Abstract
This study aimed to evaluate whether functional variants in the ankyrin repeat and kinase domain-containing 1 gene (ANKK1) and/or the dopamine receptor D2 gene (DRD2) modulate the subjective effects (reward or non-reward response to a stimulus) produced by cocaine administration. Cocaine-dependent participants (N = 47) were administered 40 mg of cocaine or placebo at time 0, and a subjective effects questionnaire (visual analog scale) was administered 15 minutes prior to cocaine administration, and at 5, 10,15, and 20 minutes following administration. The influence of polymorphisms in the ANKK1 and DRD2 genes on subjective experience of cocaine in the laboratory was tested. Participants with a T allele of ANKK1 rs1800497 experienced greater subjective ‘high’ (p = 0.00006), ‘any drug effect’ (p = 0.0003), and ‘like’ (p = 0.0004) relative to the CC genotype group. Although the variant in the DRD2 gene was shown to be associated with subjective effects, LD analysis revealed this association was driven by the ANKK1 rs1800497 variant. A participant’s ANKK1 genotype may identify individuals who are likely to experience greater positive subjective effects following cocaine exposure, including greater ‘high’ and ‘like’, and these individuals may have increased vulnerability to continue using cocaine or they may be at greater risk to relapse during periods of abstinence. However, these results are preliminary and replication is necessary to confirm these findings.
Keywords: ANKK1, subjective effects, cocaine
Introduction
The reward machinery of the brain is a critical component in the development of substance addiction. Cocaine and methamphetamine increase the levels of dopamine (DA) in synapses of the nucleus accumbens (NA) (Nestler, 2001, Nestler, 2005, Wise & Bozarth, 1987). Dopamine is one of the main neurotransmitters that mediates reward and was observed to be associated with the availability of natural reinforcers (Agmo & Berenfeld, 1990, Pfaus et al., 1990, Small et al., 2003).
Cocaine is one of the most commonly abused drugs with approximately 1.4 million individuals (aged 12 years and older) currently using cocaine in the United States 2011 (Samhsa, 2012). Cocaine acts, in part, on the dopaminergic pathway by blocking the DA transporter (encoded by the DAT1/SLC6A3 gene) and allowing more DA to remain in the synapse to activate DA receptors (Nestler, 2001, Nestler, 2005, Volkow et al., 1996). There is inter-individual variation in how people experience the effects of cocaine and some of this variation may be due to genetic influences [reviewed in (Hart et al., 2012)]. There is evidence that the nature and intensity of these subjective effects may lead to increased or decreased use, and may lead to dependence or abuse particularly when the effects are positive (Fergusson et al., 2003, Haertzen et al., 1983). With alcohol, for example, it has been shown that flushing and increased heart rate (e.g., negative reactions) after consumption of alcohol by individuals with aldehyde dehydrogenase II deficiency lead to reduced rates of use and alcoholism (Edenberg, 2007). Another study showed that positive reactions to cannabis early on (e.g., feeling happy or relaxed) led to an increased odds ratio of future dependence (Fergusson et al., 2003).
In this study, we examined rs1800497 (C→T, also known as DRD2/ANKK1 TaqIA), a functional variant that codes for a substitution of glutamate to lysine at position 713 in the ankyrin repeat and kinase domain-containing 1 (ANKK1) protein. The rs1800497 variant in the ANKK1 gene has been found to be associated with DA D2 receptor density, with the minor T allele found to be associated with lower receptor density (Jonsson et al., 1999). The T allele of rs1800497 has been shown to be associated with various psychiatric disorders, including alcohol and cocaine dependence (e.g. (Blum et al., 1991, Connor et al., 2007, Dubertret et al., 2001, Noble et al., 1993)).
Variants in the DA receptor D2 gene (DRD2) have been found to be associated with several neurological and psychiatric conditions including substance addiction and/or dependence, migraine, and schizophrenia (Blum et al., 1991, Blum et al., 1990, Comings et al., 1991, Connor et al., 2007, Glatt et al., 2009, Golimbet et al., 2003, Noble, 1994, Noble et al., 1991, Voisey et al., 2012, Yang et al., 2007, Yang et al., 2008). The rs2283265 T allele of the DRD2 gene occurs more frequently in cocaine addicts than in controls (Moyer et al., 2011), and has been shown to be associated with decreased expression of the short form (D2S) form of the D2 receptor (Moyer et al., 2011, Zhang et al., 2007).
Given the involvement of ANKK1 and DRD2 in the dopaminergic pathway, and given cocaine’s direct effect on the dopaminergic pathway, we hypothesized that genetic variation in the ANKK1 and DRD2 genes may be associated with subjective effects produced by cocaine in the laboratory.
Materials and methods
Participants
Non-treatment-seeking, cocaine-dependent participants were recruited between March 2010 - July 2012 via advertisements and were paid for their participation as part of ongoing research trials conducted by Drs. De La Garza’s and Newton’s Stimulant Addiction Research Program at the Baylor College of Medicine. A total of 47 participants signed informed consent documents in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki), had blood samples for genetic analysis collected, and were enrolled in this component of the study. All participants were administered the Mini-International Neuropsychiatric Interview (M.I.N.I.) (Sheehan et al., 1998) and Addiction Severity Index (ASI) (Mclellan et al., 1992), and met Diagnostic and Statistical Manual of Mental Disorders 4th Edition (DSM-IV) criteria for cocaine-dependence. Participants were between 18 and 55 years of age, had a history of using cocaine by the smoked or intravenous route, and had normal laboratory evaluation (comprehensive metabolic panel and complete blood count), electrocardiogram, and vital signs. Exclusion criteria included a history of head trauma, epilepsy, dependence on drugs other than cocaine and nicotine, inability to detect effects of cocaine, or the presence of any other axis I psychiatric disorder. Serious medical condition such as heart disease, acquired immunodeficiency syndrome (AIDS), and asthma were also exclusionary. Women were required to provide a negative pregnancy test prior to enrollment. Concomitant use of psychotropic medications or medications affecting blood pressure was not allowed. This study was approved by the Institutional Review Board of the Baylor College of Medicine as well as the Research and Development committee of the Michael E. DeBakey Veteran Affairs Medical Center.
Subjective effects testing
Using a double-blind, placebo-controlled, within-subjects study design, participants were administered 0 (saline) or 40 mg cocaine intravenously. Each individual participated in both conditions, one dose being administered in the morning (~9 AM) and one in the afternoon (~1 PM). Subjective effects of cocaine were measured using a visual analog scale anchored at 0 for “no effect” to a maximum of 100 for “most ever”. Ratings were obtained for ‘high’ (“How high are you right now?”), ‘any drug effect’ (“Do you feel any drug effect right now?”), ‘stimulated’ (“How stimulated do you feel right now?”), ‘good effect’ (“Does the drug have any good effects right now?”), ‘like’ (“How much do you like the drug right now?”), ‘bad effect’ (“Does the drug have any bad effects right now?”), ‘anxious’ (“How anxious do you feel right now?”), ‘desire’ (“How much do you desire the drug right now?”), ‘likely to use cocaine if had access’ (“If you had access to the drug right now how likely would you be to use it right now?”), and ‘depressed’ (“How depressed do you feel right now?”). Subjective effects ratings were obtained 15 minutes prior to dosing and at 5, 10, 15, and 20 minutes following dosing.
Genotyping
DNA was isolated from blood using the Gentra Puregene blood kit (Qiagen, Germantown, MD) according to the manufacturer’s recommendations. Genotypes were determined by a researcher who was unaware of the clinical status of the participants using 5′-fluorogenic exonuclease assays (TaqMan®, Applied Biosystems, Foster City, CA). The ANKK1 rs1800497 variant was genotyped using the TaqMan® primer-probe sets (Applied Biosystems) assay ID C_7486676_10, and the DRD2 rs2283265 variant with assay ID C__16070796_10. PCR amplifications were performed in duplicate using Platinum® quantitative PCR SuperMix-UDG (Invitrogen, Carlsbad, CA) on a ViiA7 and ViiA 7 Software v1.1 was used for data analysis (Applied Biosystems). SRY was genotyped to determine sex (Kosten et al., 2013). Ten ancestry-informative markers were genotyped to determine population structure, and the data from our cohort were compared against CEPH-HGDP samples (1,035 subjects of 51 populations) as previously described (Kosten et al., 2013).
Statistical Analyses
A repeated measures analysis of variance (ANOVA) was used to analyze the subjective effects scores over time for each subject. Subjective effect values following cocaine or saline administration were normalized to baseline values (−15 minute time point), and the saline administration subjective effect values (Supplementary Figure 2, right panels) were then subtracted from the 40 mg cocaine subjective effect values (Supplementary Figure 2, left panels) for each individual to yield the final subjective effect scores (Figure 1 and Supplementary Figure 1). Covariates included in the statistical model were gender, population structure, and years of nicotine use. Data from the two genotype groups over time were analyzed to determine if the subjective effect scores were moderated by the ANKK1 locus or the DRD2 locus using R version 2.9.1 (R_Development_Core_Team, 2009). We compared ANKK1 genotype (0 = CC genotype, 1 = CT/TT), or DRD2 genotype (0 = GG genotype, 1 = GT/TT), time, and interactions between genotypes and time. ANOVA was also used to analyze the continuous variables in the demographic data, while a Fisher’s exact test was used to analyze any categorical variables. We calculated effect size as a partial eta-squared statistic using condition or SNP variance over residual variance. The three general cut-offs for effect size are the following: a large effect is 0.14, a medium effect is 0.06, and a small effect is 0.01. Power analyses revealed that with a 47-participant sample divided into four groups and measured over six time points, and assuming a similar effect size to what we observed for ANKK1 and DRD2, there is 80% power to detect a difference within our samples at a Bonferroni-corrected alpha level of 0.05.
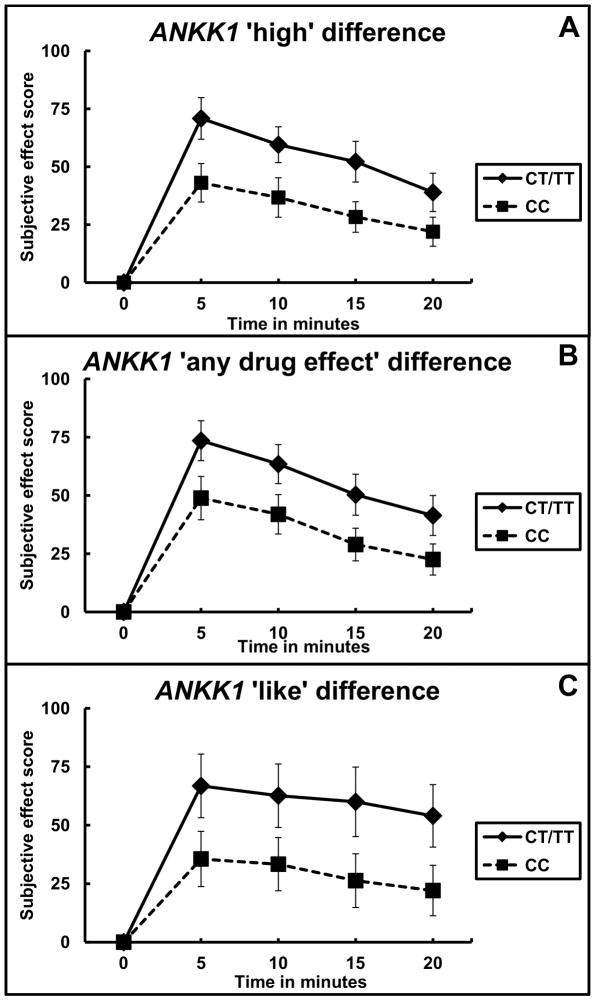
Figure 1. Subjective effect scores by ANKK1 genotype.
Subjective effect scores for ‘high’ (panel A), ‘any drug effect’ (panel B), and ‘like’ (panel C) are shown for each time point starting with baseline at time zero. Scores for each time point in the CT/TT genotype group (N = 21) are represented by the solid line, and time points for the CC genotype group (N = 26) are represented by the dashed line. Error bars indicate standard error of the mean.
To determine population structure from the AIMS data, our cohort was compared to the Centre d'Etude du Polymorphisme Humain–Human Genome Diversity Panel (CEPH-HGDP) samples as outlined in Lao et al (Lao et al., 2006). The CEPH-HGDP cohort consists of 1035 subjects from 51 different regional populations including America, Europe, the Middle East, Central and East Asia, Oceania, and sub-Saharan Africa. Proportion of population substructure for each individual was determined using the STRUCTURE 2.3.3 software (Hubisz et al., 2009, Pritchard et al., 2000) using four ancestral populations (K = 4), a burn-in period of 100,000 iterations, and 1 million Markov chain Monte Carlo replications after burn-in. The proportion of population substructure represented in each individual was included in the analysis as a covariate to eliminate population stratification effects.
Results
Demographic characteristics by treatment and ANKK1/DRD2 genetics
The genotypes of the participants included 26 CC, 18 CT, and three TT genotypes for ANKK1, and 37 GG, ten GT, and zero TT genotypes for DRD2 (see Table 1). The participants were mostly African American males (68%) with a mean age of 43.5 years. Participants used cocaine for a mean of 16 years and for a mean of 17 days in the month prior to participating in this study. The only significant difference among the demographic variables was years of nicotine use for ANKK1 (p = 0.0432) and DRD2 (p = 0.0133) (see Table 1). There were no other significant baseline differences among the genotype groups in any demographic characteristics after adjusting for multiple testing (Bonferroni, p < 0.05).
Table 1.
Demographic and clinical characteristics by ANKK1 and DRD2 genotype
| Gene |
ANKK1
|
DRD2
|
||
|---|---|---|---|---|
| TT/TC | CC | TT/TG | GG | |
| Characteristic | ||||
| N | 21 | 26 | 10 | 37 |
| Male (%) | 81 | 92.3 | 70 | 91.9 |
| African American (%) | 76.2 | 61.5 | 50 | 73 |
| Caucasian (%) | 9.5 | 11.5 | 20 | 8.1 |
| Hispanic (%) | 4.8 | 11.5 | 10 | 8.1 |
| Other (%) | 9.5 | 15.5 | 20 | 10.8 |
| Education, years (SD) | 12.5 (2) | 13 (1.6) | 13.2 (2.4) | 12.7 (1.6) |
| Age (SD) | 42.3 (6.1) | 44.5 (7) | 40.4 (7) | 44.4 (6.3) |
| Weight (SD) | 183.4 (38) | 190.9 (30) | 181.3 (41) 189.2 (32) | |
| Cocaine use, years (SD) | 16.3 (6.7) | 16.2 (8) | 17.5 (7.3) | 15.9 (7.4) |
| Cocaine use, daily (SD) | 4.9 (6.4) | 4.1 (4) | 6.5 (8.7) | 3.9 (3.7) |
| Cocaine use, past 30 days (SD) | 17.7 (7.4) | 16.1 (7.9) | 17.3 (7.3) | 16.7 (7.8) |
| Grams used per day (SD) | 2.7 (3.1) | 1.6 (1.2) | 3.5 (3.8) | 1.6 (1.3) |
| Nicotine use, years (SD)* | 17.4 (8.5) | 22.7 (7.7) | 14.9 (5.4) | 21.5 (8.6) |
The only significant difference among the demographic variables is for years of nicotine use for ANKK1 p = 0.0432 and DRD2 p = 0.0133. No significant baseline differences in any clinical characteristics were observed after adjusting for multiple testing (p > 0.05).
Linkage disequilibrium (LD) analyses
LD analysis revealed that the ANKK1 and DRD2 genes are almost in complete LD according to the D' measure based on allele frequencies where D' = 0.999 (r2 = 0.35). The distribution of participants among the genotype groups varies and is as follows: ANKK1; TT = 3, TC = 18, and CC= 26, and DRD2; TT=0, TG= 10, and GG=37. The CC genotype for ANKK1 only is observed in the presence of the GG genotype of DRD2. When both variants were included in the statistical model, the ANKK1 variant was the only significant result, indicating the effects of the variants were not independent, and the statistical significance of the DRD2 rs2283265 variant was result of the ANKK1 C-allele on which it arose.
ANKK1 genotype and subjective effects
A dominant model was used for statistical analysis that grouped participants based on the presence or absence of the ANKK1 rs1800497 minor T allele. For the subjective scores of ‘high’ there was a significant main effect of genotype (F = 16.99; df = 1,179; p = p = 0.00006, with an effect size of 0.0949) and time (F = 11.87; df = 1,179; p = 0.0007, with an effect size of 0.0663), without a significant interaction (p = 0.53). The difference from baseline between the genotype groups was the largest for ‘high’ at 5 minutes into the trial where the CT/TT genotype group had values of 70.86 ± 8.99 (s.e.m.) and the CC genotype group had values of 43.08± 8.31 (s.e.m.) (see Figure 1A).
For the subjective score of ‘any drug effect’ there was a significant main effect of genotype (F = 13.82; df = 1,175; p = p = 0.0003, with an effect size of 0.0772) and time (F = 14.71; df = 1,179; p = 0.00017, with an effect size of 0.0822), and the interaction term was not significant (p = 0.07). The largest difference from baseline between the genotype groups was at 5 minutes into the trial for ‘any drug effect’ where the CT/TT genotype group had values of 73.48 ± 8.56 (s.e.m.) and the CC genotype group had values of 48.85 ± 9.30 (s.e.m.) (see Figure 1B).
Finally, for the subjective scores of ‘like’ there was a significant main effect of genotype (F = 12.98; df = 1,179; p = 0.0004, with an effect size of 0.0725), but neither time (p = 0.26) nor the interaction term (p = 0.93) were significant. The difference from baseline between the genotype groups was the largest for ‘like’ at 5 minutes into the trial where the CT/TT genotype group had values of 66.81 ± 13.36 (s.e.m.) and the CC genotype group had values of 35.58 ± 11.80 (s.e.m.) (see Figure 1C). The other subjective effects variables did not reach the p < 0.05 level of significance and are therefore not reported.
Discussion
Analyses revealed that the ANKK1 and DRD2 genes were in almost complete LD (D' = 0.999) and that the ANKK1 variant was the only significant result when both variants were included in the statistical model. The statistical significance of the DRD2 rs2283265 variant is a result of the ANKK1 genotype on which it arose and, therefore, this discussion will focus on the ANKK1 rs1800497variant found to be associated with subjective effects of cocaine.
In this preliminary study, we found that individuals who carry the T allele of rs1800497 (originally referred to as the DRD2/ANKK1TaqIA1 allele: (Blum et al., 1990)) in the ANKK1 gene reported higher subjective effects in response to acute cocaine administration in the laboratory. Specifically, participants with genotypes reflecting lower dopamine D2 receptor density had greater subjective effects after cocaine administration than participants with genotypes associated with higher D2 receptor density.
The dopaminergic pathway in the brain, also known as the ‘reward’ or ‘motivational’ pathway, is integral to addictive and psychiatric conditions. More recently, the role of the dopaminergic pathway has been clarified as the ‘motivational’ machinery of the brain as DA release is associated with anticipation of reward, instead of being associated with the reward administration itself (Berridge, 2007). In 2007, Berridge and colleagues described the effect as DA mediating the ‘wanting’ (also known as “incentive salience”) of pleasurable effects, more than the ‘liking’ of pleasurable effects.
DA D2 receptor density has been shown to impact cocaine addiction and self-administration. Non-human primates with low DA D2 receptor density show higher frequency of cocaine self-administration (Nader et al., 2006). The brain tissues of cocaine addicts show reduced D2 receptor availability via PET scan after chronic cocaine addiction (Volkow et al., 1990), and healthy volunteers with lower dopamine D2 receptor density reported liking the effects of methylphenidate (a stimulant with some structural and pharmacologic similarities to cocaine), whereas individuals with higher dopamine D2 receptor density reported more intense unpleasant effects of methylphenidate (Volkow et al., 1999).
The ANKK1 rs1800497 T allele has been found to associate with lower DA receptor tone (Jonsson et al., 1999, Pohjalainen et al., 1998, Ritchie & Noble, 2003, Thompson et al., 1997). Low density of DA receptors also has been described in relation to the “reward deficiency syndrome” in which individuals with increased risk for substance addiction have defects in the dopaminergic pathway such as rapid degradation of DA, low dopamine receptor density, or high clearance of DA from the synapse (Comings & Blum, 2000). The hypothesis is that these individuals experience a lower level of ‘reward’ due to these factors and therefore are more habitual in seeking “artificial” means of increasing the ‘reward’ sensation.
Given its presumed function in the dopaminergic pathway, it is not surprising that the ANKK1 gene has been linked to several addictive disorders, polysubstance addiction (Blum et al., 1991, Blum et al., 1990, Cobos et al., 2007, Comings et al., 1996, Lawford et al., 2000, Munafo et al., 2004, Noble, 1994, Persico et al., 1996), and several psychiatric disorders (Comings et al., 1991, Denys et al., 2006, Glatt et al., 2009, Golimbet et al., 2003, Nisoli et al., 2007). In 1990, the ANKK1/DRD2 TaqIA variant also examined in this study (rs1800497) was one of the first genetic variants found to be associated with alcoholism (Blum et al., 1991, Blum et al., 1990), and since that time has been one of the most-studied variants in relation to neurological and psychiatric conditions, including substance abuse and dependence disorders. Initially identified as a polymorphism in the promoter region of DRD2 (as it is located 10,000 bases upstream from the DRD2 transcription start site), ANKK1/DRD2 TaqIA was later found to be a non-synonymous variant in the final exon of the subsequently discovered ANKK1 gene (Neville et al., 2004). The functional significance of this variation has not been identified although it changes an amino acid (Glu713Lys) in a concatenation of ankyrin repeats, for which this kinase is partially named.
In this preliminary study, we have shown that genetic variation in the ANKK1 gene may modify the subjective effects of cocaine, given that, in this study, individuals carrying the rs1800497 minor T allele reported greater subjective response. Since the T allele of rs1800497 in the ANKK1 gene is associated with lower DA D2 receptor tone, we speculate that when cocaine blocks the DA transporter and DA concentration in the synapse is increased, it might take less DA to saturate the reduced number DA receptors in individuals with these genetic variants, causing an increased response to the drug. More studies will be required in order to test this hypothesis.
A potential limitation of this study is that the cohort is relatively small. This should be considered a pilot study and replication in a separate cohort is needed.
In summary, individuals with the rs1800497 minor T allele in the ANKK1 gene reported increased ratings on some subjective effects of cocaine such as ‘high’ and ‘like’ in this preliminary study. These findings upon replication in future studies may help to better treat individuals who may be at higher risk of continuing to use cocaine or those at greater risk to relapse during periods of abstinence, and may help to provide insight into the mechanism underlying cocaine addiction.
Supplementary Material
Subjective effect scores for ‘high’ (panel A) and ‘any drug effect’ (panel B) are shown for each time point starting with baseline at time zero. Scores for each time point in the GT/TT genotype group (N = 10) are represented by the solid line, and time points for the GG genotype group (N = 37) are represented by the dashed line. Error bars indicate standard error of the mean.
For the subjective scores of ‘high’ there was a significant main effect of genotype (F = 11.14; df = 1,179; p = 0.001, with an effect size of 0.0622) and time (F = 11.67; df = 1,179; p = 0.0008, with an effect size of 0.0652), without a significant interaction (p = 0.89). The difference from baseline between the genotype groups was the largest for ‘high’ at 5 minutes into the trial where the GT/TT genotype group had values of 77.8+/−10.74 (s.e.m.) while the GG genotype group had lower scores of 49.46+/− 7.31 (s.e.m.) (panel A). For the subjective score of ‘any drug effect’ there was a significant main effect of genotype (F = 13.86; df = 1,175; p = 0.0003, with an effect size of 0.0774) and time (F = 14.91; df = 1,179; p = 0.0002, with an effect size of 0.0833), without a significant interaction (p = 0.59). The difference from baseline between the genotype groups was the largest for ‘any drug effect’ at 5 minutes into the trial. The GT/TT genotype group had values of 86.3 ± 8.49 (s.e.m.) while the GG genotype group had lower scores of 52.70 ± 7.69 (s.e.m.) (panel 2B). The other subjective effect variables did not reach the p < 0.05 level of significance and are therefore not reported.
Subjective effect scores for ‘high,’ ‘any drug effect,’ and ‘like’ are shown for each gene at each time point starting with baseline at time zero. The left panel (40mg) is data from the treatment condition, the right panel (saline) is data from the placebo condition. Subjective effect scores represent the subjective effect value with the baseline score (−15 time point) subtracted from each time point. Scores for each time point in the DRD2 GT/TT or ANKK1 CT/TT genotype groups (N = 10 and N=21, respectively) are represented by the solid line, and time points for the DRD2 GG and ANKK1 CC genotype groups (N = 37 and N=26, respectively) are represented by the dashed line. Error bars indicate standard error of the mean.
Acknowledgments
Funding sources: Supported by: NIH/NIDA 5 P50 DA018197-05 (TK, DN), through MD Anderson's Cancer Center Support Grant DA026120 NIH/NIDA DA026120, and the Toomim Family Fund. This material is the result of work supported with resources and the use of facilities at the Michael E. DeBakey VA Medical Center, Houston, TX.
Footnotes
Statement of conflict of interest: None declared
The authors have no conflicts of interest to declare.
References
- Agmo A, Berenfeld R. Reinforcing properties of ejaculation in the male rat: role of opioids and dopamine. Behav Neurosci. 1990;104:177–182. doi: 10.1037//0735-7044.104.1.177. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Blum K, Noble E, Sheridan P, Finley O, Montgomery A, Ritchie T, Ozkaragoz T, Fitch R, Sadlack F, Sheffield D, et al. Association of the A1 allele of the D2 dopamine receptor gene with severe alcoholism. Alcohol. 1991;8:409–416. doi: 10.1016/0741-8329(91)90693-q. [DOI] [PubMed] [Google Scholar]
- Blum K, Noble E, Sheridan P, Montgomery A, Ritchie T, Jagadeeswaran P, Nogami H, Briggs A, Cohn J. Allelic association of human dopaine D2 receptor gene in alcoholism. JAMA. 1990;26:2055–2060. [PubMed] [Google Scholar]
- Cobos JPdl, Baiget M, Trujols J, Sinol N, Volpini V, Banuls E, Calafell F, Luquero E, Rio Ed, Alvarez E. Allelic and genotypic associations of DRD2 TaqIA polymorhpism with heroin dependence in Spanish subjects: a case control study. Behavioral and Brain Functions. 2007;3:25. doi: 10.1186/1744-9081-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings D, Comings B, Muhleman D, Dietz G, Shahbahrami B, Tast D, Knell E, Kocsis P, Bumgarten R, Kovaks B, Levy D, Smith M, Borison R, Evans D, Klein D, MacMurray J, Tosk J, Sverd J, Gysin R, Flanagan S. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. JAMA. 1991;266:1793–1800. [PubMed] [Google Scholar]
- Comings D, Rosenthal R, Lesieur H, Rugle L, Muhleman D, Chiu C, Dietz G, Gade R. A study of the dopamine D2 receptor gene in pathological gambling. Pharmacogenetics. 1996;6:223–234. doi: 10.1097/00008571-199606000-00004. [DOI] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Connor J, Young R, Lawford B, Saunders J, Ritchie T, Noble E. Heavy nicotine and alcohol use in alcohol dependence is associated with D2 dopamine receptor (DRD2) Addict Behav. 2007;32:310–319. doi: 10.1016/j.addbeh.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Denys D, Nieuwerburgh FV, Deforce D, Westenberg H. Association between the dopamine D2 receptor TaqIA A2 allele and low activity COMT allele with obsessive-compulsive disorder in males. Eur Neuropsychopharmacol. 2006;16:446–450. doi: 10.1016/j.euroneuro.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Dubertret C, Gorwood P, Gouya L, Deybach J, Ades J. Association and excess of transmission of a DRD2 haplotype in a sample of French schizophrenic patients. Schizophr Res. 2001;49:203–212. doi: 10.1016/s0920-9964(00)00085-2. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT, Madden PA. Arch Gen Psychiatry. 2003;60:1033–1039. doi: 10.1001/archpsyc.60.10.1033. [DOI] [PubMed] [Google Scholar]
- Glatt S, Faraone S, Lasky-Su J, Kanazawa T, Tsuang M. Family-based association testing strongly implicates DRD2 as a risk gene for schizophrenia in Han Chinese from Taiwan. Mol Psychiatry. 2009;14:885–893. doi: 10.1038/mp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golimbet V, Aksenova M, Nosikov V, Orlova V, Kaleda V. Analysis of the linkage of the Taq1A and Taq1B loci of the dopamine D2 receptor gene with schizophrenia in patients and their siblings. Neurosci Behav Physiol. 2003:223–225. doi: 10.1023/a:1022191012698. [DOI] [PubMed] [Google Scholar]
- Haertzen CA, Kocher TR, Miyasato K. Reinforcements from the first drug experience can predict later drug habits and/or addiction: results with coffee, cigarettes, alcohol, barbiturates, minor and major tranquilizers, stimulants, marijuana, hallucinogens, heroin, opiates and cocaine. Drug Alcohol Depend. 1983;11:147–165. doi: 10.1016/0376-8716(83)90076-5. [DOI] [PubMed] [Google Scholar]
- Hart AB, de Wit H, Palmer AA. Genetic Factors Modulating the Response to Stimulant Drugs in Humans. Curr Top Behav Neurosci. 2012 doi: 10.1007/7854_2011_187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubisz MJ, Falush D, Stephens M, Pritchard JK. Inferring weak population structure with the assistance of sample group information. Mol Ecol Resour. 2009;9:1322–1332. doi: 10.1111/j.1755-0998.2009.02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson E, Nothen M, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall G. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy voluneers. Mol Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Wu G, Huang W, Harding MJ, Hamon SC, Lappalainen J, Nielsen DA. Pharmacogenetic randomized trial for cocaine abuse: disulfiram and dopamine beta-hydroxylase. Biol Psychiatry. 2013;73:219–224. doi: 10.1016/j.biopsych.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao O, Dujin Kv, Kersbergen P, Knijff Pd, Kayser M. Proportioning whole-genome single-nucleotide-polymorphism diversity for the identification of geographic population structure and genetic ancestry. The American Journal of Human Genetics. 2006;78:680–690. doi: 10.1086/501531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawford B, Young R, Noble E, Sargent J, Rowell J, Shadforth S, Zhang X, Ritchie T. The D(2) dopamine receptor A(1) allele and opioid dependence: association with heroin use and response to methadone treatment. Am J Med Genet. 2000;96:592–598. doi: 10.1002/1096-8628(20001009)96:5<592::aid-ajmg3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- McLellan A, Kushner H, Metzger D, Peters R, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Moyer RA, Wang D, Papp AC, Smith RM, Duque L, Mash DC, Sadee W. Intronic polymorphisms affecting alternative splicing of human dopamine D2 receptor are associated with cocaine abuse. Neuropsychopharmacology. 2011;36:753–762. doi: 10.1038/npp.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo M, Clark T, Johnstone E, Murphy M, Walton R. The genetic basis for smoking behavior: a systematic review and meta-analysis. Nicotine Tob Res. 2004;6:583–597. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- Nader M, Morgan D, Gage H, Nader S, Calhoun T, Buchheimer N, Ehrenkaufer R, Mach R. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. The neurobiology of cocaine addiction. Sci Pract Perspect. 2005;3:4–10. doi: 10.1151/spp05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville M, Johnstone E, RTW Identification and characterization of ANKK1: A novel kinase gene closely linked to DRD2 on chromosome band 11p23.1. Hum Mutat. 2004;23:540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Brunani A, Borgomainerio E, Tonello C, Dioni L, Briscini L, Redaelli G, molinari E, Cavagnini F, Carruba M. D2 dopamine receptor (DRD2) gene Taq1A polymorphism and the eating-related psychological traits in eating disorders (anorexia nervosa and bulimia) and obesity. Eat Weight Disord. 2007;12:91–96. doi: 10.1007/BF03327583. [DOI] [PubMed] [Google Scholar]
- Noble E. Polymorphisms of the D2 dopamine receptor gene and alcoholism and other substance use disorders. Alcohol and Alcoholism Supplement. 1994;2:35–43. [PubMed] [Google Scholar]
- Noble E, Blum K, Khalsa M, Ritchie T, Montgomery A, Wood R, Fitch R, Ozkaragoz T, Sheridan P, Anglin M. Allelic association of the D2 dopamine receptor gene with cocaine dependence. Drug Alcohol Depend. 1993;33:271–285. doi: 10.1016/0376-8716(93)90113-5. [DOI] [PubMed] [Google Scholar]
- Noble E, Blum K, Ritchie T, Montgomery A, Sheridan P. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry. 1991;48:648–654. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- Persico A, Bird G, Gabbay F, Uhl G. D2 dopamine receptor gene TaqI A1 and B1 restriction fragment length polymorphisms: enhanced frequencies in psychostimulant-preferring polysubstance abusers. Biol Psychiatry. 1996;40:776–784. doi: 10.1016/0006-3223(95)00483-1. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Nomikos GG, Wenkstern DG, Blaha CD, Phillips AG, Fibiger HC. Sexual behavior enhances central dopamine transmission in the male rat. Brain Res. 1990;530:345–348. doi: 10.1016/0006-8993(90)91309-5. [DOI] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne J, Nagren K, Lehikoinen P, anttila K, Swalathi E, Hietala J. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R_Development_Core_Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2009. [Google Scholar]
- Ritchie T, Noble E. Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochemical Research. 2003;28:73–82. doi: 10.1023/a:1021648128758. [DOI] [PubMed] [Google Scholar]
- NS, editor. SAMHSA. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings H-44. Rockville, MD: 2012. [Google Scholar]
- Sheehan D, Lecrubier Y, Sheehan K, Amorim P, Janavs J, Weiller E, Herqueta T, Baker R, Dunbar G. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Thompson J, Thomas N, Singleton A, Piggott M, Lloyd S, Perry E, Morris C, Perry R, Ferrier I, Court A. D2 dopamine receptor gene (DRD2) Taq1A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics. 1997;7:479–484. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Voisey J, Swagell C, Hughes I, van Daal A, Noble E, Lawford B, Young R, Morris C. A DRD2 and ANKK1 haplotype is associated with nicotine dependence. Psychiatry Res. 2012;196:285–289. doi: 10.1016/j.psychres.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, et al. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry. 1990;147:719–724. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Ding YS, Logan J, Dewey SL, Hitzemann R, Lieberman J. Relationship between psychostimulant-induced “high” and dopamine transporter occupancy. Proc Natl Acad Sci U S A. 1996;93:10388–10392. doi: 10.1073/pnas.93.19.10388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Yang B, Kranzler H, Zhao H, Gruen J, Luo X, Gelernter J. Association of haplotypic variants in DRD2, ANKK1, TTC12 and NCAM1 to alcohol dependence in independent case control and family samples. Hum Mol Genet. 2007;16:2844–2853. doi: 10.1093/hmg/ddm240. [DOI] [PubMed] [Google Scholar]
- Yang B, Kranzler H, Zhao H, Gruen J, Luo X, Gelernter J. Haplotypic variants in DRD2, ANKK1, TTC12 and NCAM1 are associated with comorbid alcohol and drug dependence. Alcohol Clin Exp Res. 2008;32:2117–2127. doi: 10.1111/j.1530-0277.2008.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, Xiao T, Papp A, Wang D, Sadee W. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci U S A. 2007;104:20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subjective effect scores for ‘high’ (panel A) and ‘any drug effect’ (panel B) are shown for each time point starting with baseline at time zero. Scores for each time point in the GT/TT genotype group (N = 10) are represented by the solid line, and time points for the GG genotype group (N = 37) are represented by the dashed line. Error bars indicate standard error of the mean.
For the subjective scores of ‘high’ there was a significant main effect of genotype (F = 11.14; df = 1,179; p = 0.001, with an effect size of 0.0622) and time (F = 11.67; df = 1,179; p = 0.0008, with an effect size of 0.0652), without a significant interaction (p = 0.89). The difference from baseline between the genotype groups was the largest for ‘high’ at 5 minutes into the trial where the GT/TT genotype group had values of 77.8+/−10.74 (s.e.m.) while the GG genotype group had lower scores of 49.46+/− 7.31 (s.e.m.) (panel A). For the subjective score of ‘any drug effect’ there was a significant main effect of genotype (F = 13.86; df = 1,175; p = 0.0003, with an effect size of 0.0774) and time (F = 14.91; df = 1,179; p = 0.0002, with an effect size of 0.0833), without a significant interaction (p = 0.59). The difference from baseline between the genotype groups was the largest for ‘any drug effect’ at 5 minutes into the trial. The GT/TT genotype group had values of 86.3 ± 8.49 (s.e.m.) while the GG genotype group had lower scores of 52.70 ± 7.69 (s.e.m.) (panel 2B). The other subjective effect variables did not reach the p < 0.05 level of significance and are therefore not reported.
Subjective effect scores for ‘high,’ ‘any drug effect,’ and ‘like’ are shown for each gene at each time point starting with baseline at time zero. The left panel (40mg) is data from the treatment condition, the right panel (saline) is data from the placebo condition. Subjective effect scores represent the subjective effect value with the baseline score (−15 time point) subtracted from each time point. Scores for each time point in the DRD2 GT/TT or ANKK1 CT/TT genotype groups (N = 10 and N=21, respectively) are represented by the solid line, and time points for the DRD2 GG and ANKK1 CC genotype groups (N = 37 and N=26, respectively) are represented by the dashed line. Error bars indicate standard error of the mean.