SUMMARY
Injection of the peptide hormone ghrelin stimulates food intake in mice and humans. However, mice born without ghrelin demonstrate no significant loss of appetite. This paradox suggests either that compensation develops in mice born without ghrelin or that ghrelin is not essential for appetite control. To distinguish these possibilities, we generated transgenic mice (Ghrl-DTR) that express the diphtheria toxin receptor in ghrelin-secreting cells. Injection of diphtheria toxin in adulthood ablated ghrelin cells and reduced plasma ghrelin by 80-95%. Ghrelin cell-ablated mice exhibited no loss of appetite or body weight and no resistance to a high fat diet. To stimulate food intake in mice by ghrelin injection, we had to raise plasma levels many-fold above normal. Like germline ghrelin-deficient mice, the ghrelin cell-ablated mice developed profound hypoglycemia when subjected to prolonged calorie restriction, confirming that ghrelin acts to maintain blood glucose under famine conditions.
INTRODUCTION
Ghrelin is a 28-amino acid peptide hormone secreted by specialized cells in the stomach (Kojima and Kangawa, 2005). It requires octanoylation on Ser-3 by Ghrelin-O-acyltransferase (GOAT) (Yang et al., 2008; Gutierrez et al., 2008) in order to interact with its receptor, the Growth Hormone Secretogogue Receptor (GHSR), which is present in tissues throughout the body, including the pituitary and hypothalamus (Cruz and Smith, 2008).
The discovery that ghrelin administration stimulates appetite in rodents (Tschöp et al., 2000) and humans (Wren et al., 2001) led to the hypothesis that ghrelin is an orexigenic antipode to anorexigenic leptin and raised hopes that pharmacologic inhibition of ghrelin would prove useful for curbing appetite and treating obesity (Cummings, 2006; Nass et al., 2011). The argument that ghrelin functions in vivo as an appetite-stimulating hormone was bolstered by evidence that its plasma concentration peaks before meals and falls shortly after feeding (Cummings et al., 2001; Tschöp et al., 2001; Nass et al., 2008) and that some forms of bariatric surgery – until recently the only FDA-approved treatment for reducing appetite – suppress ghrelin levels (Cummings et al., 2002), although the latter observations have been challenged (Saliba et al., 2009).
Evidence against this ghrelin hypothesis, at least in rodents, has emerged from studies of ghrelin-deficient mouse models, none of which has shown a robust reduction in appetite or body weight (Sun et al., 2003; Sun et al., 2004; Wortley et al., 2004; Zhao et al., 2010a). Unlike leptin-deficient mice, which are hyperphagic and morbidly obese (Friedman and Halaas, 1998), ghrelin-deficient mice have normal food intake and body weight. Similar negative findings were reported for mice lacking GHSR, the ghrelin receptor (Sun et al., 2004). Indeed, in mice the major function of ghrelin appears to be in the control of blood glucose. Ghrelin ablation has been demonstrated to improve glucose tolerance by increasing insulin secretion (Sun et al., 2006; Zhao et al., 2010a). Studies performed in our laboratory on Goat−/− mice revealed an essential function for ghrelin in maintaining blood glucose during periods of chronic starvation (Zhao et al., 2010a; Goldstein et al., 2011; Li et al., 2012).
The negative results with germline ghrelin knockouts might be explained if the mice develop compensation owing to the lifelong ghrelin deficiency. A precedent exists in the work of Luquet et al.(Luquet et al., 2005), who showed that ablation of AgRP/NPY neurons in the arcuate hypothalamus causes a loss of appetite when performed in adult but not neonatal mice. AgRP/NPY neurons express the GHSR and are thought to be critical for the appetite-stimulating effect of ghrelin (Cowley et al., 2003). The conditional ablation of ghrelin in an adult animal is therefore necessary to understand the influence of ghrelin levels on appetite in vivo.
In the current studies, we ask whether inhibition of ghrelin signaling in an adult mouse affects food intake or body weight. We generated transgenic mice that express the diphtheria toxin receptor (DTR) specifically on ghrelin-secreting cells (designated Ghrl-DTR mice). When injected with diphtheria toxin (DTX) in adulthood, Ghrl-DTR mice lost their ghrelin cells within 24 hr and experienced a decline in plasma ghrelin levels of 80-95%. Ghrelin levels were maintained below 80% of normal for at least four weeks and could be maintained at lower levels with repeated administrations of DTX. We found no change in food intake or body weight in the setting of ghrelin cell ablation in the short or long term.
RESULTS
Administration of DTX to Ghrl-DTR Mice Destroys Ghrelin-secreting Cells and Reduces Circulating Ghrelin
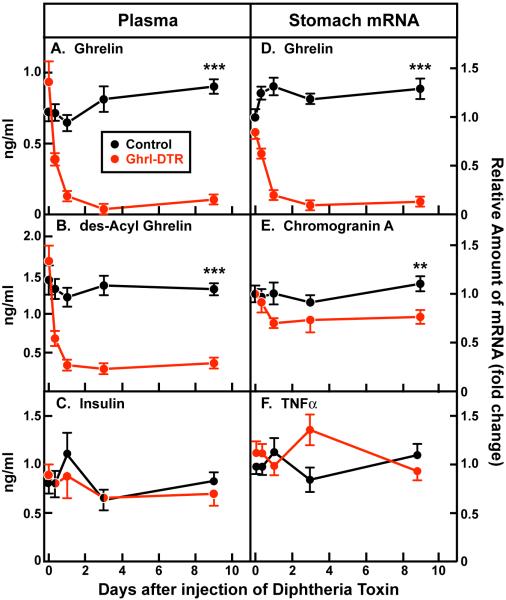
Employing the recombination strategy described in Experimental Procedures and schematized in Figure S1, we generated transgenic mice that express the simian DTR selectively in ghrelin-producing cells (Ghrl-DTR mice). When the mice reached 8 weeks of age, we administered a single dose of DTX intraperitoneally (8-10 ng/g body weight) to Ghrl-DTR mice and to control littermates. Groups of 6 mice were killed at 8, 24, 72, and 216 hr after DTX injection. The 12-hr light cycle began at 9 a.m., and all mice were killed within 30 min of 2 p.m., which corresponds to the peak of the circadian cycle of plasma ghrelin in control mice (Figure S2). Plasma was obtained for hormone measurements, and stomach tissue was harvested for mRNA measurements. In Ghrl-DTR mice, the DTX injection produced a 50% reduction in plasma ghrelin within 8 hr. By 24 hr, the level had declined to 14% of normal, and it remained at this low level through nine days (Figure 1A). The decline in des-acyl ghrelin was equally rapid, but not quite as profound, the level averaging 20% of normal through the 9 days (Figure 1B). Plasma insulin levels were unaffected by the DTX injection in either control or Ghrl-DTR mice (Figure 1C).
Figure 1. Plasma Hormones and Stomach mRNAs of Control and Ghrl-DTR Mice over 9 Days Following Injection of DTX.
Chow-fed control and Ghrl-DTR littermates (male, 8-week-old) were injected intraperitoneally with 8-10 ng/g body weight of DTX at the indicated time prior to sacrifice. To obtain groups of n=6, two identical n=3 studies were performed on consecutive days and combined into one data set. Injections of DTX were staggered such that the indicated time points were reached at 2 p.m. (the circadian maximum of ghrelin concentration) on the day of sacrifice. Uninjected controls are shown at time 0. After anesthetization by isoflurane, blood was drawn from the inferior vena cava, after which the whole stomach was divided in half longitudinally and immediately homogenized in RNA-STAT. Plasma measurements for ghrelin (A), des-acyl ghrelin (B), and insulin (C) were determined by ELISA as described in Experimental Procedures. mRNA levels for Ghrelin (D), chromogranin A (E), and TNFα (F) were measured by quantitative RT-PCR on stomach RNA and normalized to cyclophilin mRNA. Each value represents mean ± SEM of 6 mice, except the Ghrl-DTR groups at 8 hr and 3 days, which represent n=5 and n=4 mice, respectively. One mouse at the 8-hr time point and 2 mice at the 3-day time point did not respond to DTX as indicated by PCR of stomach RNA; the values for these mice were omitted from the data. **, p < 0.01; ***, p < 0.001.
The level of ghrelin mRNA in stomach extracts declined in parallel with the fall in plasma ghrelin levels (Figure 1D). The mRNA encoding chromogranin A, a protein found in all gastric endocrine cells, fell by 29% in the Ghrl-DTR mice (Figure 1E). This is consistent with the estimate that ghrelin cells account for ~20% of chromogranin A-containing cells in the stomach (Date et al., 2000). We saw no significant increase in the mRNA encoding TNFα in the stomach extracts, which suggests that DTX did not elicit an inflammatory response in the stomach (Figure 1F).
To examine the architecture of the stomach, we prepared histological sections of stomachs of control and Ghrl-DTR mice that were killed 9 days after DTX injection. The animals were part of the group studied in Figure 1. Figures 2A and D show that stomach architecture was preserved in Ghrl-DTR mice after DTX injection. In control mice we easily detected cells that stained positive for ghrelin (Figure 2B). We saw no such cells in the stomachs from the Ghrl-DTR mice (Figure 2E). Abundant chromogranin A-positive cells were observed in control and Ghrl-DTR stomachs (Figures 2C and F). We did not observe any histological signs of inflammation in these stomachs, nor did we detect any morphological changes or alterations in insulin or glucagon staining in pancreatic islets.
Figure 2. Stomach Histology of Control and Ghrl-DTR Mice 9 Days after Injection of DTX.
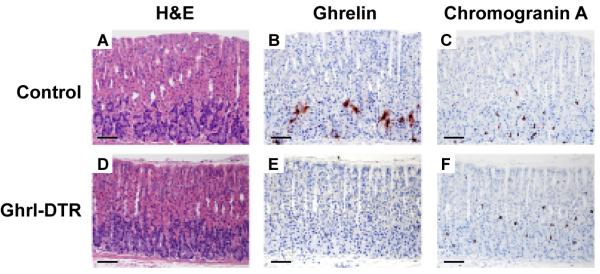
Stomachs were harvested 9 days after injection of DTX from the same control (A-C) and Ghrl-DTR (D-F) mice shown in Figure 1. Representative sections of the gastric fundus are shown. Serial sections were stained with hematoxylin and eosin (A,D), anti-ghrelin antisera (B,E), and anti-chromogranin A antisera (C,F). Magnification, 10x; scale bar, 250 μm.
Acute Ablation of Ghrelin Cells Does Not Alter Food Intake or Body Weight
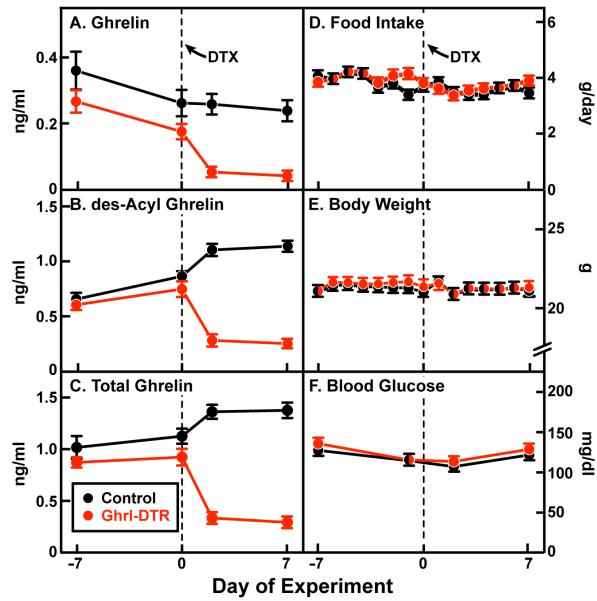
To measure daily food intake and body weight, we housed mice in individual cages for two weeks (starting on day −7). DTX was injected on day 0. Ghrelin and des-acyl ghrelin levels were measured on day −7 and day 0 before injection and on days 2 and 7 after injection (Figure 3A-C). On average, plasma ghrelin fell by 80% and des-acyl ghrelin levels by 76% in Ghrl-DTR mice relative to littermate controls. Despite these reductions in the 7 days following injection of DTX, we observed no alteration in food intake, which averaged 3.6 ± 0.1 g/day in both groups (Figure 3D) or body weight, which averaged 21.2 ± 0.3 g in both groups (Figure 3E). No differences in blood glucose levels were observed (Figure 3F).
Figure 3. Body Weight and Food Intake of Control and Ghrl-DTR Mice Before and After Ablation of Ghrelin Cells.
Chow-fed control and Ghrl-DTR littermates (male, 8-week-old) were housed individually and injected intraperitoneally with DTX on day 0. Blood was drawn by tail vein at 2:00 p.m. on the indicated day. Ghrelin (A) and des-acyl ghrelin (B) levels were measured by ELISA, and added together to give total ghrelin (C). Food intake (D) and body weight (E) were measured daily at 2 p.m. Experimenters were blinded to the genotypes of the mice for body weight and food intake measurements. Blood glucose (F) was measured by Bayer Glucometer. Dotted lines denote time of DTX injection. Each value represents mean ± SEM of 8-9 mice. One mouse that did not respond to DTX (by ghrelin and des-acyl ghrelin measurements) was excluded.
Ghrelin Deficiency Does Not Prevent Diet-Induced Obesity
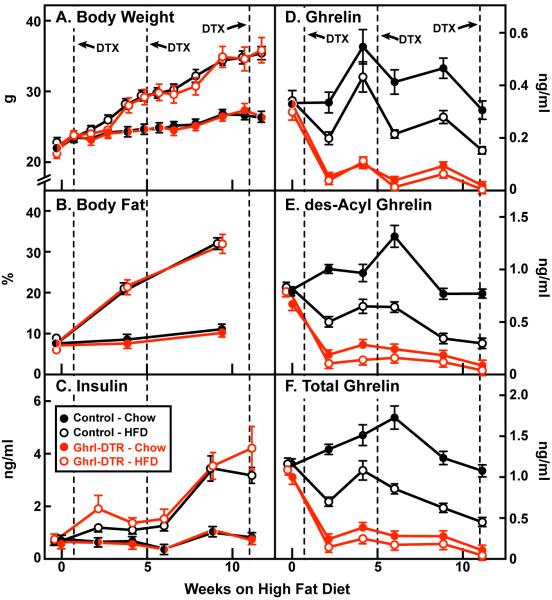
We subjected groups of 11-14 control or Ghrl-DTR mice to either a chow diet or a high fat diet (HFD) for 12 weeks. Mice were housed in cages of 4 that contained mixed genotypes. Investigators were blinded to genotype for all sample collections and assays. DTX was injected in all mice during week 1, and repeat injections were made during weeks 5 and 11 (Figure 4). Control and Ghrl-DTR mice gained equal amounts of weight on the high fat diet (Figure 4A), and they exhibited equal increases in body fat (Figure 4B). Plasma measurements of insulin, ghrelin, des-acyl ghrelin, and total ghrelin were made biweekly at 2 p.m. in all mice (Figure 4C F). Area under-the-curve calculations beginning after injection of DTX and extending until the end of the study indicate an average reduction in plasma ghrelin of 85% and 89% in chow- and HFD-fed Ghrl-DTR mice relative to chow-fed control mice (Figure 4D). Corresponding reductions in des-acyl ghrelin were 79% and 87%, respectively (Figure 4E). In control mice the HFD reduced plasma ghrelin by 38% and des-acyl ghrelin by 49% (Figures 4D and E). The significance of this finding is unknown, but is consistent with observations in humans showing that plasma ghrelin levels are significantly lower in obese subjects than in lean subjects (Shiiya et al., 2002). In the HFD-fed groups, hyperinsulinemia (> 3 ng/ml) developed in control and Ghrl-DTR mice between weeks 6 and 9 of high fat feeding (Figure 4C).
Figure 4. Body Weight of Control and Ghrl-DTR Mice during High Fat Feeding.
Control and Ghrl-DTR littermates (male, 8-week-old) were housed in cages containing 4 mice of mixed genotype. Mice were fed either a chow diet or a HFD for 12 weeks as indicated and were injected intraperitoneally with DTX at the indicated time. Body weight (A) was measured weekly. Body fat (B) was measured monthly. Blood was drawn biweekly at 2:00 p.m. Plasma levels of insulin (C), ghrelin (D), and des-acyl ghrelin (E) were measured by ELISA, and values in (D) and (E) were added together to give values for total ghrelin (F). Dotted lines denote times of DTX injection. Each value represents mean±SEM of 11-15 mice. One control mouse was incorrectly genotyped, and three Ghrl-DTR mice in the chow-fed group did not respond to DTX; the values for these mice were omitted from the data. HFD, high-fat diet.
Ghrelin Cells Are Necessary to Prevent Profound Hypoglycemia during Calorie Restriction
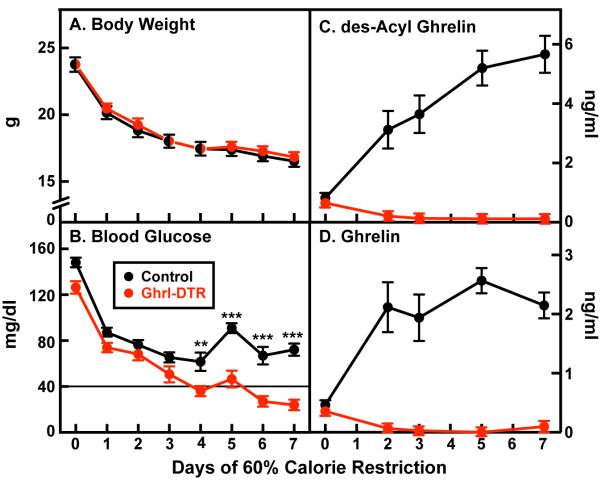
We showed previously that mice genetically deficient for acylated ghrelin (Goat−/−) become severely hypoglycemic during 60% calorie restriction (Zhao et al., 2010a; Goldstein et al., 2011; Li et al., 2012). The same was true of genetic ghrelin deficiency (Li et al., 2012). To determine whether ablation of ghrelin cells in adulthood would reproduce this result, we subjected control and Ghrl-DTR mice (n=8) to seven days of 60% calorie restriction. These animals were fed each day at 6 p.m. Blood glucose was measured at 5:30 p.m. just before feeding (Figure 5). Inasmuch as calorie-restricted wild-type and ghrelin-deficient mice consume all of their food within 1 hr (Zhao et al., 2010a), the mice in Figure 5 have been fasting for 23.5 hr when the blood is collected at 5:30 p.m. Beginning on day 4 and continuing through day 7 of calorie restriction, we observed a decline in blood sugar in Ghrl-DTR mice relative to control mice, with Ghrl-DTR mice reaching a nadir of 24 ± 4 mg/dl on day 7, while control mice averaged 72 ± 5 mg/dl (Figure 5B). These results are similar to those observed in calorie-restricted wild-type and Goat−/− mice (Zhao et al., 2010a), confirming that the presence of ghrelin during calorie restriction is necessary to protect against fasting-induced hypoglycemia. By day 7 of calorie restriction, ghrelin levels in control mice reached 2.2 ± 0.21 ng/ml, while levels in Ghrl-DTR mice were undetectable (i.e. < 0.07 ng/ml) (Figure 5D). Des-acyl ghrelin levels reached 5.7 ± 0.62 ng/ml in control animals and were 0.12 ng/ml in Ghrl-DTR mice, a reduction of 98% (Figure 5C).
Figure 5. Comparison of Control and Ghrl-DTR Mice Subjected to 60% Calorie Restriction.
Chow-fed control and Ghrl-DTR littermates (male, 8-week-old) were housed individually and injected intraperitoneally with DTX on day 0. Mice were subjected to 60% calorie restriction for 7 days as described in Experimental Procedures. (A) Body weight was measured daily at 5:30 p.m. (B) Blood was drawn from the tail vein at 5:30 p.m. every day for glucose measurement and every 2-3 days for measurement of plasma levels of des-acyl ghrelin (C) and ghrelin (D) by ELISA. Each value represents mean±SEM of 8 mice. **, p < 0.01; ***, p < 0.001.
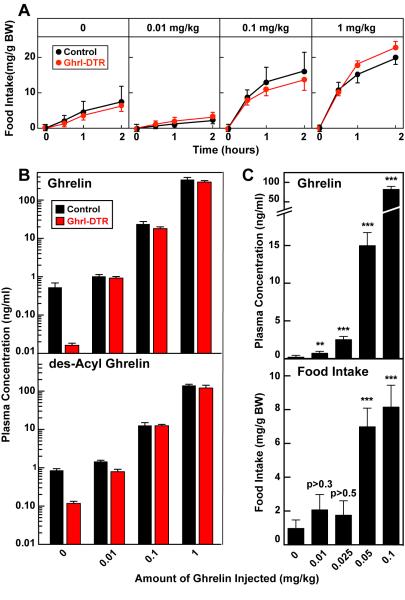
Ghrelin Administration Stimulates Food Intake Only at Supraphysiologic Doses in Control and Ghrl-DTR Mice
The failure of ghrelin cell ablation to reduce food intake in mice contrasts with many previous experiments demonstrating that injections of ghrelin cause an acute increase in food consumption (Tschöp et al., 2000; Wren et al., 2001; Kojima and Kangawa, 2005). In an attempt to reconcile these findings, we injected doses of ghrelin ranging from 0.01 to 1 mg/kg to control and Ghrl-DTR mice (Figure 6). Four days after injection of DTX, we injected the mice subcutaneously with ghrelin or saline solution and measured food intake over the following 2 hr. The experiment was initiated at 2:00 p.m. (5 hr after beginning of the light cycle), a time in which mice normally consume only small amounts of food. Injection of 0.01 mg/kg ghrelin failed to increase food intake in control and Ghrl-DTR mice (Figure 6A). At a 10-fold higher dose of ghrelin (0.1 mg/kg), there was a marked increase in food intake over the first 30 min in both control and Ghrl-DTR mice, after which there was little further increase. Another 10-fold dose increase (1 mg/kg) produced a slightly higher food intake at 30 min, and the mice continued to eat an increased amount over 2 hr. Again, the response of control and Ghrl-DTR mice was indistinguishable.
Figure 6. Food Intake of Control and Ghrl-DTR Mice Following Ghrelin Injection.
(A and B) Chow-fed control and Ghrl-DTR littermates (male, 8-12 weeks old) were housed individually and injected with DTX on day 0. On day 2, plasma ghrelin and des-acyl ghrelin were measured to verify ablation in Ghrl-DTR mice (Figure S3) and mice were randomly sorted into 8 groups of 5 mice each. On day 4 at 2:00 p.m. mice of both genotypes were injected subcutaneously with 0.1 ml of 0.15 M NaCl containing the indicated amount of ghrelin. (A) Food intake was measured at 0, 0.5, 1, and 2 hr after injection. (B) On the following day, identical injections of ghrelin were given, and blood was drawn from the tail vein 10 min after injection of the indicated concentration of ghrelin. Levels of ghrelin (top) and des-acyl ghrelin (bottom) were measured by ELISA. (C) Chow-fed control and Ghrl-DTR littermates (not injected with DTX) were randomized into 5 groups of mixed genotype (n=6). On the first day of the experiment, mice were injected subcutaneously at 2:00 p.m. with the indicated dose of ghrelin (top), and food intake (bottom) was measured at 10 and 30 min, respectively, after injection as described in A. On the following day, identical injections of ghrelin were given, and blood was drawn from the tail vein 10 min later, as described in B. **, p < 0.01; ***, p < 0.001.
We could not measure plasma ghrelin levels during the food intake experiments because it would have disrupted the feeding pattern of the mice. To obtain plasma for ghrelin determination, we repeated the saline or ghrelin injections on the next day at the same time and measured the plasma level of ghrelin and des-acyl ghrelin after 10 min. Plasma ghrelin was reduced by 97% in the saline-injected Ghrl-DTR mice as compared with controls. Injection of ghrelin at 0.01 mg/kg raised the plasma ghrelin level in the Ghrl-DTR mice so that it equaled the level in the control mice, yet there was no increase in food intake (Figures 6A and 6B). A dose of 0.1 mg/kg, which increased food intake, raised the plasma ghrelin level to 45 times above the value in saline-injected control mice. At 1 mg/kg, ghrelin injection raised the plasma ghrelin level to 650-fold above the saline-injected level (from 0.53 to 345 ng/ml) (Figure 6B, top). The values for des-acyl ghrelin paralleled those for ghrelin (Figure 6B, bottom). We measured plasma GH in the same samples and found that a ghrelin dose of 0.1 mg/kg was required to raise GH in control mice (Figure S4).
The experiment of Figures 6A and 6B indicated that in order to increase food intake acutely, plasma ghrelin must be elevated above physiological levels. To explore this phenomenon over a narrower concentration range, we repeated the ghrelin injections in control mice and measured food intake over the next 30 min (Figure 6C). On the next day, we repeated the injections and measured plasma ghrelin 10 min later. Injection of 0.025 mg/kg of ghrelin raised the plasma level of ghrelin 14 times above the level in saline-injected mice, but it did not increase food intake significantly. When the dose was raised to 0.05 mg/kg, plasma ghrelin increased 80-fold, and food intake increased by 7.2-fold. A similar 8-fold increase in food intake was observed when the ghrelin dose was further increased to 0.1 mg/kg. Thus, in this experiment, as in the experiment of Figures 6A and 6B, it was necessary to raise plasma ghrelin many fold above normal in order to observe an acute increase in food intake.
DISCUSSION
The rationale for the current experiments is based on the experimental design of Luquet, et al. (Luquet et al., 2005), who used diphtheria toxin (DTX) to ablate NPY/AgRP neurons that were engineered to express the human DTX receptor. When the neurons were ablated in neonatal mice, there was little reduction in food intake, but ablation in adult mice induced starvation. These findings could be explained if compensatory changes ameliorated the effect of ablation when performed in young animals, obscuring the true role of NPY/AgRP neurons. We reasoned that a similar type of life-long compensation might be obscuring the anorexic effects of ghrelin deficiency when the gene for ghrelin, GOAT or the ghrelin receptor is disrupted in the germline. Therefore, we engineered ghrelin-secreting cells to produce the simian DTX receptor and ablated those cells by injecting DTX in adult mice. The result was identical to the one that we obtained earlier in studies of ghrelin and GOAT germline knockout mice (Zhao et al., 2010a; Li et al., 2012). The ghrelin cell-ablated mice showed no reduction in food intake, and they gained weight as rapidly as controls when placed on a high fat diet. This study therefore rules out neonatal compensation as an explanation for the failure of ghrelin deficiency to alter food intake in mice.
It should be noted that the current studies were designed specifically to test the effect of acute ghrelin ablation on overall food intake. We did not address the previously reported effects of ghrelin on other feeding paradigms, such as stress-induced food-reward behavior and depression (Lutter et al., 2008; Chuang et al., 2011).
Although deprivation of ghrelin did not reduce food intake in the Ghrl-DTR mice, the injection of excess ghrelin increased food intake in Ghrl-DTR mice as well as in control mice. An explanation for this paradox emerged when we measured the ghrelin level 10 min after the injections (Figure 6A and 6B). In both experiments ghrelin stimulated food intake only when the plasma level was raised above the normal concentration. The highest ghrelin concentration that we have observed in the diurnal rhythm of normal mice is 0.5 to 0.6 ng/ml (Figures S2 and 6B). Even after a 24 hr fast, plasma ghrelin rises only to 0.9 ng/ml. Yet, when we injected 0.025 mg/kg ghrelin to control mice, we raised the plasma level to 2.7 ng/ml (14-fold above normal) without a significant increase in food intake. When we doubled the ghrelin dose to 0.05 mg/kg, plasma ghrelin rose by 80-fold to 16 ng/ml and food intake was significantly increased. The feeding response to supraphysiologic ghrelin levels is known to require the ghrelin receptor (Sun et al., 2004), thus indicating that the receptor is far from saturated, even at the highest ghrelin levels that are observed in normal or fasting conditions.
The requirement for supraphysiologic ghrelin levels to stimulate appetite and raise GH appears to explain the normal food intake of mice lacking ghrelin or the ghrelin receptor from birth (Sun et al., 2003; Sun et al., 2004; Wortley et al., 2004; Zhao et al., 2010a). Humans may also require supraphysiologic ghrelin levels to stimulate appetite, as shown by Lippl, et al. (Lippl et al., 2012), who infused ghrelin to normal weight volunteers and found no increase in appetite even when plasma ghrelin was raised 4-fold above control values. These researchers cited 9 previous studies in which increased food intake in humans was observed at doses of ghrelin that raised plasma ghrelin levels many fold above normal.
Thus far, the only documented essential requirement for ghrelin is to maintain life-preserving levels of blood glucose when mice have been depleted of adipose tissue through prolonged severe calorie restriction. In this respect the ghrelin cell-ablated mice behaved identically to mice that lack GOAT or ghrelin from birth (Figure 5; Zhao et al., 2010a; Li et al., 2012; Goldstein et al., 2011). In control mice, ghrelin levels rise progressively during this period, and GH levels follow. Administration of ghrelin or GH prevents the hypoglycemia, indicating that the essential role of ghrelin is prevention of hypoglycemia during periods of extreme food deprivation (Zhao et al., 2010a; Goldstein et al., 2011).
In a paper published in PLOS One, Yi, et al. (2012) recently reported that, in contrast to our findings, they did not observe selective profound hypoglycemia in germline GOAT knockout mice during prolonged calorie restriction. In our lab, we have observed selective profound hypoglycemia in calorie-restricted GOAT and ghrelin knockout mice in more than 100 experiments conducted over the past five years. As pointed out in Experimental Procedures, demonstration of profound hypoglycemia requires careful attention to the experimental protocol. Among a number of differences from our protocol, the mice studied by Yi, et al. were not housed in individual cages, had a higher starting fat mass, and were fed a higher carbohydrate diet. All of these differences would require re-standardization of the protocol and also a longer period of calorie restriction in order to observe selective profound hypoglycemia.
EXPERIMENTAL PROCEDURES
Generation of Mice with DTX-Inducible Ablation of Ghrelin-Producing Cells
All mice (C57BL/6J background unless otherwise indicated) were housed in cages with 12-hr light/12-hr dark cycles. The dark cycle began at 9:00 or 10:00 p.m. All animal experiments were performed with approval of the Institutional Animal Care and Research Advisory Committee at University of Texas Southwestern Medical Center.
To generate Ghrl-DTR mice (Figure S1), we crossed Ghrelin-Cre mice (gift of Jeffrey L Zigman, UT Southwestern) (Engelstoft et al., 2013) to inducible Diphtheria Toxin Receptor (iDTR) mice (Jackson Laboratory; #007900). The Ghrelin-Cre mice, when crossed to a reporter strain, activated reporter expression predominantly in the stomach (>96% of ghrelin cells), but not in the pancreas or brain (Engelstoft et al., 2013). The iDTR strain contains a modified Rosa26 locus in which a flox-inactivated copy of the simian Hbegf gene (commonly known as Diphtheria Toxin Receptor gene) (DTR) has been inserted (Buch et al., 2005). Expression of Cre recombinase removes a premature stop codon and activates transcription of DTR, conferring DTX sensitivity to ghrelin cells. Mice with the genotype Ghrl-Cre; iDTRf/f or Ghrl-Cre; iDTRf/+ were designated Ghrl-DTR, and littermates without the ghrelin-cre transgene (iDTRf/+ or iDTRf/f) were used as control mice.
For all experiments, DTX (Sigma, Cat. No. D0564) was dissolved in normal saline at a concentration of 1.0-1.2 μg/ml, and an injection of 200 μl was administered intraperitoneally to all mice, including controls. This corresponds to a dose of 8-10 ng DTX/g body weight. Of all mice used in these studies, 91% experienced a robust ablation of ghrelin cells after a single DTX injection; 9% showed no response to DTX administration. Partial responses were not observed in any of the injected mice.
Diets
The chow diet (Teklad Mouse/Rat Diet 7002; Harlan Teklad Global Diets) contains 3.0 kcal/g of metabolizable energy, of which 18% of calories are from fat, 49% from carbohydrates, and 33% from protein. For high fat diet (HFD) studies, mice were fed a diet containing 45% calories from fat. The diet was composed of 24 gm% fat, 41 gm% carbohydrate, and 24 gm% protein (Research Diets; Cat No. D12451). Total body fat was measured by NMR (Bruker Minispec mq7.5 NMR analyzer).
Calorie Restriction
Calorie restriction for studies of ghrelin-mediated control of blood glucose was carried out as previously described (Zhao et al., 2010a; Li et al., 2012). Essential requirements for reproducibility include: 1) baseline food intake must be measured separately for each mouse, so that the calories can be reduced by 60% for each individual mouse. This requires that the mice be housed in individual cages (our rooms are maintained at 22°C); 2) ghrelin-deficient mice will not become hypoglycemic until their total body fat is reduced below 2% of body weight, after which they are fasted for 23 hr; and 3) it is essential not to stress the mice in any way because stress lowers blood glucose in the calorie-restricted wild-type mice.
In the current studies, 8-week-old male control and Ghrl-DTR littermates were placed in individual cages for one week before initiation of calorie restriction and then fed the chow diet ad libitum. During this week, food intake was monitored to determine the average daily food consumption of each individual mouse. On day 0, all mice received a single IP injection of 8-10 ng/g DTX and were subjected to 60% calorie restriction such that each mouse was fed 40% of its average food consumption daily at 6:00 p.m. Body weight and blood glucose were measured daily at 5:30 p.m. before feeding.
Administration of Ghrelin
Mice were briefly anesthetized with isoflurane and injected subcutaneously with ~ 0.1 ml of 0.15 M sodium chloride containing the indicated concentration of ghrelin (Genscript, Cat No. RP10781-5).
Blood Collection and Hormone Measurements
For measurements in which mice were kept alive (Figures 3-6, S2, and S3), 10-30 μl of tail vein blood was collected. For measurements in which mice were sacrificed (Figures 1, S1, and S4), mice were anesthetized with isoflurane, after which 500-700 μl blood was drawn from the inferior vena cava (Figures 1, and S1) or the orbit (Figure S4). Blood samples were stored in EDTA-coated tubes containing p-hydroxymercuribenzoic acid (final concentration, 1 mM). Plasma was separated into two aliquots and stored at −80° C: one for insulin or GH measurements and the other for ghrelin/des-acyl ghrelin measurements, the latter stored in HCl (final concentration, 0.1 M). Ghrelin and des-acyl ghrelin were measured with immunoassay kits from Cayman Chemical that distinguish both forms of the peptide (Cat. No. 10006307 and No. 10008953). Insulin was measured with an ELISA kit from Crystal Chem (Cat No. 90080). GH was measured with an ELISA kit from Cayman Chemical (Cat No. 589601).
Quantitative Real-Time PCR
Total RNA was isolated from mouse kidney, liver, duodenum, and whole stomach, after which the mRNAs were subjected to real-time PCR using primers for mouse cyclophilin, ghrelin, and chromogranin A as previously described (Zhao et al., 2010b). Additional primers used (forward and reverse) were as follows: mouse TNFα, 5′-CTGAGGTCAATCTGCCCAAGTAC-3′ and 5′-CTTCACAGAGCAATGACTCCAAAG-3′; cre recombinase, 5′-GGCCCAAATGTTGCTGGAT-3′ and 5′-TGTTCGCGATTATCTTCTATATCTTCA-3′; simian HBEGF, 5′-AGGCAAGGGACTAGGGAAGA-3′ and 5′-CCACCACAGCCAGGATAGTT-3′. All reactions were done in triplicate. The relative amount of all mRNAs was calculated using the comparative threshold cycle (Ct) method. Cyclophilin mRNA was used as the invariant control.
Histology
Stomachs were fixed for 20-48 hr in 4% (v/v) paraformaldehyde in PBS. The fixed tissues were embedded in paraffin and sectioned at 4 μm. For immunohistochemistry, slides were pretreated with 0.3% (v/v) hydrogen peroxide for 15 min at room temperature, followed by antigen retrieval for 10 min at 98°C in Retrievagen A buffer (BD Biosciences). The slides were then incubated for 1 hr at room temperature in 10% (v/v) normal goat serum (NGS; Vector Labs) in PBS, followed by overnight incubation at 4°C with anti-ghrelin antiserum (1:1000 dilution in 10% NGS; Phoenix Pharmaceuticals, Code H-031-31, Lot no. 01241-3) or anti-chromogranin A antiserum (1:400 in 10% NGS; Abcam, Code ab15160, Lot no. GR121602-3). Slides were then incubated for 1 hr at room temperature with anti-rabbit antiserum (1:500 in 10% NGS; Jackson ImmunoResearch), followed by 60 min at room temperature in Streptavidin-HRP conjugate (1:750 in 10% NGS; Invitrogen). Activity was developed for 1 min with an AEC substrate kit (Invitrogen), counterstained with hematoxylin, and mounted using Aqua-Polymount (Polysciences, Inc.).
Supplementary Material
HIGHLIGHTS.
Ghrelin cells were inducibly ablated in adult mice
Loss of ghrelin cells did not reduce appetite/body weight on chow or high fat diet
Ghrelin cell-depleted mice were susceptible to starvation-induced hypoglycemia
Appetite stimulation by ghrelin occurred only at supraphysiological plasma levels
ACKNOWLEDGMENTS
We thank our colleagues Guosheng Liang for helpful advice and Jeffrey Zigman for providing Ghrelin-Cre mice; Brandon January, Min Ding, and Wenzhu Fan for excellent technical assistance; and Isis Soto for invaluable assistance with animal studies. This work was supported by grants from the NIH (HL20948-37) and the Moss Heart Foundation. M.R.M. is supported by Medical Scientist Training Program Grant ST32 GM08014 and is also a HHMI International Student Fellow.
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information including four figures can be found with this article online at__________.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Buch T, Heppner FL, Tertilt C, Heinen TJAJ, Kremer M, Wunderlich FT, Jung S, Waisman A. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nature Methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- Chuang J-C, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, Zigman JM. Ghrelin mediates stress-induced food-reward behavior in mice. J. Clin. Invest. 2011;121:2684–2692. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Cruz CRY, Smith RG. The Growth Hormone Secretagogue Receptor. Vitamins and Hormones. 2008;77:47–88. doi: 10.1016/S0083-6729(06)77004-2. [DOI] [PubMed] [Google Scholar]
- Cummings DE. Ghrelin and short- and long-term regulation of appetite and body weight. Physio. Behavior. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- Engelstoft MS, Park W-M, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nøhr MK, Pan J, Sinz CJ, Carrington PE, Akiyama TE, Jones RM, Tang C, Ahmed K, Offermanns S, Egerod KL, Zigman JM, Schwartz TW. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol. Metab. 2013 doi: 10.1016/j.molmet.2013.08.006. In-Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Zhao T-J, Li RL, Sherbet DP, Liang G, Brown MS. Surviving starvation: Essential role of the ghrelin-growth hormone axis. Cold Spring Harbor Symp. Quant. Biol. 2011;76:121–127. doi: 10.1101/sqb.2011.76.010447. [DOI] [PubMed] [Google Scholar]
- Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc. Natl. Acad. Sci. USA. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Kangawa K. Ghrelin: Structure and function. Physiol. Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- Li RL, Sherbet DP, Elsbernd BL, Goldstein JL, Brown MS, Zhao T-J. Profound hypoglycemia in starved, Ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J. Biol. Chem. 2012;287:17942–17950. doi: 10.1074/jbc.M112.358051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippl F, Erdmann J, Steiger A, Lichter N, Czogalla-Peter C, Bidlingmaier M, Tholl S, Schusdziarra V. Low-dose ghrelin infusion - evidence against a hormonal role in food intake. Regul. Pept. 2012;174:26–31. doi: 10.1016/j.regpep.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat. Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R, Farhy LS, Liu J, Prudom CE, Johnson ML, Veldhuis P, Pezzoli SS, Oliveri MC, Gaylinn BD, Geysen HM, Thorner MO. Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J. Clin. Endocrinol. Metab. 2008;93:1988–1994. doi: 10.1210/jc.2007-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R, Gaylinn BD, Thorner MO. The ghrelin axis in disease: potential therapeutic indications. Mol. Cell. Endocrinol. 2011;340:106–110. doi: 10.1016/j.mce.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba J, Wattacheril J, Abumrad NN. Endocrine and metabolic response to gastric bypass. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:515–521. doi: 10.1097/MCO.0b013e32832e1b14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S-I, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J. Clin. Endocrinol. Metab. 2002;87:240–244. doi: 10.1210/jcem.87.1.8129. [DOI] [PubMed] [Google Scholar]
- Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol. Cell. Biol. 2003;23:7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metabolism. 2006;3:379–386. doi: 10.1016/j.cmet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc. Natl. Acad. Sci. USA. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Tschöp M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J. Endocrinol. Invest. 2001;24:RC19–21. doi: 10.1007/BF03351037. [DOI] [PubMed] [Google Scholar]
- Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD, Wiegand SJ, Sleeman MW. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc. Natl. Acad. Sci. USA. 2004;101:8227–8232. doi: 10.1073/pnas.0402763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endoc. Metab. 2001;86:5992–5995. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Yi C-X, Heppner KM, Kirchner H, Tong J, Bielohuby M, Gaylinn BD, Müller TD, Bartley E, Davis HW, Zhao Y, Joseph A, Kruthaupt T, Ottaway N, Kabra D, Habegger KM, Benoit SC, Bidlingmaier M, Thorner MO, Perez-Tilve D, Tschöp MH, Pfluger PT. The GOAT-ghrelin system is not essential for hypoglycemia prevention during prolonged calorie restriction. PLoS ONE. 2012;7:e32100, 1–7. doi: 10.1371/journal.pone.0032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T-J, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc. Natl. Acad. Sci. USA. 2010a;107:7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T-J, Sakata I, Li RL, Liang G, Richardson JA, Brown MS, Goldstein JL, Zigman JM. Ghrelin secretion stimulated by β1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc. Natl. Acad. Sci. USA. 2010b;107:15868–15873. doi: 10.1073/pnas.1011116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.