Abstract
Ideal cardiovascular health is a recently defined construct by the American Heart Association (AHA) to promote cardiovascular disease reduction. Arterial stiffness is a major risk factor for cardiovascular disease. The extent to which the presence of multiple prevalent cardiovascular risk factors and health behaviors is associated with arterial stiffness is unknown. The aim of this study was to examine the association between the AHA construct of cardiovascular health and arterial stiffness, as indexed by pulse wave velocity and pulse pressure. The AHA health metrics, comprising of four health behaviors (smoking, body mass index, physical activity, and diet) and three health factors (total cholesterol, blood pressure, and fasting plasma glucose) were evaluated among 505 participants in the Maine-Syracuse Longitudinal Study. Outcome measures were carotid-femoral pulse wave velocity (PWV) and pulse pressure measured at 4 to 5-year follow-up. Better cardiovascular health, comprising both health factors and behaviors, was associated with lower arterial stiffness, as indexed by pulse wave velocity and pulse pressure. Those with at least five health metrics at ideal levels had significantly lower PWV (9.8 m/s) than those with two or less ideal health metrics (11.7 m/s) (P<0.001). This finding remained with the addition of demographic and PWV-related variables (P=0.004).
Keywords: arterial stiffness, pulse wave velocity, cardiovascular health
INTRODUCTION
Arterial stiffness is higher in persons with diabetes mellitus, obesity, and other cardiovascular risk factors and increases with blood pressure and age.1, 2 It is a major risk factor for myocardial infarction, stroke, end stage renal disease and other cardiovascular diseases (CVD).3–6 Traditionally, blood pressure (BP) is measured non-invasively by determining the pressure (mm/Hg) in the brachial artery in the upper arm and arterial stiffness is indirectly assessed by way of pulse pressure (PP) defined as systolic BP (SBP) minus diastolic BP (DBP).
However, there is increasing recognition that measures of central arterial function are more valuable predictors of vascular health outcomes than traditional BP, and pulse wave velocity (PWV) is now considered the gold standard non-invasive method for measuring aortic stiffness, and more generally arterial stiffness.7
While various indices of arterial stiffness, including PWV, have been related to risk factors for CVD, there have been few, if any, studies that have related positive health factors and behaviors to PWV. Crichton, Elias, Dore, Abhayaratna, and Robbins8 found that dairy consumption in a relatively healthy community-based population was related to lower PWV, PP and SBP, but not to DBP. This study raises the question as to whether positive health behaviors are related to lower levels of PWV and, by inference, lower levels of arterial stiffness. This can be addressed by comparing PWV values for persons with constellations of cardiovascular risk factors that are associated with reduced cardiovascular morbidity and mortality and those that place one at higher risk.
The American Heart Association (AHA) has defined ideal cardiovascular health as the presence of four health behaviors (non-smoking, regular physical activity at goal levels, body mass index (BMI) <25 kg/m2, and diet consistent with national recommendations) and three health factors (total cholesterol <200 mg/dL, BP <120/<80 mm Hg, and fasting blood glucose <100 mg/dL) at ideal level.9 This definition of cardiovascular health was designed to facilitate the monitoring of CVD and the promotion of substantial disease reduction by 2020. Two years after its introduction, Ford, Greenlund and Hong10 found that the number of ideal cardiovascular health factors and behaviors was a strong predictor of all-cause mortality and diseases of the circulatory system. Intakes of omega-3 fish oils, soy isoflavones, and fermented milk products containing bioactive peptides have been related to lower levels of arterial stiffness.11 Thus positive health behaviors do appear to play a role in lowering arterial stiffness. However, it is unknown whether the combined influence of multiple risk factors and behaviors is associated with arterial stiffness. To our knowledge this hypothesis has not been tested using a constellation of health factors to categorize individuals with respect to gradations of ideal cardiovascular health. We hypothesized that a higher number of cardiovascular health factors and behaviors at ideal levels, as defined by the AHA,9 would be related to lower levels of aortic stiffness as indexed by PWV and PP.
MATERIALS AND METHODS
Study design and participants
Data were obtained from the Maine Syracuse Longitudinal Study (MSLS) data set.12 Beginning in 1974, MSLS participants were recruited from Syracuse, New York and surrounding counties for studies of BP and cognitive performance. There were no exclusions at recruitment other than institutionalization, diagnosed psychiatric disorder and treatment for diagnosed alcoholism. Extensive data on risk factors for CVD were collected for the first time at wave 6 (2001–2006) and PWV data were obtained for the first time at wave 7 (2006–2010). This allowed for a prospective design in which cardiovascular risk factors at wave 6 were used to predict PWV at wave 7. Mean time between waves 6 and 7 was 4.68 years (s.d. = 0.61). Eight-hundred twenty two subjects were invited back to the laboratory for testing at wave 7. Six-hundred nine participants returned and completed PWV data collection. Participants were excluded for the following reasons: missing baseline data for health variables (n = 61), acute stroke at baseline (n = 28), probable dementia at baseline (n = 8), undertaking renal dialysis treatment at baseline (n = 5), inability to read English (n = 1), and prior alcohol abuse (n = 1), leaving a final sample of 505 people. Acute stroke was defined as a focal neurological deficit persisting for >24 hours and probable dementia was defined by cognitive measures, medical records and a multidisciplinary dementia review using the National Institute of Neurological Diseases and Communicative Diseases and Stroke/ Alzheimer's Disease and Related Disorders (NINCDS-ADRDA) criteria.13 The University of Maine Institutional Review Board approved this study and informed consent was obtained from all participants.
Procedure
Except for assessment of PWV at wave 7, procedures were identical at waves 6 and 7. A blood sample was obtained following a fast from midnight. Anthropometric measures and BP were taken after a light breakfast including decaffeinated tea or coffee. Body weight was measured with participants wearing light clothing to the nearest 0.1 kg, and height was measured with a vertical ruler to the nearest 0.1 cm. Brachial artery pressure was assessed at waves 6 and 7 using the traditional pressure-cuff method (Critikon Dinamap Pro Care 100, oscillometeric method). After 10 minutes of supine rest, five measurements were taken in the supine, sitting and standing positions, with a 5-minute rest between each set of measures. After reclining for 10 minutes, 5 additional BP measures in the supine position were taken and the mean of these 5 measurements provided the brachial supine BP values for the SphygmoCor® system protocol.
Information assessed via clinical interview or questionnaire included the Nurses’ Health Study Activity Questionnaire,14 the Nutrition and Health Questionnaire,15, 16 the Center for Epidemiological Studies Depression Scale (CES-D),17 and the self-rating of health.
The Independent Variable: Cardiovascular Health
Cardiovascular Health component measures were assessed as follows. Standard assay methods were employed18 to obtain the health factor measures of total cholesterol and fasting plasma glucose, as well as high density lipoprotein (HDL) and low density lipoprotein (LDL) cholesterols, and triglycerides. SBP and DBP were calculated by averaging the fifteen BP measurements. With regard to the health behaviors, BMI was calculated as weight in kilograms divided by height in meters squared. Physical activity was measured with the Nurses’ Health Study Activity Questionnaire.14 From this validated measure, the time spent engaging in various physical activities was multiplied by the MET value for each activity19 to obtain MET-hours per week, and then and summed to give total MET-hours per week. Smoking status (never, former, current) was based on self-report from the Nutrition and Health Questionnaire, as was dietary intake.15, 16 Participants are required to stipulate how frequently they consume a list of foods, with six response options (never, seldom, 1 time per week, 2–4 times per week, 5–6 times per week, ≥1 time per day). Portion or serving sizes are not stipulated; the totals are an estimate of intake in terms of times per day. For the diet metric of the Cardiovascular Health index, a Recommended Food Score (RFS)20 and a non-Recommended Food Score (non-RFS)21 were calculated. These scores were used in place of the AHA healthy diet metric for two reasons: 1) due to the availability of dietary data available from the questionnaires used, and 2) to capture a more detailed measure of dietary intakes with regard to the 2010 Dietary Guidelines for Americans.22 The RFS included 23 items: four types of fruit (fresh, dried, stewed/tinned, pure fruit juice), eight types of vegetables and legumes (peas, baked beans, lentils and dried beans, tomatoes, carrots, green vegetables, other cooked vegetables, salad/raw vegetables), five types of wholegrain cereal products (brown bread, whole wheat cereal, bran cereal, hot oat cereal, museli), two types of fish (fatty fish and other fish), two low fat milk products (semi-skimmed or skim milk), nuts and seeds, and olive oil. These items are similar to those used previously.20, 23 Consumption of any of the recommended foods at least once per week was assigned 1 point, otherwise 0 points if consumed less often.20 A total RFS out of 23 was calculated, with a high score indicating a higher consumption of recommended food items.
The non-RFS included 15 items: processed meats (combined bacon/meat pies/sausage), four whole-fat dairy products (milk, butter, hard margarine, combined cream/ice-cream), two types of refined grains (white bread, sweet rolls), solid fats/added sugars group (pizza, french fries, combined cakes/puddings/pies, carbonated soft drinks, combined fruit drinks/sports drinks, sweets, added sugar to drinks or cereal), and alcohol (males > 14 standard drinks/week, females > 7 standard drinks/week). A score of 1 was assigned for consumption of non-recommended foods at least 2 to 4 times per week; otherwise 0 points were assigned if consumed less often than this.23, 24 A total non-RFS out of 15 was calculated, with a higher value indicating a higher consumption of non-recommended food items.
The AHA definitions9 were used to determine ideal levels of cardiovascular health for smoking, BMI, physical activity, BP, total cholesterol, and fasting blood glucose. For each component, participants were given a score of 1 if they met the ideal AHA criterion, otherwise 0 points were assigned. The diet metric was split into two components (RFS and non-RFS as described above). The highest 15% of RFS (≥14/23) and the lowest ≤15% of the non-RFS ( 1/15) were given a score of 1. A total Cardiovascular Health Score (CHS) was calculated ranging from 0 (no cardiovascular health components at ideal levels) to 8 (all cardiovascular health components at ideal levels), and then categorized into low (score of 0–2), medium (3–4) or high (5–8) cardiovascular health. The scores were categorized into these three groups to create the most equal groups for comparison.
The Dependent Variable: PWV
Following BP five supine measurement as described above, PWV was assessed non-invasively in the supine position using the SphygmoCor® system (AtCor Medical, Sydney, Australia). Electrocardiogram-gated carotid and femoral waveforms were recorded using applanation tonometry. Carotid-femoral path length was measured as the difference between the surface distances joining: i) the suprasternal notch, the umbilicus and the femoral pulse, and ii) the suprasternal notch and the carotid pulse, measured in meters. Carotid-femoral transit time was estimated in 8–10 sequential femoral and carotid waveforms as the average time difference between the onset of the femoral and carotid waveforms. The foot of the pulse wave was identified using the intersecting tangent method. PWV was calculated as the carotid-femoral path length in meters divided by the carotid-femoral transit time in seconds. This is an established, widely employed, non-invasive and reproducible method to determine arterial stiffness.7 The coefficient of variation (1.79%) for serial measurements of PWV in our laboratory indicates high reproducibility of the PWV measurements.
Covariates
The data collected for covariance analyses and statistical adjustment, or to more thoroughly characterize the sample, are summarized as sample-descriptive data in the results section with units of measurement. Standard assays and diagnostic criteria were used and are fully described in a previous study.25 Self-report variables were as follows: education level (years), alcohol consumption (grams per week), cigarette smoking (number per week), and self-rated health (ranging from 1= very poor, to 5 = excellent). Depressive symptoms were measured using the CES-D.17 Prevalent CVD was defined by the self-reported presence of coronary artery disease, myocardial infarction, congestive heart failure, transient ischemic attack, or angina pectoris, and confirmed by medical records. Obesity was defined as a BMI of 30 kg/m2 or above. Waist circumference was measured using a non-extendable tape at the level of the iliac crest. Diabetes mellitus was defined as treatment with insulin, oral anti hypoglycemic diabetic agents, or by fasting glucose level of ≥126 mg/dl. Hypertension was defined as treatment for hypertension or a BP of ≥140/90.
Statistical Analysis
For the primary analysis, the predictor, the covariates in the basic model and self-rated health were based on wave 6 data (prospectively) and heart rate, height, weight, and mean arterial pressure (MAP) were taken from wave 7 as it is essential that they be taken at the time of PWV and PP assessment. After eliminating CVD variables unrelated to both PWV and the CHS, the following models were employed to adjust for confounding with the CHS in the analysis:
Preliminary analyses indicated a non-significant Age x CHS interaction (P=0.73) and a non-significant Sex x CHS interaction (P=0.32); consequently these interaction terms were not employed in the final covariate models. Three categories of cardiovascular health were formed based on the number of ideal components that were observed: low (CHS 0–2), medium (CHS 3–4), and high (CHS 5–8). Analysis of variance (SPSS) was employed to compare mean values for the groups. Linear trend analyses across values for the three CHS groups were performed when the omnibus test was statistically significant at alpha=0.05. Distributional requirements were met.
In order to examine standardized regression coefficients expressing relations between CHS and PWV mixed categorical and continuous regression analyses were performed using the linear regression feature of SPSS.
RESULTS
Table 1 displays descriptive data for the demographic, risk factor and health variables stratified by CHS group. The majority of variables shown were related to the CHS classifications. Height (P=0.31), alcohol consumption (P=0.90), and prevalence of CVD were clearly unrelated (P=0.87). The constituent variables of the CHS, characterized in Table 1 as cardiovascular health components, were related to PWV in linear regression analyses with each variable controlled for the others. Standardized regression coefficients indicated that SBP (β = 0.610, P< 0.001) and glucose (β=0.186, P<0.001) showed the highest positive relations with PWV in the CHS; the next highest magnitude of positive relations was observed for total cholesterol (β=0.078, P< 0.05), and the lowest for BMI (β= 0.003, P= 0.94). These data are shown in Supplementary Table 1. Given that the CHS requires each of these variables, none were removed from the calculation of the CHS for the primary analyses.
Table 1.
Baseline demographic, health and PWV factors according to Cardiovascular Health Score of the MSLS sample (N=505)
| Variable | Cardiovascular Health Score, 0–8
|
||||||
|---|---|---|---|---|---|---|---|
| Low 0-2 (N=113, 22.4%) | Medium 3–4 (N=269, 53.3%) | High 5–8 (N=123, 24.4%) | P-valuea | ||||
|
| |||||||
| Mean | s.d. | Mean | s.d. | Mean | s.d. | ||
| Age, years | 61.6 | 11.3 | 61.7 | 11.4 | 58.8 | 12.2 | 0.050 |
| Education, years | 14.0 | 2.7 | 14.7 | 2.7 | 15.5 | 2.6 | <0.001 |
| Cardiovascular Health components (at wave 6): | |||||||
| Smoking, # cigarettes smoked/ wk in past year | 29.3 | 64.1 | 5.6 | 26.2 | 1.1 | 12.6 | <0.001 |
| BMI, kg/m2 | 32.4 | 6.8 | 29.3 | 5.2 | 26.2 | 4.3 | <0.001 |
| Physical activity, MET-mins/wk | 582 | 1082 | 1299 | 1605 | 1776 | 1444 | <0.001 |
| Total cholesterol, mg/dl | 221.6 | 40.6 | 203.2 | 37.1 | 186.4 | 37.5 | <0.001 |
| SBP, mm Hg | 139.5 | 17.8 | 130.2 | 21.0 | 116.8 | 18.4 | <0.001 |
| DBP, mm Hg | 72.4 | 8.2 | 70.7 | 10.1 | 65.1 | 8.4 | <0.001 |
| Fasting blood glucose, mg/dl | 112.0 | 33.7 | 96.7 | 25.6 | 86.3 | 7.5 | <0.001 |
| RFS, 0–23b | 9.4 | 2.9 | 11.0 | 2.7 | 11.6 | 2.8 | <0.001 |
| non-RFS, 0-15c | 4.1 | 1.8 | 3.8 | 1.6 | 2.9 | 1.6 | <0.001 |
| PWV related variables (at wave 7): | |||||||
| PWV, m/s | 11.9 | 3.2 | 10.5 | 2.8 | 9.4 | 2.7 | <0.001 |
| Heart rate, bpm | 62.7 | 9.3 | 59.9 | 9.3 | 57.6 | 8.4 | <0.001 |
| Pulse pressure, mmHg | 59.9 | 16.5 | 52.8 | 15.3 | 46.8 | 15.2 | <0.001 |
| Mean arterial pressure, mmHg | 98.5 | 11.9 | 95.1 | 11.8 | 89.6 | 10.9 | <0.001 |
| Height, cm | 166.9 | 11.3 | 167.8 | 10.3 | 166.2 | 9.0 | 0.31 |
| Weight, kg | 90.1 | 22.1 | 82.9 | 17.5 | 73.9 | 14.7 | <0.001 |
| Additional health variables (at wave 6): | |||||||
| HDL-lipoprotein, mg/dl | 51.2 | 13.7 | 55.5 | 16.3 | 57.1 | 15.7 | 0.010 |
| LDL-lipoprotein, mg/dl | 135.3 | 35.9 | 123.0 | 32.0 | 110.2 | 31.2 | <0.001 |
| Triglycerides, mg/dl | 203.4 | 188.6 | 127.0 | 69.5 | 97.0 | 49.6 | <0.001 |
| hs-CRP, mg/l | 0.5 | 0.5 | 0.4 | 0.5 | 0.3 | 0.3 | <0.001 |
| Waist circumference, cm | 102.9 | 15.1 | 94.3 | 13.2 | 85.4 | 12.0 | <0.001 |
| Depression, CES-D scored | 8.2 | 6.6 | 6.7 | 6.2 | 6.5 | 6.1 | 0.07 |
| Self-rated health, 1–5e | 3.4 | 0.7 | 3.7 | 0.8 | 4.0 | 0.8 | <0.001 |
| Alcohol intake, g/week | 27.9 | 54.6 | 38.1 | 66.8 | 28.9 | 38.9 | 0.90 |
| Total energy intake, total serves/ day all food | 15.0 | 4.7 | 15.2 | 4.3 | 14.1 | 4.2 | 0.08 |
|
|
|||||||
| n | % | n | % | n | % | P-value | |
|
|
|||||||
| Gender | |||||||
| Males | 48 | 23.9 | 117 | 58.2 | 36 | 17.9 | 0.023 |
| Females | 65 | 21.4 | 152 | 50.0 | 87 | 28.6 | 0.023 |
| Ethnicity | |||||||
| African American | 17 | 45.9 | 17 | 45.9 | 3 | 8.1 | 0.001 |
| Other | 96 | 20.5 | 252 | 53.8 | 120 | 25.6 | 0.001 |
| CVDf | 12 | 10.6 | 24 | 8.9 | 12 | 9.8 | 0.87 |
| Diabetes mellitusg | 25 | 22.1 | 23 | 8.6 | 3 | 2.4 | <0.001 |
| Hypertensionh | 85 | 75.2 | 159 | 59.1 | 41 | 33.3 | <0.001 |
| Obesity (≥30 kg/m2) | 62 | 56.4 | 103 | 38.7 | 25 | 20.5 | <0.001 |
Abbreviations: BMI, body mass index; CES-D, Centre for Epidemiological Studies Depression Scale; CHS, Cardiovascular Health Score; CVD, cardiovascular disease; DBP, diastolic blood pressure; HDL, high-density lipoprotein; hs-CRP, high sensitivity C-reactive protein; LDL, low-density lipoprotein; MET, metabolic equivalent; MMSE, Mini-Mental State Examination; non-RFS, non-Recommended Food Score; RFS, Recommended Food Score; SBP, systolic blood pressure; s.d., standard deviation.
ANOVA for continuous variables, Chi-square for categorical variables.
RFS: higher scores indicating a higher consumption of recommended foods to increase.
non-RFS: higher scores indicating a higher consumption of foods recommended to reduce.
CES-D: higher score indicates greater number of depressive symptoms.
Self-rated health: higher score indicates better self-rated health.
Cardiovascular disease was defined as present if there was self-reported history of coronary artery disease, myocardial infarction, congestive heart failure, transient ischemic attack, or angina pectoris.
Diabetes mellitus defined as fasting plasma glucose ≥126 mg/dl or treated.
Hypertension defined as ≥140/90 mm Hg or treated.
Table 2 displays PWV (m/s) mean, P, and model R2 values for the three CHS groups and Figure 1 shows mean PWV according to CHS group (fully extended model). The omnibus comparisons among PWV means for the three CHS groups, and the tests of linear trend across the three PWV means, were all statistically significant for all three covariate models (P<0.001). Model R2 values indicate that the least gain in prediction was obtained by adding self-reported health to model 1 and model 2. Quadratic tends were not significant when adjusted for linear trend, but pair-wise comparisons of differences among means indicated that no differences were seen for the medium and high CHS groups. PWV values were significantly lower for the highest CHS group than for the low group (P values <0.05).
Table 2.
Multivariate-adjusted mean and standard error for PWV according to Cardiovascular Health Scorea (N=505) at wave 6, predicting wave 7 cfPWV
| Model | Low, CHS 0-2 (n=113, 22.4%) | Medium, CHS 3-4 (n=269, 53.3%) | High, CHS 5-8 (n=123, 24.4%) | Model R2 | P overall | P linear trend | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean | s.e. | Mean | s.e. | Mean | s.e. | ||||
| Basicb | 11.7 | 0.23 | 10.4*** | 0.15 | 9.8*** | 0.22 | 0.363 | <0.001 | <0.001 |
| Model 2c | 11.3 | 0.22 | 10.4** | 0.14 | 10.2** | 0.22 | 0.455 | 0.001 | 0.001 |
| Model 3d | 11.3 | 0.23 | 10.4** | 0.14 | 10.3* | 0.22 | 0.462 | 0.003 | 0.004 |
Abbreviations: CHS, Cardiovascular Health Score; cfPWV, carotid femoral pulse wave velocity; s.e., standard error.
significantly different from the low group, P<0.05;
significantly different from the low group, P<0.01;
significantly different from the low group, P<0.001.
Based on 8 health components: smoking, BMI, physical activity, total cholesterol, blood pressure, fasting plasma glucose, 2 dietary indices: Recommended Food Score, non-Recommended Food Score.
Basic: adjusted for age, education, gender, ethnicity.
Model 2: adjusted for Basic covariates + height, weight, heart rate, mean arterial pressure (all at wave 7).
Model 3: adjusted for Basic covariates + Model 2 covariates + self-rated health.
Figure 1.
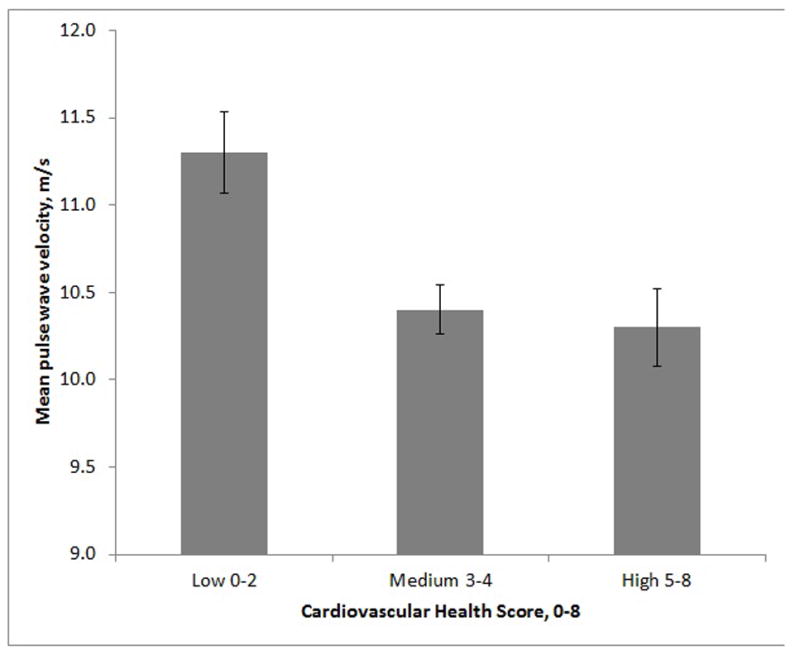
Mean pulse wave velocity according to Cardiovascular Health Score. Error bars are standard error of the mean. Data is for fully adjusted model (controlling for age, education, gender, ethnicity, height, weight, heart rate, mean arterial pressure, self-rated health).
It could be argued that the presence of SBP and DBP in the CHS biased our results toward the positive in relation to PWV. However, we controlled for MAP in model 2, and we obtained the same results when we omitted SBP and DBP values from calculation of the CHS (data not shown).
Linear regression analyses with categorical and continuous variables were done in order to examine the control variables in the model that were most strongly related to PWV when each was adjusted for the other. Standardized regression coefficients indicated that age (β =0.560, P<0.001), MAP (β =0.172, P<0.001), weight (β =0.132, P=0.003) and self-reported health (β =−0.94, P=0.01) exhibited the higher magnitude of relations with PWV; ethnicity (β = 0.035, P=0.34), gender (0.017, P=0.73), education (β =0.008, P=0.82) exhibited the lowest.
Given its traditional use as an index of arterial stiffness, analyses were repeated with PP. These data are shown in Supplementary Table 2; the results were similar to those obtained with PWV. The omnibus test and the linear regression analysis indicated significant differences among the CHS groups as for PWV.
Linear regression analyses using the CHS as a continuous variable yielded the same results for both PWV and PP, with regard to findings of significant linear and no quadratic trends (for all models). The same results were also obtained in sensitivity analyses replacing height and weight with waist circumference and then BMI.
There was no significant change in CHS from wave 6 to 7. Lipids and cigarette smoking were associated with significant but relatively minor changes (decreases) but these changes were unrelated to PWV. Diastolic BP showed an average rise of 7 mm Hg (s.e.=0.37, P<0.001). Each 1 mm Hg rise in diastolic BP (wave 6 to 7) was related to a 0.03 increment in level of PWV at wave 7 (b=0.03, P=0.03), or an estimated 0.21 increment for a 7 mmHg rise.
The CHS was unrelated to CVD prevalence at wave 6, however prevalence was very low (9.5%). The incidence of CVD (with any category resulting in a yes score for CVD) was 4 percent (20 new cases) between wave 6 and 7. All 20 individuals that developed CVD had a CHS of 5 or less (lower score indicating poorer cardiovascular health). New cases of CVD at wave 7 also had a higher mean PWV (12.7 m/s) than those who did not change between waves (PWV of 10.5 m/s) (P=0.001, data not shown).
Attrition
In order to evaluate the effects of attrition on the results, CHS and health variable values for those who were participants at both wave 6 and wave 7 were compared with values for participants who did not return for wave 7 testing. Those who dropped out of the study were older, exhibited more depressive symptoms, higher SBP and DBP, lower HDL-cholesterol and lower self-rated health, and CHS (all P<0.05, data not shown). There was no significant difference in the rate of attrition between the three CHS groups (P=0.08, data not shown).
DISCUSSION
In this prospective study, PWV decreased significantly in a linear fashion across increasing CHS categories with full adjustment for demographic and PWV-related variables, and self-rated health. Those with a higher number of ideal cardiovascular health metrics (5 or more) had significantly lower PWV than those with a low number of metrics at ideal levels (2 or less). The findings were the same for PP. These findings are consistent with our hypothesis that increasingly higher CHS (decreasing cardiovascular risk) would be associated with lower PWV. Those with a medium CHS also had significantly lower PWV than the low CHS group.
These findings were the same for all covariate models. We adjusted for all CVD risk factors in the study that were related both to PWV and CHS. Self-rated health was an important control that attenuated but did not eliminate statistically significant results obtained with the more objectively scored CHS. Self-rated health has been shown to add value to risk prediction models for cardiovascular outcomes comprising traditional risk factors,26 and for mortality risk.27 In the present study, CHS was a stronger predictor of PWV than self-rated health.
To our knowledge the present study is the first to relate cardiovascular health as defined by the AHA9 to PWV. The Coronary Artery Risk Development in Young Adults study (CARDIA) is the only published longitudinal study that we are aware of in which the association between ideal cardiovascular health and cognitive function was investigated.28 Persons with fewer ideal health metrics exhibited lower levels of psychomotor speed, executive function and verbal memory in midlife. As higher PWV is related to lower cognitive performance29, 30 and dementia,31 PWV could be an important mediator between cardiovascular health status and cognitive performance, a hypothesis that can be investigated in future studies using path analysis or structural equation modelling methods.
Comparisons of our data with other studies relating risk factors, good or bad, are important in terms of the population or clinical importance of the magnitude of the effects observed. With adjustment for the most extensive set of covariates, the low group in the present study had a PWV value of 11.3 m/s and the middle group a value of 10.3 m/s. In published reference group data for PWV for the MSLS sample1 this amount of difference (1 m/s) was seen between 50 to 59 year old and 60 to 69 year old individuals with essential hypertension, i.e. 9.3 m/s (s.d.=1.5) and 10.3 m/s (s.d.=2.3). In a recent study with data from the MSLS,8 adjusting for demographics and cardiovascular risk factors, people who seldom or never consumed dairy exhibited a mean 1.0 m/s lower PWV value relative to those that consumed dairy at least 5 to 6 times per week. Although a 1 m/s difference in PWV might be considered as modest with respect to arterial stiffness in an individual, this magnitude of difference is most certainly important from a population risk perspective. In a recent meta-analysis it was estimated that for every 1 m/s increase in PWV there were age-, sex- and cardiovascular risk factor adjusted increases of 14%, 15% and 15% in total cerebrovascular events, CVD mortality and all-cause mortality in general.6
The presence of SBP and DBP in the CHS could bias results in the positive direction for PWV in relation to CHS. However, we controlled for MAP in our regression models and, more importantly, we dealt with this issue by deleting the BP variables from the CHS and our findings with the full CHS were replicated. While it is true that cardiovascular risk factors, individually and in combination, e.g. diabetes and BP1, 2, 32 have been related to higher PWV, we feel the CHS is potentially valuable in monitoring patients because it relates a constellation of risk factors and health behaviors to PWV and thus does offer the possibility, based on patient history, to evaluate the likelihood of higher levels of arterial stiffness. Obviously studies, including trials, in which decreasing risk and improving health behaviors are related to PWV and PP are an important next step in research should other studies using the AHA ideal cardiovascular health construct replicate our findings.
Some limitations of the current study must be acknowledged. The study was prospective, the cardiovascular health data were gathered, on average 4.8 years prior to PWV, however the study was not longitudinal as we only had one measurement of PWV. Studies with longitudinal follow-up of PWV are important.
There may also have been an underestimation of the association between cardiovascular health and PWV because the participants who did not return for wave 7 PWV assessment were older, exhibited more depressive symptoms, higher SBP and DBP, lower HDL-cholesterol and lower self-rated health. As with most studies, smoking, diet and physical activity data were based on participant self-report.
Strengths of the study include a community-based sample, extensive data on cardiovascular risk factors enabling the control of potential confounding factors, the adjustment of findings for self-rated health, the use of two dietary metrics to measure adherence to the current national dietary recommendations, the inclusion of adults with a low prevalence of CVD spanning a wide age, and use of PWV as the primary indirect gold- standard measure of arterial stiffness. The constuct of cardiovascular health as defined by the AHA has not been examined in relation to arterial stiffness, or PWV before. The calculation of the CHS, with an expanded diet metric compared to that defined by the AHA, as an indicator of cardiovascular health is new. Therefore the findings of this study are novel in that they relate an indicator of cardiovascular health, ie. not cardiovascular disease, including lifestyle behaviors such as smoking, physical activity and diet, to arterial stiffness as indexed by PWV.
In conclusion, having more cardiovascular health metrics at ideal levels (indicated by a high CHS) is related to lower levels of PWV and, by inference from PWV, lower stiffness of the coronary arteries.33 However, PWV did not differ significantly between those with a high CHS (5–8 ideal health metrics) and those with a medium score (3–4 ideal health metrics). Thus improvements in PWV values reach an asymptotic level short of ideal on all health behaviors and factors. The literature identifies several promising avenues involving diet, the facilitation of physical activity and weight loss, which may impact directly upon arterial stiffness.8, 34, 35 We hope our findings with the CHS will be a stimulus to longitudinal studies using this construct and eventually controlled clinical trials relating effective management of CVD risk factors, lifestyle variables, and nutrition to arterial stiffness.
Supplementary Material
Summary Table.
What is known about this topic
|
What this study adds
|
Acknowledgments
The Maine Syracuse Longitudinal Study was supported by grants R01HL067358, and R01HL081290 from the National Heart, Lung and Blood Institute, National Institutes of Health (USA), and research grant R01AG03055 from the National Institute on Aging, National Institutes of Health (USA). GEC is supported by a National Health and Medical Research Council (NHMRC) Sidney Sax Research Fellowship (GNT1054567) (Australia).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary information is available at the Journal of Human Hypertension website (http://www.nature.com/jhh/index.html).
References
- 1.Elias MF, Dore GA, Davey A, Abhayaratna WP, Goodell AL, Robbins MA. Norms and reference values for pulse wave velocity: one size does not fit all. J Bioscience and Medicine. 2011;(4) doi: 10.5780/jbm2011.4. [DOI] [Google Scholar]
- 2.Khoshdel AR, Thakkinstian A, Carney SL, Attia J. Estimation of an age-specific reference interval for pulse wave velocity: a meta-analysis. J Hypertens. 2006;24(7):1231–1237. doi: 10.1097/01.hjh.0000234098.85497.31. [DOI] [PubMed] [Google Scholar]
- 3.Benetos A, Waeber B, Izzo J, Mitchell G, Resnick L, Asmar R, et al. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens. 2002;15(12):1101–1108. doi: 10.1016/s0895-7061(02)03029-7. [DOI] [PubMed] [Google Scholar]
- 4.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39(1):10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 5.McEniery CM, Spratt M, Munnery M, Yarnell J, Lowe GD, Rumley A, et al. An analysis of prospective risk factors for aortic stiffness in men: 20-year follow-up from the Caerphilly prospective study. Hypertension. 2010;56(1):36–43. doi: 10.1161/HYPERTENSIONAHA.110.150896. [DOI] [PubMed] [Google Scholar]
- 6.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 7.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 8.Crichton GE, Elias MF, Dore GA, Abhayaratna WP, Robbins MA. Relations between dairy food intake and arterial stiffness: pulse wave velocity and pulse pressure. Hypertension. 2012;59:1044–1051. doi: 10.1161/HYPERTENSIONAHA.111.190017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 10.Ford ES, Greenlund KJ, Hong YL. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125(8):987–995. doi: 10.1161/CIRCULATIONAHA.111.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pase MP, Grima NA, Sarris J. The effects of dietary and nutrient interventions on arterial stiffness: a systematic review. Am J Clin Nutr. 2011;93(2):446–454. doi: 10.3945/ajcn.110.002725. [DOI] [PubMed] [Google Scholar]
- 12.Elias PK, Elias MF, Robbins MA, Budge MM. Blood pressure-related cognitive decline: does age make a difference? Hypertension. 2004;44(5):631–636. doi: 10.1161/01.HYP.0000145858.07252.99. [DOI] [PubMed] [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. Reproducibility and validity of a self-administered physical-activity quesionnaire. Int J Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 15.Kaaks R, Riboli E. Validation and calibration of dietary intake measurements in the EPIC project: methodological considerations. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26(suppl 1):S15–25. doi: 10.1093/ije/26.suppl_1.s15. [DOI] [PubMed] [Google Scholar]
- 16.Kroke A, Klipstein-Grobusch K, Voss S, Moseneder J, Thielecke F, Noack R, et al. Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: comparison of energy, protein, and macronutuient intakes estimated with the doubly labeled water, urinary nitrogen, and repeated 24-h dietary recall methods. Am J Clin Nutr. 1999;70(4):439–447. doi: 10.1093/ajcn/70.4.439. [DOI] [PubMed] [Google Scholar]
- 17.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 18.Elias MF, Robbins MA, Budge MM, Elias PK, Brennan SL, Johnston C, et al. Homocysteine, folate, and vitamins B6 and B12 blood levels in relation to cognitive performance: the Maine-Syracuse study. Psychosom Med. 2006;68(4):547–554. doi: 10.1097/01.psy.0000221380.92521.51. [DOI] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Kant AK, Schatzkin A, Graubard BI, Schairer C. A prospective study of diet quality and mortality in women. JAMA. 2000;283(16):2109–2115. doi: 10.1001/jama.283.16.2109. [DOI] [PubMed] [Google Scholar]
- 21.Michels KB, Wolk A. A prospective study of variety of healthy foods and mortality in women. Int J Epidemiol. 2002;31(4):847–854. doi: 10.1093/ije/31.4.847. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Department of Agriculture and and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7. Washington, D.C: U.S. Government Printing Office; Dec, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaluza J, Hakansson N, Brzozowska A, Wolk A. Diet quality and mortality: a population-based prospective study of men. Eur J Clin Nutr. 2009;63(4):451–457. doi: 10.1038/sj.ejcn.1602968. [DOI] [PubMed] [Google Scholar]
- 24.Wengreen HJ, Neilson C, Munger R, Corcoran C. Diet quality is associated with better cognitive test performance among aging men and women. J Nutr. 2009;139(10):1944–1949. doi: 10.3945/jn.109.106427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robbins MA, Elias MF, Elias PK, Budge MM. Blood pressure and cognitive function in an African–American and a Caucasian-American sample: the Maine-Syracuse Study. Psychosom Med. 2005;67(5):707–714. doi: 10.1097/01.psy.0000171164.50990.80. [DOI] [PubMed] [Google Scholar]
- 26.May M, Lawlor DA, Brindle P, Patel R, Ebrahim S. Cardiovascular disease risk assessment in older women: can we improve on Framingham? British Women's Heart and Health prospective cohort study. Heart. 2006;92(10):1396–1401. doi: 10.1136/hrt.2005.085381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wennberg P, Rolandsson O, Jerden L, Boeing H, Sluik D, Kaaks R, et al. Self-rated health and mortality in individuals with diabetes mellitus: prospective cohort study. BMJ Open. 2012;2(1) doi: 10.1136/bmjopen-2011-000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reis JP, Loria CM, Launer LJ, Sidney S, Liu K, Jacobs DR, Jr, et al. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol. 2013;73(2):170–179. doi: 10.1002/ana.23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singer J, Trollor JN, Crawford J, O'Rourke MF, Baune BT, Brodaty H, et al. The association between pulse wave velocity and cognitive function: the Sydney Memory and Ageing Study. PLoS One. 2013;8(4):e61855. doi: 10.1371/journal.pone.0061855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51(1):99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 31.Nagai K, Akishita M, Machida A, Sonohara K, Ohni M, Toba K. Correlation between pulse wave velocity and cognitive function in nonvascular dementia. J Am Geriatr Soc. 2004;52(6):1037–1038. doi: 10.1111/j.1532-5415.2004.52277_15.x. [DOI] [PubMed] [Google Scholar]
- 32.Reference Values for Arterial Stiffness Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'establishing normal and reference values'. Eur Heart J. 2010;31(19):2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Popele NM, Mattace-Raso FU, Vliegenthart R, Grobbee DE, Asmar R, van der Kuip DA, et al. Aortic stiffness is associated with atherosclerosis of the coronary arteries in older adults: the Rotterdam Study. J Hypertens. 2006;24(12):2371–2376. doi: 10.1097/01.hjh.0000251896.62873.c4. [DOI] [PubMed] [Google Scholar]
- 34.Dengo AL, Dennis EA, Orr JS, Marinik EL, Ehrlich E, Davy BM, et al. Arterial Destiffening With Weight Loss in Overweight and Obese Middle-Aged and Older Adults. Hypertension. 2010;55(4):855–861. doi: 10.1161/HYPERTENSIONAHA.109.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards NM, Daniels SR, Claytor RP, Khoury PR, Dolan LM, Kimball TR, et al. Physical activity is independently associated with multiple measures of arterial stiffness in adolescents and young adults. Metabolism-Clinical and Experimental. 2012;61(6):869–872. doi: 10.1016/j.metabol.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


