Abstract
Background
An assortment of variables has been used in predicting anterior shoulder instability resulting from pathologic engagement of Hill-Sachs lesions on the glenoid. The glenoid track is a unique biomechanical model that relates both Hill–Sachs and bony Bankart lesions to predict shoulder engagement. We examined the glenoid track concept to determine if it provides a model that unifies glenoid rim and humeral head bone loss in predicting engagement.
Questions/purposes
In this review we addressed two questions: (1) How are humeral head and glenoid rim bony defects and their interactions quantified? (2) Why is the concept of the glenoid track important?
Methods
We performed a systematic review of the literature using PubMed (MEDLINE) and OVID for biomechanical studies and peer-reviewed articles published until March 2013. Twenty-four studies fit the inclusion criteria. These were subdivided into four anatomic studies, four studies quantifying glenohumeral bone loss, nine studies biomechanically defining shoulder engagement, six studies analyzing current treatment models, and one clinical study to be included in the final review.
Results
Data demonstrate pathologic engagement is dependent on the medial margin of the Hill–Sachs lesion traveling outside the glenoid track. The width of the glenoid track decreases accordingly if there is a glenoid defect, making engagement more likely. Most treatment models focus on widening the glenoid track before addressing Hill–Sachs lesions.
Conclusions
The glenoid track uses both glenoid and humeral head bone loss to predict subsequent risk of humeral head engagement and possible dislocation. The glenoid track shows us that restoring the track to its natural width should be among the surgeon’s first priority in restoring shoulder stability. Humeral head lesions, also known as Hill–Sachs lesions, are surgically addressed when they cause clinical symptoms. Symptoms arise when the medial margin of the defect engages the glenoid track.
Introduction
It is well established that glenoid bone loss (Bankart lesions) and humeral head bone loss (Hill–Sachs lesions) are associated with shoulder instability. The incidence of Hill–Sachs lesions in anterior shoulder instability ranges from 38% to 88% and is associated with up to 100% of recurrent dislocations [4, 12, 22]. The geometry of the glenohumeral joint surface provides less static stabilization compared with other joints because of the smaller surface area and shallowness of the glenoid in comparison with the humeral head [16]. Glenohumeral joint stability is dependent on multiple dynamic and static stabilizers whose importance changes depending on the position of the arm. When the shoulder is in the middle ROM (defined as up to 30° glenohumeral abduction, AP translation, or superoinferior translation), it is stabilized by negative intraarticular pressure along with a concavity-compression effect caused by muscle contraction on the glenoid cavity. A bony lesion that results in disruption of the negative intraarticular pressure could cause shoulder instability.
At extremes of motion such as during maximum external rotation and abduction, as seen in pitchers, the glenohumeral joint is kept congruous by the anteroinferior shoulder capsule. This end range stability can be disrupted by Bankart lesions and large Hill–Sachs lesions that engage with the anterior rim of the glenoid and lever the humerus out of congruity.
Multiple studies have attempted to relate risk of engagement with focus on either the Bankart or the Hill–Sachs size, diameter, and volume with mixed results. A potential weakness of just looking at the one humeral aspect of the joint is that it ignores the many interactions of the joint. It is intuitive that instability resulting from a Hill–Sachs injury is potentiated by glenoid bone deficiency. The relationship of Hill–Sachs defects to anterior shoulder instability is well recognized (Fig. 1A–B); however, the impact in association with glenoid bone loss is less clearly defined. There is a lot of heterogeneity in the assessment of Hill–Sachs lesions that has made quantifying and treatment decisions difficult. There has been disagreement as to whether depth, volume, or other factors is the most important factor to determine risks of further engagement or dislocation. The glenoid track concept tries to best explain this through arguing that location is the most important factor in predicting whether a lesion is clinically significant.
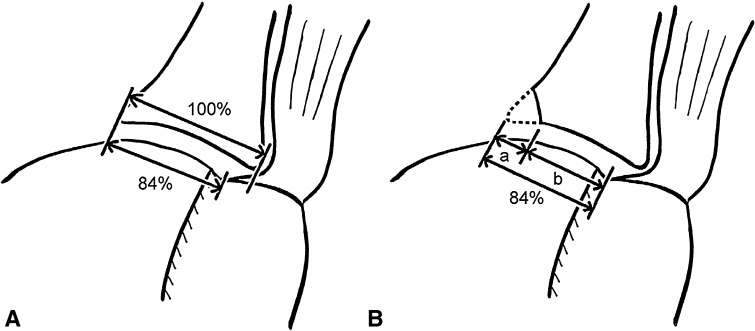
Fig. 1A–B.
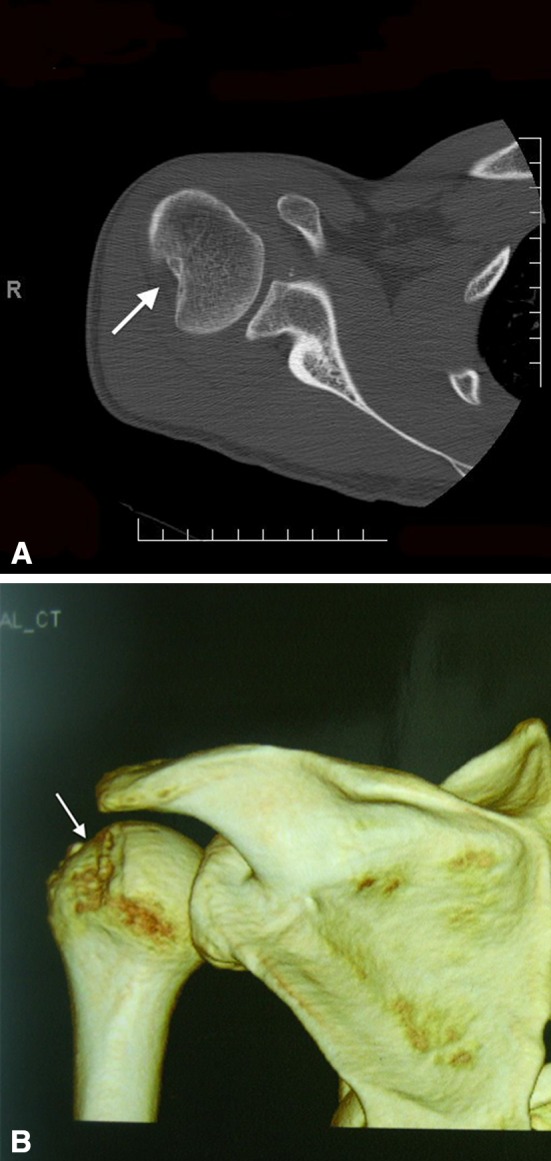
(A) This is a CT scan axial view of a right shoulder with a large Hill–Sachs lesion (denoted by the white arrowhead). (B) This is a three-dimensional CT reconstruction of the same large Hill–Sachs lesion shown in (A).
Burkhart and De Beer [2] describe the “engaging Hill–Sachs lesion” as an arthroscopic finding and is based on the arm being in a position of function (maximally externally rotated and abducted). The prevalence of engaging lesions has varied from 1.5% to 34% [2, 5, 19]. Yamamoto et al. [30] introduced the concept of the glenoid track based on the arm in a position of function to biomechanically relate both humeral head and glenoid rim bone loss to shoulder instability. The study found that as glenoid bone loss increases, the glenoid track decreases in width making it more likely that the medial aspect of the Hill–Sachs lesion can engage the glenoid rim (Fig. 2A–B).
Fig. 2A–B.
(A) This is a drawing of the glenoid track. Notice the glenoid pushes the cuff tendon at its insertion by 16% of the glenoid width, leaving the remaining 84% covering the articular surface of the humeral head. (B) This is a similar drawing as (A); however, note the large glenoid defect decreasing the total width of the glenoid track. In a case with a bony defect of the glenoid, the defect width (a) should be subtracted from the 84% length to obtain a true glenoid track width (b) in this shoulder. Reprinted with permission from Yamamoto N, Itoi E, Abe H, Minagawa H, Seki N, Shimada Y, Okada K. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16:649–656.
Using a systematic literature review of biomechanical shoulder studies, we addressed the following questions: (1) How are humeral head and glenoid rim bony defects and their interactions quantified? (2) Why is the concept of the glenoid track important? In answering these questions, we demonstrate the importance of examining the glenoid and humeral head bony loss in synergy and how this information can benefit the practicing orthopaedic surgeon.
Search Strategy and Criteria
We searched MEDLINE (through PubMed) and OVID1 up to March 2013. Articles were identified using the following queries: “Shoulder instability” or “glenoid track” or “hill sachs” or “bankart”. In addition to the PubMed and OVID searches, we also conducted a hand search of the Journal of Bone and Joint Surgery, Clinical Orthopaedics and Related Research®, and the Journal of Shoulder and Elbow Surgery for articles published up to March 2013. We identified 4399 total articles. The resulting articles were entered into EndNote® (Thomas Reuters, Carlsbad, CA, USA) for removal of duplicate articles, resulting in a total of 1503 unique references that were retained for further title and abstract review.
Articles were included in the systematic review based on the following criteria: (1) published in English; and (2) cadaver studies; or (3) a clinical study with a level I, II, III, or IV design. We specifically included biomechanical studies on humeral head and glenoid bony loss and (4) redislocation risk in clinical studies and the propensity to redislocate in cadaveric or biomechanical studies after bony repairs to the glenoid and/or the humeral head. All authors screened articles to determine inclusion or exclusion; disagreements were resolved through discussion and consensus.
We specifically excluded articles that had concurrent capsular, ligamentous, rotator cuff, or labrum injury. We also excluded Bankart or Hill–Sachs lesions in the context of concurrent arthritis, neoplasm, humeral shaft, clavicle, or scapula fracture. All authors independently verified included articles to ensure that the inclusion and exclusion criteria were being met.
A total of 87 articles were eventually identified for detailed review with the predominant patient population being young males with shoulder instability. Of these, 24 were identified as topic-pertinent cadaver and biomechanical studies (Fig. 3). Of these 24 articles, four correlated humeral head and glenoid anatomy [6, 16, 26, 28]. Four articles examined imaging strategies used to quantified humeral head and glenoid bone loss [5, 17, 21, 27]. Nine articles correlated humeral head and/or glenoid bone loss with shoulder engagement [2, 9, 10, 13, 14, 24, 29–31]. Six articles defined current concepts in Bankart and Hill–Sachs repair and treatment [3, 7, 8, 11, 15, 25]. Finally, one article relates the glenoid track concept to current clinical practices [18].
Fig. 3.
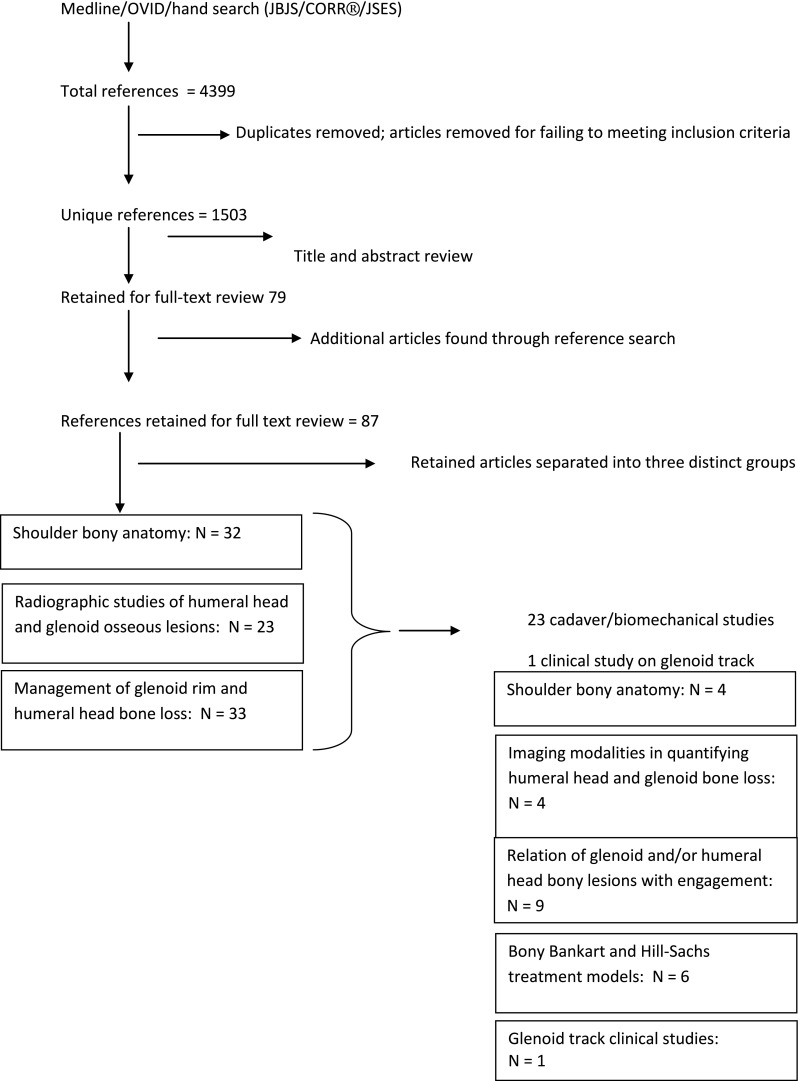
A flow diagram shows the methods of article selection for study inclusion. JBJS = Journal of Bone and Joint Surgery; CORR® = Clinical Orthopaedics and Related Research®; JSES = Journal of Shoulder and Elbow Surgery.
Results
How Are Humeral Head and Glenoid Rim Bony Defects and Their Interactions Quantified?
Huysmans et al. [9] demonstrated the shape of the inferior glenoid to be a true circle in 39 of 40 cadaveric scapulae (Table 1). Glenoid loss can be calculated using the circle method in which a line is drawn about the long axis of the glenoid with a best-fit circle placed about the inferior border of the glenoid. Glenoid bone loss can be calculated through the difference between expected and actual diameter of the glenoid. Itoi et al. [10] showed that CT scans are better than axillary or West Point views in assessing glenoid bone loss. Itoi et al. also showed that a glenoid defect at 45° from the longitudinal axis of the glenoid of 21% can result in a loss of 50% of width at the inferior one-fourth of the glenoid, resulting in instability.
Table 1.
Shoulder anatomy
| Study | Year | Sample size | Study purpose | Conclusions |
|---|---|---|---|---|
| Curtis et al. [6] | 2006 | 20 (cadaver study) | Define the rotator cuff footprint and relate to major landmarks | A consistent pattern of rotator cuff attachment tendons to the humerus was delineated |
| Kelkar et al. [16] | 2001 | 9 (cadaver study) | Define the relation between articular surface geometry and contact patterns | Small translations of the humeral head position on the glenoid are possible; translation depends on joint congruence and humeral external rotation |
| Soslowsky et al. [26] | 1992 | 9 (cadaver study) | Determine contact areas in a normal glenohumeral joint | Contact areas are greatest at midelevation positions and positions of function |
| Warner et al. [28] | 1998 | 10 (cadaver study) | Define the articular contact patterns of a normal glenohumeral joint | Shoulder abduction resulted in increased joint congruence and increased contact area increasing |
Kodali et al. [17] demonstrated that with two-dimensional CT, individual observers are more accurate in determining depth rather than width measurements of Hill–Sachs lesions. Coronal views gave the largest percentage error at 19.2% ± 13.6%, whereas sagittal views the least at 11.8% ± 8.2%. A defect that is more perpendicular to the image plane makes it easier for an observer to select an image that depicts the maximum depth and width; however, in defects relatively parallel to the image plane, the observer is likely to select an image that represents maximum depth or width but not both.
Cho et al. [5] used CT scans to determine engaging Hill–Sachs lesions are larger and more horizontally orientated to the humeral shaft. Burkhart and De Beer [2] describe similar findings in which engaging Hill–Sachs lesions are positioned more horizontal to the humeral shaft than nonengaging lesions.
Kurokawa et al. [18] used CT to demonstrate that Hill–Sachs engagement does not depend on width of the humeral lesion, but on the location of the lesion’s medial margin. In a series of 100 cases with a Hill–Sachs lesion, 7% had engaging lesions. Three percent of these had wide Hill–Sachs lesions, whereas 4% had narrow but medially located Hill–Sachs lesions. All engaging lesions had substantial (greater than 20% of the affected glenoid width) glenoid bone loss (Table 2).
Table 2.
Imaging modalities in quantifying humeral head and glenoid bone loss
| Study | Year | Sample size | Study purpose | Conclusions |
|---|---|---|---|---|
| Kodali et al. [17] | 2011 | 6 (anatomic bone substitutes) | Assess the accuracy of CT scan in measuring Hill–Sachs lesions | Hill–Sachs width is underestimated in all planes with CT scans; sagittal and axial plane measurements are the most accurate in evaluating defects |
| Sugimoto [27] | 2004 | 6 (cadaver study) | Clarify the reliability of ultrasound in evaluating a Bankart lesion | Ultrasound with axillary approach is useful in diagnosing Bankart lesions |
| Cho et al. [5] | 2011 | 107 (prospective cohort) | Judge the diagnostic validity of three-dimensional CT to predict engagement of Hill–Sachs lesions | Engaging lesions were larger in size and horizontally orientated to the humeral shaft than non-engaging lesions on three-dimensional CT scan |
| Richards et al. [21] | 1994 | 8 (cadaver study) | Can MRI be used to differentiate Hill–Sachs lesions from the anatomic groove on the posterior humerus? | Hill–Sachs are best differentiated by means of a more cephalic position along the longitudinal humeral axis |
In addition to noninvasive preoperative imaging, intraoperative findings help quantify bone loss. Burkhart and De Beer [2] and Burkhart et al. [3] used intraoperative findings to calculate glenoid bone loss while using the bare spot as the anatomic center of the glenoid. Huysman et al. [9] describe using the bare spot and a calibrated probe to identify global glenoid bone loss but note the exact center of the bare spot can sometimes be difficult to determine resulting in measurement error.
Why Is the Concept of the Glenoid Track Important?
Early studies of contact areas at the glenohumeral joint demonstrate a complex interaction of the glenoid and humeral head. Kaar et al. [14] and Sekiya et al. [24] note that increasing the size of a humeral bony defect increases the risk of engagement; however, a defect of up to 25% in isolation does not necessarily result in engagement. With elevation and abduction, the humeral head dramatically migrates from an inferior region to a superior-central posterior region of the glenoid and contact area increased [26, 28]. Kurokawa et al. [18] noted that all of their engaging Hill–Sachs lesions also had significant glenoid bone loss (Table 3). The importance of glenoid surface area was further characterized by Saito et al. [23] and Ji et al. [13] who found the major direction of glenoid defects was in a more anterior portion rather than anteroinferior in patients with recurrent shoulder dislocation (Table 4).
Table 3.
Relation of glenoid and/or Humeral head bony lesions with engagement
| Study | Year | Sample size | Study purpose | Conclusions |
|---|---|---|---|---|
| Ji et al. [13] | 2012 | 44 (cadaver-controls) 24 (cohort) |
Define the most common location of glenoid bony defects in recurrent dislocations based on three-dimensional CT | Most common location of glenoid defect was at the 3:20 position; glenoid bone defects were anterior as opposed to anteroinferior in recurrent dislocators |
| Sekiya et al. [24] | 2012 | 9 (cadaver study) | Does a 25% Hill–Sachs defect affect glenohumeral translation? | A 25% Hill–Sachs defect in isolation with an intact capsule does not cause recurrent instability |
| Yamamoto et al. [30] | 2007 | 9 (cadaver study) | Determine the articulating surface of the humeral head with the glenoid through the arm ROM | Glenoid track is 84% of the glenoid width; if the medial margin of a Hill–Sachs lesion is more medial than the track, it will engage |
| Burkhart and De Beer [2] | 2000 | 194 patients (case series) | Analyze Bankart repairs to determine factors that influence instability | Significant bone loss is best treated with open repairs and bone grafting |
| Kaar et al. [14] | 2010 | 8 (cadaver study) | Test hypothesis that glenohumeral stability decreases with increasing larger Hill–Sachs lesions | Glenohumeral stability decreases at five-eighths radius defect of the humeral head |
| Yamamoto et al. [29] | 2009 | 8 (cadaver study) | Determine the critical size of an anterior glenoid defect on anterior stability | Loss of 20% of anterior glenoid (3 o’clock position) width results in instability significant enough to warrant reconstruction |
| Yamamoto et al. [31] | 2010 | 13 (cadaver study) | Determine critical size of bony Bankart lesion and determine stabilizing effects of bone grafting | Bankart defects of ≥ 19% (6 mm) of the glenoid length remain unstable after repair and procedures to reconstruct glenoid concavity should be considered |
| Huysmans et al. [9] | 2006 | 40 (cadaver study) | Determine if the inferior glenoid is shaped as a circle and is the bare spot the center of the circle | Inferior glenoid is a true circle; however, the bare spot is an approximation of the circle’s center; the bare spot can be used for referencing glenoid bone loss |
| Itoi et al. [10] | 2003 | 12 (cadaver study) | What is the critical size of glenoid defects that results in instability? | A 21% defect results in 50% loss of inferior one-fourths glenoid width and resulting instability |
Table 4.
Bankart and Hill–Sachs treatment models
| Study | Year | Sample size | Study purpose | Conclusions |
|---|---|---|---|---|
| Elkinson et al. [7] | 2012 | 8 (cadaver study) | Examine effects of remplissage on glenohumeral stability as an adjunct to Bankart repairs with Hill–Sachs defects of 15%–30% | After Bankart repair, no additional stability was provided with remplissage of Hill–Sachs lesions of 15% and ROM was diminished; for Hill–Sachs lesions of 30%, stability was provided by remplissage |
| Kazel et al. [15] | 2005 | 14 (cadaver study) | Can a bone tamp be used to correct a Hill–Sachs defect using a technique of humeroplasty? | Humeroplasty significantly reduced width, length, depth and volume of “small” Hill–Sachs lesions |
| Giles et al. [8] | 2012 | 8 (cadaver study) | Comparison between 3 “moderate-large” Hill–Sachs repair techniques; humeral head allografting (HHA), partial resurfacing arthroplasty (PRA), and remplissage | All improved stability; HHA most resembled intact shoulders; PRA does not fully prevent engagement; remplissage decreases ROM |
| Itoi et al. [11] | 2000 | 16 (cadaver study) | Determine the effect of various sized Bankart lesions on shoulder stability before/after repair | Glenoid defects with width of ≥ 21% of glenoid length increase the likelihood of instability and limit ROM after Bankart repair |
| Sekiya et al. [25] | 2009 | 9 (cadaver study) | Assess effects of size and orientation of Hill–Sachs defect on stability and osteoarticular repair | Defects as small as 12.5% could affect stability if defects in line with anterior glenoid and stability was reduced with increasing size; large humeral head osseous defects (> 37.5%) may benefit from osteoarticular grafting |
| Burkhart et al. [3] | 2002 | 194 patients (case series) | Analyze Bankart repairs to determine factors that influence instability | Significant bone loss is best treated with open repairs and bone grafting |
Yamamoto et al. [30] defined the glenoid track using cadaveric models to map the path of the glenoid rim on the humeral head as the arm moved to maximum abduction, extension, and external rotation. It was noted that the width of the glenoid track was 84% of the total glenoid width because there was overlap laterally with the rotator cuff attachment. Additional glenoid defects are subtracted from 84% to give the corrected glenoid width (Fig. 4A–B).
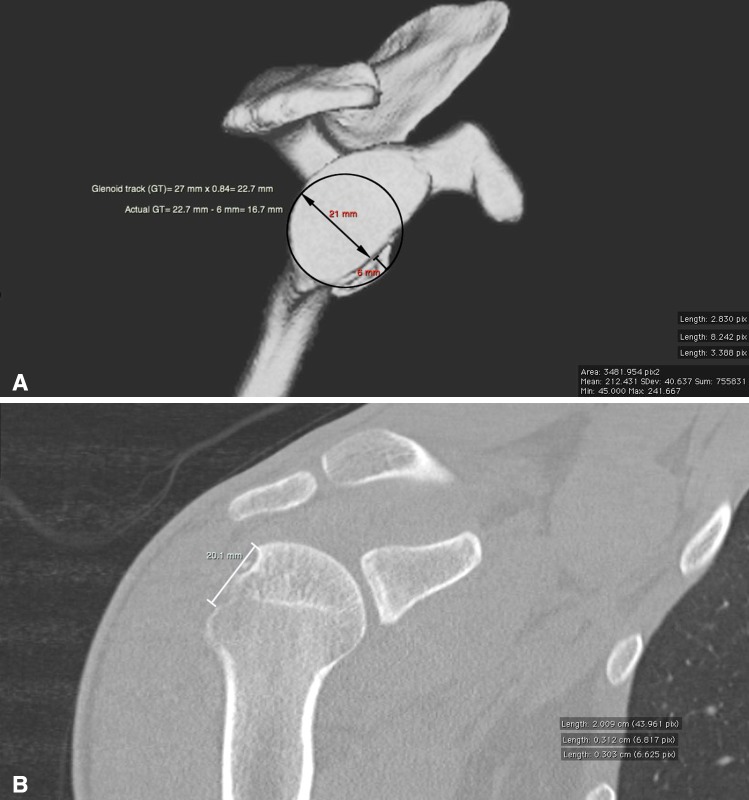
Fig. 4A–B.
(A) A coronal view CT of the right shoulder that corresponds to the largest size of the Hill–Sachs lesion measuring 20.1 mm at the medial margin. (B) This is a three-dimensional CT reconstruction of the glenoid showing anterior bone loss. Glenoid track (GT) width = 27 mm x 0.84 = 22.7 mm; actual GT width = 22.7 mm − 6 mm = 16.7 mm; X = actual GT = 16.7 mm; Y = medial margin of Hill–Sachs lesion = 20.1 mm. If Y > X, lesion will engage 20.1 mm > 16.7 mm ⇒ engagement.
In sharp contrast to humeral bony defects, Yamamoto et al. [31] and Itoi et al. [10] note that anterior glenoid defects greater than 19% the width of the total glenoid length remain unstable even after Bankart repair and require additional bone grafting for stability (Table 5).
Table 5.
Glenoid track clinical studies
| Study | Year | Sample size | Study purpose | Conclusions |
|---|---|---|---|---|
| Kurokawa et al. [18] | 2013 | 100 (case series) | Use the glenoid track concept to clarify prevalence of engaging shoulder lesions | 2 types of engaging Hill–Sachs lesions–wide and large and narrow, but medially located; these lesions are dependent on location of the medial margin |
Discussion
An assortment of variables has been used in predicting anterior shoulder joint instability resulting from pathologic engagement of Hill–Sachs lesions on the glenoid. However, controversy still exists over the best method to predict shoulder engagement. Most studies assessing shoulder instability concentrate on bone deficits of the humerus or the glenoid in isolation. These studies overlook the interaction of the two lesions through the arc of ROM and how this may influence instability. The glenoid track is the first model to determine, in a dynamic way, how bone loss on both sides of the joint can lead to instability. In this review, we sought to further examine the concept of the glenoid track and addressed the following questions: (1) How are humeral head and glenoid rim bony defects and their interactions quantified? (2) Why is the concept of the glenoid track important?
There were several limitations to our study. First, Yamamoto et al.’s [30] initial study is the only biomechanical study of the glenoid track concept to date. All other biomechanical studies focused on either glenoid or humeral head bony defects in isolation from which we had to extrapolate results to apply to our study. Second, the dominance of cadaveric studies in our study can reflect bias because they do not fully simulate in vivo conditions. Third, biomechanical and anatomic studies are often not reported in a unified manner, making synthesis of results difficult. Despite the limited biomechanical and sparse clinical studies regarding the glenoid track concept, we believe the systematic nature of our search encompasses current ideas on the combined effects of glenoid rim and humeral head bony defects on shoulder engagement. Lastly, although a rigorous assessment of the topic using systematic review methods was used, this topic does not easily lend itself to the systematic review methodologies. In answering our second question, we hoped to educate the reader rather than simply answer a testable question.
Previous studies have produced multiple models in predicting glenohumeral bone loss but have failed to accurately predict shoulder engagement [20]. However, it has been noted that lesions in line with the anterior glenoid in a position of function [5, 25], or medially located, are more likely to result in instability [18, 30]. In isolated studies of humeral head bone loss, Sekiya et al. [25] determined that humeral head defects of up to 25% in isolation have significantly less anterior translation before dislocation. However, large osteoarticular defects, greater than 37.5%, could benefit from an allograft transplant to restore shoulder stability. In a similar study, Kaar et al. [14] determined that humeral head defects that are five-eighths total width of the humeral head radius showed a significant decrease in stability. Similarly, in isolated studies of the glenoid, Yamamoto et al. [29] determined that lesions greater than 20% result in possible engagement. However, to accurately predict shoulder engagement, it is vital to assess both glenoid and humeral head bone loss.
Yamamoto et al.’s [30] concept of the glenoid track takes both humeral head and glenoid lesions into consideration when assessing a shoulder with anterior instability. In following the pathway of the humerus on the glenoid through functional ROM, several important concepts were appreciated. First, bone loss from the glenoid results in a smaller track making it more likely for the humerus to engage and dislocate. Second, humeral lesions located medially are at increased risk of engagement. Although both of these findings have been validated through prior research, the glenoid track concept best unifies these concepts. Recent clinical trials have shown promise to the concept of the glenoid track. Kurokawa et al. [18] noted that in the setting of glenoid bone loss, shoulder engagement depended on the location of the medial margin of the humeral lesion. Kurokawa et al. calculated an occupancy ratio of the Hill–Sachs lesion in the glenoid track with the medial margin of the rotator footprint a fixed point and the two variables being the medial margin of the Hill–Sachs lesion along with the glenoid track width. Per their formula, engaging lesions score greater than a 100% occupancy ratio indicating an increased risk of shoulder instability or dislocation (Table 6).
Table 6.
Occupancy ratio
| Occupancy ratio (OR) = ([maximum distance from medial margin of the Hill–Sachs lesion to medial margin of the rotator footprint]/glenoid track width) * 100 |
The use of the glenoid track concept can potentially help guide surgical decision-making. Previous studies have focused on the isolated treatment of Hill–Sachs and bony Bankart lesions. Giles et al. [8], Sekiya et al. [25], Elkinson et al. [7], and Kazel et al. [15] all studied various methods of isolated Hill–Sachs repair, whereas Itoi et al. [10] and Yamamoto et al. [29] both studied various methods of isolated bony Bankart repair. The importance of addressing the glenoid in the setting of humeral deficits was demonstrated by Kurokawa et al. [18] who used a Latarjet procedure to increase the width of the glenoid without treatment of the concurrent Hill–Sachs lesions. They showed that after the Hill–Sachs lesions were placed inside the glenoid track, they became nonengaging. Their study classified engaging Hill–Sachs lesions into two specific types: wide and large versus narrow and medially located. The formula demonstrates that the risk of engagement does not depend on the width of the Hill–Sachs lesion but on the location of its medial margin [1, 31] (Fig. 5). In the context of glenohumeral bony lesions, glenoid rim bone grafting could be considered a primary method to return a Hill–Sachs lesion inside the glenoid track with the various methods of Hill–Sachs repair a secondary option in cases of extensive glenohumeral bone loss (Fig. 6A–C).
Fig. 5.
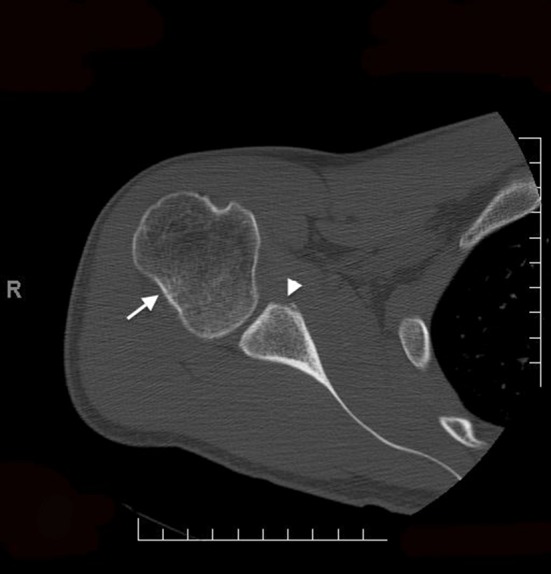
An axial view CT scan of the right shoulder showing a Hill–Sachs lesion (white arrow) with corresponding anterior glenoid bone loss (white arrowhead).
Fig. 6A–C.
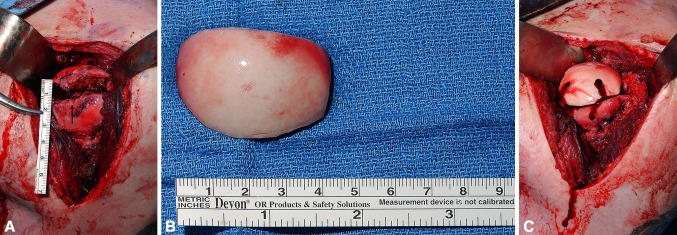
(A) Intraoperative photograph of humeral head showing a large Hill–Sachs lesion indicated with a black arrowhead. (B) Fresh osteochondral humeral head allograft prepared for filling the Hill–Sachs defect. (C) The humeral head allograft is fit into final position affixed with headless compression titanium screws.
Orthopaedic surgeons can use this information in several ways. First, if the Hill–Sachs lesion is particularly wide or medially located, we can predict engagement resulting in shoulder instability and may push for earlier interventions anticipating additional interventions such as having to address the Hill–Sachs lesion with or without glenoid repair. Second, if the Hill–Sachs lesion does not fall out of the glenoid track, then it can be ignored while the glenoid defect can be focused on. Further exploration of the topic may allow for more precise predictions based on imaging, but the best assessment of shoulder stability is done under direct visualization in the operating room.
Although early research is promising, further clinical studies to validate and refine the glenoid track concept are needed to better predict shoulder engagement. Further studies are needed into the predicting ability of the glenoid track. Can the glenoid track be modified to meet the sport-specific demands of various athletes? Although the clinical patient population in our study was predominantly younger males with shoulder instability, it would be fair to extrapolate to other patient populations with similar lesions because there is little reason to believe the glenoid track concept would change with age or sex. However, further research is needed into how various shoulder pathologies and anatomic variants influence and affect the glenoid track. How does modification of soft tissue restraints alter the glenoid track? Further research is also needed into clinical application of the glenoid track such as assessing the outcomes of isolated glenoid repairs in cases with glenohumeral bone loss. When does an isolated glenoid repair placing an engaging Hill–Sachs inside the glenoid track negate the need for further humeral head repairs? Does artificially widening the glenoid track (greater than 84%) have any deleterious effects? (Fig. 7).
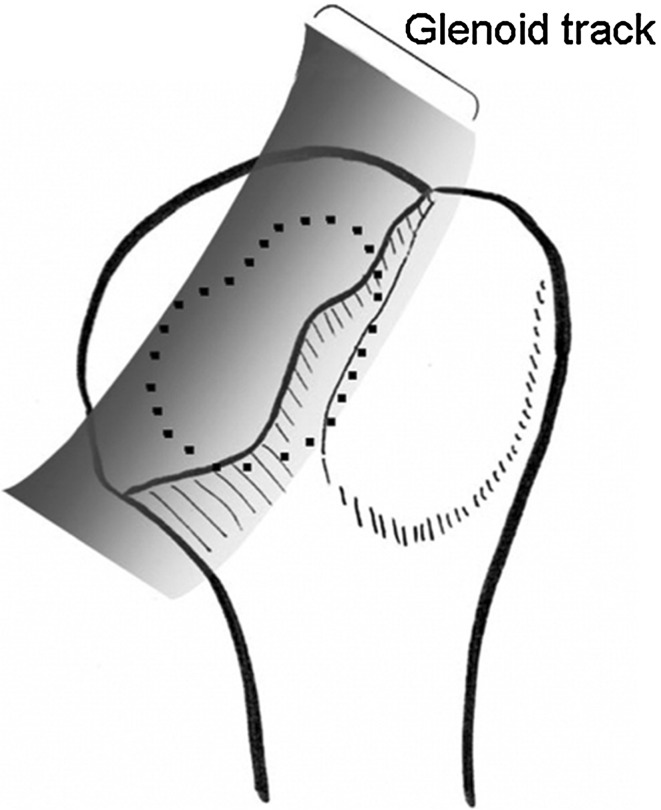
Fig. 7.
When the arm is elevated, the glenoid created a zone of contact (gray zone) along the rim of the humeral head. We defined this zone as a “glenoid track.” Reprinted with permission from Yamamoto N, Itoi E, Abe H, Minagawa H, Seki N, Shimada Y, Okada K. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16:649–656.
The glenoid track concept incorporates both humeral head and glenoid rim bony loss to predict the likelihood of humeral head engagement and shoulder instability. Humeral head bony defects are likely to engage if they lie outside the track, and restoring the track to its natural width should be a priority when reestablishing shoulder stability. Further studies are needed to validate and refine the glenoid track concept, but initial studies show it to be a potentially powerful method to predict risk of engagement and plan surgical interventions.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
The views expressed in this article are those of the author(s) and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government.
References
- 1.Beran MC, Donaldson CT, Bishop JY. Treatment of chronic glenoid defects in the setting of recurrent anterior shoulder instability: a systematic review. J Shoulder Elbow Surg. 2010;19:769–780. doi: 10.1016/j.jse.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill–Sachs lesion. Arthroscopy. 2000;16:677–694. doi: 10.1053/jars.2000.17715. [DOI] [PubMed] [Google Scholar]
- 3.Burkhart SS, Debeer JF, Tehrany AM, Parten PM. Quantifying glenoid bone loss arthroscopically in shoulder instability. Arthroscopy. 2002;18:488–491. doi: 10.1053/jars.2002.32212. [DOI] [PubMed] [Google Scholar]
- 4.Calandra JJ, Baker CL, Uribe J. The incidence of Hill–Sachs lesions in initial anterior shoulder dislocations. Arthroscopy. 1989;5:254–257. doi: 10.1016/0749-8063(89)90138-2. [DOI] [PubMed] [Google Scholar]
- 5.Cho SH, Cho NS, Rhee YG. Preoperative analysis of the Hill–Sachs lesion in anterior shoulder instability: how to predict engagement of the lesion. Am J Sports Med. 2011;39:2389–2395. doi: 10.1177/0363546511398644. [DOI] [PubMed] [Google Scholar]
- 6.Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(609):e1. doi: 10.1016/j.arthro.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Elkinson I, Giles JW, Faber KJ, Boons HW, Ferreira LM, Johnson JA, Athwal GS. The effect of the remplissage procedure on shoulder stability and range of motion: an in vitro biomechanical assessment. J Bone and Joint Surg Am. 2012;94:1003–1012. doi: 10.2106/JBJS.J.01956. [DOI] [PubMed] [Google Scholar]
- 8.Giles JW, Elkinson I, Ferreira LM, Faber KJ, Boons H, Litchfield R, Johnson JA, Athwal GS. Moderate to large engaging Hill–Sachs defects: an in vitro biomechanical comparison of the remplissage procedure, allograft humeral head reconstruction, and partial resurfacing arthroplasty. J Shoulder Elbow Surg. 2012;21:1142–1151. doi: 10.1016/j.jse.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Huysmans PE, Haen PS, Kidd M, Dhert WJ, Willems JW. The shape of the inferior part of the glenoid: a cadaveric study. J Shoulder Elbow Surg. 2006;15:759–763. doi: 10.1016/j.jse.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Itoi E, Lee SB, Amrami KK, Wenger DE, An KN. Quantitative assessment of classic anteroinferior bony Bankart lesions by radiography and computed tomography. Am J Sports Med. 2003;31:112–118. doi: 10.1177/03635465030310010301. [DOI] [PubMed] [Google Scholar]
- 11.Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82:35–46. doi: 10.2106/00004623-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Itoi E, Yamamoto N, Omori Y. Glenoid track. In: Di Giacomo G, Costantini A, De Vita A, de Gasperis N, editors. Shoulder Instability: Alternative Surgical Techniques. New York, NY, USA: Springer; 2011. [Google Scholar]
- 13.Ji JH, Kwak DS, Yang PS, Kwon MJ, Han SH, Jeong JJ. Comparisons of glenoid bony defects between normal cadaveric specimens and patients with recurrent shoulder dislocation: an anatomic study. J Shoulder Elbow Surg. 2012;21:822–827. doi: 10.1016/j.jse.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Kaar SG, Fening SD, Jones MH, Colbrunn RW, Miniaci A. Effect of humeral head defect size on glenohumeral stability: a cadaveric study of simulated Hill–Sachs defects. Am J Sports Med. 2010;38:594–599. doi: 10.1177/0363546509350295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazel MD, Sekiya JK, Greene JA, Bruker CT. Percutaneous correction (humeroplasty) of humeral head defects (Hill–Sachs) associated with anterior shoulder instability: a cadaveric study. Arthroscopy. 2005;21:1473–1478. doi: 10.1016/j.arthro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Kelkar R, Wang VM, Flatow EL, Newton PM, Ateshian GA, Biglani LU, Pawluk RJ, Mow VC. Glenohumeral mechanics: a study of articular geometry, contact, and kinematics. J Shoulder Elbow Surg. 2001;10:73–84. doi: 10.1067/mse.2001.111959. [DOI] [PubMed] [Google Scholar]
- 17.Kodali P, Jones MH, Polster J, Miniaci A, Fening SD. Accuracy of measurement of Hill–Sachs lesions with computed tomography. J Shoulder Elbow Surg. 2011;20:1328–1334. doi: 10.1016/j.jse.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Kurokawa D, Yamamoto N, Nagamoto H, Omori Y, Tanaka M, Sano H, Itoi E. The prevalence of a large Hill–Sachs lesion that needs to be treated. J Shoulder Elbow Surg. 2013 Mar 1 [Epub ahead of print]. [DOI] [PubMed]
- 19.Pagnani MJ. Open capsular repair without bone block for recurrent anterior shoulder instability in patients with and without bony defects of the glenoid and/or humeral head. Am J Sports Med. 2008;36:1805–1812. doi: 10.1177/0363546508316284. [DOI] [PubMed] [Google Scholar]
- 20.Provencher MT, Bhatia S, Ghodadra NS, Grumet RC, Bach BR, Jr, Dewing CB, LeClere L, Romeo AA. Recurrent shoulder instability: current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg Am. 2010;92(Suppl 2):133–151. doi: 10.2106/JBJS.J.00906. [DOI] [PubMed] [Google Scholar]
- 21.Richards RD, Sartoris DJ, Pathria MN, Resnick D. Hill–Sachs lesion and normal humeral groove: MR imaging features allowing their differentiation. Radiology. 1994;190:665–668. doi: 10.1148/radiology.190.3.8115607. [DOI] [PubMed] [Google Scholar]
- 22.Rowe CR. Acute and recurrent anterior dislocations of the shoulder. Orthop Clin North Am. 1980;11:253–270. [PubMed] [Google Scholar]
- 23.Saito H, Itoi E, Sugaya H, Minagawa H, Yamamoto N, Tuoheti Y. Location of the glenoid defect in shoulders with recurrent anterior dislocation. Am J Sports Med. 2005;33:889–893. doi: 10.1177/0363546504271521. [DOI] [PubMed] [Google Scholar]
- 24.Sekiya JK, Jolly J, Debski RE. The effect of a Hill–Sachs defect on glenohumeral translations, in situ capsular forces, and bony contact forces. Am J Sports Med. 2012;40:388–394. doi: 10.1177/0363546511425018. [DOI] [PubMed] [Google Scholar]
- 25.Sekiya JK, Wickwire AC, Stehle JH, Debski RE. Hill–Sachs defects and repair using osteoarticular allograft transplantation: biomechanical analysis using a joint compression model. Am J Sports Med. 2009;37:2459–2466. doi: 10.1177/0363546509341576. [DOI] [PubMed] [Google Scholar]
- 26.Soslowsky LJ, Flatow EL, Bigliani LU, Pawluk RJ, Ateshian GA, Mow VC. Quantitation of in situ contact areas at the glenohumeral joint: a biomechanical study. J Orthop Res. 1992;10:524–534. doi: 10.1002/jor.1100100407. [DOI] [PubMed] [Google Scholar]
- 27.Sugimoto K. Ultrasonographic evaluation of the Bankart lesion. J Shoulder Elbow Surg. 2004;13:286–290. doi: 10.1016/j.jse.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Warner JJ, Bowen MK, Deng XH, Hannafin JA, Arnoczky SP, Warren RF. Articular contact patterns of the normal glenohumeral joint. J Shoulder Elbow Surg. 1998;7:381–388. doi: 10.1016/S1058-2746(98)90027-1. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto N, Itoi E, Abe H, Kikuchi K, Seki N, Minagawa H, Tuoheti Y. Effect of an anterior glenoid defect on anterior shoulder stability: a cadaveric study. Am J Sports Med. 2009;37:949–954. doi: 10.1177/0363546508330139. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto N, Itoi E, Abe H, Minagawa H, Seki N, Shimada Y, Okada K. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16:649–656. doi: 10.1016/j.jse.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto N, Muraki T, Sperling JW, Sperling JW, Cofield RH, Itoi E, Walch G, Steinmann SP. Stabilizing mechanism in bone-grafting of a large glenoid defect. J Bone Joint Surg Am. 2010;92:2059–2066. doi: 10.2106/JBJS.I.00261. [DOI] [PubMed] [Google Scholar]