Abstract
We hypothesized that clinical risk factors could be identified within 2 weeks of onset of severe (stage 3 or 4) acute gut GVHD for identifying a patient population with a very poor outcome. Among 1,462 patients who had allogeneic hematopoietic cell transplantation (HCT) between January 2000 and December 2005, 116 (7.9%) developed stage 3–4 gut GVHD. The median time to onset of stage 3–4 gut GVHD was 35 (4–135) days after allogeneic HCT. Eighty-five of the 116 patients (73%) had corticosteroid-resistance before or within 2 weeks after the onset of stage 34 gut GVHD. Significant risk factors for mortality included corticosteroid-resistance (HR=2.93; p=0.0005), age >18 years (HR=4.95; p=0.0004), increased serum bilirubin (HR 2.53; p=0.0001), and overt gastrointestinal bleeding (HR 2.88; p=0.0004). Among patients with stage 3–4 gut GVHD, the subgroup with 0, 1 or 2 risk factors had a favourable prognosis, whereas the subgroup with 3 or 4 risk factors had a dismal prognosis. This information should be considered in designing future studies of severe gut GVHD and in counseling patients about prognosis.
Keywords: GVHD, gastrointestinal, allogeneic HCT
INTRODUCTION
Acute graft-versus-host disease (GVHD) is one of the most important complications to occur after allogeneic hematopoietic cell transplantation (HCT). In the most severe and life-threatening cases, the triad of skin, gut, and liver involvement has come to be dominated by gastrointestinal GVHD. Acute GVHD of the skin is generally not life-threatening as both systemic and topical therapies are usually effective, and isolated skin GVHD does not influence mortality.1 The incidence of stage 3–4 liver GVHD (peak total serum bilirubin > 6 mg/dL) is now 2%.2 While the incidence of severe gastrointestinal GVHD (stage 3–4, or peak diarrheal volumes over 1.5 liters per day) has also decreased during the past decade, treatment remains unsuccessful in most cases,2 and the gastrointestinal tract is involved in virtually all fatal cases of acute GVHD.
Two distinct phenotypes of gut GVHD can be identified—upper gut (limited to stage 1) and mid-lower gut—that differ in presentation, natural history, response to therapy, and risk of mortality. The upper gut phenotype generally does not progress to the mid-lower gut phenotype.3,4 The upper gut phenotype presents with persistent loss of appetite, satiety, nausea, vomiting, and weight loss, with variable amounts of diarrhea, usually less than 500 mL per day.5,6 The presentation can be indolent, and therapy with prednisone at doses of 1 mg/kg/day plus topical oral corticosteroid is effective.3,7,8
The mid-lower gut phenotype of GVHD presents with secretory, protein-rich diarrhea and abdominal pain resulting from gut distention.9,10 In severe cases, the entire small intestine and colon are edematous and inflamed, with diarrheal volumes in excess of 1.5 liters per day and evidence of mucosal ulceration and bleeding. This clinical picture may be accompanied by culture-negative fever, jaundice, low systemic vascular resistance, and high cardiac output. Most patients with severe mid-lower gut GVHD require prolonged hospitalization for supportive care including total parenteral nutrition and pain control. Although outcomes are typically poor, the standard for initial therapy is prednisone at 2 mg/kg/day, with addition of other immune suppressive therapies when treatment with prednisone fails to control the disease.11
We hypothesized that a detailed analysis of the early clinical findings and response to initial therapy in patients with severe (stage 3–4) gut GVHD would identify patients at very high risk for mortality. Identification of risk factors for mortality could serve at least three highly useful purposes. First, this information could be used to assist physicians and patients in making decisions to implement more aggressive treatment or to change course toward palliative care. Second, the results could be used to formulate eligibility criteria and to stratify patients for enrollment in investigational studies testing new approaches for treating severe gut GVHD. Third, the profile of risk factors could be used as a tool to compare and interpret the results of different studies evaluating new treatment for severe gut GVHD. To test our hypothesis, we retrospectively analyzed 116 consecutive patients with stage 3 or 4 acute GVHD of the gastrointestinal tract to characterize their clinical course and to identify risk factors for mortality.
PATIENTS AND METHODS
Patients
The study included consecutive patients from a well-characterized database who had allogeneic HCT at the Fred Hutchinson Cancer Research Center (FHCRC) between January 2000 and December 2005 and developed stages 3 and 4 gut GVHD.7 Patients had given consent allowing the use of medical records for research, as approved by the FHCRC Institutional Review Board.
Preparative regimens and post-transplant immunosuppressive regimens
High-intensity (myeloablative) conditioning regimens were based on total body irradiation (TBI) or busulfan. After myeloablative conditioning, most patients received a calcineurin inhibitor (cyclosporine or tacrolimus) in combination with either methotrexate or mycophenolate mofetil (MMF) for GVHD prophylaxis as previously described.12,13 After reduced-intensity conditioning, a calcineurin inhibitor was administered in combination with MMF for posttransplant immunosuppression.14,15
Assessment of GVHD
The diagnosis of acute gut GVHD was based on clinical signs, exclusion of other causes of diarrhea >500 ml/day (Stage ≥ 1) and, if available, endoscopic evaluation including mucosal biopsy with characteristic histologic findings of GVHD and no infection identified.2,16 Gut biopsies were not done in all patients to establish a diagnosis of gut GVHD. The most common reasons for not obtaining a gut biopsy were 1) the presence of low platelet counts which were unsupportable with transfusions or 2) the diagnosis of GVHD was already established by biopsies of the skin with negative stool studies for infection and CMV-negative serology in both donor and recipient. Presentation with isolated nausea and vomiting without biopsy documentation of GVHD was not considered sufficient for the diagnosis. Acute GVHD was staged and graded according to established criteria.17,18
Treatment of GVHD and supportive care
Treatment of acute GVHD for the time period covered by this study has been previously described.7 Briefly, after a diagnosis of acute GVHD was confirmed, treatment was started with an initial prednisone-equivalent corticosteroid dose of 1 or 2 mg/kg/day at the discretion of the attending physician,7 and any prior treatment with calcineurin inhibitors or MMF was continued. Second-line therapy was started with other immunosuppressive agents at the discretion of the attending physician if patients had an unsatisfactory response to first-line therapy with corticosteroids. In patients treated initially with low-dose corticosteroids, alternative immunosuppressive agents were added only after an unsuccessful attempt to control GVHD with higher doses of corticosteroids.
Supportive care after allogeneic HCT has been previously described.7 Briefly, levofloxacin was administered to prevent bacterial infections during neutropenia. Fluconazole 400 mg/day was administered until day 75 to prevent fungal infection. Voriconazole was substituted to prevent mold infection at the discretion of the attending physician. Neutropenic fever was treated with broad-spectrum antibiotics, and if fever persisted, antifungal therapy was changed. Antimicrobial treatment was administered until fever and neutropenia resolved. Acyclovir was administered to prevent activation of herpes simplex virus and varicella-zoster virus. Blood samples were tested weekly for cytomegalovirus activation, and preemptive therapy with ganciclovir was started when results were positive. Trimethoprim-sulfamethoxazole was given to prevent Pneumocystis jiroveci pneumonia. Platelet transfusions were given for platelet counts <10,000/mm3.
Study design
Values for peak and nadir of clinical and laboratory parameters were assigned for each consecutive 14-day interval starting at 14 days before the diagnosis of stage 3 or 4 acute gut GVHD and continuing to the resolution of symptoms, end of follow-up or death. A peak grade or organ stage of acute GVHD was also assigned for each 14-day interval. The parameters for which data were collected are summarized in Supplementary Table 1. Other data collected for this analysis included demographics, the regimen used for GVHD prophylaxis, the severity of the regimenrelated toxicity, dose of corticosteroids for treatment of GVHD (prednisone-equivalent 1 or 2 mg/kg/day) and cause of death.
Corticosteroid-resistant GVHD was defined as progression at 2 days, the absence of improvement at 7 days, incomplete response at 14 days during prednisone-equivalent treatment at 2 mg/kg or higher,11 or development of stage 3 or 4 gut GVHD during treatment with a prednisone-equivalent dose of 2 mg/kg or higher for skin, liver or lower stage of gut GVHD. Complete response was defined as resolution of all manifestations of acute gastrointestinal GVHD for at least 14 days at any time after onset of stage 3–4 gut GVHD, regardless of the number of previous lines of treatment.
Statistical analysis
Survival and progression-free survival after the onset of stage 3–4 GVHD were estimated using the Kaplan-Meier method. Cumulative incidence curves for GVHD, mortality, infection and secondary therapy were estimated by methods previously described.19 Unadjusted and adjusted hazard ratios for time-to-event endpoints were estimated by Cox regression, treating death and recurrent malignancy as competing events when appropriate. Unadjusted and adjusted odds ratios for binary endpoints (prolonged hospitalization) were estimated by using logistic regression.
RESULTS
Patient characteristics
Between January, 2000 and December, 2005, 1462 patients underwent allogeneic hematopoietic cell transplantation. A total of 116 (7.9%) patients developed stage 3 or 4 acute GVHD of the gastrointestinal tract by day 135 (Table 1). The median patient age was 48 (range, 1–74) years. The cumulative incidence of stage 3 or 4 gut GVHD was 11.7% after reduced-intensity conditioning and 6.4% after myeloablative conditioning.
Table 1.
Patient characteristics
| Patients with stage 3–4 GI GVHD (n=116) N (%) |
|
|---|---|
| Patient age | |
| ≤18 years | 14 (12) |
| 19–39 years | 23 (20) |
| 40–59 years | 62 (53) |
| ≥ 60 years | 17 (15) |
| Donor/patient sex | |
| Female/male | 32 (28) |
| Other | 84 (72) |
| Diagnosis | |
| AML | 36 (31) |
| ALL | 6 (5) |
| MDS/MPD | 25 (22) |
| CML | 9 (8) |
| CLL | 5 (4) |
| HD | 7 (6) |
| NHL | 17 (15) |
| MM | 4 (3) |
| Other | 7 (6) |
| Donor type and HLA status | |
| Related donors HLA-identical | 47 (40) |
| HLA-mismatched | 0 |
| Unrelated donors HLA-matched | 44 (38) |
| HLA-mismatched | 25 (22) |
| Cell source | |
| Cord blood | 0 |
| Bone Marrow | 20 (17) |
| PBSC | 96 (83) |
| Conditioning regimen | |
| Reduced intensity | 48 (41) |
| Myeloablative | 68 (59) |
| Previous autologous HCT | |
| No | 97 (84) |
| Yes | 19 (16) |
| GVHD prophylaxis | |
| CNI | 5 (4) |
| CNI/Methotrexate | 54 (47) |
| CNI/MMF | 57 (49) |
| Other | 0 |
Abbreviations: ALL acute lymphoid leukemia, AML- acute myeloid leukemia, CLL- chronic lymphocytic leukemia, CML- chronic myeloid leukemia, CNI- calcineurin inhibitor, GI- gastrointestinal, GVHD- graft-vs-host disease, HCT- hematopoietic cell transplantation, HD- Hodgkin’s disease, HLA- human leukocyte antigen, MDS- myelodysplastic syndrome, MM- multiple myeloma, MMF- mycophenolate mofetil, NHL- non-Hodgkin’s lymphoma, PBSCperipheral blood stem cells.
Clinical characteristics of stage 3–4 acute GI GVHD
The median onset of stage 3–4 gut GVHD was 35 (4–135) days after HCT. Ninety-eight of the 116 patients (84%) developed either stage 3 or 4 gut GVHD within the first 2 weeks after the onset of diarrhea. The median time to onset was significantly later after a reduced-intensity vs. myeloablative conditioning regimen (54.5 (6–122) vs. 20.5 (4–135) days, respectively (p<0.0001). During the first 14-day interval, 42 patients (36%) had peak stage 3 and 74 patients (64%) had peak stage 4 gut GVHD (Table 2). Eleven of the 42 patients (26%) with peak stage 3 gut GVHD during the first 14-day interval later had progression to stage 4. Concomitant liver and skin GVHD were observed in 59 (50%) and 73 (63%) of patients, respectively. Twenty-three patients (20%) had isolated gut GVHD with no skin or liver involvement. During the first 14-day interval, the mean peak volume of diarrhea was 1954 (SD, 1391) mL/day, and the median serum albumin concentration was 2.1 (range 1.1–3.6) g/dL. Five patients were treated for Clostridium difficile infection during the first 14-day interval. Five patients were diagnosed with CMV enteritis (n=5) during subsequent intervals.
Table 2.
Clinical characteristics during the first 14 days after onset of stage 3–4 gut GVHD
| Characteristic | ||
|---|---|---|
| Peak stage of gut GVHD, n (%) | Stage 3 | 42 (36) |
| Stage 4 | 74 (64) | |
| Skin GVHD, n (%) Stage 1–4 | 59 (50) | |
| Liver GVHD, n (%) Stage 1–4 | 73 (63) | |
| Mean peak daily volume of diarrhea during interval; mL (SD) | 1954 (1391) | |
| Anorexia, nausea or vomiting, n (%) | 90 (78) | |
| Median nadir of serum albumin, g/dL (range) | 2.1 (1.1–3.6) | |
| Cramps, n (%) | 54 (47) | |
| Melena or blood, n (%) | 66 (57) | |
| Melena/blood and cramps, n (%) | 46 (40) | |
Response to therapy
First-line therapy for acute GVHD in all patients was corticosteroids. By the end of the first 14-day period after onset of stage 3–4 gut GVHD, 85 of the 116 patients (73%) were corticosteroid-resistant. Forty-five patients (39%) had received high-dose prednisone for skin, liver or lower stage of gut GVHD and were corticosteroid-resistant at onset of progression to stage 3–4 gut GVHD. Another 40 patients (34%) developed corticosteroid resistance during the first 14-day interval after onset of stage 3–4 gut GVHD. Sixteen and 12 of these 40 patients were assessed as corticosteroid-resistant after 2 and 7 days of treatment, respectively.
Sixty-one of the 116 patients (53%) had a first CR by the end of the first 14-day period (n=31) or a subsequent 14-day time period after additional therapy (n=30). The mean time interval to CR after the onset of stage 3–4 gut GVHD was 39 days (range 14 to 322). Gut GVHD recurred at a mean of 45 (range 14 to 112) days in 36 patients who had a CR. Of the 36 patients who had a relapse of gut GVHD, 22 had a second CR, which persisted until the end of follow-up in all but two patients.
Mortality of patients with stage 3–4 gut GVHD
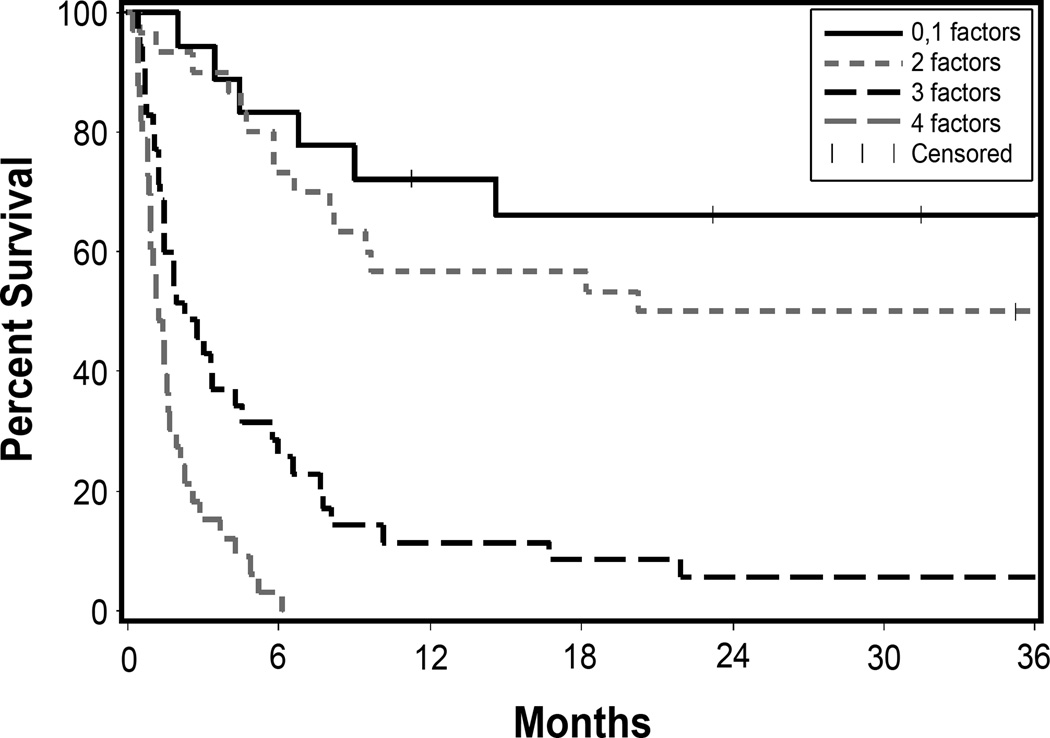
Overall survival at 2 years after the onset of stage 3 or 4 gut GVHD was 25% for the 116 patients. In the multivariate analysis of risk factors identified within 14 days after onset of stage 3–4 gut GVHD, corticosteroid-resistance (HR=2.93; [1.6–5.3], p=0.0005), adult age (>18 years) at HCT (HR=4.95; [2.0–12], p=0.0004), increased total serum bilirubin (>3.0 mg/dL) (HR 2.53; [1.6–4.0], p=0.0001), and overt gastrointestinal bleeding (HR 2.88; [1.6–5.2], p= 0.0004) were most significantly related to mortality (Table 3). Overall survival at 2 years after onset of corticosteroid-resistant stage 3 or 4 gut GVHD was 55% for 11 pediatric patients. Grade 3 endoscopic abnormalities in the upper gastrointestinal tract showed an association with an increased risk of mortality (HR 2.18, [1.0–4.7], p=0.05). Increasing numbers of the 4 most statistically significant risk factors increased the risk of mortality (Figure 1 and Supplementary Table 2). No long-term survival was observed when all four of these risk factors were present. The severity of regimen-related toxicity, endoscopic grade in the colon, histopathological grades, intensity of the conditioning regimen, conditioning with high-dose TBI, disease status and prior CMV infection were not associated with statistically significant differences in survival after the onset of stage 3–4 gut GVHD. Causes of death were GVHD (n=49), infection (n=16), relapse (n=13) and other (n=11).
Table 3.
Risk factors for mortality*
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Stage at onset | ||||
| 3 (n=42) | 1.0 | 0.001 | 1.0 | 0.83 |
| 4 (n=74) | 2.07 (1.3–3.3) | 1.07 (0.6–1.9) | ||
| Corticosteroid-resistance before or during the 14-day interval | ||||
| No (n=31) | 1.0 | <0.0001 | 1.0 | 0.0005 |
| Yes (n=85) | 2.81 (1.6–4.8) | 2.93 (1.6–5.3) | ||
| Age at transplant | ||||
| ≤18 (n=14) | 1.0 | 0.0003 | 1.0 | 0.0004 |
| >18 (n=102) | 3.57 (1.6–8.2) | 4.95 (2.0–12) | ||
| Bilirubin | ||||
| ≤ 3.0 (n=57) | 1.0 | <0.0001 | 1.0 | 0.0001 |
| > 3.0 (n=59) | 2.81 (1.8–4.3) | 2.53 (1.6–4.0) | ||
| Albumin | ||||
| >1.6 (n=84) | 1.0 | <0.0001 | 1.0 | 0.26 |
| ≤ 1.6 (n=32) | 2.69 (1.7–4.2) | 1.35 (0.8–2.3) | ||
| GI bleed | ||||
| No (n=50) | 1.0 | <0.0001 | 1.0 | 0.0004 |
| Yes (n=66) | 3.46 (2.2–5.5) | 2.88 (1.6–5.2) | ||
| TBI (≥1200 cGy) | ||||
| No (n=94) | 1.0 | 0.03 | 1.0 | 0.24 |
| Yes (n=22) | 0.50 (0.3–0.9) | 0.69 (0.4–1.3) | ||
| Conditioning | ||||
| MA (n=68) | 1.0 | 0.68 | ||
| NMA (n=48) | 1.09 (0.7–1.7) | |||
| Patient CMV | ||||
| − (n=55) | 1.0 | 0.57 | ||
| + (n=61) | 1.13 (0.7–1.7) | |||
| Disease risk** | ||||
| Low (n=40) | 1.0 | 0.10 | ||
| High (n=76) | 1.46 (0.9–2.3) | |||
| Regimen related toxicity | ||||
| No (n=57) | 1.0 | 0.40 | ||
| Yes (n=59) | 0.84 (0.6–1.3) | |||
| Upper endoscopy*** (clinical) | ||||
| 0–2 (n=64) | 1.0 | 0.006 | 1.0 | 0.05 |
| 3+ (n=11) | 2.84 (1.4–5.6) | 2.18 (1.0–4.7) | ||
| Upper endoscopy*** (biopsy) | ||||
| 0–2 (n=69) | 1.0 | 0.08 | ||
| 3+ (n=6) | 2.35 (1.0–5.5) | |||
| Lower endoscopy*** (clinical) | ||||
| 0–2 (n=35) | 1.0 | 0.58 | ||
| 3+ (n=11) | 1.23 (0.6–2.5) | |||
| Lower endoscopy*** (biopsy) | ||||
| 0–2 (n=26) | 1.0 | |||
| 3+ (n=20) | 1.43 (0.7–2.7) | 0.28 | ||
| CT scan*** | ||||
| 0,1 (n=28) | 1.0 | |||
| 2 (n=11) | 0.55 (0.2–1.3) | 0.16 | ||
All covariates are based on the ‘worst’ assessment during the 14 days before or after the onset of stage 3–4 gut GVHD.
For disease risk: lower risk is CML chronic phase, MDS RA/RARS, and acute leukemia in remission; all others are higher risk.
Results were available for upper endoscopy in 75 patients, for lower endoscopy in 46 patients and CT scan in 39 patients (term for missing included in multivariate model).
Figure 1. Overall survival of patients with stage 3–4 gut GVHD according to the number of risk factors.
The risk factors include: 1) serum bilirubin >3.0, 2) corticosteroid-resistance, 3) age >18 years at transplant and 4) gastrointestinal bleeding. Risk factors are based on events occurring within the first 14 days after onset of stage 3–4 gut GVHD. (0–1 risk factors: n=18; 2 risk factors: n=30; 3 risk factors: n=35; 4 risk factors: n=33).
DISCUSSION
Our results show that clinical characteristics within 14 day after the onset of stage 3–4 gut GVHD can be used to identify subgroups of patients with distinctly different outcomes. The subgroup with 0–1 or 2 risk factors had a more favorable prognosis (6 month overall survival 83% and 73%, respectively), whereas the subgroup with 3 or 4 risk factors had a dismal prognosis (6 month overall survival 26% and 0, respectively). We used an interval of 14 days after the onset of gut GVHD to identify risk factors that predicted a poor outcome. In many patients, GVHD was identified as resistant to corticosteroids before the onset of stage 3–4 gut GVHD. Observation for the full 14 days after the onset of stage 3–4 gut GVHD was needed to define corticosteroid-resistance only in patients who had an incomplete response. Other investigators have identified clinical markers that predict mortality at the onset of GVHD.20,21 Our study differs from others by virtue of its focus on patients with stage 3–4 GVHD.
Severe gut GVHD has conventionally been considered to include both stage 3 and 4. The severe abdominal cramping or appearance of blood or melena that defines stage 4 disease likely reflects profound mucosal damage. Endoscopy with biopsy of the upper and lower gastrointestinal tract is useful for diagnosing acute GVHD. We observed worse survival in patients with extensive, confluent erosions or ulcerations (grade 3) of the upper but not the lower gastrointestinal tract. We did not include endoscopic grade of GVHD in upper gastrointestinal tract as one of the risk factors in the assessment of overall survival based on cumulative risks because this data was not available for many patients and the other 4 risk factors were much stronger predictors.
Neither endoscopic examination of the upper gastrointestinal tract nor sigmoidoscopy examines the jejunum, ileum, and cecum, which are the sites of the most severe gut involvement with acute GVHD.22 The yield for diagnosis of gut GVHD is highest when the entire colon and ileum are examined and biopsied. Endoscopic findings and histology offer complementary information.23 Because the normal ileum delivers only 1.2 liters of fluid into the colon per day, diarrheal volumes over 1.5 liters are indicative of severe ileal dysfunction.
Regenerating islet-derived 3α (REG3α) has been associated with presence of gut GVHD and could distinguish GVHD-associated diarrhea from non-GVHD causes.24,25 Blood levels of REG3α were significantly higher when gut biopsies showed mucosal denudation compared to less severe GVHD. REG3α concentrations correlated with survival, but the strongest correlation was observed when clinical factors such as advanced clinical stage and severe histologic damage of the gut mucosa were added to the assessment of risk for a poor outcome. In the current retrospective analysis, we have shown that clinical factors alone are highly predictive of outcome among patients with severe gut GVHD.
Histologic evidence of crypt loss has been correlated with volume of diarrhea and severity of gastrointestinal symptoms.26 Histologic severity of gut GVHD was observed to be an important prognostic factor especially when used in association with blood concentrations of REG3α. However, we and others have not previously observed an association between histological grade of GVHD and outcome.16,23 In this current report, an increased histologic grade of GVHD in the upper gut was associated with increased mortality only as a trend in the univariate analysis. The accuracy of histologic diagnosis of GVHD is limited by inability to biopsy the most severely involved mucosa, sampling error, and infrequency of apoptotic crypt lesions.16,23
Stage 3–4 gut GVHD is frequently resistant to corticosteroid treatment. McMillan et al. noted a higher incidence of corticosteroid-treatment failure in patients with gut GVHD than in other patients.27 Outcomes after therapy for corticosteroid-resistant acute GVHD have been reviewed by Pidala et al.28 Although certain agents appear to have some efficacy, response rates and survival rates have varied but are generally poor.29 Randomized clinical trials have failed to demonstrate that any agent substantially improves outcomes of corticosteroid-resistant or refractory GVHD, and survival remains poor in recent pilot studies of novel agents.30
The risk factors identified in our study should allow better selection and stratification of patients for novel treatment strategies. Risk factors for decreased survival identified in other studies of corticosteroid-resistant GVHD include grade III-IV disease, involvement of gut or liver, serum bilirubin ≥ 3.0 mg/dL and prior use of MMF as treatment for GVHD.31–35 Age < 18 years has also been identified as a favorable risk factor in some other studies of GVHD.31,32,34,35 Considerable variation has been noted in the reported outcomes of treatment for corticosteroid-resistant GVHD.28 Although some of this variability might be explained by the agent being tested or by differences in the definition of corticosteroid-resistant GVHD, differences in the prevalence of the risk factors for survival that we have identified are likely to have an important role. The risk factors for survival that we have identified could be used to inform the design of future studies aimed at improving treatment for gut GVHD.
In summary, we have identified risk factors for mortality related to severe gut GVHD which can be identified within 2 weeks of onset. We did not attempt to describe the full spectrum of gut GVHD. Instead, our goal was to develop a tool for identifying those patients with gut GVHD who have a very poor prognosis. We see several directions for future research toward improving survival of patients with severe gut GVHD: 1) clinical trials of novel therapies to blunt both cellular and innate immune reactions after the onset of GVHD symptoms; 2) clinical trials to modulate epithelial and endothelial tight junctions affected by the inflammatory milieu and stimulation of epithelial regeneration to restore mucosal integrity; 3) consideration of reconditioning and transplanting either an autologous or allogeneic hematopoietic cell graft for patients who otherwise would have a very poor survival.36–39 Very little progress has been made in improving therapy for severe gut GVHD during the past 30 years. The results of this study should be considered in designing future studies of severe gut acute GVHD and in counseling patients about prognosis.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Helen Crawford and Bonnie Larson for their excellent support in typing the manuscript. We are also very thankful for the excellent care provided to patients and families by the inpatient and outpatient physicians, nursing teams, and support staff at the Fred Hutchinson Cancer Research Center and at the University of Washington Medical Center.
Financial Disclosure: This work was supported in part by National Institutes of Health Grants CA018029, HL036444, CA078902 and CA015704. The funding body played no part in the design of the study, collection and analysis of data, or the decision to publish. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its subsidiary Institutes and Centers.
Footnotes
Conflicts of Interest Disclosure: The authors declare no competing financial interests in relation to the work described.
AUTHORS CONTRIBUTIONS
C.C-L. designed the study, extracted and analyzed the data and wrote the manuscript.
P.J.M. designed the study, analyzed the data and edited the manuscript.
G.B.M. designed the study, analyzed the data and edited the manuscript.
B.E.S. conducted the statistical analysis.
H.S. analyzed the data and edited the manuscript.
R.A.N. designed the study, analyzed the data and wrote the manuscript.
P.J.M., G.B.M. F.R.A., H.J.D., M.M., R.S. and R.A.N. served as GVHD or Gastroenterology attending physicians and edited the manuscript.
Supplementary information is available at BMT's website.
REFERENCES
- 1.Leisenring WM, Martin PJ, Petersdorf EW, Regan AE, Aboulhosn N, Stern JM, et al. An acute graft-versus-host disease activity index to predict survival after hematopoietic cell transplantation with myeloablative conditioning regimens. Blood. 2006;108:749–755. doi: 10.1182/blood-2006-01-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hockenbery DM, Cruickshank S, Rodell TC, Gooley T, Schuening F, Rowley S, et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood. 2007;109:4557–4563. doi: 10.1182/blood-2006-05-021139. [DOI] [PubMed] [Google Scholar]
- 4.Baehr PH, Levine DS, Bouvier ME, Hockenbery DM, Gooley TA, Stern JG, et al. Oral beclomethasone dipropionate for treatment of human intestinal graft-versus-host disease. Transplantation. 1995;60:1231–1238. [PubMed] [Google Scholar]
- 5.Wakui M, Okamoto S, Ishida A, Kobayashi H, Watanabe R, Yajima T, et al. Prospective evaluation for upper gastrointestinal tract acute graft-versus-host disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;23:573–578. doi: 10.1038/sj.bmt.1701613. [DOI] [PubMed] [Google Scholar]
- 6.Wu D, Hockenbery DM, Brentnall TA, Baehr PH, Ponec RJ, Kuver R, et al. Persistent nausea and anorexia after marrow transplantation: a prospective study of 78 patients. Transplantation. 1998;66:1319–1324. doi: 10.1097/00007890-199811270-00010. [DOI] [PubMed] [Google Scholar]
- 7.Mielcarek M, Storer BE, Boeckh M, Carpenter PA, McDonald GB, Deeg HJ, et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood. 2009;113:2888–2894. doi: 10.1182/blood-2008-07-168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castilla C, Perez-Simon JA, Sanchez-Guijo FM, Diez-Campelo M, Ocio E, Perez-Persona E, et al. Oral beclomethasone dipropionate for the treatment of gastrointestinal acute graft-versus-host disease (GVHD) Biol Blood Marrow Transplant. 2006;12:936–941. doi: 10.1016/j.bbmt.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz JM, Wolford JL, Thornquist MD, Hockenbery DM, Murakami CS, Drennan F, et al. Severe gastrointestinal bleeding after hematopoietic cell transplantation, 1987–1997: incidence, causes, and outcome. Am J Gastroenterol. 2001;96:385–393. doi: 10.1111/j.1572-0241.2001.03549.x. [DOI] [PubMed] [Google Scholar]
- 10.Ross WA, Couriel D. Colonic graft-versus-host disease (Review) Current Opinion in Gastroenterology. 2005;21:64–69. [PubMed] [Google Scholar]
- 11.Martin PJ, Schoch G, Fisher L, Byers V, Anasetti C, Appelbaum FR, et al. A retrospective analysis of therapy for acute graft-versus-host disease: Initial treatment. Blood. 1990;76:1464–1472. [PubMed] [Google Scholar]
- 12.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 13.Nash RA, Johnston L, Parker P, McCune JS, Storer B, Slattery JT, et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11:495–505. doi: 10.1016/j.bbmt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 14.McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 15.Maris MB, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 16.Ponec RJ, Hackman RC, McDonald GB. Endoscopic and histologic diagnosis of intestinal graft-versus-host disease after marrow transplantation. Gastrointest Endosc. 1999;49:612–621. doi: 10.1016/s0016-5107(99)70390-1. [DOI] [PubMed] [Google Scholar]
- 17.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, Klingemann H-G, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 19.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.MacMillan ML, Defor TE, Weisdorf DJ. What predicts high risk acute graft-versus-host disease (GVHD) at onset?: identification of those at highest risk by a novel acute GVHD risk score. Br J Haematol. 2012;157:732–741. doi: 10.1111/j.1365-2141.2012.09114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robin M, Porcher R, de Castro R, Fisher G, de Latour RP, Ribaud P, et al. Initial liver involvement in acute GVHD is predictive for nonrelapse mortality. Transplantation. 2009;88:1131–1136. doi: 10.1097/TP.0b013e3181bc2583. [DOI] [PubMed] [Google Scholar]
- 22.McDonald GB, Sale GE. The human gastrointestinal tract after allogeneic marrow transplantation in humans. In: Sale GE, Shulman HM, editors. The Pathology of Bone Marrow Transplantation. New York: Masson, Inc.; 1984. pp. 77–103. [Google Scholar]
- 23.Kreisel W, Dahlberg M, Bertz H, Harder J, Potthof K, Deibert P, et al. Endoscopic diagnosis of acute intestinal GVHD following allogeneic hematopoietic SCT: a retrospective analysis in 175 patients. Bone Marrow Transplant. 2012;47:430–438. doi: 10.1038/bmt.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrara JL, Harris AC, Greenson JK, Braun TM, Holler E, Teshima T, et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118:6702–6708. doi: 10.1182/blood-2011-08-375006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris AC, Ferrara JL, Braun TM, Holler E, Teshima T, Levine JE, et al. Plasma biomarkers of lower gastrointestinal and liver acute GVHD. Blood. 2012;119:2960–2963. doi: 10.1182/blood-2011-10-387357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melson J, Jakate S, Fung H, Arai S, Keshavarzian A. Crypt loss is a marker of clinical severity of acute gastrointestinal graft-versus-host disease. Am J Hematol. 2007;82:881–886. doi: 10.1002/ajh.20976. [DOI] [PubMed] [Google Scholar]
- 27.MacMillan ML, Weisdorf DJ, Wagner JE, Defor TE, Burns LJ, Ramsay NK, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 28.Pidala J, Anasetti C. Glucocorticoid-refractory acute graft-versus-host disease (Review) Biol Blood Marrow Transplant. 2010;16:1504–1518. doi: 10.1016/j.bbmt.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Jamani K, Russell JA, Daly A, Stewart D, Savoie L, Duggan P, et al. Prognosis of grade 3–4 acute GVHD continues to be dismal. Bone Marrow Transplant. 2013;48:1359–1361. doi: 10.1038/bmt.2013.59. [DOI] [PubMed] [Google Scholar]
- 30.Xhaard A, Rocha V, Bueno B, de Latour RP, Lenglet J, Petropoulou A, et al. Steroid-refractory acute GVHD: lack of long-term improved survival using new generation anticytokine treatment. Biol Blood Marrow Transplant. 2012;18:406–413. doi: 10.1016/j.bbmt.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Arai S, Margolis J, Zahurak M, Anders V, Vogelsang GB. Poor outcome in steroid-refractory graft-versus-host disease with antithymocyte globulin treatment. Biol Blood Marrow Transplant. 2002;8:155–160. doi: 10.1053/bbmt.2002.v8.pm11939605. [DOI] [PubMed] [Google Scholar]
- 32.Bay JO, Dhedin N, Goerner M, Vannier JP, Marie-Cardine A, Stamatoullas A, et al. Inolimomab in steroid-refractory acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation: retrospective analysis and comparison with other interleukin-2 receptor antibodies. Transplantation. 2005;80:782–788. doi: 10.1097/01.tp.0000173995.18826.de. [DOI] [PubMed] [Google Scholar]
- 33.Carpenter PA, Lowder J, Johnston L, Frangoul H, Khoury H, Parker P, et al. A phase II multicenter study of visilizumab, humanized anti-CD3 antibody, to treat steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:465–471. doi: 10.1016/j.bbmt.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Perales MA, Ishill N, Lomazow WA, Weinstock DM, Papadopoulos EB, Dastigir H, et al. Long-term follow-up of patients treated with daclizumab for steroid-refractory acute graft-vs-host disease. Bone Marrow Transplant. 2007;40:481–486. doi: 10.1038/sj.bmt.1705762. [DOI] [PubMed] [Google Scholar]
- 35.Bordigoni P, Dimicoli S, Clement L, Baumann C, Salmon A, Witz F, et al. Daclizumab, an efficient treatment for steroid-refractory acute graft-versus-host disease. Br J Haematol. 2006;135:382–385. doi: 10.1111/j.1365-2141.2006.06321.x. [DOI] [PubMed] [Google Scholar]
- 36.Passweg JR, Orchard K, Buergi A, Gratwohl A, Powles R, Goldman J, et al. Autologous/syngeneic stem cell transplantation to treat refractory GvHD. Bone Marrow Transplant. 2004;34:995–998. doi: 10.1038/sj.bmt.1704658. [DOI] [PubMed] [Google Scholar]
- 37.Taniguchi Y, Yoshihara S, Hoshida Y, Inoue T, Fujioka T, Ikegame K, et al. Recovery from established graft-vs-host disease achieved by bone marrow transplantation from a third-party allogeneic donor. Exp Hematol. 2008;36:1216–1225. doi: 10.1016/j.exphem.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Ustun C, JillelLa A, Shah R, Sterling K, Deremer D, Savage N, et al. Second allo-SCT from a different donor can improve severe steroid-resistant gut GVHD (Letter to the Editor) Bone Marrow Transplant. 2010;45:1658–1660. doi: 10.1038/bmt.2010.17. [DOI] [PubMed] [Google Scholar]
- 39.Taniguchi Y, Ikegame K, Yoshihara S, Sugiyama H, Kawase I, Ogawa H. Treatment of severe life-threatening graft-versus-host disease by autologous peripheral blood stem cell transplantation using a nonmyeloablative preconditioning regimen (Review) Haematologica. 2003;88:ELT06. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.