Abstract
Aims—To develop a rapid PCR ELISA procedure for the detection of mutations in K-ras in a microtitre plate format, and to evaluate the assay for the detection of these mutations in human colorectal cancer.
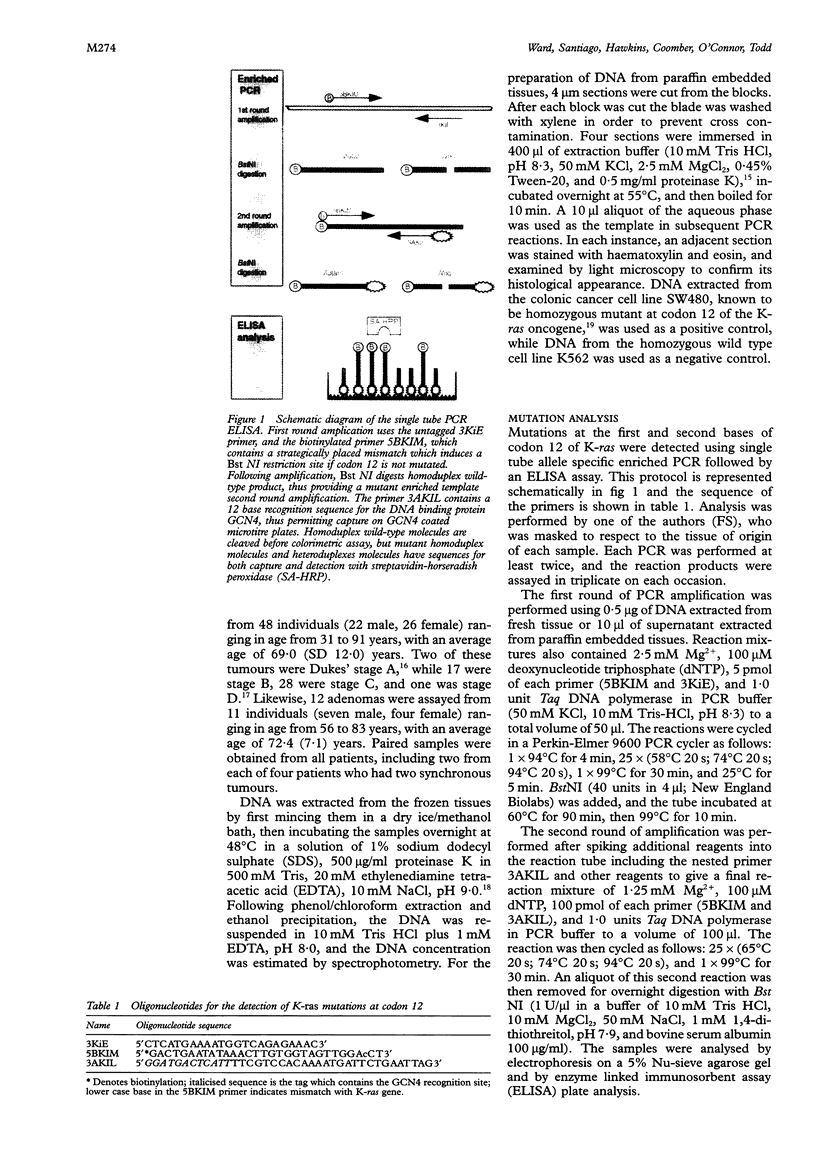
Methods—An enriched PCR method was used with labelled primers, and PCR product was captured on GCN4 coated immunoassay plates. Detection of biotinylated mutant product was performed by colorimetric assay with streptavidin-horseradish peroxidase. The assay was used to determine K-ras status in a series of 60 human colorectal neoplasms, together with paired normal colonic mucosa. Results from gel electrophoretic analysis were compared with ELISA results.
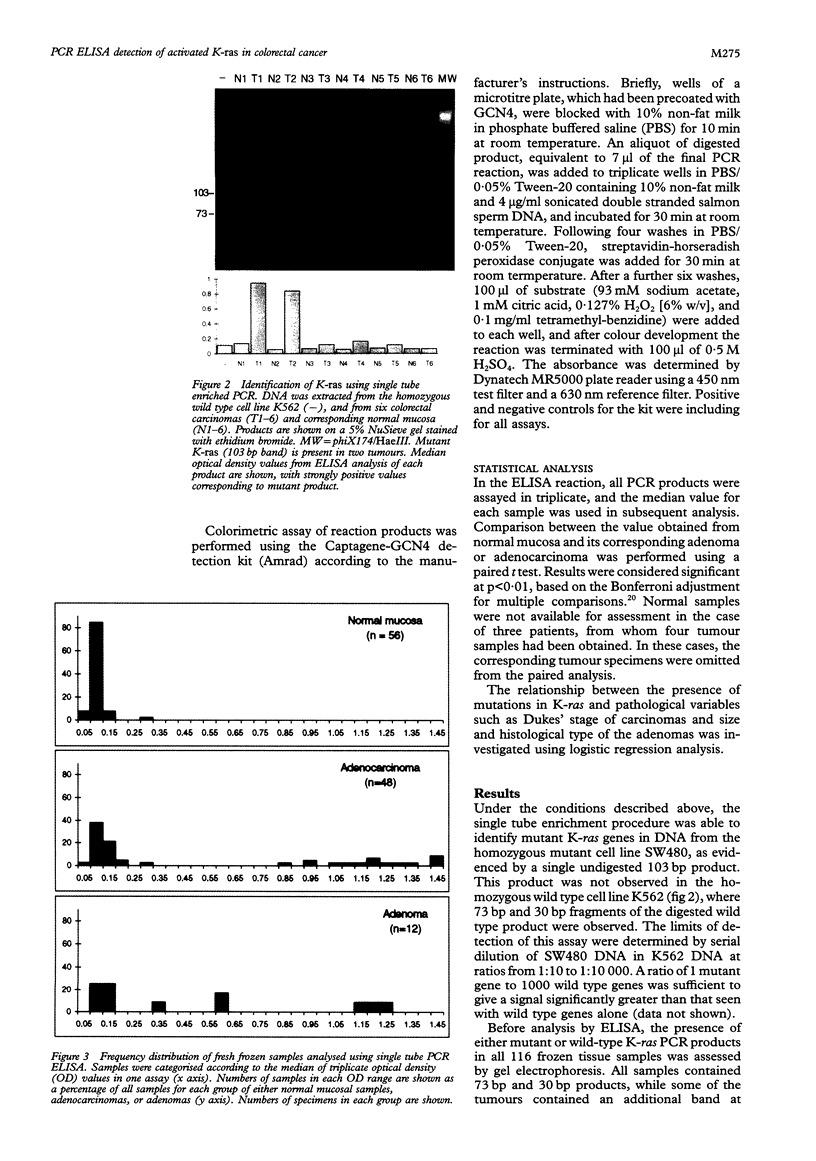
Results—The assay proved reliable in detecting K-ras mutations in DNA extracted from both fresh and paraffin embedded colorectal tumours. ELISA results were comparable with results from gel electrophoresis. Mutations of K-ras were detected in 16 of 48 adenocarcinomas and five of 12 adenomas but no mutations were detected in normal mucosa. There was a highly significant difference (p<0·0005) between optical density values for carcinomas with mutant K-ras and their paired normal data. Adenomas did not show the clear distinction between positive and negative results seen with carcinomas.
Conclusions—This assay provides a rapid and reliable means of detecting mutations in codon 12 of the K-ras oncogene. The single tube format colorimetric analysis in microtitre plates and clear discrimination between mutant and wild type genes makes the assay suitable for automation. The occurrence of intermediate results in the case of adenomas provides support for the hypothesis that mutations of K-ras occur early in the course of colorectal carcinogenesis.
Keywords: K-ras
Keywords: colorectal neoplasia
Keywords: point mutation
Keywords: ELISA
Keywords: PCR
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Seeburg P. H., McGrath J. P., Hayflick J. S., Edman U., Levinson A. D., Goeddel D. V. Activation of Ki-ras2 gene in human colon and lung carcinomas by two different point mutations. Nature. 1983 Aug 11;304(5926):507–513. doi: 10.1038/304507a0. [DOI] [PubMed] [Google Scholar]
- DUKES C. E., BUSSEY H. J. The spread of rectal cancer and its effect on prognosis. Br J Cancer. 1958 Sep;12(3):309–320. doi: 10.1038/bjc.1958.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlen T., Dubeau L. Detection of ras point mutations by polymerase chain reaction using mutation-specific, inosine-containing oligonucleotide primers. Biochem Biophys Res Commun. 1989 Apr 28;160(2):441–447. doi: 10.1016/0006-291x(89)92452-2. [DOI] [PubMed] [Google Scholar]
- Finkelstein S. D., Sayegh R., Christensen S., Swalsky P. A. Genotypic classification of colorectal adenocarcinoma. Biologic behavior correlates with K-ras-2 mutation type. Cancer. 1993 Jun 15;71(12):3827–3838. doi: 10.1002/1097-0142(19930615)71:12<3827::aid-cncr2820711207>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Goelz S. E., Hamilton S. R., Vogelstein B. Purification of DNA from formaldehyde fixed and paraffin embedded human tissue. Biochem Biophys Res Commun. 1985 Jul 16;130(1):118–126. doi: 10.1016/0006-291x(85)90390-0. [DOI] [PubMed] [Google Scholar]
- Haliassos A., Chomel J. C., Grandjouan S., Kruh J., Kaplan J. C., Kitzis A. Detection of minority point mutations by modified PCR technique: a new approach for a sensitive diagnosis of tumor-progression markers. Nucleic Acids Res. 1989 Oct 25;17(20):8093–8099. doi: 10.1093/nar/17.20.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruban R. H., van Mansfeld A. D., Offerhaus G. J., van Weering D. H., Allison D. C., Goodman S. N., Kensler T. W., Bose K. K., Cameron J. L., Bos J. L. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993 Aug;143(2):545–554. [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Barbacid M. Oncogene detection at the single cell level. Oncogene. 1988 Dec;3(6):647–651. [PubMed] [Google Scholar]
- Levi S., Urbano-Ispizua A., Gill R., Thomas D. M., Gilbertson J., Foster C., Marshall C. J. Multiple K-ras codon 12 mutations in cholangiocarcinomas demonstrated with a sensitive polymerase chain reaction technique. Cancer Res. 1991 Jul 1;51(13):3497–3502. [PubMed] [Google Scholar]
- McLellan E. A., Owen R. A., Stepniewska K. A., Sheffield J. P., Lemoine N. R. High frequency of K-ras mutations in sporadic colorectal adenomas. Gut. 1993 Mar;34(3):392–396. doi: 10.1136/gut.34.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky D., Tokino T., Hamilton S. R., Kinzler K. W., Levin B., Frost P., Vogelstein B. Identification of ras oncogene mutations in the stool of patients with curable colorectal tumors. Science. 1992 Apr 3;256(5053):102–105. doi: 10.1126/science.1566048. [DOI] [PubMed] [Google Scholar]
- Sidransky D., Von Eschenbach A., Tsai Y. C., Jones P., Summerhayes I., Marshall F., Paul M., Green P., Hamilton S. R., Frost P. Identification of p53 gene mutations in bladder cancers and urine samples. Science. 1991 May 3;252(5006):706–709. doi: 10.1126/science.2024123. [DOI] [PubMed] [Google Scholar]
- Soh K., Yanagisawa A., Hiratsuka H., Sugano H., Kato Y. Variation in K-ras codon 12 point mutation rate with histological atypia within individual colorectal tumors. Jpn J Cancer Res. 1993 Apr;84(4):388–393. doi: 10.1111/j.1349-7006.1993.tb00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork P., Loda M., Bosari S., Wiley B., Poppenhusen K., Wolfe H. Detection of K-ras mutations in pancreatic and hepatic neoplasms by non-isotopic mismatched polymerase chain reaction. Oncogene. 1991 May;6(5):857–862. [PubMed] [Google Scholar]
- Tada M., Omata M., Kawai S., Saisho H., Ohto M., Saiki R. K., Sninsky J. J. Detection of ras gene mutations in pancreatic juice and peripheral blood of patients with pancreatic adenocarcinoma. Cancer Res. 1993 Jun 1;53(11):2472–2474. [PubMed] [Google Scholar]
- Todd A. V., Ireland C. M., Iland H. J. Allele-specific enrichment: a method for the detection of low level N-ras gene mutations in acute myeloid leukemia. Leukemia. 1991 Feb;5(2):160–161. [PubMed] [Google Scholar]
- Todd A. V., Ireland C. M., Radloff T. J., Kronenberg H., Iland H. J. Analysis of N-ras gene mutations in acute myeloid leukemia by allele specific restriction analysis. Am J Hematol. 1991 Nov;38(3):207–213. doi: 10.1002/ajh.2830380310. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Yamagata S., Muto T., Uchida Y., Masaki T., Sawada T., Tsuno N., Hirooka T. Lower incidence of K-ras codon 12 mutation in flat colorectal adenomas than in polypoid adenomas. Jpn J Cancer Res. 1994 Feb;85(2):147–151. doi: 10.1111/j.1349-7006.1994.tb02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]