Abstract
We performed this study to evaluate the role of Interleukin-17 (IL-17) and Interleukin-18 (IL-18) in insulin resistance during normal pregnancy. This descriptive cross sectional study was carried out on 97 healthy pregnant women including 32, 25, and 40 individuals in the first, second, and third trimesters, respectively, and on 28 healthy non pregnant women between the autumn of 2012 and the spring of 2013. We analyzed the serum concentrations of IL-17 and IL-18 by using the enzyme linked immunosorbent assay (ELISA). Insulin resistance was measured by homeostasis model assessment of insulin resistance equation. No significant differences between the demographic data of the pregnant and non pregnant groups were observed. Insulin resistant in pregnant women was significantly higher than the controls (p=0.006). Serum IL-17 concentration was significantly different in non pregnant women and pregnant women in all gestational ages (p<0.05). Serum IL-18 level was significantly lower in subjects with first, second, and third trimesters of pregnancy in compared to non pregnant women (p<0.05). No significant correlations were found between serum IL-17 and IL-18 levels with insulin resistance (r=0.08, p=0.34 vs. r=0.01, p=0.91, respectively). Our data suggested that IL-17 and IL-18 do not appear to attribute greatly to pregnancy deduced insulin resistance during normal pregnancy.
Keywords: Insulin resistance, Interleukin-17, Interleukin-18, Pregnancy
INTRODUCTION
Pregnancy is associated with insulin resistance and glucose metabolism disorders. There is an oncoming augmentation in maternal insulin secretary response to glucose and a variety of other stimuli during the course of gestation (1). Insulin resistance may facilitate supply of expedient nutrients solely of glucose to fetus for fetal metabolism and growth (2). The exact mechanism related to insulin resistance during pregnancy is still unknown.
Immune system plays an important role during pregnancy which induces the development of normal pregnancy and ensures the development of complications. Due to the association between obstetric outcomes and immunologic responses, the early recognition of immunologic alterations would be invaluable in prevention of adverse pregnancy outcomes (3). In recent years, many studies have tried to find determinants of insulin resistance during normal pregnancy, revealing that the fat tissue and placenta are capable of secreting many hormones and cytokines (4).
Recent studies have been revealed that cytokines including interlukine-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (IL1-β) play a fundamental role in insulin resistance (5,6). Interleukin-17 (IL-17) and IL-18 are both inflammatory cytokines. There are contradictory results about the role of IL-17 and IL-18 during normal pregnancy (7,8). However, the immunologic profile of human during pregnancy needs to be more clearly defined. Since evidence regarding this issue is seems to be inadequate, we aimed to investigate the serum levels of IL-17 and IL-18 and its possible association with insulin resistance during normal pregnancy.
MATERIALS AND METHODS
Study design
The target subjects of this descriptive cross-sectional study consisted of pregnant and non pregnant women aged 15 to 35 years who were recruited from the outpatient clinic of the division of Obstetrics and Gynecology at the Medical University of Jahrom between the autumn of 2012 and the spring of 2013. Of them, 97 pregnant women with different gestational ages (first trimester: 32; second trimester: 25; and third trimester: 40) and 28 non pregnant women as controls were compared with each other. All groups were frequency matched by age, sex, and body mass index (BMI). Healthy subjects were included in the analysis of this study that had no history of pre gestational diabetes. None of the patients and the controls received hormone replacement therapy, significant liver damage and/or renal dysfunction and history of a sex hormone dependent disease. All of the participants consented to donate biological specimens for this study. The study protocol was approved by the Research Ethics Committee of Jahrom University of Medical Sciences and all of the subjects gave their written informed consent.
Biochemical analysis
Maternal height and pregnancy weight were measured in subjects and BMI was calculated according to these measurements. Blood samples were taken after 8 hours fasting time, then immediately the serum was separated by centrifugation at 2,500 rpm for a period of 10 minutes. The samples were processed directly or in a week following preservation at -70℃. Glucose measurements (intra-assay coefficient of variation (9) 2.1%, inter-assay CV 2.6%) were done using the glucose oxidase method. Serum insulin was determined by enzyme linked immunosorbent assay (ELISA) using commercial kit (IBL-IB79167). Insulin resistance was evaluated through glucose and insulin concentrations by using the homeostasis model assessment of insulin resistance (HOMA-IR) equation (10).
Serum IL-17 was measured by commercial ELISA kit (Bendermedsystems, Austria: Cat. No. BMS2017) follows company instruction. Serum IL-18 was measured by ELISA (Bendermedsystems, Austria: Cat. No. BMS267INST), According to company instruction as the same method for serum IL-17.
Statistical analysis
With regarding to our parent study (11) and based on a power of 90% to find significant differences between subjects (p=0.05, two-tailed), 20 subjects were needed to require for each group. To raise the credit of this research, the number of subjects in each group was increased as following. All data were reported as mean±standard deviation (SD). Maternal heigh, pregnancy weight, BMI and systolic and diastolic blood pressure, and insulin resistance data were analyzed with one way analysis of variance (ANOVA). Serum IL-17, IL-18, and insulin concentration data were analyzed with non-parametric kruskal-wallis test followed by Mann Whitney U-test. Correlations were calculated by using liner correlation (Pearson). All the statistical analyses were performed using SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL, USA). A two-sided p-value of 0.05 or less was considered to be statistically significant.
RESULTS
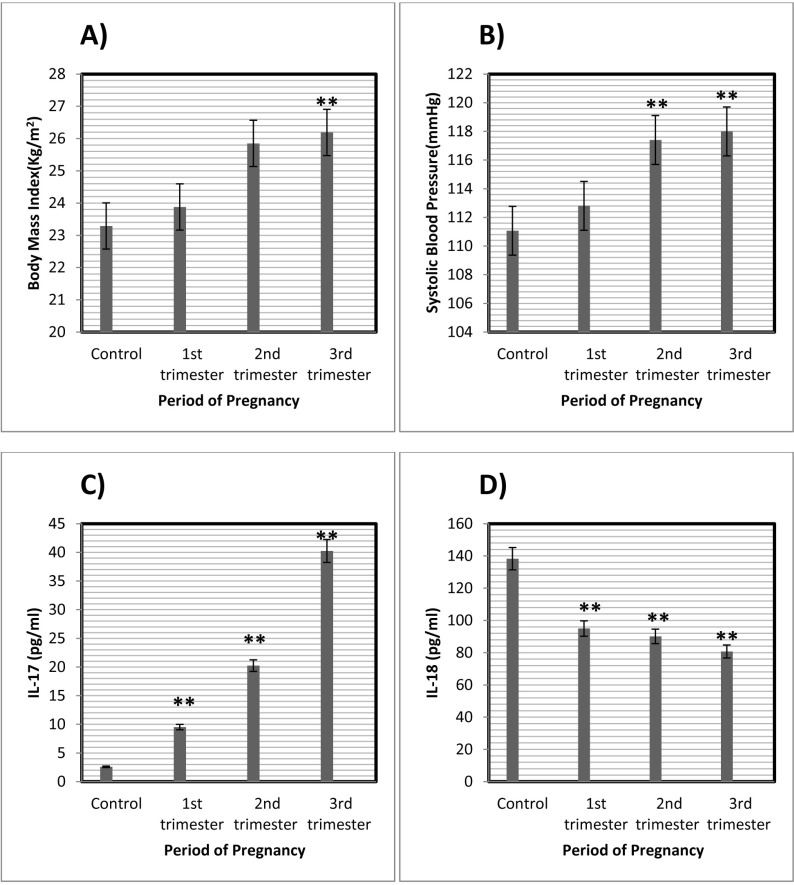
Ninety seven pregnant women and 28 non pregnant women finished the study and no one was excluded from it. The baseline characteristics and clinical data of participants are summarized in Table I. BMI was significantly increased in the third trimester of pregnancy (26.19±3.55) in compared to controls (23.29±2.73; p=0.001) and to women at first trimester of pregnancy (23.88±3.94; p=0.011) (Table II and Fig. 1A).
Table I.
Clinical and laboratory characteristic of patients and control
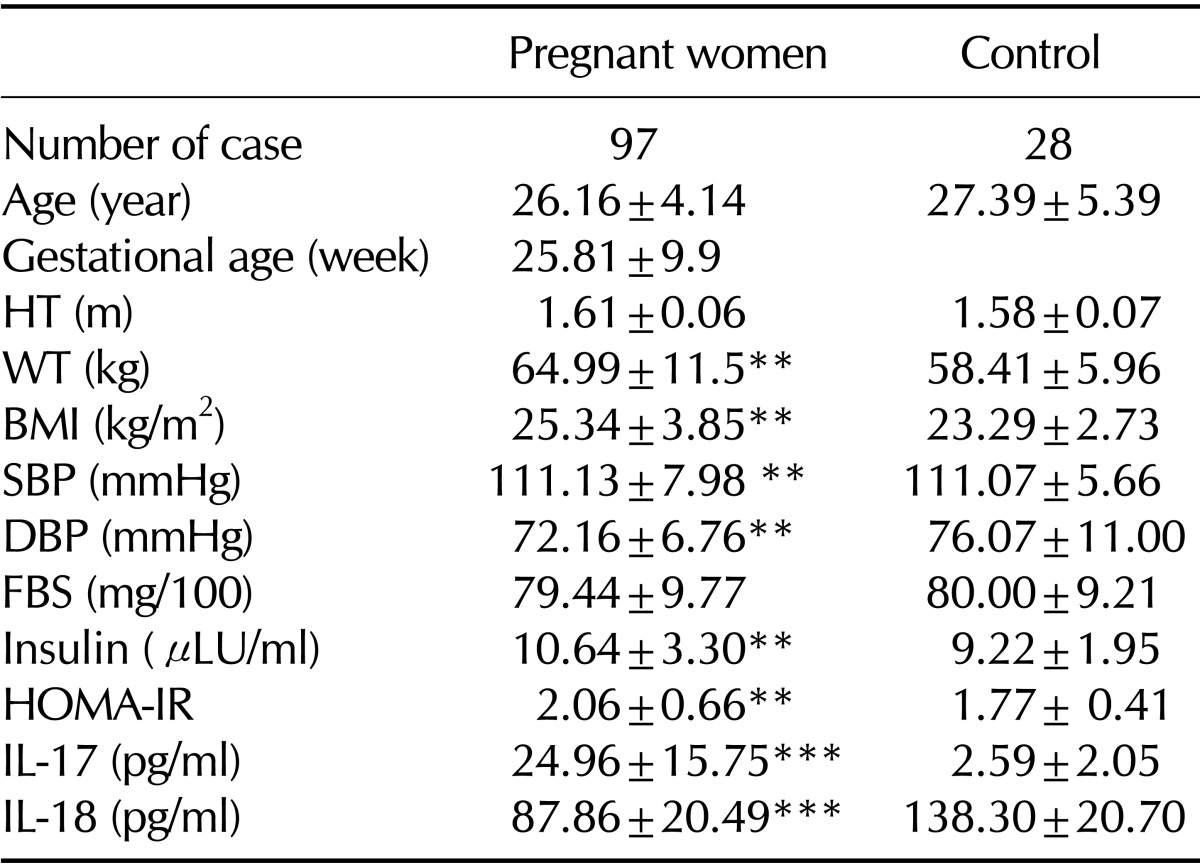
BMI: body mass index; HT: Height of women; WT: weight of body; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FBS: Fasting blood sugar; HOMA-IR: homeostasis model assessment of insulin resistance; IL-17: Interleukin 17; IL-18: Interleukin 18. *p<0.05 (control); **p<0.01 (control); ***p<0.001 (control)
Table II.
Clinical and laboratory characteristics of pregnant women with different gestational ages
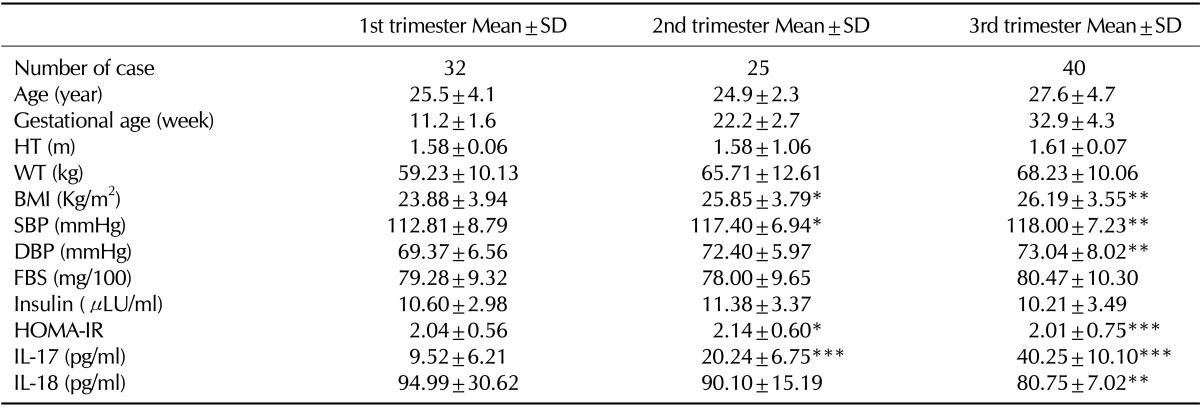
BMI: body mass index; HT: Height of women; WT: weight of body; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FBS: Fasting blood sugar; HOMA-IR, homeostasis model assessment of insulin resistance; IL-17: Interleukin 17; IL-18: Interleukin 18. *p<0.05; **p<0.01; ***p<0.001 (Significantly different from pregnant women in first trimester)
Figure 1.
(A) Body mass index (BMI) in patients in different trimesters of pregnancy and controls. BMI were significantly higher in 3rd trimesters compared with the control (**p<0.01). (B) Systolic blood pressure in different trimesters of pregnancy and controls. Systolic blood pressure were significantly higher in the 2nd and 3rd trimesters as compared with control group (**p<0.01). (C) Serum IL-17 level in different trimesters of pregnancy and control. Serum IL-17 level were significantly higher in the 1st, 2nd, and 3rd trimesters of pregnancy as compared with control (**p<0.01). (D) Serum IL-18 level in different trimesters of pregnancy. IL-18 level significantly were lower in the 1st, 2nd, and 3rd trimesters of pregnancy as compared with control (**p<0.01). *p<0.05.
Systolic blood pressure in subjects during third and second trimester of pregnancy was significantly different in compared to subjects in first trimester of pregnancy and non pregnant women (Table II and Fig. 1B). Serum IL-17 concentration was significantly different in non pregnant women and pregnant women in all gestational ages (Table II and Fig. 1C). Serum IL-18 level was significantly lower in subjects with first, second, and third trimesters of pregnancy in compared to non pregnant women (Table II). Alternatively, serum IL-18 level decreased by increasing in the gestational age during pregnancy (Fig. 1D). Insulin resistant in pregnant women was significantly higher than the controls (p=0.006). However, there was no significant difference in insulin resistance of women with different gestational ages (Table II).
There was no significant correlation between gestational ages with BMI (r=0.02, p=0.740), systolic blood pressure (r=-0.09, p=0.37) and serum IL-17 level (r=-0.10, p=0.24). On the other hand, there was a positive significant correlation between gestational ages and diastolic blood pressure (r=0.27, p=0.002), Insulin resistance (r=0.25, p=0.004). Also in pregnant women, there was a positive significant correlation between serum IL-17 level and BMI (r=0.30, p=0.0002) and systolic blood pressure (r=0.40, p=0.0001). A negative significant correlation was found between serum IL-18 level and BMI (r=-0.25, p=0.004) and systolic blood pressure (r=-0.30, p=0.001) and gestational ages (r=-0.31, p=0.0005). No significant correlations were found between serum IL-17 and IL-18 levels with insulin resistance (r=0.08, p=0.34 vs. r=0.01, p=0.91, respectively).
DISCUSSION
To our knowledge, this is the first survey aimed to investigate the association of serum IL-17 and IL-18 levels with insulin resistance during the normal pregnancy. Based on the results obtained from this study, serum IL-17 level in pregnant women was significantly higher in compared to control group. In contrast, data showed serum IL-18 level in pregnant women was significantly lower in compared to the controls. Although findings of present study demonstrated a significant correlation between gestational ages and serum IL-18 level in pregnant women, on the other hand, obtained data revealed no significant correlation between serum IL-17 level and gestational ages. Additionally, data showed no significant correlation between serum IL-17 and IL-18 levels with insulin resistance.
BMI of patients in the third trimester of pregnancy was significantly greater than the BMI of first trimester of pregnancy and non pregnant women. These results were in consonance with the data founded by previous researches (11). A common complication during pregnancy is glucose metabolism disorder which its pathology is related to deficiency of insulin secretion and insulin resistance. During the present study, it was observed that insulin resistance in pregnant women was significantly higher in compared to non pregnant women. And, insulin resistance increased with further increase in pregnancy period. In consonance to previous studies (11,12) no significant differences between gestational ages and insulin resistance were observed in the present study. On the other hand, data of present study revealed significant correlation between insulin resistance with gestational ages during pregnancy. Kirwan et al. (3) showed that insulin resistance during the late pregnancy was significantly increased as compared to the early pregnancy and/or non pregnant women. The findings of present study did not entirely correlate with Kirwan's study. The exact reasons behind these differences are not completely clear; life style between eastern and western societies, differences in dietary composition, variability between insulin assays in different experimental studies, sampling time during pregnancy, and discrepancies in the population studied may have played a role (13,14).
Based on T-cell lineage, cytokines are classified into helper T lymphocyte 1 (Th1) or helper T lymphocyte 2 (Th2). Th1 cells are characterized by inducing cell-mediated immunity and production of interferon-γ (IFN-γ) against intracellular pathogens, while Th2 cells produce stimulate humoral immunity and interleukin-4 (IL-4) against parasitic helminthes and extracellular pathogens. This paradigm was maintained until 2006, when the third T-cell subset, known as helper T lymphocyte 17 (Th17), was recognized (15,16). Th17 was reported with its major signature of releasing IL-17 (17,18). Growing reports in recent years on function and role of Th17 show that this subset of CD4 T cells play an important role in infiltration and recruitment of inflammatory cells against intercellular fungi and parasites (19) and recently in certain Th1 mediated autoimmune diseases such as multiple sclerosis and rheumatoid arthritis (20). Previous studies reported that Th1 cell-derived cytokines in normal pregnant women are decreased and Th2 cell-derived cytokines increased in normal pregnancy (21). In this study serum IL-17 concentration in pregnant women is significantly higher than non pregnant women and showed no significant correlation between IL-17 and gestational age. Serum IL-17 level ascends with increasing in pregnancy period. The data of present study is correlated with previous studies which showed the high level of IL-17 during pregnancy (22). A recent review study showed that the reciprocal development of pathways between the imbalance of Th17/Treg development, between Th17/Treg subsets, and between Th1/Th17 subsets has been reported in the recurrent pregnancy loss (23). These data suggest that the elevated secretion of IL-17 in normal pregnancy and might contribute the inflammatory response. However, elevated IL-17 secretion alone could not explain the excessive inflammatory response.
One of the IL-1 families is IL-18 cytokine with mixed properties, capable of inducing both a Th1 and a Th2 response related to the immunologic context. IL-18 was originally cloned from activated macrophages as interferon (IFN)-γ-inducing factor and was identified as a molecule of circulating in endotoxin-challenged mice followed by bacterial priming (24). IL-18 is critical in host defense against severe infections through the deduction of other effector molecules and cells and/or cytokines. The synergistic actions of IL-18 and other cytokines including IL-12 are important for the induction of a Th1 immune response, while IL-18 alone has capacity to deduce a Th2 immune response (25). These findings suggest that IL-18 may act as regulatory cytokines in normal pregnancy. In this study, serum IL-18 concentration in pregnant women is significantly lower than non pregnant women and showed negative significant correlation between IL-18 and gestational age. Serum IL-18 level descends with increasing in pregnancy period. Similarly to present study, Ekelund et al. (26) found low level of IL-18 in women with symptoms of preterm delivery. However in a study by Sakai et al. (27) in normal pregnancy an enhanced IL-18 secretion by non-stimulated peripheral blood mononuclear cells was founded. These differences might explain the down-regulation of IL-18 in normal pregnancy. The exact mechanisms behind these discrepancies are not entirely clear, therefore, further studies are needed to clarify these points.
Insulin resistance occurs in obesity. Th1 mediated cytokines including TNF-α, IL-6 and IL-1 are key mediators of the response to pathogens. They initiate metabolic changes to provide nutrients for the immune system. Recently, Jagannathan-Bogdan et al., (28) showed the expansion of both Th1 and Th17 subsets including IL-17 and a decrease in the T regulatory subset are associated with the inflammation and insulin resistance in metabolic status. On the other hand, Esposito et al. (29) showed that serum IL-18 concentrations are increased in obese women and decrease after weight loss. Additionally, serum IL-18 concentrations correlated with surrogate indexes of insulin resistance, such as fasting insulin levels and the waist-to-hip ratio (WHR), suggesting that the augmentation in serum IL-18 levels is related not only to obesity but also to insulin resistance. In the present study, we did not find any correlation between maternal IL-17 and IL-18 with insulin resistance. Although, we did not find any study in medicine literature to show the association of IL-17 and IL-18 with insulin resistance during pregnancy, but our findings were in contrast with previous studies which showed the significant roles of IL-17 and IL-18 on insulin resistance in non pregnant population (29,30,31). We contemplate, that this discrepancy was probably referred to the different biochemical assays for determination of serums IL-17 and IL-18, and differences in survey population. Moreover, several other variables may influence on cytokine levels during pregnancy including pregnancy body mass index, prior preterm delivery status, and maternal age (31).
The present study is simple in analysis and design, but examines a continuously and well-described collected of pregnant and non pregnant women. Gestational ages was determined conventionally and affirmed by ultrasonographic measurements. The information on outcomes was obtained before knowledge of IL-17 and IL-18 levels and thereafter any misclassification can lonely are of non differential type. Limitation-wise, the number of pregnant and non pregnant women is inadequate for comprehensive subgroup analyses and then associations in relation to subgroups need larger samples.
In current investigation, it could not find correlation between IL-17 and IL-18 concentrations with insulin resistance. In conclusion, the findings of present study suggest that IL-17 and IL-18 do not appear to contribute greatly to pregnancy induced insulin resistance in healthy pregnancy.
ACKNOWLEDGEMENTS
Hereby, the authors acknowledge the authorities of Jahrom University of Medical Sciences for financial support.
Abbreviations
- IL-17
interleukin-17
- IL-18
interleukin-18
- ELISA
enzyme linked immunosorbent assay
- IL-6
interlukine-6
- TNF-α
tumor necrosis factor-alpha
- IL1-β
interleukin-1 beta
- BMI
body mass index
- Th
helper T lymphocyte
- IFN-γ
interferon-γ
- WHR
waist-to-hip ratio
Footnotes
The authors have no financial conflict of interest.
References
- 1.Hadden DR, McLaughlin C. Normal and abnormal maternal metabolism during pregnancy. Semin Fetal Neonatal Med. 2009;14:66–71. doi: 10.1016/j.siny.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Baranyi E, Winkler G. Diabetes and pregnancy. Orv Hetil. 2011;152:1635–1640. doi: 10.1556/OH.2011.29192. [DOI] [PubMed] [Google Scholar]
- 3.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, Kalhan SC, Catalano PM. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–2213. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 4.Gwozdziewiczova S, Lichnovska R, Hrebicek J. Tumor necrosis factor alfa (TNFalpha) and insulin resistance. Cesk Fysiol. 2004;53:167–175. [PubMed] [Google Scholar]
- 5.Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51(12):3391–3399. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- 6.Zinman B, Hanley AJ, Harris SB, Kwan J, Fantus IG. Circulating tumor necrosis factor-alpha concentrations in a native Canadian population with high rates of type 2 diabetes mellitus. J Clin Endocrinol Metab. 1999;84:272–278. doi: 10.1210/jcem.84.1.5405. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed M, Gaffen SL. IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev. 2010;21:449–453. doi: 10.1016/j.cytogfr.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roland L, Gagne A, Belanger MC, Boutet M, Julien P, Bilodeau JF. Plasma interleukin-18 (IL-18) levels are correlated with antioxidant vitamin coenzyme Q(10) in preeclampsia. Acta Obstet Gynecol Scand. 2010;89:360–366. doi: 10.3109/00016340903576020. [DOI] [PubMed] [Google Scholar]
- 9.Shearman AM, Cupples LA, Demissie S, Peter I, Schmid CH, Karas RH, Mendelsohn ME, Housman DE, Levy D. Association between estrogen receptor alpha gene variation and cardiovascular disease. JAMA. 2003;290:2263–2270. doi: 10.1001/jama.290.17.2263. [DOI] [PubMed] [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Jahromi AS, Zareian P, Madani A. Association of insulin resistance with serum interleukin-6 and TNF-alpha levels during normal pregnancy. Biomark Insights. 2011;6:1–6. doi: 10.4137/BMI.S6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jahromi AS, Zareian P, Madani A. Insulin resistance and interleukin-1β during normal pregnancy. Asian J Biochem. 2011;6:366–372. doi: 10.4137/BMI.S6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clapp JF. Effects of Diet and Exercise on Insulin Resistance during Pregnancy. Metab Syndr Relat Disord. 2006;4:84–90. doi: 10.1089/met.2006.4.84. [DOI] [PubMed] [Google Scholar]
- 14.Manley SE, Luzio SD, Stratton IM, Wallace TM, Clark PM. Preanalytical, analytical, and computational factors affect homeostasis model assessment estimates. Diabetes Care. 2008;31:1877–1883. doi: 10.2337/dc08-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 16.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu S, Cao X. Interleukin-17 and its expanding biological functions. Cell Mol Immunol. 2010;7:164–174. doi: 10.1038/cmi.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louten J, Boniface K, de Waal Malefyt R. Development and function of TH17 cells in health and disease. J Allergy Clin Immunol. 2009;123:1004–1011. doi: 10.1016/j.jaci.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Peck A, Mellins ED. Breaking old paradigms: Th17 cells in autoimmune arthritis. Clin Immunol. 2009;132:295–304. doi: 10.1016/j.clim.2009.03.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sallusto F, Lanzavecchia A. Human Th17 cells in infection and autoimmunity. Microbes Infect. 2009;11:620–624. doi: 10.1016/j.micinf.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, Clerici M. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106:127–133. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63:601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 23.Saito S, Nakashima A, Ito M, Shima T. Clinical implication of recent advances in our understanding of IL-17 and reproductive immunology. Expert Rev Clin Immunol. 2011;7:649–657. doi: 10.1586/eci.11.49. [DOI] [PubMed] [Google Scholar]
- 24.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–264. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53–72. doi: 10.1016/s1359-6101(00)00015-0. [DOI] [PubMed] [Google Scholar]
- 26.Ekelund CK, Vogel I, Skogstrand K, Thorsen P, Hougaard DM, Langhoff-Roos J, Jacobsson B. Interleukin-18 and interleukin-12 in maternal serum and spontaneous preterm delivery. J Reprod Immunol. 2008;77:179–185. doi: 10.1016/j.jri.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Sakai M, Shiozaki A, Sasaki Y, Yoneda S, Saito S. The ratio of interleukin (IL)-18 to IL-12 secreted by peripheral blood mononuclear cells is increased in normal pregnant subjects and decreased in pre-eclamptic patients. J Reprod Immunol. 2004;61:133–143. doi: 10.1016/j.jri.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Jagannathan-Bogdan M, McDonnell ME, Shin H, Rehman Q, Hasturk H, Apovian CM, Nikolajczyk BS. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol. 2011;186:1162–1172. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esposito K, Pontillo A, Ciotola M, Di Palo C, Grella E, Nicoletti G, Giugliano D. Weight loss reduces interleukin-18 levels in obese women. J Clin Endocrinol Metab. 2002;87:3864–3866. doi: 10.1210/jcem.87.8.8781. [DOI] [PubMed] [Google Scholar]
- 30.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escobar-Morreale HF, Botella-Carretero JI, Villuendas G, Sancho J, San Millan JL. Serum interleukin-18 concentrations are increased in the polycystic ovary syndrome: relationship to insulin resistance and to obesity. J Clin Endocrinol Metab. 2004;89:806–811. doi: 10.1210/jc.2003-031365. [DOI] [PubMed] [Google Scholar]