Abstract
Dual time point FDG PET imaging (DTPI) has been considered helpful for discrimination of benign and malignant disease, and staging lymph node status in patients with pulmonary malignancy. However, DTPI for benign disease has been rarely reported, and it may show a better description of metabolic status and extent of benign infectious disease than early imaging only. The authors report on the use F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) imaging with additional delayed imaging on a 52-year-old man with sparganosis and a 70-year-old man with tuberculous meningitis. To the best of our knowledge, this is the first report on dual time point PET/CT imaging in patients with cerebral sparganosis and tuberculous meningitis.
Keywords: Dual time point imaging, F-18 FDG PET/CT, Cerebral sparganosis, Tuberculous meningitis
Introduction
Lately, positron emission tomography (PET) using F-18 fluorodeoxyglucose (FDG), a glucose analogue, has been demonstrated to be useful in differentiating benign from malignant processes. In general, malignant tumors show higher FDG uptake than benign lesions due to over-expression of glucose membrane-transporters and glycolysis-pathway-control hexokinase in cancer cells [1]. However, false-positive and false-negative results of PET examination may still occur [2–4]. A tumor focus smaller than 4–5 mm in diameter may escape detection by current PET scanners. Benign processes such as infection, inflammation, and granulomatous diseases may cause enhanced FDG uptake. This is mainly due to increased glucose utilization by activated macrophages and inflammatory cells [5].
It has been proposed that dual time point FDG PET imaging (DTPI) may be helpful in remedying these difficulties for the evaluation of pulmonary nodules [6]. Data from an additional delayed PET scan will be acquired after a certain interval from the time of the initial imaging. If one adopts the criterion that more than a 10% increase of standardized uptake value (SUV) between the initial and delayed scan signals malignancy, significant improvement in the diagnostic accuracy for the detection of malignant pulmonary nodules has been observed [7]. DTPI has also been applied to stage lymph node status in patients with pulmonary malignancy, and DTPI showed better specificity, positive predictive value and accuracy [8].
However, DTPI in benign disease has been rarely reported, [9] and it may show a better description of metabolic status and extent of benign infectious disease than early (at 1 h after administration of FDG) imaging. To the best of our knowledge, this is the first report on using DTPI in patients with cerebral sparganosis and tuberculous meningitis (TBM).
Case Reports
Case 1
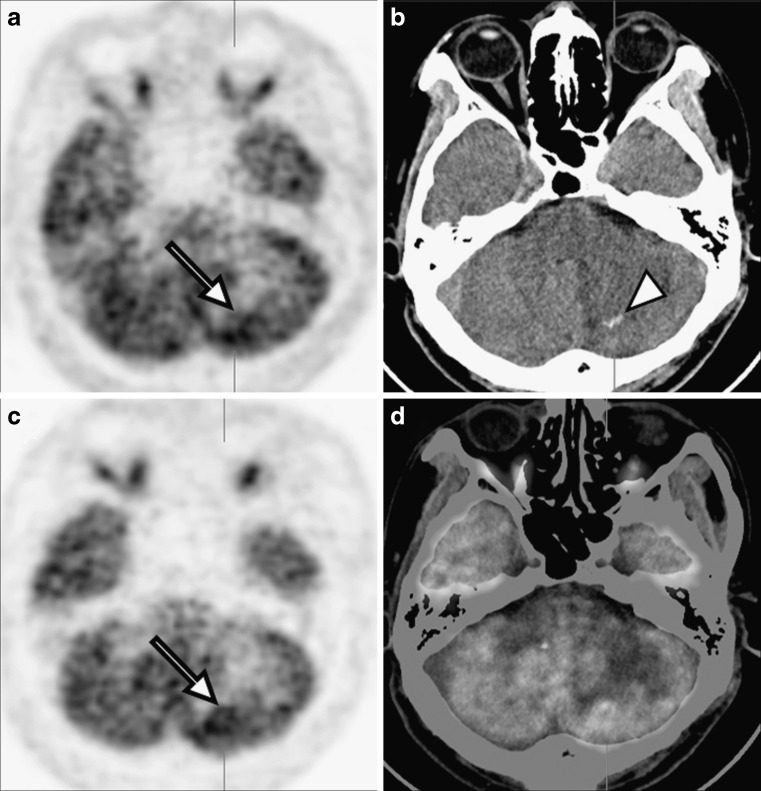
A 52-year-old man presented with headache and vomiting, and he had a history of left frontal lobe partial resection due to gemistocytic astrocytoma 6 months previously. Gadolinium-enhanced brain magnetic resonance imaging (MRI) scan showed multiple nodular lesions with ring enhancement in left cerebellar hemisphere and prominent peripheral edema without significant mass effect (Fig. 1). These lesions were considered as new or metastatic brain tumors, and no tumor recurrence was detected in the left frontal lobe, the previous operation site. Subsequently, brain FDG PET/CT demonstrated low metabolic activity in the left cerebellar hemisphere [maximal SUV of early imaging (SUV1): 6.3] and a 1-cm sized, serpiginously shaped calcification (HU: 70–90) in the computed tomography (CT) component of PET/CT (Fig. 2). Delayed FDG PET/CT imaging at 2 h after administration of FDG showed an increase in metabolic activity [maximal SUV of delayed imaging (SUV2): 8.7] of the lesion and allowed a slightly clearer discrimination of disease. The metabolic activity of normal gray matter of the temporal lobe in delayed imaging [mean SUV: 8.2; maximal SUV: 10.3; volume of interest: 0.23 cm3] was higher than the metabolism in early imaging [mean SUV: 6.03; maximal SUV: 7.43; volume of interest: 0.22 cm3]. Excision biopsy of this cerebellar lesion revealed parasite infestation of sparaganum, and anti-sparganum IgG antibody was positive on cerebrospinal fluid (CSF) examination. After treatment with surgical resection and praziquantel, the patient became symptom-free and follow-up brain CT revealed no enhancing lesion in left cerebellar hemisphere.
Fig. 1.
a T1-weighted, b T2-weighted and c, d gadolinium-enhanced brain MRI scans showed multiple nodular lesions with ring enhancement (arrows) in left cerebellar hemisphere and prominent peripheral edema (arrowhead)
Fig. 2.
a Early PET, b early CT, c delayed PET and d delayed fusion images of brain FDG PET/CT demonstrated focus of hypermetabolic lesion, showing increment of metabolic activity in delayed imaging at 2 h after administration of FDG (arrows). The 1-cm sized, serpiginously shaped calcification (HU: 70–90) was also observed in the CT component of PET/CT (arrowhead)
Case 2
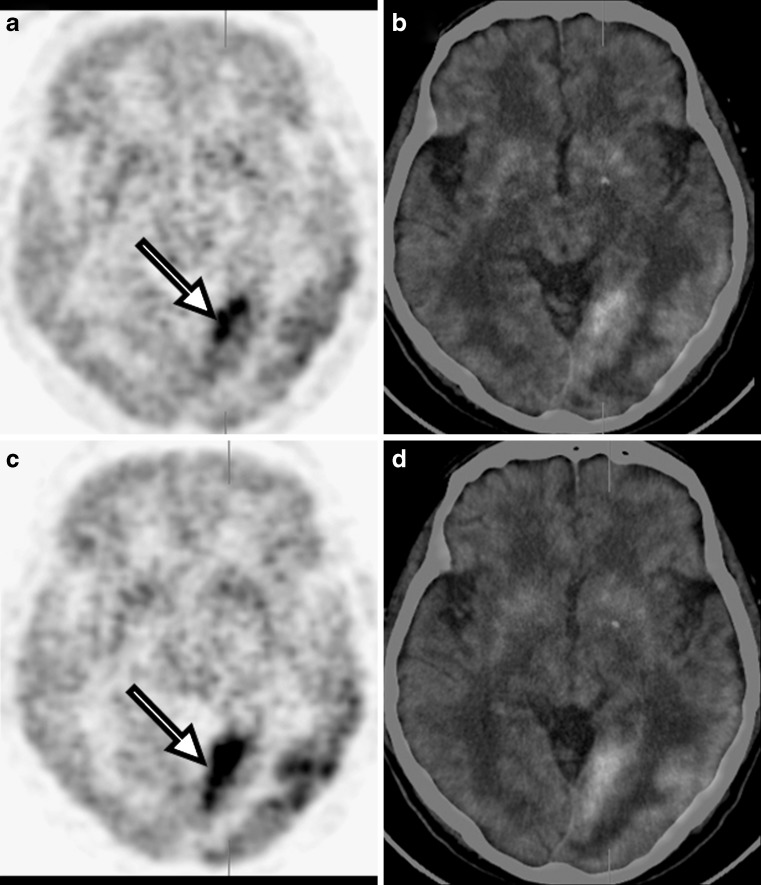
A 70-year-old man presented with confused mental status. Gadolinium-enhanced brain MRI scan showed multiple nodular enhancing lesions in the subpial area or meningeal surface with localized meningeal enhancement in left temporal and occipital lobes (Fig. 3). Tuberculous meningitis (TBM) or parasite diseases were suspected. On CSF examination, the CSF contained a normal white blood cell (WBC) count, normal protein, normal adenosine deaminase (ADA), and elevated glucose (92.9 mg/dl), not consistent with tuberculosis. CSF was negative for malignant cells, polymerse chain reaction (PCR) for tuberculosis, acid-fast bacilli (AFB) stain and AFB culture. Subsequently, brain FDG PET/CT demonstrated geographic hypermetabolism along gray matter of left temporal and occipital lobes (SUV1: 7.1) and delayed FDG PET/CT imaging at 2 h after administration of FDG demonstrated this lesion more clearly (SUV2: 10.2; Fig. 4). The metabolic activity of normal gray matter of the temporal lobe in delayed imaging (mean SUV: 2.99; maximal SUV: 4.23; volume of interest: 0.35 cm3) was higher than the metabolism in early imaging (mean SUV: 2.36; maximal SUV: 2.97; volume of interest: 0.37 cm3). Excision biopsy of left occipital meninges, metabolically the most active area, revealed chronic granulomatous inflammation with caseous necrosis, consistent with tuberculosis. Follow-up brain MRI after treatment with anti-tuberculosis medication, revealed no enhancing area of the left temporal and occipital lobes, but postoperative localized cystic encephalomalacia in left occipital lobe.
Fig. 3.
a T1-weighted, b T2-weighted and c, d gadolinium-enhanced brain MRI scans showed vasogenic edema (arrowhead) and multiple nodular enhancing lesions in subpial area or meningeal surface with localized meningeal enhancement (arrows) in left temporal and occipital lobes
Fig. 4.
a Early PET, b early fusion, c delayed PET and d delayed fusion images of brain FDG PET/CT demonstrated geographic hypermetabolism along gray matter of the left temporal and occipital lobes, showing an increment of metabolic activity in delayed imaging at 2 h after administration of FDG (arrows)
Discussion
Cerebral sparganosis, caused by infestation by the larval form of the pseudophyllidean cestode (a form of tapeworm), is common in South-east Asia, Korea, China and Japan, where uncooked or partially cooked freshwater fish, frog legs or snakes are eaten [10]. In our patient with sparganosis, the lesion was not clearly demonstrated on early imaging due to low metabolic activity. In delayed imaging, however, the lesion became more distinct showing elevated metabolism. Serpiginous shaped calcification was also detected on the CT component of PET/CT. Several studies have reported that punctuate calcification is one of the characteristic findings in cerebral sparganosis on CT scan [11, 12]. As seen in our patient, this punctuate calcification may be observed in the CT component of PET/CT and diagnostic accuracy of sparganosis with FDG PET/CT may be improved when regarding this finding.
Tuberculosis is still a serious international health problem, with about 8 million new cases and 3 million deaths per year [13]. Extra-pulmonary tuberculosis constitutes 15% of all tuberculous locations and includes TBM, which occurs in 4% of all cases [14]. The disease occurs by spreading secondarily to the CNS years after the initial pulmonary infection, with reactivation of the bacillus by the hematogenous route. CNS spread results in two interrelated pathological processes, in the form of TBM or intracranial tuberculomas. The most commonly seen form is meningitis, followed by tuberculomas [15]. The imaging manifestations of CNS tuberculosis are very pleomorphic and can mimic parenchymal cerebral mass lesions or meningeal processes of a different nature [16]. Gadolinium-enhanced, T1-weighted imaging is the standard MRI technique used for evaluating meningeal disease and is routinely used in most centers [17]. The role of FDG PET or PET/CT in TBM was not fully elucidated [18]. In our patient with TBM, FDG PET could show elevated metabolic activity and delayed imaging was useful in showing more distinctly the extent of disease.
Spence et al. [19] had reported that the SUV of normal gray matter showed an increase over a 90-min time point after administration of FDG, and then decreased at later times. In contrast with normal gray matter, the SUV of tumor showed a continuous increase over the course of the study, and tumor-to-background ratio also increased over the time. In our patient with TBM, the incease in SUV of the lesion was greater than that of normal gray matter (44% and 24%, respectively), allowing clear discrimination of disease. However, in the patient with cerebral sparganosis, the increase in SUV of the lesion was lower than that of normal gray matter (38% and 46%, respectively) and discrimination of the lesion was difficult, even in delayed imaging. This difference in SUV increase could reflect the stage of inflammation, such as acute or chronic, but further study is needed.
DTPI has been considered helpful for the discrimination of benign and malignant disease, and the staging of lymph node status in patients with pulmonary malignancy [6–8]. As regards our cases, DTPI may be also useful for showing the description of metabolic status and the extent of benign infectious disease. Further study evaluating the role of DTPI in benign infectious disease is required.
Acknowledgements
This paper was supported by Wonkwang University in 2010.
References
- 1.Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan CN, Wolf AP. Metabolic trapping as a principle of radiopharmaceutical design: some factors responsible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J Nucl Med. 1978;19:1154–1161. [PubMed] [Google Scholar]
- 2.Gupta NC, Tamim WJ, Graeber GG, Bishop HA, Hobbs GR. Mediastinal lymph node sampling following positron emission tomography with fluorodeoxyglucose imaging in lung cancer staging. Chest. 2001;120:521–527. doi: 10.1378/chest.120.2.521. [DOI] [PubMed] [Google Scholar]
- 3.Konishi J, Yamazaki K, Tsukamoto E, Tamaki N, Onodera Y, Otake T, et al. Mediastinal lymph node staging by FDG-PET in patients with non-small cell lung cancer: analysis of false-positive FDG-PET findings. Respiration. 2003;70:500–506. doi: 10.1159/000074207. [DOI] [PubMed] [Google Scholar]
- 4.Takamochi K, Yoshida J, Murakami K, Niho S, Ishii G, Nishimura M, et al. Pitfalls in lymph node staging with positron emission tomography in non-small cell lung cancer patients. Lung Cancer. 2005;47:235–242. doi: 10.1016/j.lungcan.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Kubota R, Kubota K, Yamada S, Tada M, Ido T, Tamahashi N. Microautoradiographic study for the differentiation of intratumoral macrophages, granulation tissues and cancer cells by the dynamics of fluorine-18-fluorodeoxyglucose uptake. J Nucl Med. 1994;35:104–112. [PubMed] [Google Scholar]
- 6.Zhuang H, Pourdehnad M, Lambright ES, Yamamoto AJ, Lanuti M, Li P, et al. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med. 2001;42:1412–1417. [PubMed] [Google Scholar]
- 7.Matthies A, Hickeson M, Cuchiara A, Alavi A. Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. J Nucl Med. 2002;43:871–875. [PubMed] [Google Scholar]
- 8.Nishiyama Y, Yamamoto Y, Kimura N, Ishikawa S, Sasakawa Y, Ohkawa M. Dual-time-point FDG-PET for evaluation of lymph node metastasis in patients with non-small-cell lung cancer. Ann Nucl Med. 2008;22:245–250. doi: 10.1007/s12149-007-0103-2. [DOI] [PubMed] [Google Scholar]
- 9.Burdick MJ, Jolles PR, Grimes MM, Henry DA. Mediastinal hibernoma simulates a malignant lesion on dual time point FDG imaging. Lung Cancer. 2008;59:391–394. doi: 10.1016/j.lungcan.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Kim DG, Paek SH, Chang KH, Wang KC, Jung HW, Kim HJ, et al. Cerebral Sparganosis: clinical manifestations, treatment and outcome. J Neurosurg. 1996;85:1066–1071. doi: 10.3171/jns.1996.85.6.1066. [DOI] [PubMed] [Google Scholar]
- 11.Moon WK, Chang KH, Cho SY, Han MH, Cha SH, Chi JG, et al. Cerebral sparganosis: MR imaging versus CT features. Radiology. 1993;188:751–757. doi: 10.1148/radiology.188.3.8351344. [DOI] [PubMed] [Google Scholar]
- 12.Song T, Wang WS, Zhou BR, Mai WW, Li ZZ, Guo HC, et al. CT and MR Characteristics of Cerebral Sparganosis. AJNR Am J Neuroradiol. 2007;28:1700–1705. doi: 10.3174/ajnr.A0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vera-Cabrera L, Rendon A, Diaz-Rodriguez M, Handzel V, Laszlo A. Dot Blot for detection of antidiacyltrehalose antibodies in tuberculous patients. Clin Diagn Lab Immunol. 1999;6:686–689. doi: 10.1128/cdli.6.5.686-689.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacut JY, Dupon M, Paty MC. Tuberculoses extra-pulmonaires: revue et possibilités de diminution des délais d’intervention thérapeutique. Med Mal Infect. 1995;25:304–320. doi: 10.1016/S0399-077X(05)80589-X. [DOI] [Google Scholar]
- 15.Bayindir C, Mete O, Bilgic B. Retrospective study of 23 pathologically proven cases of central nervous system tuberculomas. Clin Neurol Neurosurg. 2006;108:353–357. doi: 10.1016/j.clineuro.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Morgado C, Ruivo N. Imaging meningo-encephalic tuberculosis. Eur J Radiol. 2005;55:188–192. doi: 10.1016/j.ejrad.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Oztoprak I, Gümüs C, Oztoprak B, Engin A. Contrast medium-enhanced MRI findings and changes over time in stage I tuberculous meningitis. Clin Radiol. 2007;62:1206–1215. doi: 10.1016/j.crad.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Walot I, Miller BL, Chang L, Mehringer CM. Neuroimaging findings in patients with AIDS. Clin Infect Dis. 1996;22:906–919. doi: 10.1093/clinids/22.6.906. [DOI] [PubMed] [Google Scholar]
- 19.Spence AM, Muzi M, Mankoff DA, O’Sullivan SF, Link JM, Lewellen TK, et al. 18F-FDG PET of gliomas at delayed intervals: improved distinction between tumor and normal gray matter. J Nucl Med. 2004;45:1653–1659. [PubMed] [Google Scholar]