Abstract
Background
Conditional survival measures change in the risk of mortality given that a patient has survived a defined period of time. This has yet to be reported for chondrosarcoma of bone. This information should be of interest to the clinician and helpful in counseling patients with chondrosarcoma.
Questions/purposes
Our questions include the following: (1) Does the conditional survival of patients with local/regional chondrosarcoma improve with each additional year of survival? (2) Does the conditional survival of patients with metastatic chondrosarcoma improve with each additional year of survival? (3) Does tumor location, use of radiation, or patient age affect conditional survival? (4) Can chondrosarcoma ever be considered cured?
Methods
We used the Surveillance, Epidemiology, and End Results Program database maintained by the National Cancer Institute to identify 2138 patients with chondrosarcoma of bone from 1973 to 2009. We used an actuarial life table analysis to explore differences in 5-year cause-specific survival estimates conditional on 1 to 5 years of survival. The cohort was stratified by grade, location (axial versus extremity), use of radiation, and age. Finally, we expanded the analysis to include survival estimates 20 years after diagnosis conditional on survival for 5 and 10 years.
Results
The estimated survival for all grades of local/regional chondrosarcoma improved from baseline with each year of survival after diagnosis. At 5 years after diagnosis, local/regional Grade 1 chondrosarcoma displayed higher conditional survival than Grade 2 and 3 local/regional chondrosarcoma (97.2% [95% confidence interval {CI}, 95.2%–98.4%] versus 92.8% [95% CI, 89.5%–95.0%], p = 0.006 and 83.8% [95% CI, 69.9%–91.7%], p = 0.012). Estimated survival improved from baseline with each year of survival for all grades of metastatic chondrosarcoma. Conditional survival estimates for Grade 3 axial tumors failed to improve from baseline to 5 years after diagnosis (52.9% versus 70.2%, p > 0.05) compared with Grade 3 extremity tumors at baseline and 5 years after diagnosis (58.1% versus 95.8%, p < 0.0001) The 20-year conditional survival estimates reveal that a cancer-specific risk of mortality exists even 10 years after diagnosis, suggesting that although the conditional survival increases considerably over time, it cannot be considered cured.
Conclusions
The 5-year conditional survival estimate for patients with chondrosarcoma improved with each additional year of survival regardless of grade, site, age, or use of radiation. At 10 years after diagnosis, deaths attributable to cancer were still present, and patients should be aware of this small long-term risk.
Level of Evidence
Level II, prognostic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
Chondrosarcoma is the second most common primary sarcoma of bone, ranking behind only osteosarcoma [8]. Surgical resection is the principle modality of treatment for chondrosarcoma, which is inherently resistant to both radiation and chemotherapy [18, 23, 37, 40]. Estimates of survival are most frequently presented as a single, static 5- or 10-year overall survival value determined at the time of diagnosis. Such estimates fail to take into consideration changes in prognostic risk factors with time and that the chances of survival likely improve the longer an individual survives after diagnosis [25, 31].
Conditional survival measures the risk of mortality as a function of time taking into consideration the dynamic nature of prognostic risk factors [31, 34]. Such a measure is not only intriguing for the clinical scientist, but it also provides a valuable tool for patient counseling [6, 9, 13, 17, 24, 26, 41]. In this fashion, conditional survival provides a dynamic and relevant estimate of survival while also individualizing counseling for patients based on demographic and cancer-specific characteristics [25, 34]. Beyond counseling, conditional survival may also help guide disease surveillance protocols, which could be of particular value when advanced imaging or expensive diagnostic testing is suggested [13, 34].
Consistently reported tumor and patient factors that negatively influence survival in the current literature include increased patient age, high tumor grade, inadequate surgical resection, axial tumor location, local recurrence, and the use of adjuvant therapy including radiation or chemotherapy [3, 5, 11, 16, 19, 21, 36]. Although these factors provide a basis for current counseling, they provide only static estimates of survival at the time of diagnosis and do not take into consideration prognostic change over time. To our knowledge, the conditional survival of chondrosarcoma has yet to be defined.
Our questions include the following: (1) Does the conditional survival of patients with local/regional chondrosarcoma improve with each additional year of survival? (2) Does the conditional survival of patients with metastatic chondrosarcoma improve with each additional year of survival? (3) Does tumor location, use of radiation, or patient age affect conditional survival? (4) Can chondrosarcoma ever be considered cured?
Patients and Methods
We used the Surveillance, Epidemiology, and End Results (SEER) program database to capture all recorded patients with the diagnosis of chondrosarcoma from 1973 to 2009. The SEER program began with eight state and regional cancer registries in 1973 and has since added centers to currently include 18 racially, economically, geographically, and socially diverse areas representing 26% of the US population [28]. The SEER program database is publicly available and does not contain unique individual identifiers such as name, date of birth, or Social Security number. As such, our institutional review board determined that this investigation did not meet the definition of human subjects’ research and did not require a formal review. We used SEER*Stat (Version 8.0.4; National Cancer Institute, Bethesda, MD, USA) for all database queries, conditional survival calculations, and calculation of 95% confidence intervals (CIs) using a logarithmic transformation to reduce skewing. SE was provided by SEER*Stat and was used to perform unpaired Student’s t-test (two-tailed) for point estimates of survival. Probability values < 0.05 were considered statistically significant.
Within SEER, age is recorded as a categorical variable in 5-year intervals. We limited our investigation to patients aged 20 to 85 + years, because chondrosarcoma is unusual in children and adolescents. The histologic diagnosis was based on the International Classification of Diseases for Oncology, Third Revision [12]. We included all patients with a diagnosis of “chondrosarcoma, not otherwise specified” with a location of “bones and joints” to eliminate chondrosarcomas originating in the soft tissue. We excluded all subtypes of malignant cartilage lesions reported in SEER (juxtacortical chondrosarcoma, chondroblastoma, malignant, myxoid chondrosarcoma, mesenchymal chondrosarcoma, clear cell chondrosarcoma, and dedifferentiated chondrosarcoma). Tumor grade is recorded in the SEER database as I, II, III, and IV. We classified low-grade (Grade I) lesions as Grade 1 and intermediate (Grade II) lesions as Grade 2. Because Grade III and IV tumors represented a constellation of high-grade chondrosarcoma, we combined these tumors as Grade 3. We identified a total of 2138 patients with chondrosarcoma, not otherwise specified, eligible for analysis from 1973 to 2009, and the number of patients with a given tumor grade as well as local/regional or metastatic disease at the time of diagnosis were identified (Table 1).
Table 1.
Number of patients with chondrosarcoma in the Surveillance, Epidemiology, and End Results Program database, 1973–2009
| Years after diagnosis | Grade 1 | Grade 2 | Grade 3 | |||
|---|---|---|---|---|---|---|
| Local/regional | Metastatic | Local/regional | Metastatic | Local/regional | Metastatic | |
| 0 | 925 | 24 | 855 | 56 | 231 | 47 |
| 5 | 603 | 9 | 481 | 13 | 73 | 3 |
| 10 | 339 | 3 | 224 | 5 | 31 | 2 |
| 20 | 107 | 1 | 54 | 3 | 5 | 0 |
We separated the patients by the presence of metastatic disease at diagnosis. Patients with localized or regional disease were coded as local/regional, whereas those with metastatic disease at the time of diagnosis were coded as metastatic. It is important to note that in the SEER program database, metastatic designation is only made at the time of diagnosis. Furthermore, the ability to radiographically detect metastatic disease has most certainly changed during the time period of this study, which coincides with significant advances in cross-sectional imaging. Because of this concern, a sensitivity analysis was performed to compare the current time period under investigation, 1973–2009, with a more recent time period, 1990–2009, when cross-sectional imaging was more readily available. This analysis failed to show any difference in conditional survival between the two time periods, justifying the inclusion of the entire cohort. Patients who were unstaged or had a blank entry were excluded.
For all portions of the analysis, we separated the tumors by grade. We calculated a 5-year survival estimate at the time of diagnosis and a revised 5-year survival estimate conditional on 1, 2, 3, 4, and 5 years of survival. We also calculated 20-year survival estimates from the time of diagnosis. The 20-year conditional survival estimates were made at baseline and conditional on surviving 5 and 10 years after diagnosis.
We stratified the data in several ways to investigate previously reported risk factors for poor outcomes in chondrosarcoma. First, we separated patients into those with tumors involving the extremities (the long and short bones of the upper and lower extremities) or axial skeleton (pelvis, spine, and chest wall). Next, we compared patients who had surgery only with those who had surgery and radiation. Our initial statistical analysis revealed that axial tumors received radiation with a disproportionate frequency, 51.4% in Grade 1, 57.5% in Grade 2, and 45.1% in Grade 3, compared with their overall frequency in local/regional disease, 35.7% in Grade 1, 39.1% in Grade 2, and 35.5% in Grade 3 chondrosarcoma. We therefore limited our analysis of the use of radiation to tumors with an axial location. Finally, we stratified by age using 60 years as a cutoff, which is supported by previous literature that reports increased age as a risk factor for mortality [16, 21, 36]. The number of patients older than 60 years of age as well as the number of patients with axial tumors who received radiation were identified (Table 2).
Table 2.
Number of patients with chondrosarcoma in the Surveillance, Epidemiology, and End Results Program database by age (axial and extremity) and use of radiation (axial only), 1973–2009
| Grade and years after diagnosis | Radiation | Age (years) | ||
|---|---|---|---|---|
| Yes | No | < 60 | ≥ 60 | |
| Grade 1 | ||||
| 0 years | 37 | 293 | 682 | 243 |
| 5 years | 19 | 188 | 470 | 133 |
| Grade 2 | ||||
| 0 years | 65 | 269 | 594 | 261 |
| 5 years | 27 | 143 | 369 | 112 |
| Grade 3 | ||||
| 0 years | 23 | 59 | 132 | 99 |
| 5 years | 9 | 17 | 54 | 19 |
We used an actuarial life table method to determine cause-specific cancer survival. Any mortality resulting from cancer recurrence or metastatic spread qualified as cause-specific. We limited our investigation to “first primary only” to capture patients who had only a diagnosis of chondrosarcoma. In this, we were able to eliminate any patients who had an additional malignancy to more accurately reflect cancer-specific death attributable to chondrosarcoma. The SEER registry uses data abstracted from death certificates to determine the cause of death, which is coded as either “cancer” or “other causes.” Individuals were censored if the cause of death was attributed to something other than cancer. Patients with missing or unknown causes of death were excluded.
Results
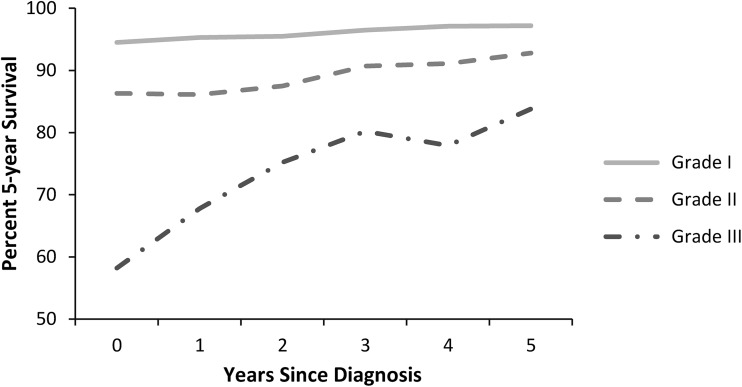
The 5-year conditional survival estimates for local/regional disease improved with each additional year of survival after diagnosis for all grades (Fig. 1). Estimated 5-year survival at baseline was 94.5% (95% CI, 92.7%–95.9%) for Grade 1, 86.3% (95% CI, 83.4%–88.6%) for Grade 2, and 58.2% (95% CI, 50.7%–64.9%) for Grade 3 chondrosarcoma (p < 0.0001 for Grade 1 versus Grade 2 and Grade 3). The 5-year conditional survival estimate for patients who survived 5 years after diagnosis failed to increase in Grade 1 (94.5% versus 97.2% [95% CI, 95.2%–98.4%], p > 0.05), whereas improvements were noted in Grade 2 (86.3% versus 92.8% [95% CI, 89.5%–95.0%], p < 0.0001) and Grade 3 (58.2% versus 83.8% [95% CI, 69.9%–91.7%], p < 0.0001) chondrosarcoma. Conditional survival for patients who survived 5 years after diagnosis was better for patients with Grade 1 chondrosarcoma as compared with patients with Grade 2 or 3 chondrosarcoma (97.2% [95% CI, 95.2%–98.4%] versus 92.8% [95% CI, 89.5%–95.0%], p = 0.006 and 83.8% [95% CI, 69.9%–91.7%], p = 0.012).
Fig. 1.
A graph shows the cause-specific conditional survival of patients with local/regional chondrosarcoma. Each point represents the 5-year survival given the patient has survived 0 to 5 years after diagnosis. Grade 1, 2, and 3 chondrosarcoma were separately analyzed.
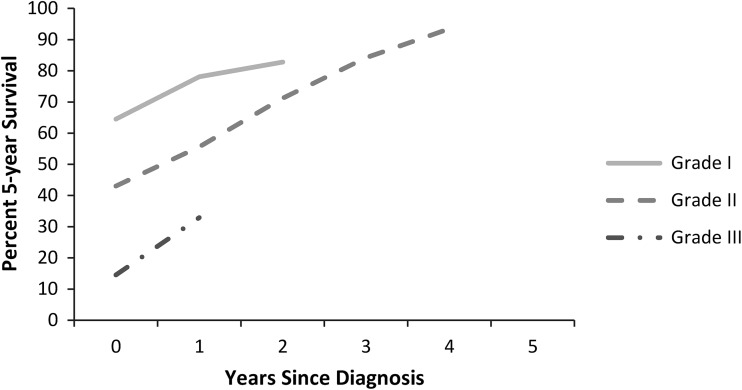
For metastatic disease, 5-year conditional survival estimates improved with each additional year of survival after diagnosis for all grades (Fig. 2). Metastatic chondrosarcoma was rare in Grades 1 and 2 tumors and lethal in Grade 3 tumors, therefore limiting the cohort available for analysis. Although sample size limited statistical comparison, there was a more pronounced increase in conditional survival in metastatic disease when compared with local/regional disease.
Fig. 2.
A graph shows the cause-specific conditional survival of patients with metastatic chondrosarcoma at the time of diagnosis. Each point represents the 5-year survival given the patient has survived 0 to 5 years after diagnosis. Grade 1, 2, and 3 chondrosarcoma were separately analyzed. Data points were omitted when < 15 subjects were available for analysis.
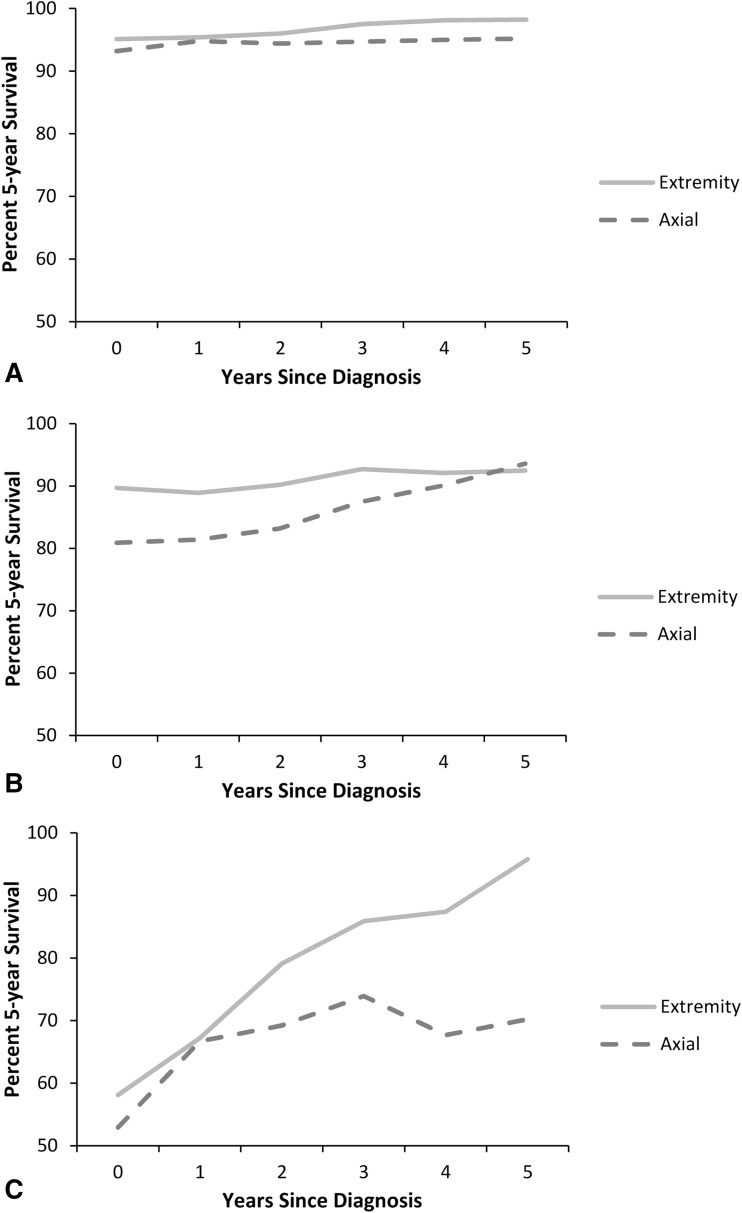
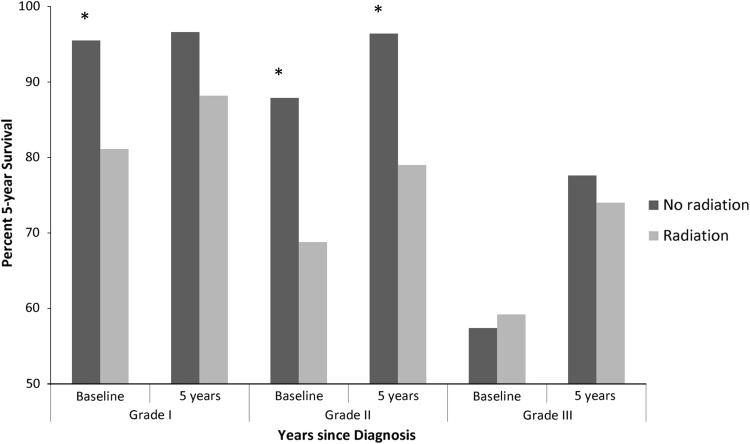
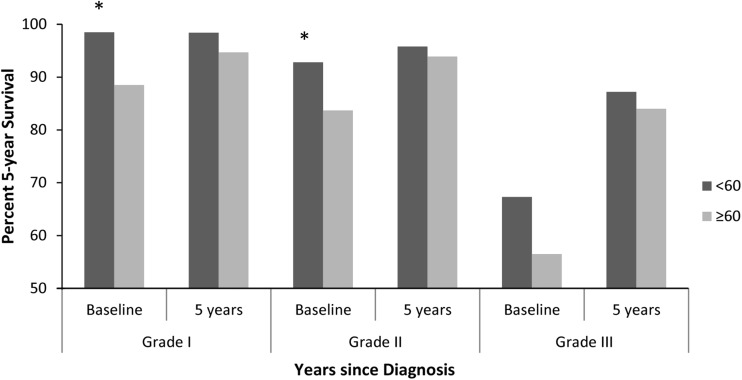
Conditional survival improved with each additional year of survival after diagnosis irrespective of tumor location. The 5-year conditional survival estimates in Grade 1 chondrosarcoma were unchanged at baseline compared with patients who survived 5 years after diagnosis (94.5% [95% CI, 92.7%–95.9%] versus 97.2% [95% CI, 95.2%–98.4%], p > 0.05). In Grade 1 extremity tumors, conditional survival was unchanged at baseline compared with having survived 5 years after diagnosis (95.1% [95% CI, 92.7%–96.7%] versus 98.2% [95% CI, 95.7%–99.3%], p > 0.05). For Grade 1 axial tumors, conditional survival was unchanged at baseline compared with having survived 5 years after diagnosis (93.2% [95% CI, 89.5%–95.6%] versus 95.2% [95% CI, 90.6%–97.6%], p > 0.05) (Fig. 3A). There was no difference in 5-year conditional survival estimates at baseline or 5 years after diagnosis when comparing extremity and axial tumor location in Grade 1 chondrosarcoma. In Grade 2 lesions, axial tumors had a poorer estimated 5-year survival at baseline compared with extremity tumors (80.9% [95% CI, 75.5%–85.1%] versus 89.7% [95% CI, 86.1%–92.4%], p = 0.002), but this difference was no longer present conditionally on having survived 5 years after diagnosis (93.6% [95% CI, 88.0%–96.7%] versus 92.5% [95% CI, 88.0%–95.4%], p > 0.05) (Fig. 3B). The most striking difference in conditional survival estimates between axial and extremity tumor location was observed in Grade 3 chondrosarcoma (Fig. 3C). Although estimated 5-year survival was nearly equivalent at baseline for axial and extremity tumors (52.9% [95% CI, 40.3%–64.0%] versus 58.1% [95% CI, 47.8%–67.0%], p > 0.05), conditional survival was substantially lower in axial lesions 5 years after diagnosis compared with extremity lesions (70.2% [95% CI, 44.9%–85.5%] versus 95.8% [95% CI, 73.9%–99.4%], p = 0.021). The 5-year survival estimate in patients with Grade 3 extremity lesions improved from baseline compared with having survived 5 years after diagnosis (58.1% [95% CI, 47.8%–67.0%] versus 95.8% [95% CI, 73.9%–99.4%], p < 0.0001). The estimated 5-year survival at baseline for patients with an axial tumor location was generally greater when treated with surgery alone as compared with surgery plus radiation in all tumor grades (Fig. 4). This difference was less pronounced in Grade 1 axial chondrosarcoma treated with surgery alone versus surgery plus radiation (95.5% [95% CI, 92.0%–97.5%] versus 81.1% [95% CI, 62.5%–91.1%], p = 0.043) as compared with Grade 2 axial chondrosarcoma (87.9% [95% CI, 82.6%–91.6%] versus 68.8% [95% CI, 54.0%–79.8%], p = 0.006). The generally improved conditional survival in patients treated with surgery alone remained after surviving 5 years after diagnosis. Similarly, the estimated survival was greater in patients < 60 years of age in all grades at baseline but nearly equivalent conditional on having survived 5 years after diagnosis (Fig. 5).
Fig. 3A–C.
A graph shows the cause-specific conditional survival of patients with (A) Grade 1, (B) Grade 2, and (C) Grade 3 chondrosarcoma in either an extremity or axial location. Each point represents the 5-year survival given the patient has survived 0 to 5 years after diagnosis.
Fig. 4.
A graph shows the cause-specific conditional survival at baseline and 5 years of survival after diagnosis for patients with axial chondrosarcoma treated with surgery alone or surgery and radiation together. Asterisks denote statistical significance with a p value of < 0.05 considered statistically significant.
Fig. 5.
A graph shows the cause-specific conditional survival at baseline and 5 years of survival after diagnosis for patients ≥ 60 years of age and patients < 60 years of age. Asterisks denote statistical significance with a p value of < 0.05 considered statistically significant.
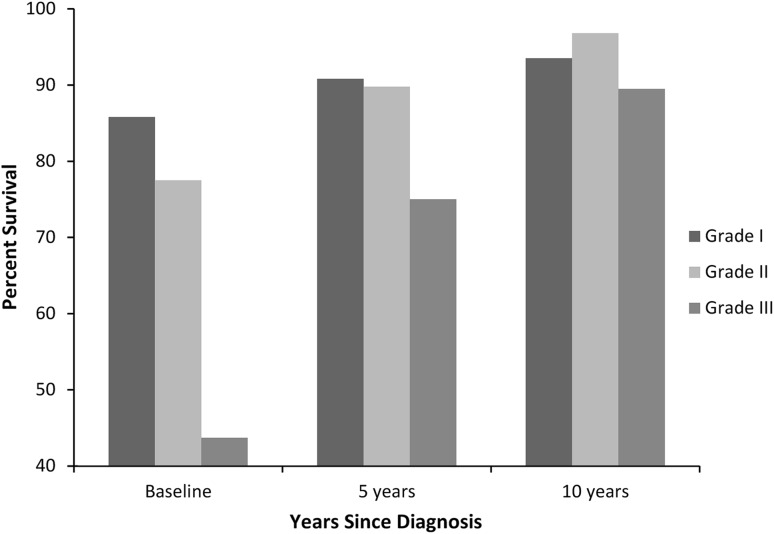
Looking at more distant followup, 20-year survival estimates were calculated at baseline and additionally conditional on 5 and 10 years of survival after diagnosis (Fig. 6). An improvement was seen in Grade 2 chondrosarcoma, whereas the 20-year survival estimate increased from 77.5% (95% CI, 73.2%–81.1%) at baseline to 96.8% (95% CI, 92.3%–98.7%) conditional on 10 years of survival after diagnosis (p < 0.0001). This improvement was also seen in Grade 3 chondrosarcoma (43.7% [95% CI, 34.1%–52.8%] versus 89.5% [95% CI, 70.8%–96.5%], p < 0.0001). The 20-year conditional survival estimates for patients who survived 10 years after diagnosis was approximately 90% and was equivalent for all grades but failed to reach 100% for any grade of chondrosarcoma.
Fig. 6.
A graph shows the 20-year survival estimates in patients with chondrosarcoma at baseline and conditional on 5 and 10 years of survival after diagnosis. At 10 years after diagnosis, the 20-year survival estimate was statistically equivalent for all grades of chondrosarcoma.
Discussion
Conditional survival provides an estimate of the risk of mortality given that a patient has survived a defined period of time after diagnosis. Studies using conditional survival have repeatedly shown that prognostic factors change with time [6, 9, 13, 17, 24–26, 31, 34, 41]. Conditional survival has been calculated for numerous other malignancies including bone sarcoma, melanoma, lung, gastric, ovarian, brain, squamous cell, rectal, and bladder cancers [7, 13, 17, 24, 26, 34, 38, 39, 41], but to our knowledge, conditional survival for chondrosarcoma has yet to be reported in the literature. Using the SEER database to identify patients with chondrosarcoma from 1973 to 2009, we found that 5-year conditional survival estimates improved with each additional year of survival after diagnosis in patients with both local/regional and metastatic disease regardless of age. Tumor location did not have a lasting effect on conditional survival in Grade 1 chondrosarcoma, but axial Grade 3 chondrosarcoma showed substantially lower survival compared with extremity chondrosarcoma even in patients who lived 5 years after diagnosis. The 20-year cause-specific conditional survival estimates for patients who survived 10 years after diagnosis failed to reach 100% for any grade of chondrosarcoma.
There are several limitations to the present study that warrant further discussion. Although the SEER database provides highly reliable demographic data from regions that comprise nearly 26% of the US population [2, 4, 28], the generalizability of the data has been questioned because the population in these areas tends to be more affluent with less unemployment compared with the nation as a whole [30]. Even with these concerns, the SEER database remains one of the most reliable and complete databases for long-term cancer research for a multitude of cancer types [7, 13, 17, 24, 26, 34, 38, 39, 41]. Determining the cause of death in a cause-specific survival analysis is inherently important. SEER*Stat uses an algorithm based on death certificates, which may be inaccurate or difficult to interpret if multiple causes of death are listed. However, this method for survival analysis has been used in several similar prior investigations [7, 16, 17, 25, 26, 38]. Additionally, the accuracy of histologic diagnosis, which is difficult in cartilaginous bone lesions even in the hands of experts [35], cannot be confirmed. Grade and staging are therefore dependent on the accuracy of information in the database. Several important staging criteria, including compartmentalization of bone sarcoma, are not universally reported and their use precludes investigations over long time periods. Although SEER does not include any data on surgical margins, the use of radiation is typically reserved for patients with inadequate surgical resection and has been shown to negatively influence overall survival [18, 23, 36]. We acknowledge that factors other than inadequate surgical margins, including patient age, comorbidities, facility treatment protocols, and anatomic location, may play a role in choosing whether to use radiation. In the present study, axial tumors received radiation therapy with disproportionate frequency compared with their overall incidence. Most importantly, we were unable to obtain information regarding local recurrence and metastatic spread, both of which have been shown to affect overall survival in patients with chondrosarcoma in previous studies [11, 19]. SEER does not collect these data and only designates metastatic disease at the time of diagnosis. This limitation prevented us from distinguishing between disease-free survival and cause-specific survival, although this may be less important in chondrosarcoma as compared with other sarcomatous lesions of bone where radiation and chemotherapy are more frequently used and provide a significant source of morbidity as well as the potential for secondary malignancy. Lastly, the time period for this study spans nearly four decades. Over this time period, the evolution of cross-sectional imaging may have enhanced the ability to detect metastatic lesions, and patients early in the study period may have been inappropriately categorized as having local/regional disease when indeed metastatic lesions were present. If metastatic lesions were consistently missed early in the study period and subsequently classified as local/regional, one would expect a difference in survival given the relatively poor prognosis of metastatic disease identified in this study. A sensitivity analysis comparing the time periods 1973–2009 and 1990–2009 failed to identify a difference in survival regardless of tumor grade.
We found that the conditional survival of patients with local/regional chondrosarcoma improved with each additional year of survival after diagnosis for Grade 2 and 3 chondrosarcomas at the time of diagnosis, whereas conditional survival failed to improve annually in Grade 1 chondrosarcomas. Recent literature has advocated for intralesional treatment of contained, Grade 1, extremity chondrosarcoma, suggesting that functional results may be superior to wide resection without an increase in local recurrence or mortality [1, 10, 20, 27]. Our findings confirm that Grade 1 chondrosarcoma has a very low likelihood of oncologic failure, and treatment decisions that optimize functional outcomes may be justified. Despite the dramatic increase in conditional survival over time, Grade 2 and 3 lesions continued to have lower 5-year conditional survival estimates at 5 years after diagnosis compared with Grade 1 lesions, consistent with previous literature that has demonstrated tumor grade as an important predictor of survival [3, 5, 15, 16, 19, 21, 22, 36]. The initial steep increase in conditional survival in patients with Grade 3 lesions at the time of diagnosis may be partially explained by a greater number of early deaths in Grade 3 lesions compared with Grade 1 and 2 lesions. In contrast, the better prognosis at diagnosis in low-grade tumors limits the rate and amount of increase in conditional survival over time compared with the more aggressive high-grade lesions [6, 42]. Interestingly, the shape of the conditional survival curve in Grade 3 chondrosarcoma more closely approximates the conditional survival curves in osteosarcoma and Ewing’s sarcoma [26], suggesting that the conditional survival of high-grade bone sarcoma behaves similarly regardless of histologic subtype.
In metastatic chondrosarcoma, estimated 5-year survival improved with each additional year of survival after diagnosis in all grades. Furthermore, the rate of increase in conditional survival was more marked across all grades compared with local/regional disease. Although low numbers limit extensive analysis in this subgroup, the steeper conditional survival slope in metastatic disease conveys a more positive outlook with each additional year of survival.
With respect to tumor location, conditional survival was equivalent in Grade 1 lesions with differences noted in Grade 2 lesions at baseline that became equivalent by 5 years after diagnosis. In Grade 3 lesions, the baseline estimated 5-year survival was poor for both axial and extremity lesions but grew more divergent with time because extremity lesions displayed improved conditional survival 5 years after diagnosis as compared with baseline. This improvement was not noted in Grade 3 axial lesions. This finding has interesting implications. First, the similarity of baseline survival rates suggests that high-grade chondrosarcoma carries a poor initial prognosis regardless of the primary site. The substantial early mortality is likely reflective of the biological aggressiveness of the tumor and high likelihood of systemic failure regardless of the success of surgical excision. In contrast, as an individual survives into the future, the location of the primary tumor becomes more important because extremity tumors clearly carried a more favorable prognosis than axial tumors. This may be explained by increased local recurrence resulting from anatomic constraints of the pelvis limiting the adequacy of primary resection and options for subsequent salvage procedures, larger tumors at the time of diagnosis, and a higher likelihood of metastasis [15, 19, 21, 22, 32]. This observation, combined with the lack of similar findings in lower grade tumors, is an important area for further study. In patients with an axial tumor location, the addition of radiation therapy to the treatment regimen decreased conditional survival at both baseline and 5 years after diagnosis. Radiation therapy has been shown to have relatively little effect on chondrosarcoma and has essentially been limited to situations in which complete tumor resection is not possible or where inadequate margins are obtained, often secondary to anatomic constraints [18, 23]. Previous research has demonstrated that inadequate tumor excision has resulted in reduced overall and disease-free survival [15, 22, 33]. Similarly, we found a suggestion that the use of radiation may result in diminished survival estimates at baseline and after 5 years of survival in patients with axial chondrosarcoma. Chondrosarcoma typically affects individuals in the fifth to seventh decade of life [8], and several studies have identified increased age as a risk factor for decreased overall survival [16, 36]. In the present study, estimated 5-year survival was poorer at baseline in patients with Grade 1 or 2 chondrosarcoma who were ≥ 60 years of age. However, by 5 years after diagnosis, survival estimates were essentially equivalent across all grades regardless of age. This finding suggests that although older patients may have more early deaths even with low-grade disease, the effect of age on chondrosarcoma-related mortality decreases over time.
The best opportunity for cure in patients with chondrosarcoma is surgical resection with wide margins [14]. Cure, by definition, suggests lifetime disease-free survival, which leads to the question: can chondrosarcoma ever truly be cured? When looking at 20-year conditional survival estimates, survival improved with each additional year of survival after diagnosis in all grades and most dramatically in Grade 3 chondrosarcoma. The 20-year conditional survival for patients who survived 10 years after diagnosis was nearly equivalent for all grades of chondrosarcoma, although conclusions should be made with caution given the relatively small number of patients who were available for analysis at 10 years after diagnosis. No grade exhibited 100% cause-specific survival, implying that a mortality risk still exists for patients with chondrosarcoma even after 10 years of survival. The National Comprehensive Cancer Network (NCCN) currently recommends prolonged clinical and radiologic followup, including imaging of the primary site and chest, every 6 to 12 months for the first 2 years followed by annual imaging in low-grade chondrosarcoma. Similarly, the NCCN recommends clinical and radiologic followup every 3 to 6 months for the first 5 years followed by annual examinations and imaging for a minimum of 10 years [29]. Our data support this general protocol of gradually increasing the periodicity of surveillance over time and caution that cancer-related mortality can occur in some patients even after 10 years of survival.
Conditional survival estimates provide valuable and, with respect to chondrosarcoma, optimistic information for the physician and patient alike. Several specific results of this study, namely the elimination of age as a negative prognostic factor by 5 years after diagnosis as well as the aggressive nature of axial Grade 3 chondrosarcoma, provide information that can help individualize patient counseling. Additionally, patients with a poor prognosis at baseline such as those with Grade 3 chondrosarcoma or metastatic disease have the greatest increase in conditional survival for each additional year of survival after diagnosis. The high survival rates of low-grade chondrosarcoma imply that long-term functional outcomes are extremely important because the likelihood of oncologic failure is low. Although each year of survival portends an improved prognosis regardless of stage, grade, location, use of radiation, and patient age, the risk of cause-specific mortality still exists as far out as 10 years after diagnosis. These findings can help counsel patients on their individual prognosis in a temporal fashion as well as guide long-term surveillance in patients with chondrosarcoma.
Acknowledgments
We thank Yubo Gao, PhD for his statistical review of this article.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aarons C, Potter BK, Adams SC, Pitcher JD, Jr, Temple HT. Extended intralesional treatment versus resection of low-grade chondrosarcomas. Clin Orthop Relat Res. 2009;467:2105–2111. doi: 10.1007/s11999-008-0691-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambs A, Warren JL, Bellizzi KM, Topor M, Haffer SC, Clauser SB. Overview of the SEER–Medicare Health Outcomes Survey linked dataset. Health Care Financ Rev. 2008;29:5–21. [PMC free article] [PubMed] [Google Scholar]
- 3.Angelini A, Guerra G, Mavrogenis AF, Pala E, Picci P, Ruggieri P. Clinical outcome of central conventional chondrosarcoma. J Surg Oncol. 2012;106:929–937. doi: 10.1002/jso.23173. [DOI] [PubMed] [Google Scholar]
- 4.Bach PB, Guadagnoli E, Schrag D, Schussler N, Warren JL. Patient demographic and socioeconomic characteristics in the SEER-Medicare database—applications and limitations. Med Care. 2002;40:19–25. doi: 10.1097/00005650-200208001-00003. [DOI] [PubMed] [Google Scholar]
- 5.Bjornsson J, McLeod RA, Unni KK, Ilstrup DM, Pritchard DJ. Primary chondrosarcoma of long bones and limb girdles. Cancer. 1998;83:2105–2119. doi: 10.1002/(SICI)1097-0142(19981115)83:10<2105::AID-CNCR9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 6.Bleyer A, Choi M, Fuller CD, Thomas CR, Jr, Wang SJ. Relative lack of conditional survival improvement in young adults with cancer. Semin Oncol. 2009;36:460–467. doi: 10.1053/j.seminoncol.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Choi M, Fuller CD, Thomas CR, Jr, Wang SJ. Conditional survival in ovarian cancer: results from the SEER dataset 1988–2001. Gynecol Oncol. 2008;109:203–209. doi: 10.1016/j.ygyno.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clin Orthop Relat Res. 2007;459:40–47. doi: 10.1097/BLO.0b013e318059b8c9. [DOI] [PubMed] [Google Scholar]
- 9.Davis FG, McCarthy BJ, Freels S, Kupelian V, Bondy ML. The conditional probability of survival of patients with primary malignant brain tumors—Surveillance, Epidemiology and End Results (SEER) data. Cancer. 1999;85:485–491. doi: 10.1002/(SICI)1097-0142(19990115)85:2<485::AID-CNCR29>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.Donati D, Colangeli S, Colangeli M, Di Bella C, Bertoni F. Surgical treatment of grade I central chondrosarcoma. Clin Orthop Relat Res. 2010;468:581–589. doi: 10.1007/s11999-009-1056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorenza F, Abudu A, Grimer RJ, Carter SR, Tillman RM, Ayoub K, Mangham DC. Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg Br. 2002;84:93–99. doi: 10.1302/0301-620X.84B1.11942. [DOI] [PubMed] [Google Scholar]
- 12.Fritz A, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S, editors. International Classification of Diseases for Oncology, 3rd Revision. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 13.Fuller CD, Wang SJ, Thomas CR, Jr, Hoffman HT, Weber RS, Rosenthal DI. Conditional survival in head and neck squamous cell carcinoma: results from the SEER dataset 1973–1998. Cancer. 2007;109:1331–1343. doi: 10.1002/cncr.22563. [DOI] [PubMed] [Google Scholar]
- 14.Gelderblom H, Hogendoorn PC, Dijkstra SD, van Rijswijk CS, Krol AD, Taminiau AH, Bovee JV. The clinical approach towards chondrosarcoma. Oncologist. 2008;13:320–329. doi: 10.1634/theoncologist.2007-0237. [DOI] [PubMed] [Google Scholar]
- 15.Gitelis S, Bertoni F, Picci P, Campanacci M. Chondrosarcoma of bone. The experience at the Istituto Ortopedico Rizzoli. J Bone Joint Surg Am. 1981;63:1248–1257. [PubMed] [Google Scholar]
- 16.Giuffrida AY, Burgueno JE, Koniaris LG, Gutierrez JC, Duncan R, Scully SP. Chondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER database. J Bone Joint Surg Am. 2009;91:1063–1072. doi: 10.2106/JBJS.H.00416. [DOI] [PubMed] [Google Scholar]
- 17.Johnson DR, Ma DJ, Buckner JC, Hammack JE. Conditional probability of long-term survival in glioblastoma: a population-based analysis. Cancer. 2012;118:5608–5613. doi: 10.1002/cncr.27590. [DOI] [PubMed] [Google Scholar]
- 18.Krochak R, Harwood AR, Cummings BJ, Quirt IC. Results of radical radiation for chondrosarcoma of bone. Radiother Oncol. 1983;1:109–115. doi: 10.1016/S0167-8140(83)80014-0. [DOI] [PubMed] [Google Scholar]
- 19.Lee FY, Mankin HJ, Fondren G, Gebhardt MC, Springfield DS, Rosenberg AE, Jennings LC. Chondrosarcoma of bone: an assessment of outcome. J Bone Joint Surg Am. 1999;81:326–338. doi: 10.1302/0301-620X.81B5.9588. [DOI] [PubMed] [Google Scholar]
- 20.Leerapun T, Hugate RR, Inwards CY, Scully SP, Sim FH. Surgical management of conventional grade I chondrosarcoma of long bones. Clin Orthop Relat Res. 2007;463:166–172. doi: 10.1097/BLO.0b013e318146830f. [DOI] [PubMed] [Google Scholar]
- 21.Lin PP, Alfawareh MD, Takeuchi A, Moon BS, Lewis VO. Sixty percent 10-year survival of patients with chondrosarcoma after local recurrence. Clin Orthop Relat Res. 2012;470:670–676. doi: 10.1007/s11999-011-2059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcove RC, Mike V, Hutter RV, Huvos AG, Shoji H, Miller TR, Kosloff R. Chondrosarcoma of the pelvis and upper end of the femur. An analysis of factors influencing survival time in one hundred and thirteen cases. J Bone Joint Surg Am. 1972;54:561–572. [PubMed] [Google Scholar]
- 23.McNaney D, Lindberg RD, Ayala AG, Barkley HT, Hussey DH. Fifteen year radiotherapy experience with chondrosarcoma of bone. Int J Radiat Oncol Biol Phys. 1982;8:187–190. doi: 10.1016/0360-3016(82)90512-0. [DOI] [PubMed] [Google Scholar]
- 24.Merrill RM, Henson DE, Barnes M. Conditional survival among patients with carcinoma of the lung. Chest. 1999;116:697–703. doi: 10.1378/chest.116.3.697. [DOI] [PubMed] [Google Scholar]
- 25.Merrill RM, Hunter BD. Conditional survival among cancer patients in the United States. Oncologist. 2010;15:873–882. doi: 10.1634/theoncologist.2009-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller BJ, Lynch CF, Buckwalter JA. Conditional survival is greater than overall survival at diagnosis in patients with osteosarcoma and Ewing’s sarcoma. Clin Orthop Relat Res. 2013;471:3398–3404. doi: 10.1007/s11999-013-3147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohler DG, Chiu R, McCall DA, Avedian RS. Curettage and cryosurgery for low-grade cartilage tumors is associated with low recurrence and high function. Clin Orthop Relat Res. 2010;468:2765–2773. doi: 10.1007/s11999-010-1445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Cancer Institute. National Cancer Institute Fact Sheet. Available at: http://www.cancer.gov/cancertopics/factsheet/disparities/cancer-health-disparities. Accessed June 3, 2013.
- 29.National Comprehensive Cancer Network. Available at: www.nccn.org/index.asp. Accessed December 28, 2012.
- 30.Nattinger AB, McAuliffe TL, Schapira MM. Generalizability of the Surveillance, Epidemiology, and End Results Registry population: factors relevant to epidemiologic and health care research. J Clin Epidemiol. 1997;50:939–945. doi: 10.1016/S0895-4356(97)00099-1. [DOI] [PubMed] [Google Scholar]
- 31.Parsons HM, Habermann EB, Tuttle TM, Al-Refaie WB. Conditional survival of extremity soft-tissue sarcoma: results beyond the staging system. Cancer. 2011;117:1055–1060. doi: 10.1002/cncr.25564. [DOI] [PubMed] [Google Scholar]
- 32.Pring ME, Weber KL, Unni KK, Sim FH. Chondrosarcoma of the pelvis. A review of sixty-four cases. J Bone Joint Surg Am. 2001;83:1630–1642. [PubMed] [Google Scholar]
- 33.Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;391:224–233. doi: 10.1097/00003086-200110000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Rueth NM, Groth SS, Tuttle TM, Virnig BA, Al-Refaie WB, Habermann EB. Conditional survival after surgical treatment of melanoma: an analysis of the Surveillance, Epidemiology, and End Results database. Ann Surg Oncol. 2010;17:1662–1668. doi: 10.1245/s10434-010-0965-8. [DOI] [PubMed] [Google Scholar]
- 35.Skeletal Lesions Interobserver Correlation among Expert Diagnosticians Study Group Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones. J Bone Joint Surg Am. 2007;89:2113–2123. doi: 10.2106/JBJS.F.01530. [DOI] [PubMed] [Google Scholar]
- 36.Soderstrom M, Ekfors TO, Bohling TO, Teppo LH, Vuorio EI, Aro HT. No improvement in the overal survival of 194 patients with chondrosarcoma in Finland in 1971–1990. Acta Orthop Scand. 2003;74:344–350. doi: 10.1080/00016470308540851. [DOI] [PubMed] [Google Scholar]
- 37.Staals EL, Bacchini P, Bertoni F. Dedifferentiated central chondrosarcoma. Cancer. 2006;106:2682–2691. doi: 10.1002/cncr.21936. [DOI] [PubMed] [Google Scholar]
- 38.Wang SJ, Emery R, Fuller CD, Kim JS, Sittig DF, Thomas CR. Conditional survival in gastric cancer: a SEER database analysis. Gastric Cancer. 2007;10:153–158. doi: 10.1007/s10120-007-0424-9. [DOI] [PubMed] [Google Scholar]
- 39.Wang SJ, Fuller CD, Emery R, Thomas CR. Conditional survival in rectal cancer: a SEER database analysis. Gastrointest Cancer Res. 2007;1:84–89. [PMC free article] [PubMed] [Google Scholar]
- 40.Wyman JJ, Hornstein AM, Meitner PA, Mak S, Verdier P, Block JA, Pan J, Terek RM. Multidrug resistance-1 and P-glycoprotein in human chondrosarcoma cell lines: expression correlates with decreased intracellular doxorubicin and in vitro chemoresistance. J Orthop Res. 1999;17:935–940. doi: 10.1002/jor.1100170619. [DOI] [PubMed] [Google Scholar]
- 41.Xing Y, Chang GJ, Hu CY, Askew RL, Ross MI, Gershenwald JE, Lee JE, Mansfield PF, Lucci A, Cormier JN. Conditional survival estimates improve over time for patients with advanced melanoma: results from a population-based analysis. Cancer. 2010;116:2234–2241. doi: 10.1002/cncr.24966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu XQ, Baade PD, O’Connell DL. Conditional survival of cancer patients: an Australian perspective. BMC Cancer. 2012;12:460. doi: 10.1186/1471-2407-12-460. [DOI] [PMC free article] [PubMed] [Google Scholar]