Abstract
The evolution of self-fertilization is one of the most commonly traversed transitions in flowering plants, with profound implications for population genetic structure and evolutionary potential. We investigated factors influencing this transition using Witheringia solanacea, a predominantly self-incompatible species within which self-compatible genotypes have been identified. We showed that self-compatibility in this species segregates with variation at the S-locus as inherited by plants in F1 and F2 generations. To examine reproductive assurance and the transmission advantage of selfing, we placed self-compatible and self-incompatible genotypes in genetically replicated gardens and monitored male and female reproductive success, as well as selfing rates of self-compatible plants. Self-compatibility did not lead to increased fruit or seed set, even under conditions of pollinator scarcity, and the realized selfing rate of self-compatible plants was less than 10%. Self-compatible plants had higher fruit abortion rates, consistent with previous evidence showing strong inbreeding depression at the embryonic stage. Although the selfing allele did not provide reproductive assurance under observed conditions, it also did not cause pollen discounting, so the transmission advantage of selfing should promote its spread. Given observed numbers of S-alleles and selfing rates, self-compatibility should spread even under conditions of exceedingly high initial inbreeding depression.
Keywords: embryonic inbreeding depression, mating system, pollen discounting, reproductive assurance, seed discounting, self-fertilization, self-incompatibility
INTRODUCTION
Mating systems determine how genes combine into genotypes, and therefore strongly affect population genetic structure (Hamrick and Godt 1996, Glémin et al. 2006) and evolutionary potential (Takebayashi and Morrell 2001, Goldberg et al. 2010, Igic and Busch 2013, Wright et al. 2013). The mating systems of flowering plants have been the subject of especially intense scrutiny because of the many ways in which sexual organs can be deployed across individuals and populations, the prevalence of animal-mediated mating, the array of morphological and biochemical adaptations known to affect plant mating systems, and the repeated evolution of largely self-fertilizing species from outcrossing ancestors (Darwin 1877, Stebbins 1957, Richards 1986). The dynamics of plant mating systems have attracted theoretical attention ever since Fisher (1941) pointed out the substantive advantage of an allele capable of self-fertilization. Inbreeding depression is generally credited as the only force large enough to counter the automatic transmission advantage of selfing, although numerous other factors influence mating system dynamics (reviewed by Goodwillie et al. 2005). In particular, pollen discounting, the extent to which selfing variants lose outcross siring opportunities, may nullify the automatic transmission advantage of selfing (Nagylaki 1976, Holsinger et al. 1984). Seed discounting, the loss of outcrossed seed production due to selfed seed production, can also reduce the benefit of selfing, especially in cases where selfing does not provide reproductive assurance (Lloyd 1992, Busch and Delph 2012). Genetic factors, including the genetic basis of inbreeding depression (Goodwillie et al. 2005) and genetic control of the mating system (Latta and Ritland 1993, Uyenoyama and Vallejo-Marín 2004, Porcher and Lande 2005), also influence the evolution of selfing from outcrossing.
Most empirical studies of plant mating system evolution have contrasted closely related outcrossing and self-fertilizing taxa, or have focused on species showing quantitative variation in morphological traits that affect selfing rates. Such studies have generated valuable insights about the evolution of mating systems, including the roles of reproductive assurance and inbreeding depression in facilitating or retarding the spread of self-fertilization (reviewed by Eckert at al. 2006, Winn et al. 2011). However, both of these approaches have limitations. Evolutionary models of mating systems often employ a structure that describes the conditions under which a selfing variant can invade an outcrossing population (e.g., Lloyd 1992, Porcher and Lande 2005). The study of closely related selfing and outcrossing taxa allows us to observe results of the transition in mating system, but not how selection acts on novel selfing variants. Studies of species with quantitative control over the mating system do allow direct observation of selection on selfing rate, but are not directly applicable to the case of invasion by a single selfing allele. When possible, it will be valuable to observe selection on the mating system in species where alleles segregating at a single locus have contrasting effects on the selfing rate (Kohn and Barrett 1994, Busch et al. 2010) as this scenario likely resembles the initial transition from obligate outcrossing to selfing in many hermaphroditic species of flowering plants (Igic et al. 2008).
The breakdown of genetically-enforced self-incompatibility provides a useful opportunity to study invasion of an historically outcrossing population by an allele that permits self-fertilization. Self-incompatibility systems have evolved independently several times within the flowering plants, and therefore have diverse genetic and biochemical foundations, but in general the female reproductive tract rejects pollen that fails to be recognized as non-self (de Nettancourt 2001). The simplest systems possess a multi-allelic S-locus encompassing linked genes that govern male and female function, with pollen rejected according to its haploid genotype. In the widespread S-RNase based self-incompatibility system ancestral to flowering plants (Igic and Kohn 2001) a variety of mutational mechanisms have permitted self-fertilization, including duplication of the S-locus, loss-of-function mutations at the S-locus, and mutations at unlinked modifier genes required for functional self-incompatibility reactions (Stone 2002, Igic et al. 2008). The relative importance of these various mutational mechanisms in natural populations is unknown, in part because subsequent loss-of-function mutations accumulate rapidly following the causal one (Igic et al. 2008), but theory predicts that selection favors S-linked mutations under a wider range of conditions than unlinked ones (Porcher and Lande 2005). No matter the mutational mechanism, an allele conferring self-compatibility would confront strong inbreeding depression, often leading to a realized selfing rate substantially lower than the primary selfing rate at the time of fertilization (Husband and Schemske 1996).
The evolution of self-fertilization plays out in an ecological as well as genetic context because autonomous selfing provides reproductive assurance when pollinators are scarce (Baker 1955, Stebbins 1957, Lloyd 1979, Morgan et al. 2005). Within species, selfing rates often increase in small or peripheral populations, or in populations where pollinators are less abundant (e.g., Rick et al. 1979, Barrett and Shore 1987, Barrett et al. 1989, Fausto et al. 2001, Kalisz et al. 2004, Busch 2005, Moeller 2006). Unfortunately, population size, geographic location, and pollinator abundance are frequently conflated in natural populations, so that it can be challenging to isolate the effect of these factors individually. Experimental arrays established across a range of habitats can control for population size and location, permitting a focus on the role of pollinator abundance in reproductive assurance (Busch 2005, Moeller and Geber 2005).
In this study, we sought to evaluate the transmission advantage and reproductive assurance provided by a selfing allele in an ancestrally self-incompatible (SI) species. We first sought to establish whether the breakdown of self-incompatibility was consistent with a null allele at the S-locus by examining segregation of self-incompatibility phenotypes and S-locus genotypes in progeny created from a cross between self-incompatible and self-compatible (SC) individuals. We then established nine genetically replicated gardens, with each garden containing SI and SC genotypes. We evaluated reproductive assurance by contrasting maternal reproductive success of SI and SC variants under conditions of pollinator abundance and pollinator scarcity, and we sought evidence for embryonic inbreeding depression by examining dates to abscission for flowers that do not produce ripe fruit. The transmission advantage of selfing was estimated using microsatellite markers to determine the paternal reproductive success of SI and SC variants. We compare our results to phenotypic and genetic models that consider both reproductive assurance and the transmission advantage of selfing (Porcher and Lande 2005, Busch and Delph 2012).
MATERIALS AND METHODS
Study system and inheritance of self-compatibility
Witheringia solanacea is a long-lived shrub, 0.5-2 m in height, with a Central American distribution. In Costa Rica, it is found primarily between 1000 and 1500 m.a.s.l. (meters above sea level) in the central highlands and Pacific slope, extending to sea level in the southwest. Population size generally declines with increasing elevation. The species flowers and fruits year-round. The small (~ 1 cm) flowers have anthers that form a cone surrounding the stigma. Anthers gradually dehisce over the course of a day, and individual flowers persist for about two days. W. solanacea possesses an S-RNase-based gametophytic self-incompatibility system, with an estimated number of 30 S-alleles in the Monteverde region (Stone and Pierce 2005). SC individuals have been documented in several populations, including but not limited to those with small population sizes at the periphery of the species range (Bohs 2000, Stone and Pierce 2005, Stone et al. 2006). SC individuals set fruit autonomously in a pollinator-free greenhouse although morphological modifications are not evident.
To determine whether self-compatibility co-segregates with the S-locus, we created F1 and F2 generations from a cross between SC and SI individuals, and monitored segregation of S-alleles and the incompatibility phenotype. The SC individual originated from Vara Blanca, Costa Rica. It consistently produces fruit autonomously and accepts self-pollen as revealed by fluorescence microscopy. The SI individual originated from Monteverde, Costa Rica and rejects self-pollen as indicated by fluorescence microscopy as well as lack of autonomous fruit set. S-alleles for parental plants were obtained by RT-PCR on stylar mRNA as previously described (Stone and Pierce 2005). The SI parental plant was heterozygous for S1 and S4, and the SC parental plant amplified for only the S23 allele. The crossing results reveal whether the SC plant was homozygous for S23 or heterozygous for S23 and a null-allele (S0). Allele-specific primers for the three amplifying alleles were developed (Table 1). Twenty progeny from the F1 generation were evaluated for their ability to produce fruit autonomously. We genotyped these progeny for alleles S1, S4, and S23 by extracting genomic DNA from fresh leaf tissue with the Qiagen DNeasy Plant Mini Kit (Germantown, Maryland), and using allele-specific PCR. PCR was carried out in 25μL volumes in standard buffer with 1.5 mM MgCl2, 0.8mM dNTPs, 0.5μM each primer, 2 U Taq polymerase, and 20 ng genomic DNA. Cycling conditions consisted of an initial 1 min denaturation at 94°C, 40 cycles each with 15 s denaturation at 94°C, 1 min annealing at 58°C, and 1 min extension at 72°C, followed by a 7 min extension at 72°C. The F2 generation was created by crossing two SC F1 individuals, one of which amplified only for S1 and the other of which amplified only for S4. Fourteen F2 individuals were evaluated for autonomous fruit production and genotyped for alleles S1 and S4. We predicted that if self-compatibility in this species is governed by a non-functional allele at the S-locus, self-incompatibility should re-emerge in the F2 for individuals inheriting both functional S-alleles.
Table 1.
Primers used to amplify S-alleles and microsatellite loci from genomic DNA of W. solanacea.
| Primer | Sequence | Amplifies | Tm | bp |
|---|---|---|---|---|
| WsolS1L | GGGGTGAGATGTTCAATGCT | S-RNase S1 | 60.4 | 175 |
| WsolS1R | TGTCTTTTAAGCGCAAGGCTA | 58.7 | ||
| WsolS4L | GACTTGCTCGTGGAAGAAGC | S-RNase S4 | 62.4 | 227 |
| WsolS4R | GTTATTGCCCTGACGGTGTT | 60.4 | ||
| WsolS23L | AGTCGAACGGACCAACGTAT | S-RNase S23 | 60.4 | 158 |
| WsolS23R | CCCGGAACAATTCTCTCTGA | 60.4 | ||
| WsolCTA3L | ATATCTACAAAGAAACCAAAACACACA | microsatellite | 59.4 | 145-160 |
| WsolCTA3R | CTCTCTTTACATTTGCTTTATCACAA | 58.2 | ||
| WsolCTB11L | CGTCAGCCCATTACCATCTC | microsatellite | 60.5 | 172-181 |
| WsolCTB11R | TCAGTTCATGGTTGTGTGAGC | 59.7 | ||
| WsolCTE2L | GCCTCAATTACAGCAGGTACAG | microsatellite | 58.9 | 192-202 |
| WsolCTE2R | GGGTTCTTCAACTTCAAAGAAAA | 58.8 | ||
| WsolCTF2L | GCCTTTGATTAGACGTG | microsatellite | 54.8 | 286-296 |
| WsolCTF2R | CCCTTGAGAGTTTGTCTAAGT | 58.7 |
Establishment of common gardens
Each garden contained 18 individual plants, an array size within the range of natural population sizes. Genotypes were clonally propagated in August, 2009. SI genotypes were collected at a spacing of at least 20 m from populations in the Monteverde Reserve (1550 m.a.s.l.), the Monteverde Community (1400 m.a.s.l.), and the UGA field station at San Luis de Monteverde (1100 m.a.s.l.). A total of 12 SI genotypes were used in the experiment, four from each of the three original populations. Each SI genotype was represented once per garden. SC genotypes were identified from previous collections housed in an insect-free greenhouse at Colby College. The SC genotypes set fruit autonomously, repeatedly, over a period of years during which SI plants did not produce fruit. Two of the SC genotypes originated from Vara Blanca, Costa Rica, in a small population previously documented for SI/SC polymorphism (Bohs 2000, Stone et al. 2006). The other two SC genotypes originated from the Monteverde region: one from the Monteverde Community and one from San Luis. To ensure representation of SC genotypes, two replicates each of the two local SC genotypes were planted in each garden.
Each genotype was propagated from cuttings 8-10 cm tall and 1-1.5 cm thick. Cuttings were first established in 0.5 L plastic bags and later transplanted into 3-gallon pots. All soil was obtained from the A layer of an andisol soil at a single locality in San Luis. Cuttings were fertilized monthly using 10:30:10 liquid fertilizer with 1% micronutrients. We established nine garden sites in three transects spanning the range of elevations at which this species is normally found in the Monteverde area (Table 2). The eastern transect descends lower in elevation than the other two, because suitable habitat extends to lower elevation in the San Luis valley. Gardens were 8 × 5 m, with plants established in a 6 × 3 grid at 1 m spacing. SC genotypes were distributed evenly, as were SI genotypes originating from the three natural populations. Bottoms of pots were removed so that plant roots could extend into the soil, and competing vegetation was cut back at two-month intervals. Six castor bean (Ricinus communis L.) plants were planted amongst the W. solanacea plants to provide partial shade. Gardens were fenced to exclude deer and cattle.
Table 2.
Location of experimental arrays.
| Name | Transect | Latitude | Longitude | Elevation (m.a.s.l.) |
|---|---|---|---|---|
| Casa de los Murcielagos | East | 10° 17.050’ | -84° 47.559’ | 1120 |
| Pasto El Brillante | East | 10° 17.191’ | -84° 47.335’ | 1280 |
| Reserva Monteverde | East | 10° 18.266’ | -84° 47.750’ | 1520 |
| Bajo Tigre | Central | 10° 18.308’ | -84° 48.892’ | 1360 |
| Monteverde Institute | Central | 10° 18.388’ | -84° 48.498’ | 1430 |
| Cerro Plano Arriba | Central | 10° 18.796’ | -84° 48.379’ | 1500 |
| Valle Escondido | West | 10° 18.496’ | -84° 49.102’ | 1330 |
| Cabañas los Pinos | West | 10° 18.805’ | -84° 48.823’ | 1430 |
| Estación Biológica | West | 10° 19.054’ | -84° 48.552 | 1510 |
Pollinator visitation and female fitness
We monitored pollinator visitation and marked pedicels to record fruit set during two rainy seasons (June- mid August 2010 and 2011) and two dry seasons (January 2011and 2012). We recorded pollinator activity by focusing on a single plant in each garden for 30-minute periods between 0900 and 1200, which has previously been shown to represent peak visitor activity. We monitored the plant in each garden with the largest number of flowers that day. Observers rotated among sites. A floral visitor was recorded as a pollinator if it contacted floral organs, and visits were counted as distinct if they were separated by more than 3 minutes. We recorded pollinator visitation at one to two-day intervals at each site, for a total of 277.5 hours of observation during the rainy seasons and 74.5 hours during the dry seasons. During the rainy season of 2010, we also devoted 23 half-hour observation periods to observing pollinators at natural populations of plants 500 m from the array at the Monteverde Reserve.
Individual plants typically produced zero to four new flowers each day. To obtain estimates of flower production, we counted the number of new flowers produced at two-day intervals for two western transects during the 2010 rainy season and the 2011 dry season. We marked up to two flowers per day to monitor fruit set, choosing the flowers from different inflorescences. To mark pedicels, we attached a noose of thread sealed by a small piece of laboratory tape labeled with the date. Pollinators appeared to be unaffected by the floral markers. We monitored plants weekly, collecting marked fruits when ripe. During the rainy season of 2011, we recorded presence or absence of all 4093 marked flowers at two-day intervals so that we could detect timing of abscission for flowers that did not produce ripe fruit. We counted seeds from 4-7 fruits per genotype per garden, for a total of 1428 fruits.
We carried out a small-scale experiment to compare fitness of progeny from SC and SI genotypes. For each ramet grown in each garden, we planted five seeds per cell in each of four randomized blocks, for a total of 3240 seeds. The seeds were planted in local soil in August 2011, placed on a bench in ambient conditions under a translucent plastic cover at the Monteverde Reserve and watered from below. Germination was recorded weekly. At 20 weeks, the largest surviving plant in each cell was transplanted into a 0.5 mL black plastic bag filled with local soil, placed on the bench in four randomized blocks, watered from above, and fertilized weekly. Plants were transplanted into 3-gallon pots at 28 weeks, and harvested at one year. We recorded survivorship, stem thickness, height, and reproductive status.
Statistical analysis of pollinator visitation and female function
We tested hypotheses concerning pollinator visitation using a split-plot ANOVA with season and elevation as main effects, and sites nested within elevation. Elevation was treated as a random effect and tested over site nested within elevation. Season was treated as a fixed effect and tested over the interaction of season by site nested within elevation (Sokal and Rohlf 1995). Pollinator visits per observation period were square-root transformed before analysis. A Mann-Whitney U-test was used to compare pollinator visits per observation period of the natural population versus the experimental array within the Monteverde Cloud Forest Reserve.
We tested hypotheses concerning fruit set using a split-plot ANOVA, with season and mating type as fixed effects and genotype nested within mating type. Mating type was tested over genotype, and genotype within mating type was tested over the residual. Two interaction terms were included: season by mating type and season by genotype within mating type. Both season and the interaction between season and mating type were tested over the interaction of season by genotype (Sokal and Rohlf 1995). To evaluate whether seed number varied by mating type, we used a nested ANOVA with genotype nested within mating type. Seed counts were first square-root transformed, then averaged by individual plant before analysis. A Mann-Whitney U-test was used to compare median days to abscission for SI and SC plants. We evaluated the hypothesis that germination differed by mating type using ANOVA on mean seeds germinated for each maternal genotype across the nine gardens. Block was included as a random factor, and mating type was tested over genotype nested within mating type. Post-germination fitness components for progeny of SI and SC plants were compared using a series of Mann-Whitney U-tests on mean values for each maternal genotype grown in all gardens. A Fisher’s exact test was used to test whether probability of reaching reproductive maturity after one year was independent of maternal mating type. Statistical analyses were carried out using Stata 12.0 (StataCorp 2011).
Male fitness and selfing rate
We estimated paternity and selfing rates using seedlings grown from seeds produced during the 2010 rainy season. Seeds from all mothers in all nine gardens were planted in Fafard superfine germination mix (Sun-Gro Horticulture, Agawam, Massachusetts) and watered from above in the greenhouse at Colby College. Seedlings were harvested and air-dried when the leaves were about 1 cm long. DNA was extracted using the Qiagen DNeasy 96 Plant Kit and amplified using touchdown PCR for microsatellite loci WsolcaA3, WsolcaE2, and WsolgataA6 as described previously (Stone et al. 2010). Four additional loci were also amplified (Table 1). We used the software PatQuest (Smouse and Meagher 1994; available at http://eggg.standrews.ac.uk/software/) to create matrices of genetic segregation probabilities for each progeny, given its maternal parent and the possible male parents. PatQuest assigns fractional paternity to seedlings having multiple possible sires and conducts a maximum-likelihood estimate of total contribution of each sire to the progeny array. Analyses were conducted on 20-39 seedlings per garden from the seven gardens with greater than 50% germination rates. Of the 184 seedlings analyzed, 145 had genotypes consistent with fathers established in the array, 129 had a single most likely father, and 103 had only one possible father. Fractional paternity for each genet was averaged across all gardens. We allocated paternity for the local SC genotypes equally across the two ramets of these genotypes planted in each garden. We used the segregation matrices generated by PatQuest to determine selfing rates from 79 seedlings of SC mothers. In 77 of these cases, the seedlings were unambiguously selfed or outcrossed. For the two seedlings with possible outcross or self sires, we assigned self-fertilization according to the probability that that the mother was the sire.
Transmission advantage of selfing
According to the classical phenotypic model that incorporates seed and pollen discounting (Lloyd 1992, reformulated by Busch and Delph 2012), an increase in the selfing rate is favored whenever:
where δ = inbreeding depression, y = selfed seeds, xx = outcrossed seeds produced by SI plants, xs = outcrossed seeds produced by SC plants, px = outcrossed seeds sired by SI plants, ps = outcrossed seeds sired by SC plants, (xx − xs)/y = seed discounting, and (px − ps)/y = pollen discounting. We calculated the average number of outcross seeds per day produced by SI and SC plants during the rainy and dry seasons by multiplying median flower number times average fruit set times the outcrossing rate for each mating type times average number of seeds per fruit. We gain more precise estimates by calculation than by direct count because idiosyncratic environmental factors affected size of individual plants substantially in our gardens, and proportional fruit set controls for this variation. Outcrossed seeds sired were calculated by multiplying the total number of outcrossed seeds produced times the paternal reproductive success by mating type times the outcrossing rate for each mating type.
RESULTS
Inheritance of self-compatibility
In the F1 generation, SI segregated with possession of two functional S-alleles, and SC segregated with possession of a single S-allele (Table 3). In the F2 generation, created by crossing two SC parents, about ¼ of the progeny regained SI, consistent with single locus control of the breakdown of SI. SI segregated with the presence of two functional S-alleles (Table 3).
Table 3.
S-locus genotypes and SI phenotypes for progeny from experimental crosses. The F1 was created by crossing an SI individual of genotype S1S4 and an SC individual of genotype S23S0, where S0 is a null allele. The F2 was created by crossing F1 individuals of genotypes S1S0 and S4S0.
| Generation | Genotype | Phenotype | Number observed |
|---|---|---|---|
| F1 | S1S0 | SC | 3 |
| F1 | S4S0 | SC | 9 |
| F1 | S1S23 | SI | 4 |
| F1 | S4S23 | SI | 4 |
| F2 | S0S0 | SC | 4 |
| F2 | S1S0 | SC | 3 |
| F2 | S4S0 | SC | 2 |
| F2 | S1S4 | SI | 5 |
Pollinator visitation
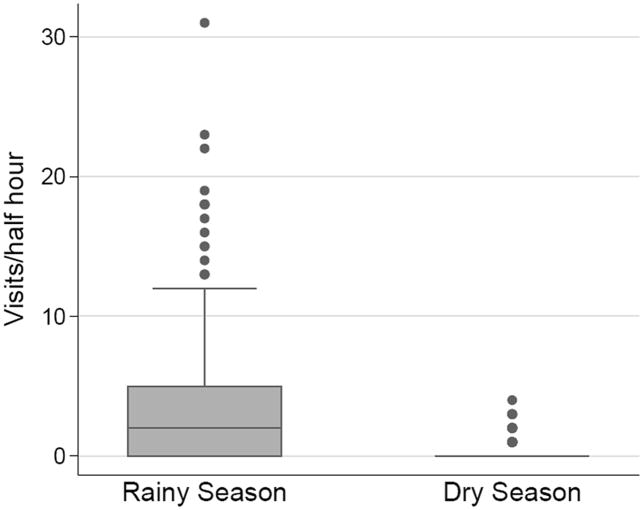
During the rainy season, pollinator visitation to focal plants ranged from 2 -14 visits/hr, with a mean of 6.2 visits per hour across all sites and both years. During the dry season, pollinator visitation ranged from 0 - 2.6 visits/hr, with a mean of 0.8 visits per hour. The most abundant pollinators were halictid bees in the genus Lasioglossum. There was no consistent relationship between pollinator visitation rate and elevation (Table 4; F2, 8 = 1.96, P = 0.20), but pollinator visitation was dramatically lower during the dry season than the rainy season (Fig. 1; Table 4; F1, 6 = 47.5; P = 0.0005). During the rainy season of 2010, pollinators visited plants of a natural population in the Monteverde Reserve at a rate of 3.4 visits per hour, significantly less than the mean rate of 13.4 visits/hr to the nearby garden array (U = 4.4, n1=23, n2=25, P < 0.0001).
Table 4.
Rates of pollinator visitation to single well-flowered plants within arrays containing 18 individuals of Witheringia solanacea. N = number of 30-minute observation periods.
| Elevation | Year | Season | N | Visits/half hour Mean ± SD |
|---|---|---|---|---|
| Low | 2010 | rainy | 74 | 3.35 ± 0.36 |
| Low | 2011 | rainy | 108 | 3.06 ± 0.38 |
| Mid | 2010 | rainy | 72 | 3.14 ± 0.42 |
| Mid | 2011 | rainy | 107 | 0.82 ± 0.12 |
| High | 2010 | rainy | 86 | 4.49 ± 0.40 |
| High | 2011 | rainy | 108 | 4.31 ± 0.53 |
| Low | 2011 | dry | 26 | 0.65 ± 0.17 |
| Low | 2012 | dry | 21 | 0.33 ± 0.13 |
| Mid | 2011 | dry | 26 | 0.27 ± 0.17 |
| Mid | 2012 | dry | 20 | 0.05 ± 0.05 |
| High | 2011 | dry | 27 | 0.52 ± 0.18 |
| High | 2012 | dry | 29 | 0.45 ± 0.18 |
See Table 2 for array locations.
Figure 1.
Box plot showing pollinator visitation to focal plants within experimental gardens over two rainy and two dry seasons. N=555 (rainy seasons); N=149 (dry seasons).
Female fitness
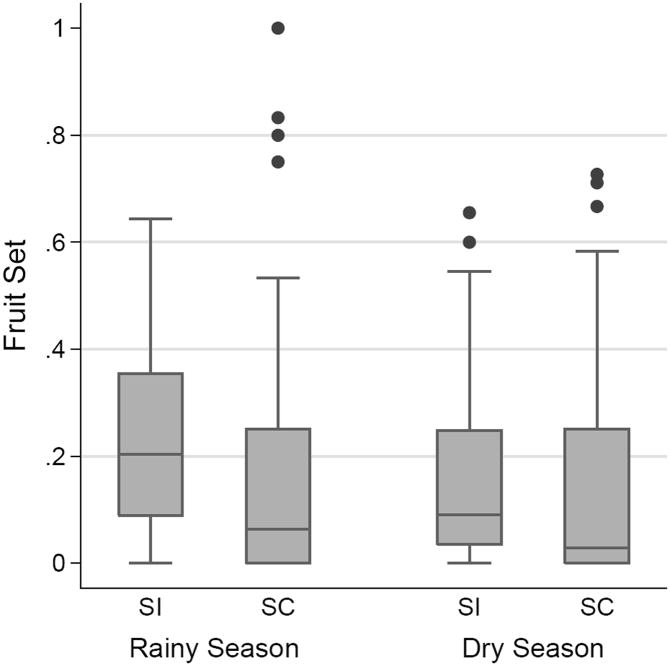
Across all plants, sites, and seasons, an average of 18.6% of flowers developed into ripe fruits. Fruit set was significantly higher in the rainy season than the dry season (Fig. 2; F1, 16 = 31.68, P < 0.0001). Fruit set varied significantly by genotype (F16, 421 = 3.92, P < 0.0001), but SC and SI genotypes did not significantly differ overall (F1, 16 = 0.65, P = 0.84). Mating type and season did interact significantly (Fig. 2; Table 5; F1, 16 = 5.76, P = 0.0289), with SI plants setting relatively more fruits during the rainy season. Ripe fruits contained 68 seeds on average. Seeds per fruit varied by maternal genotype (F16, 261 = 9.42, P < 0.0001), but not by mating type (F1, 16 = 0.47, P = 0.50).
Figure 2.
Fruit set for SI and SC genotypes of W. solanacea planted in nine replicated garden arrays. Box plots constructed using fruit set for each plant in each array over two field seasons, with N = 197, 96, 115, and 49 from left to right.
Table 5.
Flower number, fruit set and paternity estimates by season and mating type. Flower numbers are medians of plants in the six westernmost gardens during the first rainy and dry seasons. Fruit sets are averages of means for each plant in each garden across two rainy and two dry seasons. Paternity is the average proportion of seeds sired by each genotype within mating types across gardens during the rainy season of 2010.
| Mating Type |
Rainy Flowers/ Day (N) |
Rainy Fruit Set Mean ± SE (N) |
Dry Flowers/ Day (N) |
Dry Fruit Set Mean ± SE (N) |
Paternity Mean ± SE (N) |
|---|---|---|---|---|---|
| SI | 1.7 (65) |
0.23 ± 0.01 (197) |
0.8 (59) |
0.15 ± 0.02 (115) |
0.052 ± 0.013 (12) |
| SC | 2.6 (30) |
0.15 ± 0.02 (96) |
0.8 (22) |
0.15 ± 0.03 (49) |
0.062 ± 0.018 (6) |
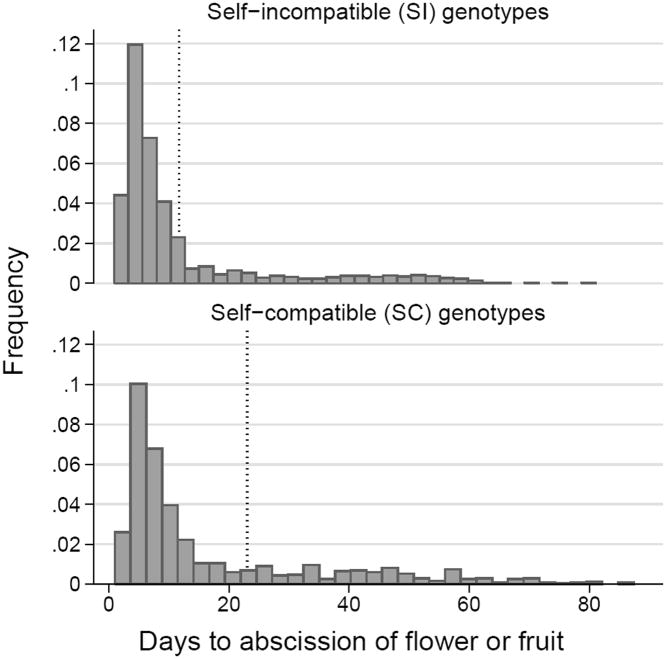
The mean number of days required to produce a ripe fruit was 56 (SE = 0.23). For flowers that did not produce fruit, days to abscission had a highly skewed distribution (Fig. 3). Half of all flowers that did not set fruit abscised within 7-8 days for both mating types. The 75th percentile for SI plants was 13 days, and for SC plants was 23 days. Proportionally more fruits on SC than on SI plants were initiated but subsequently aborted (Fig. 3; U = 2.00, n1=96, n2=46, P = 0.0451).
Figure 3.
Frequency distribution of days to abscission for 3038 marked flowers that did not develop into ripe fruits during the rainy season of 2011. Dashed lines indicate the 75th percentiles for abscission dates of SI and SC genotypes. Median time from flower to ripe fruit was 56 days. Proportionally more fruits on SC than SI plants were initiated but subsequently aborted.
Mean seed germination was 0.51 (SD = 0.19), and the proportion of germinated seedlings that survived to 20 weeks was also 0.51 (SD = 0.27). Neither germination (F1, 16 = 1.67, P = 0.2145) nor survival to 20 weeks (F1, 16 = 0.34 P = 0.5672) varied by mating type. The grand mean height at 20 weeks was 3.1 cm (SD = 0.82). Following transplantation, 71% of plants survived to 28 weeks, and 94% of those survived to one year. The grand mean height at one year was 19.0 cm (SD = 3.45), and the mean stem diameter was 5.8 mm (n = 82, SD = 1.20). No growth or survivorship measures differed by mating type (n1 = 12, n2 = 6, P > 0.09 for all comparisons). Of the 92 surviving progeny of SI mothers, four were reproductively mature at one year; of the 38 surviving progeny of SC mothers, eight were reproductively mature at one year. Progeny of SC mothers were more likely than progeny of SI mothers to reach reproductive maturity at one year of age (Fisher’s exact test; P = 0.005).
Male fitness and selfing rate
The proportion of seedlings sired by each individual ranged from 0.000 to 0.143, with means of 0.052 (SD=0.044) for SI plants and 0.062 (SD=0.044) for SC plants, a non-significant difference (U = 0.75, n1=12, n2=6, P = 0.45). SI plants were not observed to produce selfed progeny. The estimated selfing rate of SC mothers was 0.076.
DISCUSSION
The reproductive assurance provided by self-fertilization has long been recognized as an important force promoting its spread in flowering plants, especially for autonomous self-fertilization in environments where pollen or pollinators are limiting (Darwin 1876, Baker 1955). We found that mutations permitting autonomous self-fertilization in W. solanacea did not provide reproductive assurance: seed discounting was complete (Table 6). During the dry seasons, when pollinators were scarce, flower production and fruit set for the two mating types was equal. During the rainy seasons, when pollinators were abundant, SC plants produced about 50% more flowers but had about 50% lower fruit set than SI plants. Pollinator visitation to experimental arrays did not systematically decline with elevation, as it does in natural populations (Stone and Jenkins 2008). Pollinators are evidently responding to abundance of floral resources and other habitat qualities, rather than to elevation per se. Supplemental pollinations carried out during dry seasons have demonstrated strong pollen limitation for fruit set across the range of elevations and population sizes in the Monteverde region, at similar pollinator visitation rates to those observed here (Stone and Jenkins 2008). Pollen limitation of fruit set has not been evaluated during the rainy season.
Table 6.
Selfed seeds per day per SC plant (y), outcross seeds per day produced per SI (xx) and SC (xs) plant and sired by the average SI (px) and SC (ps) plant, seed discounting per selfed seed (SD = (xx - xs)/y), pollen discounting per selfed seed (PD = (px − ps)/y), and threshold inbreeding depression (δ) favoring the spread of an SC mutant for conditions prevailing during rainy and dry seasons. Calculated from values presented in Table 5, the mean of 68 seeds per fruit, and the measured selfing rate of 0.076. Male and female outcrossing rates are equal when weighted by the twelve SI and six SC ramets per garden. Dry season values were calculated using genetic analysis of seeds harvested during the rainy season.
| Season | y | xx | xs | SD | px | ps | PD | δ |
|---|---|---|---|---|---|---|---|---|
| Rainy | 2.0 | 26.4 | 24.4 | 1.0 | 24.3 | 28.5 | -2.1 | 1.6 |
| Dry | 0.6 | 8.2 | 7.5 | 1.0 | 7.5 | 8.8 | -2.1 | 1.6 |
| Dry* | 1.2 | 8.2 | 6.9 | 1.0 | 7.7 | 7.7 | 0 | 0.5 |
Dry values were calculated according to a scenario with a doubled selfing rate and no negative pollen discounting.
Even when increased selfing does not provide reproductive assurance, it is still predicted to spread by transmission advantage if it does not cause a decline in male outcross reproductive success, i.e., pollen discounting (Nagylaki 1976, Holsinger et al. 1984). Pollen discounting is classically envisioned as ranging from zero to one, but may in fact be negative if changes in floral display or morphology simultaneously promote self-fertilization and pollen export (Johnson et al. 2009). Our experiment demonstrated negative pollen discounting: SC plants had higher siring success than SI plants (Table 6). Individual flowers of W. solanacea do not display obvious morphological modifications associated with mating type, but SC plants produce many more flowers during the rainy season than do SI plants. SC plants also abort more immature fruits than do SI plants. Negative pollen discounting in this system is likely a side effect of embryonic inbreeding depression which causes fruit abortion and subsequent re-allocation of resources to flower production. Counter-intuitively, because of its effects on fruit abortion and re-allocation to flower production, the SC allele is currently selected more through male than female function. Also surprising is that the SC allele is most favored during the season with high pollinator visitation, because this is the season in which plentiful water permits abundant flowering, fruit set, and high outcross siring success.
Given our estimates of seed and pollen discounting, the threshold level of inbreeding depression permitting a selfing allele to invade exceeds one (Table 6), so selection should strongly favor the spread of the selfing allele. Several caveats apply to this prediction. First, genetic analysis was carried out only for seeds collected during the rainy season. Male reproductive success and selfing rate of SC morphs almost certainly differ across seasons. In particular, SC plants should lose their siring advantage over SI plants during the dry season when the two morphs produce the same number of flowers. It also seems likely that selfing rate increases during the dry season, explaining the parity in fruit set of SC and SI morphs under conditions of pollinator scarcity. If we assume equal siring success and a doubled selfing rate during the dry season, seed discounting remains complete and threshold inbreeding depression is reduced to 0.5 (Table 6). The dry season extends over about four months in Monteverde (Clark et al. 2000), so selection overall should still favor the SC allele. A second caveat is that our finding of negative pollen discounting during the rainy season relies upon the validity of our paternity estimates. Although it is reasonable to think that higher flower production would lead to greater male reproductive success, it is prudent to recognize that siring success varied among genotypes within each mating type, and that there was no statistically significant difference between mating types in siring success overall. If paternal reproductive success is equivalent by mating type, pollen discounting returns to zero and the SC allele would be favored according to the classical model only if inbreeding depression fell below 0.5.
As a complement to phenotypic models that treat inbreeding depression as a fixed entity, it is useful to consider models that incorporate the evolution of inbreeding depression. Porcher and Lande (2005) evaluate conditions permitting the breakdown of gametophytic self-incompatibility by invasion of SC alleles acting either at the S-locus or at a modifier locus. In general, an SC mutant invades more easily under limited numbers of S-alleles, pollen limitation, lack of genetic load linked to the S-locus, and low background genetic load due to nearly additive mildly deleterious mutations. Mutations at the S-locus spread more easily than those at modifier loci. Under models of static inbreeding depression, a non S-linked mutation can invade when δ < 1/2, but a linked mutant can invade if δ <; 2/3 because heterozygotes for SC will reject only pollen bearing the SI allele (Charlesworth and Charlesworth 1979). When inbreeding depression is allowed to evolve, purging of deleterious recessive mutations makes the spread of an SC allele even more permissive, however, its success depends upon the primary selfing rate (Porcher and Lande 2005). With a finite number of S-alleles and inbreeding depression due primarily to recessive lethals, transmission advantage favors the spread of S-linked SC mutations under any initial inbreeding depression when selfing rates are non-zero but less than ~ 0.2 or greater than ~ 0.8. Higher selfing rates favor the spread of SC due to rapid purging of genetic load; lower selfing rates favor its spread because they enhance the transmission advantage of SC pollen. Restricted but realistic combinations of parameter values in this model can also lead to stable polymorphism.
To apply the dynamic model of Porcher and Lande to W. solanacea, we require estimates of the primary selfing rate, before seed maturation and germination. We previously conducted a pollen-chase study which allowed us to estimate early-acting inbreeding depression by comparing fruit and seed set for flowers receiving bud-self pollinations relative to those receiving bud-outcross pollinations (Stone et al. 2010). We can therefore estimate primary selfing rate for our SC genotypes using the method of Maki (1993), as re-stated by Husband and Schemske (1996):
where r = primary selfing rate, rm = measured selfing rate after germination, and δ = inbreeding depression for seed production and germination. The SC genotypes from Vara Blanca had an early-acting inbreeding depression of only 0.02, so primary selfing rate was nearly identical to their measured selfing rate of 17%. Genotypes from Monteverde had nearly complete early-acting inbreeding depression, yielding estimates of primary selfing rate for the two local SC genotypes at 21% and 76%. Given our observed number of 30 S-alleles (Stone and Pierce 2005), if primary selfing rates are below about 20%, an S-linked SC mutant should be able to invade even though inbreeding depression due to recessive mutations is nearly absolute. In contrast, an SC mutant unlinked to the S-locus would be impervious to strong inbreeding depression only under very high selfing rates, where purging is most effective.
Our genetic crossing study used a SC genotype from the Vara Blanca population, rather than an indigenous SC genotype from Monteverde, so we cannot say whether SC plants at Monteverde bear S-linked null alleles. We suspect that the one with the lower selfing rate does not because it produces fruit autonomously even though fluorescence microscopy reveals its capacity to reject self-pollen (Stone et al. 2006). The other SC genotype from MV consistently rejects self-pollen, and is more likely to possess a null S-allele. Presumably, as in other species, loss-of-function mutations arise independently in different populations and SC has the potential to evolve independently in each (e.g., Rick et al. 1979, Husband and Barrett 1993, Shimizu et al. 2008, Busch et al. 2011). For W. solanacea, self-compatibility is most pronounced in small, peripheral populations (Stone et al. 2006). The population at Vara Blanca is tiny; we have found only seven plants there despite intensive searching, but both SC and SI individuals persist (Bohs 2000). The Monteverde population contains hundreds of plants, and only four SC individuals have so far been identified. We are currently engaged in a large-scale study to estimate selfing rates of small and large populations across the 200-km range of the species in Costa Rica.
Given certain caveats, phenotypic and genetic models predict that the SC allele in W. solanacea should spread even when it is not selected by its capacity to provide reproductive assurance. Although embryonic inbreeding depression is a formidable barrier, the Vara Blanca population demonstrates that purging of early-acting genetic load is possible. An S-linked SC allele confers substantial transmission advantage, and should spread when primary selfing rates are relatively low, as observed in the Monteverde populations, or high, as in populations on the periphery. Given the selective advantage of SC alleles, it is not clear why they are not more common. Indeed, this is a conundrum faced by Porcher and Lande (2005). Intermediate selfing rates together with high embryonic inbreeding depression may tip the balance in favor of SI alleles in larger populations.
Although the breakdown of self-incompatibility is one of the most commonly traversed transitions in the history of flowering plants, polymorphic species are uncommonly identified. To our knowledge, ours is the first study to evaluate selection on a loss-of-function S-allele within replicated SI populations. Our results show that the automatic transmission advantage of selfing strongly favors the SC allele, even in the face of high inbreeding depression, because increased selfing does not decrease siring success. Enhanced breakdown of SI in small or peripheral populations may be caused as much by transmission advantage due to few S-alleles or by rapid purging of genetic load as by reproductive assurance. For the many lineages of flowering plants in which self-compatibility arises through a loss-of-function mutation, the transmission advantage of selfing can be a powerful force promoting its spread.
Acknowledgments
We are grateful to the staff of the UGA Costa Rica in San Luis, to the Reserva Biológica Bosque Nuboso Monteverde, and to the landowners who permitted us to establish gardens. We thank technicians Bryan Prelgovisk, Martha Garro Cruz, and Guadalupe Cruz Rodriguez; undergraduate field assistants M. Bienkowski, W. Bloomhardt, S. Doyle, E. Griffoul, B. Hummel, K. Lebling, C. Reichler, B. Rhodes, and M. Russell; and laboratory assistants E. Anderson and C. Tsujiura. Thomas Meagher and Randall Downer helped with use of PatQuest. Comments by Associate Editor Mark Johnston and two anonymous reviewers greatly improved the manuscript. This research was supported by NSF DEB-0841482 and by NCRR (5P20RR016463-12) and the NIGMS (8 P20 GM103423-12) from the NIH.
Footnotes
DATA ARCHIVING
Data available from the Dryad Digital Repository: doi:10.5061/dryad.f8539
LITERATURE CITED
- Baker HG. Self-compatibility and establishment after “long-distance” dispersal. Evolution. 1955;9:347–348. [Google Scholar]
- Barrett SCH, Shore JS. Variation and evolution of breeding systems in the Turnera ulmifolia L. complex (Turneraceae) Evolution. 1987;41:340–354. doi: 10.1111/j.1558-5646.1987.tb05802.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Shore JS, Morgan MT, Husband BC. The dissolution of a complex genetic polymorphism: the evolution of self-fertilization in tristylous Eichhornia paniculata (Pontederiaceae) Evolution. 1989;43:1398–1416. doi: 10.1111/j.1558-5646.1989.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Bohs L. Insights into the Witheringia solanacea (Solanaceae) complex in Costa Rica. I. Breeding systems and crossing studies. Biotropica. 2000;32:70–79. [Google Scholar]
- Busch JW. The evolution of self-compatibility in geographically peripheral populations of Leavenworthia alabamica (Brassicaceae) Am J Bot. 2005;92:1503–1512. doi: 10.3732/ajb.92.9.1503. [DOI] [PubMed] [Google Scholar]
- Busch JW, Herlihy CR, Gunn L, Werner WJ. Mixed mating in a recently derived self-compatible population of Leavenworthia alabamica (Brassicaceae) Am J Bot. 2010;97:1005–1013. doi: 10.3732/ajb.1000032. [DOI] [PubMed] [Google Scholar]
- Busch JW, Joly S, Schoen DJ. Demographic signatures accompanying the evolution of selfing in Leavenworthia alabamica. Mol Biol Evol. 2011;28:1717–1729. doi: 10.1093/molbev/msq352. [DOI] [PubMed] [Google Scholar]
- Busch JW, Delph LF. The relative importance of reproductive assurance and automatic selection as hypotheses for the evolution of self-fertilization. Ann Bot. 2012;109:553–562. doi: 10.1093/aob/mcr219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B. The evolution and breakdown of S-allele systems. Heredity. 1979;43:41–55. [Google Scholar]
- Clark KL, Lawton RO, Butler PR. The physical environment. In: Nadkarni NM, Wheelwright NT, editors. Monteverde: Ecology and conservation of a tropical cloud forest. Oxford University Press; Oxford, U.K: 2000. pp. 15–38. [Google Scholar]
- Darwin C. Effects of cross and self fertilization in the vegetable kingdom. John Murray; London, U.K: 1876. [Google Scholar]
- Darwin C. The different forms of flowers of flowers on plants of the same species. John Murray; London, U.K: 1877. [Google Scholar]
- de Nettancourt D. Incompatibility and incongruity in wild and cultivated plants. Springer-Verlag; Berlin: 2001. [Google Scholar]
- Eckert CG, Samis KE, Dart S. Reproductive assurance and the evolution of uniparental reproduction in flowering plants. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford University Press; Oxford, U.K: 2006. pp. 183–203. [Google Scholar]
- Fausto JA, Jr, Eckhart VM, Geber MA. Reproductive assurance and the evolutionary ecology of self-pollination in Clarkia xantiana (Onagraceae) Am J Bot. 2001;88:1794–1800. [PubMed] [Google Scholar]
- Fisher RA. Average excess and average effect of a gene substitution. Ann Eugen. 1941;11:53–63. [Google Scholar]
- Glémin S, Bazin E, Charlesworth D. Impact of mating systems of patterns of sequence polymorphism in flowering plants. Proc R Soc B. 2006;273:3011–3019. doi: 10.1098/rspb.2006.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EE, Kohn JR, Lande R, Robertson KA, Smith SA, Igic B. Species selection maintains self-incompatibility. Science. 2010;330:493–495. doi: 10.1126/science.1194513. [DOI] [PubMed] [Google Scholar]
- Goodwillie C, Kalisz S, Eckert CG. The evolutionary enigma of mixed mating systems in plants: Occurrence, theoretical explanations, and theoretical evidence. Ann Rev Ecol Evol Syst. 2005;36:47–79. [Google Scholar]
- Hamrick JL, Godt MJ. Effects of life history traits on genetic diversity in plant species. Phil Trans R Soc Lond B. 1996;351:1291–1298. [Google Scholar]
- Holsinger KE, Feldman MW, Christiansen FB. The evolution of self-fertilization in plants: A population genetic model. Am Nat. 1984;124:446–453. [Google Scholar]
- Husband BC, Barrett SCH. Multiple origins of self-fertilization in tristylous Eichhornia paniculata (Pontederiaceae): Inferences from style morph and isozyme variation. J Evol Biol. 1993;6:591–608. [Google Scholar]
- Husband BC, Schemske DW. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution. 1996;50:54–70. doi: 10.1111/j.1558-5646.1996.tb04472.x. [DOI] [PubMed] [Google Scholar]
- Igic B, Kohn JR. Evolutionary relationships among self-incompatibility RNases. Proc Nat Acad Sci USA. 2001;98:13167–13171. doi: 10.1073/pnas.231386798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igic B, Busch JW. Is self-fertilization an evolutionary dead-end? New Phytol. 2013;198:386–397. doi: 10.1111/nph.12182. [DOI] [PubMed] [Google Scholar]
- Igic B, Lande R, Kohn JR. Loss of self-incompatibility and its evolutionary consequences. Int J Plant Sci. 2008;169:93–104. [Google Scholar]
- Johnston MO, Porcher E, Cheptou P-O, Eckert CG, Elle E, Geber MA, Kalisz S, Kelly JK, Moeller DA, Vallejo-Marín M, Winn AW. Correlations among fertility components can maintain mixed mating in plants. Am Nat. 2009;173:1–11. doi: 10.1086/593705. [DOI] [PubMed] [Google Scholar]
- Kalisz S, Vogler DW, Hanley KM. Context-dependent autonomous self-fertilization yields reproductive assurance and mixed mating. Nature. 2004;430:884–887. doi: 10.1038/nature02776. [DOI] [PubMed] [Google Scholar]
- Kohn JR, Barrett SCH. Pollen discounting and the spread of a selfing variant in tristylous Eichhornia paniculata: evidence from experimental populations. Evolution. 1994;48:1576–1594. doi: 10.1111/j.1558-5646.1994.tb02197.x. [DOI] [PubMed] [Google Scholar]
- Latta R, Ritland K. Models for the evolution of selfing under alternative modes of inheritance. Heredity. 1993;71:1–10. [Google Scholar]
- Lloyd DG. Some reproductive factors affecting the selection of self-fertilization in plants. Am Nat. 1979;113:67–79. [Google Scholar]
- Lloyd DG. Self- and cross-fertilization in plants. II. The selection of self-fertilization. Int J Plant Sci. 1992;153:370–380. [Google Scholar]
- Maki M. Outcrossing and fecundity advantage of females in gynodioecious Chionographis japonica var. kurohimensis (Liliaceae) Am J Bot. 1993;80:629–634. [Google Scholar]
- Moeller DA, Geber MA. Ecological context of the evolution of self-pollination in Clarkia xantiana: population size, plant communities, and reproductive assurance. Evolution. 2005;59:786–799. doi: 10.1554/04-656. [DOI] [PubMed] [Google Scholar]
- Moeller DA. Geographic structure of pollinator communities, reproductive assurance, and the evolution of self-pollination. Ecology. 2006;87:1510–1522. doi: 10.1890/0012-9658(2006)87[1510:gsopcr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Morgan MT, Wilson WG, Knight TM. Plant population dynamics, pollinator foraging, and the selection of self-fertilization. Am Nat. 2005;166:169–183. doi: 10.1086/431317. [DOI] [PubMed] [Google Scholar]
- Nagylaki T. A model for the evolution of self-fertilization and vegetative reproduction. J Theor Biol. 1976;58:55–58. doi: 10.1016/0022-5193(76)90138-7. [DOI] [PubMed] [Google Scholar]
- Porcher E, Lande R. Loss of gametophytic self-incompatibility with evolution of inbreeding depression. Evolution. 2005;59:46–60. [PubMed] [Google Scholar]
- Richards AJ. Plant breeding systems. Chapman & Hall; London, U.K: 1986. [Google Scholar]
- Rick CM, Forbes JF, Tanksley SD. Evolution of mating systems in Lycopersicon hirsutum as deduced from genetic variation in electrophoretic and morphological characters. Pl Syst Evol. 1979;132:279–298. [Google Scholar]
- Shimizu KK, Shimizu-Inatsugi R, Tsuchimatsu T, Purugganan MD. Independent origins of self-compatibility in Arabidopsis thaliana. Mol Ecol. 2008;17:704–714. doi: 10.1111/j.1365-294X.2007.03605.x. [DOI] [PubMed] [Google Scholar]
- Smouse PE, Meagher TR. Genetic analysis of male reproductive contributions in Chamaelirium luteum (L.) Gray (Liliaceae) Genetics. 1994;136:313–322. doi: 10.1093/genetics/136.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. 3. Freeman; New York: 1995. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 12. College Station; Texas: 2011. [Google Scholar]
- Stebbins GL. Self fertilization and population variability in the higher plants. Am Nat. 1957;91:337–354. [Google Scholar]
- Stone JL. Molecular mechanisms underlying the breakdown of gametophytic self-incompatibility. Q Rev Biol. 2002;77:17–32. doi: 10.1086/339200. [DOI] [PubMed] [Google Scholar]
- Stone JL, Pierce SE. Rapid recent radiation of S-RNase lineages in Witheringia solanacea (Solanaceae) Heredity. 2005;94:547–555. doi: 10.1038/sj.hdy.6800657. [DOI] [PubMed] [Google Scholar]
- Stone JL, Sasuclark MA, Blomberg CP. Variation in the self-incompatibility response within and among populations of the tropical shrub Witheringia solanacea (Solanaceae) Am J Bot. 2006;93:592–598. doi: 10.3732/ajb.93.4.592. [DOI] [PubMed] [Google Scholar]
- Stone JL, Jenkins EG. Pollinator abundance and pollen limitation of a Solanaceous shrub at premontane and lower montane sites. Biotropica. 2008;40:55–61. [Google Scholar]
- Stone JL, Wilson EE, Kwak AS. Embryonic inbreeding depression varies among populations and by mating system in Witheringia solanacea (Solanaceae) Am J Bot. 2010;97:1328–1333. doi: 10.3732/ajb.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi N, Morrell PL. Is self-fertilization an evolutionary dead end? Revisiting an old hypothesis with genetic theories and a macroevolutionary approach. Am J Bot. 2001;88:1143–1150. [PubMed] [Google Scholar]
- Uyenoyama MK, Vallejo-Marín On the evolutionary costs of self-incompatibility: incomplete reproductive compensation due to pollen limitation. Evolution. 2004;58:1924–1935. doi: 10.1111/j.0014-3820.2004.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Winn AA, Elle E, Kalisz S, Cheptou P-O, Eckert CG, Goodwillie C, Johnston MO, Moeller DA, Ree RH, Sargent RD, Vallejo- Marín M. Analysis of inbreeding depression in mixed-mating plants provides evidence for selective interference and stable mixed mating. Evolution. 2011;65:3339–3359. doi: 10.1111/j.1558-5646.2011.01462.x. [DOI] [PubMed] [Google Scholar]
- Wright SI, Kalisz S, Slotte T. Evolutionary consequences of self-fertilization in plants. Proc R Soc B. 2013;280:20130133. doi: 10.1098/rspb.2013.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]