward sensitivity are due to the activational (transient, reversible) effects of sex steroids (Dudley et al., 1979, Gentry & Wade, 1976a, Gentry & Wade, 1976b, Gentry et al., 1976), few studies on this subject have been able to disentangle the contributions of sex chromosome complement, gonadal sex (organizational hormone effects – permanent effects of gonadal hormones exerted during perinatal or pubertal periods (Arnold, 2009b)) and activational hormone effects. The “Four Core Genotypes” (FCG) model (Arnold & Chen, 2009, De Vries et al., 2002) provides a system for detecting all three types of sex-biasing factors (Arnold, 2009b). In one example of the use of this model to investigate motivated behaviors, XX mice showed faster formation of food-reinforced instrumental habits, compared to XY mice, independent of their type of gonad (Quinn et al., 2007).
Here, we measured the consumption and motivation for a palatable food reward in mice from the FCG model. Specifically, voluntary consumption of freely-available sweetened condensed milk (SCM) of differing concentrations was assessed in mice before and after calorie restriction. We then examined behavior in an instrumental conditioning paradigm in which access to the SCM reward was contingent upon lever responses.
Materials and methods
Subjects
Fifty-four adult FCG mice on a C57BL/6J genetic background were generated as previously described (Arnold, 2009a, Gioiosa et al., 2008). In FCG mice, the testis-determining gene Sry is deleted from the Y chromosome, producing the Ȳ chromosome whereby XȲ mice are gonadal females. Insertion of a Sry transgene onto an autosome results in the XȲSry gonadal male (called XYM). Because the Sry transgene is located on an autosome, it segregates independently from the sex chromosomes. Mating the XYM to normal WT XX female results in four types of offspring: XX and XY gonadal males (XXM, XYM) and XX and XY gonadal females (XXF, XYF). Differences between XX and XY mice are attributable to effects of sex chromosome complement, and differences between gonadal males and females are attributed to the effects of Sry (either direct effects of Sry on tissues in which it is expressed, or more likely indirect effects mediated by differential effects of testicular vs. ovarian secretions).
Gonadal males and females were separately group-housed and maintained on a 14/10 h light/dark schedule (lights on at ~7 am). The experimental protocols employed were consistent with the NIH “Guide for the Care and Use of Laboratory Animals” and approved by the Chancellor’s Animal Research Committee at UCLA.
Gonadectomy
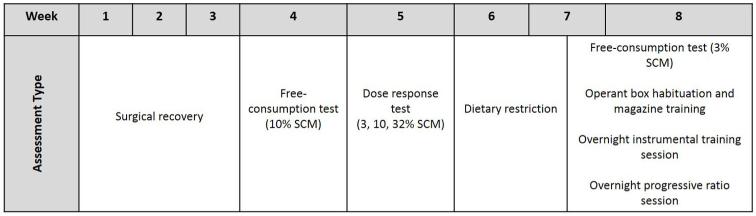
The experimental timeline is presented in Figure 1. At approximately 75 days of age, mice were gonadectomized and were allowed to recover for 4-5 weeks before behavioral phenotyping commenced. Mice were gonadectomized to remove any possible group differences in circulating levels of gonadal hormones, which might have confounded effects of sex chromosome complement. Subjects were divided into 5 cohorts (~N=12 per cohort, with all genotypes being represented in each cohort) to enable staggering of the behavioral assessments.
Figure 1.
Experimental timeline. Details of each procedure are provided in text (see methods section).
Consumption of a freely-available, palatable solution of SCM was assessed in FCG mice that were otherwise maintained on an ad libitum chow diet (Figure 1). These measures were collected during the light phase. Across 8 consecutive days, mice were placed in individual cages that were equipped with 2 bottles: one containing water and the other a 10% SCM solution (v/v, in water). Position of the bottles (either on the left or right side) was alternated daily to avoid development of a positional bias. Mice were allowed to freely consume from the bottles for 2 h and the amount of water and 10% SCM solution consumed (in grams) determined for each subject. Food was not available during the consumption test. Four daily consumption values were excluded from the analysis (XXM=2; XYM=2) as they were either abnormally low (negligible compared with daily intake; likely due to sipper malfunction) or abnormally high (almost twice the normal intake; likely due to leakage).
After completion of this stage, consumption of SCM solution was assessed at three different concentrations (3, 10, and 32%; v/v, in water) using the same 2-bottle procedure described above with the order of concentration presentation counterbalanced across groups and consumption of each concentration assessed across 2 consecutive days.
Next, mice underwent a dietary restriction (see below) and consumption of the 3% SCM solution, which produced the most robust sex differences (see results below), assessed using the same 2-bottle procedure described above.
In line with a common practice in free-consumption study, total amount of solution consumed (in grams) was divided by body weight (in grams) to account for potential differences in body size between experimental groups (referred to as normalized intake). However, because the appropriateness of this approach is currently under debate and because there were significant body weight differences between the genotypes in the current study, total raw intake (in grams not normalized to body weight) is also reported (referred to as raw intake).
Dietary Restriction
Following the initial free-consumption assessments, mice were dietary restricted to maintain a body weight approximately 90% of that prior to the dietary restriction. Body weight and amount of chow needed to maintain a 90% body weight was recorded daily.
Mouse operant testing apparatus
Standard mouse aluminum and Plexiglas operant conditioning chambers with a photocell-equipped pellet delivery magazine flanked by two retractable levers on one side and a curved panel with five photocell-equipped apertures on the opposite side (Med-Associates Inc., Mount Vernon, VT) were used for instrumental assessment of behavior. Operant boxes were housed inside of a sound-attenuating cubicle, with background white noise broadcasted and the environment illuminated with a house light (a light diffuser that was located outside of the operant chamber but within the cubicle). The reinforcer magazine was photocell-equipped and could be internally illuminated. Magazine receptacles were modified such that liquid solutions (10 μl of a 3% SCM solution) were dispensed by a remotely-operated infusion pump (Med-Associates Inc., Mount Vernon, VT), located outside of the testing cubicle, into a small well located within the magazine receptacle.
Operant testing
Following the 12 days of dietary restriction, mice were habituated to the operant conditioning box in the absence of reward. During this 30 min session, white noise was on and all light cues off. The following day, mice were trained to obtain rewards from the magazine. During this 1-h training session, mice retrieved liquid rewards (10 μl of a 3% SCM solution) delivered on a variable-time 60 s schedule via a remotely-operated infusion pump into the photocell-equipped magazine. The house light was on throughout the session and reward delivery was accompanied by 5-s illumination of a cue light located inside the magazine.
One day following completion of magazine training, mice were trained to press a lever for a single SCM reward (10 μl of a 3% SCM solution) in a single, 12 h instrumental session which is capable of capturing learning within a single, continuous session (versus fragmented learning that occurs across multiple training days). These testing sessions were conducted during the dark phase of the cycle (1900 h – 700 h). One lever (counterbalanced across the genotypes) was extended for the entire duration of the session. Presses, or depressions, of the lever triggered an auditory signal (0.1 s beep) and resulted in the delivery of the reward (10 μl of a 3% SCM solution) and a 5 s illumination of the magazine light. The schedule of reinforcement delivery changed over the course of the session: reinforcers for the first 50 lever responses were delivered under a fixed-ratio one (FR1) schedule, the next 50 reinforcers delivered on a variable-ratio two (VR2) schedule, and the remaining delivered on a variable-ratio five (VR5) schedule. Sessions terminated when either 12 h had lapsed or 400 rewards had been earned, whichever occurred first. The number and time of rewards and lever presses were recorded for each subject.
Finally, mice were tested under a progressive-ratio schedule of reinforcement approximately 36 h after completing the initial instrumental session. Similar to the instrumental assessments conducted above, these sessions were completed during the dark cycle (1900 h – 700 h). During these 12-h sessions, response requirements to obtain a reward increased by two presses for each subsequent reinforcer (e.g. first reinforcer delivered after one lever press; second reinforcer delivered after three lever presses; third reinforcer delivered after five lever presses; etc.).
Due to technical problems (clogging of the reinforcer delivery lines), data collected in the first cohort of twelve mice (FXX=3; MXX=3; FXY=4; MXY=2) were excluded from the analysis. To prevent the lines from becoming clogged in subsequent cohorts, a single infusion of 10 μl of 3% SCM solution was given once per hour for the entire duration of the 12-h operant sessions.
Subjects were fed during the light cycle 1-2 h after completing the behavioral sessions. When overnight testing was scheduled, animals were given 60% of their daily food allotment (approximately 10 hours before beginning of instrumental testing) in order to ensure sufficient levels of motivation for the overnight instrumental testing. The morning after the overnight instrumental sessions, subjects received 140% of their usual daily food allotment. On habituation and magazine training sessions, water was not available in the operant boxes; however, during the 12-h instrumental sessions, mice had free access to water located in the testing cubicle.
Statistical analyses
Intake of the SCM solution was measured for each subject and was expressed as the raw volume consumed, as well as the volume corrected for individual body weight. In the Results section, only outcomes for the raw values (not corrected for body weight) are summarized. Unless otherwise mentioned, analysis of the values corrected for body weight yielded the same pattern of outcomes.
Statistical analyses were performed using SPSS (v.14.0; Chicago, IL, USA). Dependent measures were analyzed using an ANOVA with sex (referring to gonadal sex, determined by the presence or absence of the Sry transgene) and chromosome complement (referring to sex chromosome complement, XX vs. XY) as between subject factors. When assumptions of sphericity were violated, degrees of freedom were adjusted using the Greenhouse-Geisser procedure. Two-tailed paired or unpaired t-tests were run for post-hoc analyses, where appropriate.
Results
Free reward consumption under ad libitum feeding
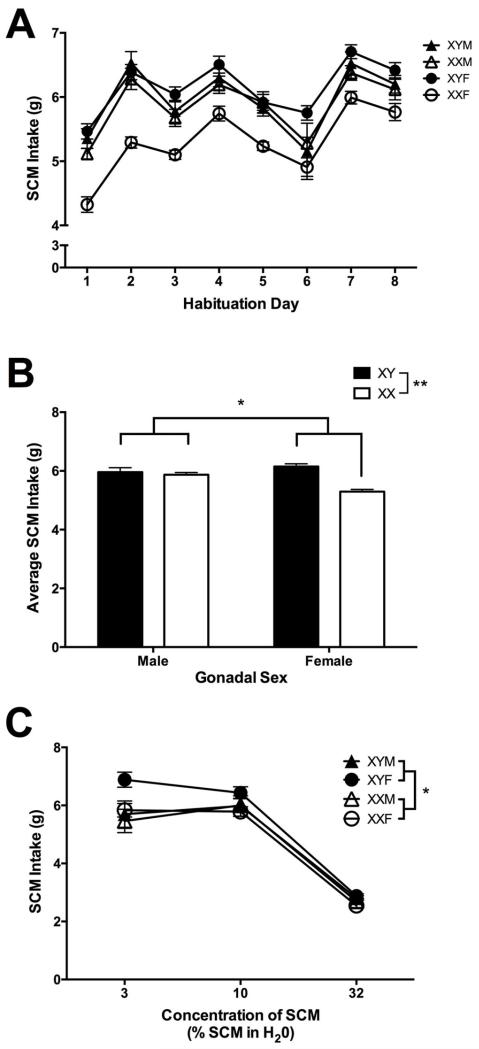
First, consumption of the 10% SCM solution in the FCG mice across the 8 d assessment was examined. There were measurable changes in SCM intake across days (Figure 2A; Day: F(5,232)=88.72, p≤0.001; Day × Sex: F(5,232)=3.2, p≤0.01; Day × Chromosome complement: F(5,232)=2.9, p<0.01). Nonetheless, significant differences among the FCG mice were detected for daily SCM intake (Figure 2B), but not for water intake (Table 1), such that a main effect of chromosome complement (F(1,46)=24.2; p<0.001), a main effect of gonadal sex (F(1,46)=7.1; p=0.01) and a significant chromosome complement by gonadal sex interaction (F(1,46)=11.8; p=0.001) were detected for raw SCM intake. Overall, XY mice consumed greater amounts of the 10% SCM solution than XX mice across the 8 consecutive observations (XY: 6.11 g +/− 0.07; XX: 5.61 g +/− 0.08), but the difference was substantially greater in females than males, leading to the chromosome complement by gonadal sex interaction. Gonadal male mice consumed more SCM than gonadal female mice (Male: 5.995 g +/− 0.08; Female: 5.72 g +/− 0.07) (Figure 2B), but the difference was driven almost entirely by XX mice.
Figure 2.
Consumption of a 10% SCM solution (measured in grams) in the 2-bottle, free-consumption test across the eight assessment days (Panel A) and the average (mean +/− SEM) of those eight days (Panel B) plotted as a function of chromosome complement (XX – open symbols; XY – close symbols) and gonadal sex (male – triangles; female – circles). Average consumption (mean +/− SEM) of differing concentrations of SCM solution (measured in grams) plotted as a function of chromosome complement (XX – open symbols; XY – close symbols) and gonadal sex (male – triangles; female – circles) (Panel C). * p<0.05, ** p<0.01; SCM – sweetened condensed milk; M – male; F – female.
Table 1.
Average water intake (± SEM) across the eight day free-choice consumption test in FCG mice.
| Genotype | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 |
|---|---|---|---|---|---|---|---|---|
| XXF | 0.136+/− 0.027 |
0.058+/− 0.015 |
0.125+/− 0.028 |
0.067+/− 0.019 |
0.117+/− 0.021 |
0.175+/− 0.045 |
0.133+/− 0.028 |
0.150+/− 0.051 |
| XYF | 0.112+/− 0.031 |
0.061+/− 0.017 |
0.076+/− 0.029 |
0.088+/− 0.019 |
0.088+/− 0.022 |
0.165+/− 0.041 |
0.129+/− 0.017 |
0.171+/− 0.037 |
| XXM | 0.083+/− 0.021 |
0.117+/− 0.047 |
0.108+/− 0.031 |
0.067+/− 0.019 |
0.083+/− 0.024 |
0.133+/− 0.014 |
0.083+/− 0.034 |
0.108+/− 0.023 |
| XYM | 0.106+/− 0.018 |
0.146+/− 0.055 |
0.131+/− 0.035 |
0.100+/− 0.032 |
0.092+/− 0.018 |
0.115+/− 0.015 |
0.085+/− 0.019 |
0.146+/− 0.035 |
Next, SCM intake at three different concentrations (3, 10 and 32%) was examined. SCM intake was affected in all mice by concentration (Figure 2C; Concentration: F(2,91)=407.4, p≤0.001). Further, consistent with our initial observations with the 10% SCM solution, there was a main effect of chromosome complement (chromosome complement: F(1,50)=12.6, p=0.001) and a significant chromosome complement by concentration interaction F(2,85)=3.4,p=0.04), without a significant effect of gonadal sex (gonadal sex: F(1,50)<10) or gonadal sex by concentration interaction (F(2,85)<1), for the amount of SCM solution consumed. As presented in Figure 2C, XY mice consumed more SCM than their XX counterparts at all concentrations assessed.
Free reward consumption under caloric restriction
Following dietary restriction, SCM intake increased in all subjects, and the effect of the chromosomal complement, that we previously detected, was no longer significant (chromosome complement F(1,50)<1; gonadal sex F(1,50)<1; chromosome complement x gonadal sex F(1,50)=2.4, p=0.13). An analysis of body weights following induction of dietary restriction indicated that XXF mice had the largest weight loss (~15% of their free-feeding weights) while all others group were comparable to one another (~10% decline from free-feeding weights); the difference persisted throughout the period of food restriction. Because of the greater weight loss in this group of mice, one would potentially expect XXF mice to consume more SCM than the other groups; however, when intake of a 3% SMC solution prior to food restriction was compared to that after dietary restriction, there was no evidence that intake in XXF increased more than the other genotypes.
Operant Behavioral Assessments
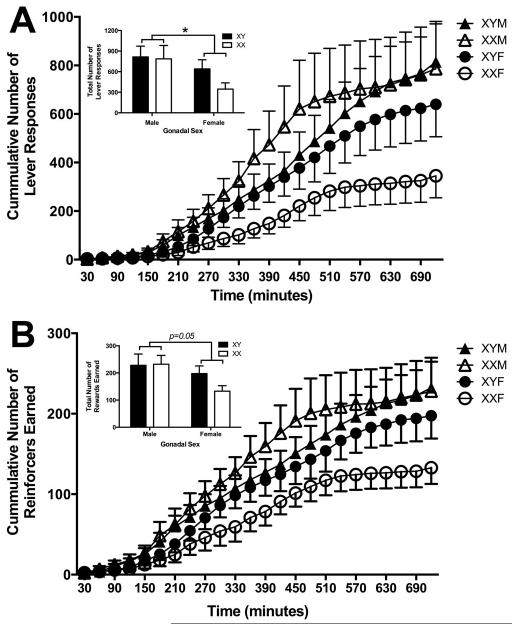
All mice retrieved all rewards delivered during the magazine training session, so the instrumental behavior of mice in the 12 h behavioral sessions was next examined. Gonadal sex, but not chromosome complement, had a significant effect on the total number of active presses made (Figure 3A; gonadal sex: F(1,28) =4.2, p=0.05; chromosome complement: F(1,28) =1.0, p=0.31; gonadal sex x chromosome complement: F(1,28) <1) and rewards earned (Figures 3B; gonadal sex: F(1,28) =4.1, p≤0.05; chromosome complement: F(1,28) =1.1, p=0.29; gonadal sex x chromosome complement: F(1,28) <1). Female mice made fewer lever presses and earned fewer reinforcers than male mice (Lever Presses: female: 518 +/− 92; male: 803 +/− 117; reinforcers earned: female: 170 +/− 20; male: 230 +/− 24; mean +/− SEM)
Figure 3.
Behavioral data in the 12 h overnight instrumental session plotted as a function of chromosome complement (XX – open symbols; XY – closed symbols) and gonadal sex (male – triangles; female – circles). Panel A presents the cumulative number of lever responses made across the 12 h session (time – mins); inset in Panel A is the average total number of lever responses made (mean +/− SEM) in gonadal male (left bars) and gonadal female (right bars) mice as a function of their chromosome complement (XX – open bars; XY – closed bars). Panel B presented the cumulative number of SCM reinforcers earned across the 12 h session (time – mins); inset in Panel B is the average total number of SCM reinforcers earned (mean +/− SEM) in gonadal male (left bars) and gonadal female (right bars) mice as a function of their chromosome complement (XX – open bars; XY – closed bars). *<0.05; M – male; F – female.
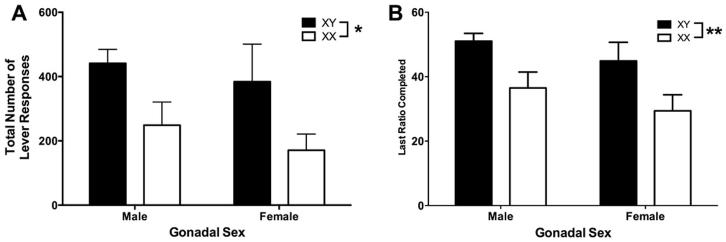
Under the progressive ratio schedule of reinforcement, a significant effect of chromosome complement, but not gonadal sex, was detected on the number of active lever presses (Figure 4A; chromosome complement: F(1,28)=5.5; p=0.02; sex: F(1,28)<1; chromosome complement by gonadal sex: F(1,28)<1) and the final ratio achieved (Figure 4B; chromosome complement: F(1,28)=9.3; p=0.005; gonadal sex: F(1,28)<1; chromosome complement by gonadal sex: F(1,28)<1). XY mice made twice as many lever presses and reached 46% higher ratios than XX mice (Lever presses: XY: 411 +/− 63; XX: 207 +/− 42; final ratio achieved: XY: 47.79 +/− 3.21; XX: 32.69 +/− 3.52; mean +/− SEM). During the progressive ratio test, individual mice obtained fewer than half of the reinforcers they voluntarily earned during the fixed ratio testing procedure, indicating that satiety was not a factor in determining the point at which they stopped responding; rather, it was the motivation to engage in effortful reward-seeking that determined their final ratio achieved. In contrast with the instrumental learning session, the effects found using the progressive ratio assessment was consistent with those from the free consumption testing conducted prior to restriction, namely, a strong influence of chromosomal complement.
Figure 4.
Behavioral data collected during the 12 h overnight instrumental session under a progressive ratio schedule of reinforcement presented as a function of chromosome complement (XX – open symbols; XY – closed symbols) and gonadal sex (male – left bars; female – right bars). Panel A presents the average total number of lever responses (mean +/− SEM) made during the 12 h session; Panel B presents the average ratio achieved (mean +/− SEM). *p <0.05, ** p<0.01.
Discussion
This study, which was conducted in gonadectomized adult mice, shows that both gonadal sex and sex chromosome complement influence ingestion of a highly palatable food item (SCM) but do so in ways that are only observable in a model system that specifically separates these two sex-biasing factors that are normally confounded. We first measured free consumption of a 10% SCM solution in mice being fed chow on an ad libitum schedule; in the case where intake was likely related to palatability rather than caloric intake. Under those conditions, both gonadal sex (gonadal males > gonadal females) and sex chromosome complement (XY>XX) affected SCM intake, but the two factors affected the impact of the other. Specifically, XY mice consumed more SCM than did XX mice, but this effect was much larger in gonadal females than in gonadal males. Similar effects were not observed for intake of simultaneously-available but non-palatable option (water).
When a 3% SCM solution was offered, the main effect of chromosome complement was exaggerated. Conversely, when the solution was increased to 32%, this difference disappeared. The effect of sex chromosome complement was also not detected when the mice were placed on mild dietary restriction and then offered free-access to the SCM; under these conditions, consumption was likely increasingly mediated to a greater degree by calorie-seeking than by palatability-seeking. The most likely explanation of these data is that XY, relative to XX, mice demonstrate elevated sensitivity to the rewarding impact of the SCM solution, a hypothesis we evaluated using an operant conditioning paradigm.
Instrumental learning was examined under dietary restriction because it is common for large proportions of subjects to fail to acquire lever pressing for food when no restriction is imposed; that said, the level of restriction used here was quite minimal. Under these conditions, chromosome complement had no influence on the initial acquisition or performance of the operant response, much as it did not affect free access of SCM in a calorie-restricted state; rather, gonadal sex had the largest impact on instrumental performance of mice. Gonadally-female mice made fewer lever responses and, as a consequence, earned fewer rewards than gonadally-male mice. However, behavior using a progressive ratio schedule of reinforcement, which allows for a direct measure of reward strength and of motivation to obtain to the reinforcer (Hodos, 1961), indicated a strong influence of chromosome complement on instrumental responding. Mice with one X and one Y chromosome, relative to those with two X chromosomes, have twice the number of lever responses and almost 50% higher break points, suggesting that they exhibit greater motivation and/or reward value of the SCM solution. A recent study of diurnal wheel-running behavior indicated that XX mice have longer run durations than XY mice (Kuljis et al., 2013), so it is unlikely that the higher numbers of lever presses made by XY mice during the progressive ratio test were a simple effect of greater levels of overall activity.
Body weights and degree of body weight change in response to introduction of a dietary restriction also varied among groups, likely due sex chromosome complement effects on chow intake, adiposity and metabolism (Chen et al., 2012, Chen et al., 2013, Link et al., 2013). Notably, body weight and weight loss during restricted access to chow were unlikely to account for the observed effects of sex chromosome complement on motivation / reward. During the free access experiments, when mice were not under dietary restriction, main effects of sex chromosome complement and gonadal sex were observed, irrespective of whether intake was normalized to body weight or not. During the operant conditioning tests, which were conducted in mice maintained on a restricted access chow diet, degree of body weight reduction was greatest in XXF but responding for the SCM was greatest in XYM. Thus, body weight or weight loss variables, differences that are essentially unavoidable in FCG mice, did not systematically explain the effects of sex chromosome complement observed here.
Implications for Reward Pursuit and Consumption
There is precedent for an effect of sex chromosome complement on motivated reward-seeking behaviors. In one experiment, the acquisition of instrumental responses for a sucrose reinforcer was measured in FCG mice (on an MF1 strain background) maintained on a restricted-access diet (Quinn et al., 2007). In that study, no main effects of gonadal sex or sex chromosome complement were noted when acquisition of an instrumental response (a nose poke) was measured. Despite differences in the palatable reinforcer used and the reinforcement schedule, their results are remarkably similar to ours, as we also noted no sex chromosome complement effects on operant learning for the SCM solution in hungry FCG mice. Quinn et al. also found that XX mice, irrespective of gonadal sex, developed a habitual (automatic) pattern of lever pressing more readily than did XY mice. Our data suggest that responding during the acquisition phase does not necessarily reveal the motivational differences or sensitivity to reward strength that are being influenced by sex chromosome complement; if these differences in motivation were apparent (though not measured), they could, in principle, have led to sex chromosome complement-sensitive rates of habit formation
Another study from the same group examined instrumental learning for an orally-consumed alcohol solution in MF1 FCG mice (Barker et al., 2010). Ethyl alcohol has both reinforcing and caloric value, much like sucrose and SCM solutions; however, unsweetened alcohol solutions lack for palatability. In this study, the acquisition of an operant response for alcohol reinforcement was not sensitive to sex chromosome complement, but the development of a habitual pattern of alcohol responding was. In this case, it was XY mice that exhibited accelerated habit formation, relative to XX mice.
These two studies, as well as the current one, used calorie-restricted FCG mice, to evaluate reward-seeking instrumental behaviors; because of procedural differences between them, it is impossible to directly evaluate commonalities and discrepancies in the outcomes. Nevertheless, they each demonstrate a key observation, that sex chromosome complement, irrespective of gonadal sex and independent of the activational effects of sex hormones, can play a significant role in controlling reward pursuit and/or consumption. These results, which clearly require more systematic study using common training and testing protocols, suggest that sex chromosome complement – in addition to gonadal sex and circulating sex hormones – is an important sex-biasing factor contributing to sex differences in sugar and fat intake, and possibly in drug and alcohol consumption (Carroll & Anker, 2010, Carroll et al., 2004, Carroll et al., 2008, Lynch et al., 2002, Ngun et al., 2011, Roth et al., 2004, Wetherington, 2007).
Implications for Metabolic Disease and Obesity
Earlier studies indicated that sex chromosome complement influences body weight, adiposity, feeding, lipid accumulation in liver, and insulin levels; in each case, XX mice exhibited a risk phenotype compared to XY or XO mice (Chen et al., 2012, Chen et al., 2013, Link et al., 2013). Here, we found that XY mice, relative to XX, were prone to consuming more SCM solution and to exhibiting a heightened sensitivity to the reward value of SCM; we did not study the physiological consequences of long-term consumption of a fat/sugar-rich diet.
The earlier studies (Chen et al., 2012, 2013) involved long-term recording of food intake and physiology, while the studies here reflected relatively short-term assessments of behavior. A key difference between earlier observations, and the current data, reflects the time scale across which the observations were made, and both sex chromosome and hormonal effects may depend upon the length of exposure to various food stimuli and over the period of time across which the metabolic and physiological changes are allowed to occur.
The collection of studies on metabolism / feeding of FCG mice nevertheless underscores the prevailing view that a diverse array of motivational factors and associated brain systems are responsible for controlling food intake in various settings. For example, seeking food for its nutritional/caloric value (usually in a nutrient-deprived state) differs, both behaviorally and neurobiologically, from seeking and consuming reward solely for its palatability and/or reward value (Berridge et al., 2010, Blouet & Schwartz, 2010, Carr, 1996, Dileone et al., 2003, Hull, 2011, Kelley, 2004, Kelley et al., 2005a, Kelley et al., 2005b, Smith & Berridge, 2007, Wise, 2013). In addition, various combinations of nutrient qualities to food (sugar, fat, salt, protein, etc.) depend upon partially distinctive biological systems. Each of these factors is independently susceptible to modulation by different sex-biasing factors (Hull, 2011), likely leading to the complex array of observations regarding sex chromosome complement influences on eating and food intake in these various settings.
Conclusions
Our studies add to the literature indicating that sex chromosome complement can have a significant sex-biasing influence on reward-related behaviors, possibly contributing to the sexual dimorphism observed in eating and overeating, as well as in the pursuit of non-food reinforcers. While the use of an SCM food does not allow us to dissect sex chromosome complement effects on palatability- vs. calorie-seeking, nor does it allow us to differently explore how sex-biasing factors influence responses to sugars vs. fats, it does set the stage for more systematic studies of these topics. Alternate rewards, like saccharin – which is a palatable but non caloric stimulus, can be used to evaluate some of these issues. Additionally, these studies should be extended to other chemical rewards (like drugs of abuse) whose effects are generally not dependent upon oral ingestion and not influenced by taste systems, but whose hedonic and reward value is the major determinant of pursuit and consumption. In doing so, a more comprehensive and mechanistic view of the biological and psychological processes through which sex chromosome genes act to influence ingestion can be determined.
Acknowledgements
This manuscript is dedicated to the memory of Dr. Emanuele Seu, who conducted the experiments but tragically passed away suddenly before the manuscript could be written. Thanks to Karen Reue and Rebecca McClusky for advice and assistance. Supported by NIH grants 1R01DA038152 (JDJ), F31DA028812 (SMG), and 1R01NS043196 and 1R01DK083561 (APA).
Footnotes
The authors have no apparent or real conflicts of interest to report.
References
- Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol. 2009a;21:377–386. doi: 10.1111/j.1365-2826.2009.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Hormones and behavior. 2009b;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Torregrossa MM, Arnold AP, Taylor JR. Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:9140–9144. doi: 10.1523/JNEUROSCI.0548-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain research. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouet C, Schwartz GJ. Hypothalamic nutrient sensing in the control of energy homeostasis. Behavioural brain research. 2010;209:1–12. doi: 10.1016/j.bbr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Carr KD. Feeding, drug abuse, and the sensitization of reward by metabolic need. Neurochem Res. 1996;21:1455–1467. doi: 10.1007/BF02532386. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Hormones and behavior. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends in pharmacological sciences. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behavioural pharmacology. 2008;19:435–460. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, Reue K. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8:e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, McClusky R, Itoh Y, Reue K, Arnold AP. X and Y chromosome complement influence adiposity and metabolism in mice. Endocrinology. 2013;154:1092–1104. doi: 10.1210/en.2012-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. Psychobiological traits in the risk profile for overeating and weight gain. Int J Obes (Lond) 2009;33(Suppl 2):S49–53. doi: 10.1038/ijo.2009.72. [DOI] [PubMed] [Google Scholar]
- Davis C, Patte K, Levitan R, Reid C, Tweed S, Curtis C. From motivation to behaviour: a model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite. 2007;48:12–19. doi: 10.1016/j.appet.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Davis C, Strachan S, Berkson M. Sensitivity to reward: implications for overeating and overweight. Appetite. 2004;42:131–138. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Davis C, Woodside DB. Sensitivity to the rewarding effects of food and exercise in the eating disorders. Compr Psychiatry. 2002;43:189–194. doi: 10.1053/comp.2002.32356. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life sciences. 2003;73:759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Kurth C, Holden-Wiltse J, Saari J. Food preferences in human obesity: carbohydrates versus fats. Appetite. 1992;18:207–221. doi: 10.1016/0195-6663(92)90198-f. [DOI] [PubMed] [Google Scholar]
- Dudley SD, Gentry RT, Silverman BS, Wade GN. Estradiol and insulin: independent effects on eating and body weight in rats. Physiol Behav. 1979;22:63–67. doi: 10.1016/0031-9384(79)90405-0. [DOI] [PubMed] [Google Scholar]
- Gentry RT, Wade GN. Androgenic control of food intake and body weight in male rats. J Comp Physiol Psychol. 1976a;90:18–25. doi: 10.1037/h0077264. [DOI] [PubMed] [Google Scholar]
- Gentry RT, Wade GN. Sex differences in sensitivity of food intake, body weight, and running-wheel activity to ovarian steroids in rats. J Comp Physiol Psychol. 1976b;90:747–754. doi: 10.1037/h0077246. [DOI] [PubMed] [Google Scholar]
- Gentry RT, Wade GN, Roy EJ. Individual differences in estradiol-induced behaviors and in neural 3H-estradiol uptake in rats. Physiol Behav. 1976;17:195–200. doi: 10.1016/0031-9384(76)90063-9. [DOI] [PubMed] [Google Scholar]
- Gioiosa L, Chen X, Watkins R, Klanfer N, Bryant CD, Evans CJ, Arnold AP. Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice. Horm Behav. 2008;53:124–130. doi: 10.1016/j.yhbeh.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hull EM. Sex, drugs and gluttony: how the brain controls motivated behaviors. Physiology & behavior. 2011;104:173–177. doi: 10.1016/j.physbeh.2011.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neuroscience and biobehavioral reviews. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. The Journal of comparative neurology. 2005a;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiology & behavior. 2005b;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuljis DA, Loh DH, Truong D, Vosko AM, Ong ML, McClusky R, Arnold AP, Colwell CS. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology. 2013;154:1501–1512. doi: 10.1210/en.2012-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link JC, Chen X, Arnold AP, Reue K. Metabolic impact of sex chromosomes. Adipocyte. 2013;2:74–79. doi: 10.4161/adip.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Ngun TC, Ghahramani N, Sanchez FJ, Bocklandt S, Vilain E. The genetics of sex differences in brain and behavior. Frontiers in neuroendocrinology. 2011;32:227–246. doi: 10.1016/j.yfrne.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Bale TL. Stress and sex influences on food-seeking behaviors. Obesity (Silver Spring) 2008;16:1539–1544. doi: 10.1038/oby.2008.221. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex chromosome complement regulates habit formation. Nat Neurosci. 2007;10:1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- Richard JM, Castro DC, Difeliceantonio AG, Robinson MJ, Berridge KC. Mapping brain circuits of reward and motivation: In the footsteps of Ann Kelley. Neuroscience and biobehavioral reviews. 2012 doi: 10.1016/j.neubiorev.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neuroscience and biobehavioral reviews. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Small DM. Individual differences in the neurophysiology of reward and the obesity epidemic. Int J Obes (Lond) 2009;33(Suppl 2):S44–48. doi: 10.1038/ijo.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiol Behav. 2009;97:551–560. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherington CL. Sex-gender differences in drug abuse: a shift in the burden of proof? Experimental and clinical psychopharmacology. 2007;15:411–417. doi: 10.1037/1064-1297.15.5.411. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dual roles of dopamine in food and drug seeking: the drive-reward paradox. Biological psychiatry. 2013;73:819–826. doi: 10.1016/j.biopsych.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]