Abstract
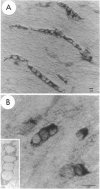
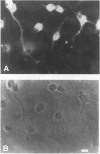
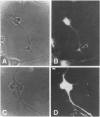
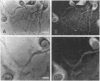
We present evidence that the microtubule-associated protein tau is present in oligodendrocytes (OLGs), the central nervous system cells that make myelin. By showing that tau is distributed in a pattern similar to that of myelin basic protein, our results suggest a possible involvement of tau in some aspect of myelination. Tau protein has been identified in OLGs in situ and in vitro. In interfascicular OLGs, tau localization, revealed by monoclonal antibody Tau-5, was confined to the cell somata. However, in cultured ovine OLGs with an exuberant network of processes, tau was detected in cell somata, cellular processes, and membrane expansions at the tips of these processes. Moreover, in such cultures, tau appeared localized adjacent to or coincident with myelin basic protein in membrane expansions along and at the ends of the cellular processes. The presence of tau mRNA was documented using fluorescence in situ hybridization. The distribution of the tau mRNA was similar to that of the tau protein. Western blot analysis of cultured OLGs showed the presence of many tau isoforms. Together, these results demonstrate that tau is a genuine oligodendrocyte protein and pave the way for determining its functional role in these cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbarese E. Spatial distribution of myelin basic protein mRNA and polypeptide in quaking oligodendrocytes in culture. J Neurosci Res. 1991 Jul;29(3):271–281. doi: 10.1002/jnr.490290302. [DOI] [PubMed] [Google Scholar]
- Behar L., Marx R., Sadot E., Barg J., Ginzburg I. cis-acting signals and trans-acting proteins are involved in tau mRNA targeting into neurites of differentiating neuronal cells. Int J Dev Neurosci. 1995 Apr;13(2):113–127. doi: 10.1016/0736-5748(95)00001-w. [DOI] [PubMed] [Google Scholar]
- Benjamins J. A., Iwata R., Hazlett J. Kinetics of entry of proteins into the myelin membrane. J Neurochem. 1978 Oct;31(4):1077–1085. doi: 10.1111/j.1471-4159.1978.tb00150.x. [DOI] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion J. P., Guilleminot J., Couchie D., Flament-Durand J., Nunez J. Both adult and juvenile tau microtubule-associated proteins are axon specific in the developing and adult rat cerebellum. Neuroscience. 1988 Apr;25(1):139–146. doi: 10.1016/0306-4522(88)90013-9. [DOI] [PubMed] [Google Scholar]
- Brophy P. J., Boccaccio G. L., Colman D. R. The distribution of myelin basic protein mRNAs within myelinating oligodendrocytes. Trends Neurosci. 1993 Dec;16(12):515–521. doi: 10.1016/0166-2236(93)90196-s. [DOI] [PubMed] [Google Scholar]
- Colman D. R., Kreibich G., Frey A. B., Sabatini D. D. Synthesis and incorporation of myelin polypeptides into CNS myelin. J Cell Biol. 1982 Nov;95(2 Pt 1):598–608. doi: 10.1083/jcb.95.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiTella M., Feiguin F., Morfini G., Cáceres A. Microfilament-associated growth cone component depends upon Tau for its intracellular localization. Cell Motil Cytoskeleton. 1994;29(2):117–130. doi: 10.1002/cm.970290204. [DOI] [PubMed] [Google Scholar]
- Dobrowolski Z., Osińska H., Mossakowska M., Baryłko B. Ca2+-calmodulin-dependent polymerization of actin by myelin basic protein. Eur J Cell Biol. 1986 Oct;42(1):17–26. [PubMed] [Google Scholar]
- Drubin D. G., Kirschner M. W. Tau protein function in living cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer C. A., Benjamins J. A. Organization of oligodendroglial membrane sheets. I: Association of myelin basic protein and 2',3'-cyclic nucleotide 3'-phosphohydrolase with cytoskeleton. J Neurosci Res. 1989 Oct;24(2):201–211. doi: 10.1002/jnr.490240211. [DOI] [PubMed] [Google Scholar]
- Gillespie C. S., Wilson R., Davidson A., Brophy P. J. Characterization of a cytoskeletal matrix associated with myelin from rat brain. Biochem J. 1989 Jun 15;260(3):689–696. doi: 10.1042/bj2600689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Crowther R. A., Garner C. C. Molecular characterization of microtubule-associated proteins tau and MAP2. Trends Neurosci. 1991 May;14(5):193–199. doi: 10.1016/0166-2236(91)90105-4. [DOI] [PubMed] [Google Scholar]
- Gozes I., Richter-Landsberg C. Identification of tubulin associated with rat brain myelin. FEBS Lett. 1978 Nov 1;95(1):169–172. doi: 10.1016/0014-5793(78)80076-3. [DOI] [PubMed] [Google Scholar]
- Himmler A., Drechsel D., Kirschner M. W., Martin D. W., Jr Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains. Mol Cell Biol. 1989 Apr;9(4):1381–1388. doi: 10.1128/mcb.9.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmler A. Structure of the bovine tau gene: alternatively spliced transcripts generate a protein family. Mol Cell Biol. 1989 Apr;9(4):1389–1396. doi: 10.1128/mcb.9.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B., Behar T., Dubois-Dalcq M. Cell shape and motility of oligodendrocytes cultured without neurons. Cell Tissue Res. 1986;244(1):27–38. doi: 10.1007/BF00218378. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Kowall N. W., Kosik K. S. Axonal disruption and aberrant localization of tau protein characterize the neuropil pathology of Alzheimer's disease. Ann Neurol. 1987 Nov;22(5):639–643. doi: 10.1002/ana.410220514. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee G., Cowan N., Kirschner M. The primary structure and heterogeneity of tau protein from mouse brain. Science. 1988 Jan 15;239(4837):285–288. doi: 10.1126/science.3122323. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991 Feb 8;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Lichtenberg B., Mandelkow E. M., Hagestedt T., Mandelkow E. Structure and elasticity of microtubule-associated protein tau. Nature. 1988 Jul 28;334(6180):359–362. doi: 10.1038/334359a0. [DOI] [PubMed] [Google Scholar]
- Loomis P. A., Howard T. H., Castleberry R. P., Binder L. I. Identification of nuclear tau isoforms in human neuroblastoma cells. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8422–8426. doi: 10.1073/pnas.87.21.8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migheli A., Butler M., Brown K., Shelanski M. L. Light and electron microscope localization of the microtubule-associated tau protein in rat brain. J Neurosci. 1988 Jun;8(6):1846–1851. doi: 10.1523/JNEUROSCI.08-06-01846.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesti N. M., Barra H. S. The interaction of myelin basic protein with tubulin and the inhibition of tubulin carboxypeptidase activity. Biochem Biophys Res Commun. 1986 Apr 29;136(2):482–489. doi: 10.1016/0006-291x(86)90466-3. [DOI] [PubMed] [Google Scholar]
- Papasozomenos S. C., Binder L. I., Bender P. K., Payne M. R. Microtubule-associated protein 2 within axons of spinal motor neurons: associations with microtubules and neurofilaments in normal and beta,beta'-iminodipropionitrile-treated axons. J Cell Biol. 1985 Jan;100(1):74–85. doi: 10.1083/jcb.100.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasozomenos S. C., Binder L. I. Phosphorylation determines two distinct species of Tau in the central nervous system. Cell Motil Cytoskeleton. 1987;8(3):210–226. doi: 10.1002/cm.970080303. [DOI] [PubMed] [Google Scholar]
- Pereyra P. M., Horvath E., Braun P. E. Triton X-100 extractions of central nervous system myelin indicate a possible role for the minor myelin proteins in the stability in lamellae. Neurochem Res. 1988 Jun;13(6):583–595. doi: 10.1007/BF00973301. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S. E., Warrington A. E., Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993 Jun;3(6):191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Riederer Beat M., Innocenti Giorgio M. Differential Distribution of Tau Proteins in Developing Cat Cerebral Cortex and Corpus Callosum. Eur J Neurosci. 1991 Oct;3(11):1134–1145. doi: 10.1111/j.1460-9568.1991.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Sadot E., Marx R., Barg J., Behar L., Ginzburg I. Complete sequence of 3'-untranslated region of Tau from rat central nervous system. Implications for mRNA heterogeneity. J Mol Biol. 1994 Aug 12;241(2):325–331. doi: 10.1006/jmbi.1994.1508. [DOI] [PubMed] [Google Scholar]
- Selden S. C., Pollard T. D. Phosphorylation of microtubule-associated proteins regulates their interaction with actin filaments. J Biol Chem. 1983 Jun 10;258(11):7064–7071. [PubMed] [Google Scholar]
- Shin R. W., Iwaki T., Kitamoto T., Tateishi J. Hydrated autoclave pretreatment enhances tau immunoreactivity in formalin-fixed normal and Alzheimer's disease brain tissues. Lab Invest. 1991 May;64(5):693–702. [PubMed] [Google Scholar]
- Steiner B., Mandelkow E. M., Biernat J., Gustke N., Meyer H. E., Schmidt B., Mieskes G., Söling H. D., Drechsel D., Kirschner M. W. Phosphorylation of microtubule-associated protein tau: identification of the site for Ca2(+)-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J. 1990 Nov;9(11):3539–3544. doi: 10.1002/j.1460-2075.1990.tb07563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Steward O., Banker G. A. Getting the message from the gene to the synapse: sorting and intracellular transport of RNA in neurons. Trends Neurosci. 1992 May;15(5):180–186. doi: 10.1016/0166-2236(92)90170-d. [DOI] [PubMed] [Google Scholar]
- Szuchet S., Arnason B. G., Polak P. E. Separation of ovine oligodendrocytes into two distinct bands on a linear sucrose gradient. J Neurosci Methods. 1980 Oct;3(1):7–19. doi: 10.1016/0165-0270(80)90030-8. [DOI] [PubMed] [Google Scholar]
- Szuchet S., Dumas M. An in-vitro approach to the study of oligodendrocytes and their involvement in multiple sclerosis. Neurol Clin. 1983 Aug;1(3):729–755. [PubMed] [Google Scholar]
- Szuchet S., Polak P. E., Yim S. H., Arvanitis D. Plasma membrane of cultured oligodendrocytes: III. Relatedness to myelin. Glia. 1988;1(2):141–150. doi: 10.1002/glia.440010206. [DOI] [PubMed] [Google Scholar]
- Szuchet S., Polak P. E., Yim S. H. Mature oligodendrocytes cultured in the absence of neurons recapitulate the ontogenic development of myelin membranes. Dev Neurosci. 1986;8(4):208–221. doi: 10.1159/000112254. [DOI] [PubMed] [Google Scholar]
- Szuchet S., Stefansson K., Wollmann R. L., Dawson G., Arnason B. G. Maintenance of isolated oligodendrocytes in long-term culture. Brain Res. 1980 Oct 27;200(1):151–164. doi: 10.1016/0006-8993(80)91101-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp B. D., Moench T., Pulley M., Barbosa E., Tennekoon G., Griffin J. Spatial segregation of mRNA encoding myelin-specific proteins. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7773–7777. doi: 10.1073/pnas.84.21.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouyiouklis D. A., Brophy P. J. Microtubule-associated protein MAP1B expression precedes the morphological differentiation of oligodendrocytes. J Neurosci Res. 1993 Jun 15;35(3):257–267. doi: 10.1002/jnr.490350305. [DOI] [PubMed] [Google Scholar]
- Wang Y., Loomis P. A., Zinkowski R. P., Binder L. I. A novel tau transcript in cultured human neuroblastoma cells expressing nuclear tau. J Cell Biol. 1993 Apr;121(2):257–267. doi: 10.1083/jcb.121.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Brophy P. J. Role for the oligodendrocyte cytoskeleton in myelination. J Neurosci Res. 1989 Apr;22(4):439–448. doi: 10.1002/jnr.490220409. [DOI] [PubMed] [Google Scholar]
- Yim S. H., Szuchet S., Polak P. E. Cultured oligodendrocytes. A role for cell-substratum interaction in phenotypic expression. J Biol Chem. 1986 Sep 5;261(25):11808–11815. [PubMed] [Google Scholar]