Abstract
Background
Backside damage of the polyethylene in TKA is a potential source of debris. The location of the tibial post in posterior-stabilized implants may influence micromotion, and thus affect backside damage, as may surface roughness.
Questions
We used implant retrieval analysis to (1) examine if there were differences in backside damage among three modern posterior-stabilized implants attributable to variable surface roughness; (2) determine if the location of damage on the tibial post affected the pattern of backside damage; and (3) determine if demographics influenced backside damage.
Methods
We identified 403 posterior-stabilized tibial retrieved inserts (147 NexGen®, 152 Optetrak®, 104 Genesis® II). The damage on the surfaces of the tibial posts was previously graded. The backside of the inserts (divided into quadrants) were scored for evidence of damage. The total quadrant damage was compared for each implant group, the relationship between post face damage and location of damage on the backside was determined for each implant group, and total backside damage was compared among the three implant groups.
Results
No correlation was found between the location of damage on the post and location of damage on the backside of the implant for any of the three groups. The Genesis® II polyethylene implants, which articulate with a highly polished tibial tray, showed a significantly lower total backside damage score (p < 0.01) when compared with the other two implant groups. The Genesis® II and Optetrak® showed significantly more damage in the posterior quadrants of the implants (p < 0.01) when compared with the anterior quadrants. A linear regression analysis revealed that lower tibial tray surface roughness was correlated with decreased damage.
Conclusions
An implant design with a highly polished tibial tray was associated with decreased backside damage. However, tibial post design and location did not influence the location of backside damage.
Clinical Relevance
Our study showed that a highly polished tibial tray was associated with decreased damage to the backside of polyethylene inserts independent of post design and location. These findings should be taken into consideration when new generations of implants are designed.
Introduction
The use of modular tibial components in a TKA allows for improved soft tissue tensioning, easier revision, and transmission of forces across the knee [1, 2, 20]. However, modular tibial components create a nonarticular surface between the UHMWPE insert and the underlying metal tray, which contributes to wear debris and subsequent osteolysis [7, 11, 15, 21, 22].
It has been reported that the magnitude of backside wear is correlated with the amount of micromotion between the insert and the tray [5, 6, 18, 19, 23]. The locking mechanism is a key factor in determining the amount of this motion [14, 23]. Other design features such as the surface finish of the tibial tray, insert and tray design, articular geometry of the insert, and polyethylene manufacturing techniques also affect backside wear [10, 16]. Finally, surgical technique and final alignment also may contribute to backside wear.
Previous studies of retrieved posterior-stabilized implants showed the location and extent of tibial post damage to be design-dependent [8, 9]. Post damage reflects the loads applied on the posterior-stabilized constraint, which in turn could influence insert-tray micromotion and therefore backside wear. As such, we used implant retrieval analysis to (1) examine if there were differences in backside damage among three modern posterior-stabilized TKA implants owing to variable surface roughness (NexGen®, Zimmer, Warsaw, IN, USA; Optetrak®, Exactech, Gainesville, FL, USA; and Genesis® II, Smith & Nephew, Memphis, TN, USA); (2) determine if the location of damage on the tibial post affected the pattern of backside damage; and (3) determine if demographics influenced backside damage.
Materials and Methods
We analyzed the damage on the tibial post and undersurface of retrieved polyethylene posterior-stabilized tibial inserts from the retrieval laboratory at the authors’ institution. The implants analyzed included 147 NexGen®, 152 Optetrak®, and 104 Genesis® II implants. The location of the posts in these three implants influence post damage as previously described [8]. The Optetrak® has the most anteriorly placed tibial post relative to the dwell point of the tibiofemoral articular surface, whereas the Genesis® II has the most posteriorly positioned post. The tibial post of the NexGen® is wider (14.9 mm) than those of the Genesis® II (13.5 mm) and the Optetrak® (13.2 mm) implants.
The Nexgen® metal tibial tray is made from cobalt-chromium alloy and the superior surface that mates against the insert is manufactured with a blasted surface finish. The surface roughness (Ra) is 1.73 μm as reported by the manufacturers (Oral communication. Angela LoSchiavo. Zimmer. April 13, 2013). The polyethylene inserts had been gamma-irradiated in an inert environment [8] and are locked in place by mating with a dovetail on the tibial tray and snapping inside a peripheral rim. The Optetrak® tibial trays are a titanium alloy [8]. The surface roughness (Ra) according to the manufacturer is 0.76 μm (Oral communication. Jeffrey Bates. Exactech. April 15, 2013). The tray has four holes, two on each plateau, to accommodate cancellous bone screws. The tibial locking mechanism includes a continuous peripheral rim around the tibial tray, posterior feet, and a central mushroom intended to prevent micromotion and liftoff. The polyethylene inserts had been compression-molded and gamma-irradiated in an inert environment. The Genesis® II tibial trays are cobalt-chromium alloy, and the superior surface is provided with a polished finish. The manufacturer-reported surface roughness (Ra) is approximately 0.05 to 0.08 μm (Oral communication. Gregory Schack. Smith & Nephew. April 7, 2013). The locking mechanism incorporates a partial peripheral capture. The polyethylene inserts had been sterilized by ethylene oxide gas in an inert environment [8].
Implants were retrieved at revision surgeries performed at the authors’ institution between December 1997 and February 2010. Institutional review board approval was obtained to review patient demographics: age, sex, BMI, reason for revision, and length of time of implantation. The patients’ ages and sex distributions were similar among the three groups, although patient BMI was significantly lower for the NexGen® group than for the Optetrak® group (30.4 ± 6.8 kg/m2 versus 32.3 ± 6.6 kg/m2, respectively; p < 0.01) (Table 1). The BMI of the patients in the Genesis® II group was 31.1 ± 7.4 kg/m2 which was between the BMI of the other two groups. The reasons for revision also were similar among the three groups (Table 2). The Optetrak® polyethylene implants had been implanted for a longer time than the Genesis® II implants (3.2 ± 8.5 years versus 1.8 ± 2.0 years, respectively; p < 0.01). The mean length of time of implantation for the NexGen® was between those of the other two groups at 2.1 ± 2.1 years.
Table 1.
Patient demographics
| Implant type | Age of patient (years), mean ± SD |
Sex | BMI (kg/m2), mean ± SD |
Length of time of implantation (years), mean ± SD |
|---|---|---|---|---|
| Genesis® II, Smith & Nephew, Memphis, TN, USA | 66.2 ± 11.3 | 47.1% males, 52.9% females | 31.1 ± 7.4 | 1.8 ± 2.0 |
| NexGen®, Zimmer, Warsaw, IN, USA | 66.3 ± 10.3 | 40.1% males, 59.1% females | 30.4 ± 6.8 | 2.1 ± 2.1 |
| Optetrak®, Exactech, Gainesville, FL, USA | 65.5 ± 11.4 | 37.5% males, 62.5% females | 32.3 ± 6.6 | 3.2 ± 8.5 |
Table 2.
Indications for revision among the three implants
| Implant Type | Infection, number (%) | Loosening, number (%) | Instability, number (%) | Stiffness, number (%) | Other, number (%) | Total number |
|---|---|---|---|---|---|---|
| Genesis® II, Smith & Nephew, Memphis, TN, USA | 41 (41.8) | 15 (15.3) | 10 (10.2) | 21 (21.4) | 11 (11.2) | 98 |
| NexGen®, Zimmer, Warsaw, IN, USA | 47 (32.6) | 27 (18.8) | 41 (28.5) | 24 (16.7) | 5 (3.5) | 144 |
| Optetrak®, Exactech, Gainesville, FL, USA | 48 (33.8) | 13 (9.2) | 43 (30.3) | 22 (15.5) | 16 (11.3) | 142 |
Some implants reported in the current study were used in a previous study of the relationship between post location and post damage [8]. These included 117 of the 147 NexGen®, 103 of the 152 Optetrak®, and 58 of the 104 Genesis® II implants. In the previous study, the Knee Society radiographic evaluation system was used to assess coronal and sagittal alignment of the femoral and tibial components in a representative sample [8]. There was no difference in radiographic component positioning among the three groups. The backside surfaces of the retrieved components also were scored for damage. Each backside surface was divided into quadrants (anteromedial, posteromedial, anterolateral, and posterolateral) and received a damage score as described by Hood et al. [13]. The amount, type, and location of surface damage were recorded for each backside quadrant. Damage types assessed were burnishing, scratching, pitting, abrasion, delamination, third-body debris, and surface deformation. Each damage mode was scored on a scale of 0 to 3. A score of 0 meant that the damage mode was absent, Grade 1 was assigned if the damage mode occurred on less than 10% of the post face or backside quadrant, Grade 2 if present on 10% to 50%, and Grade 3 if the damage mode was present on more than 50% of the section. The total possible score for each backside quadrant is 21. Backside wear scores from each quadrant were summed to create a total backside wear score with total possible score of 84. Post damage scores were obtained from the study by Dolan et al. [8]. The damage scores were determined using the same method as the quadrant grading, with post surfaces (anterior, posterior, medial, lateral, and top) having a possible wear score of 21 each and a possible total post score of 103 [8].
To determine if there was a relationship between the amount of wear on the backside and the amount of wear on the tibial post, damage scores for the backside were combined in anterior (anteromedial + anterolateral), posterior (posteromedial + posterolateral), medial (anteromedial + posteromedial), and lateral (anterolateral + posterolateral) halves.
Statistical Analysis
Descriptive data are presented as means ± SDs for continuous variables and frequencies are presented as percentages for categorical and discrete variables. To determine if differences existed in the backside damage among the three implants, the total damage scores were compared using one-way ANOVA. Patient demographics among implants also were compared using one-way ANOVA.
Linear regression analysis was used to compare post damage with backside damage. Damage comparisons were completed for anterior post versus anterior backside, anterior post versus posterior backside, posterior post versus anterior backside, posterior post versus posterior backside, medial post versus medial backside, medial post versus lateral backside, lateral post versus medial backside, and lateral post versus lateral backside.
Statistical analysis of the damage scores and locations on the backside of the tibial inserts were completed using one-way ANOVA with Dunn’s post hoc analysis.
Results
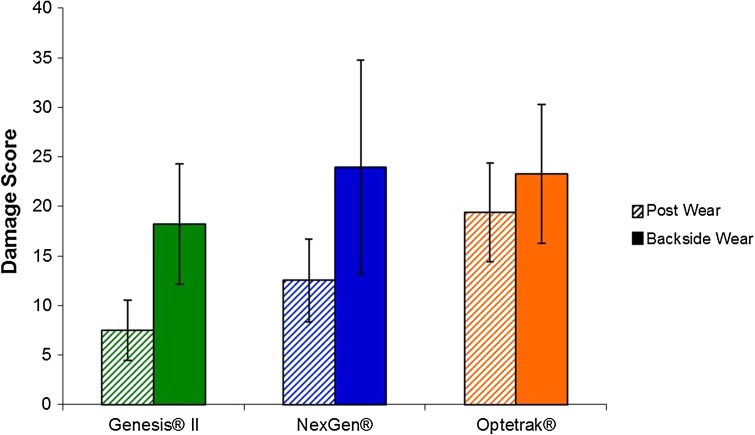
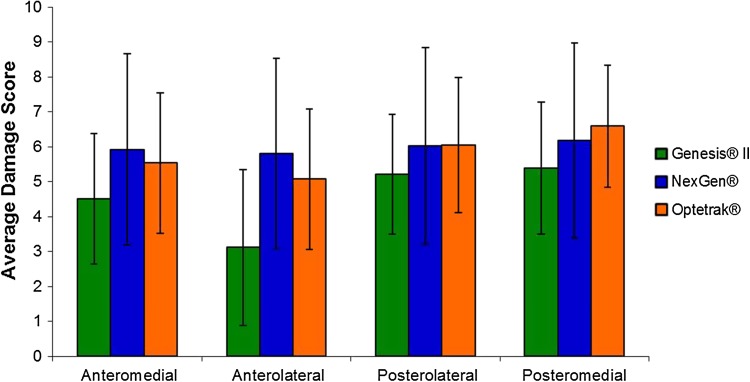
Surface roughness was associated with backside damage. The Genesis® II polyethylene implants showed a lower backside total damage score compared with the Optetrak® and NexGen® implants (18.2 ± 6.1, 23.3 ± 10.8, and 23.9 ± 7.0, respectively; p < 0.01) (Fig. 1). The Genesis® II and Optetrak® showed higher damage scores in the posterior quadrants of the implants (p < 0.01), whereas the NexGen® had similar amounts of damage in all quadrants (Fig. 2). The Genesis® II showed more damage in the anteromedial quadrant compared with the anterolateral quadrant (p < 0.001). For all three implants, the predominant types of surface damage were burnishing (NexGen®, 78% of implants; Optetrak®, 97% of implants; Genesis® II, 77% of implants), scratching (NexGen®, 88% of implants; Optetrak®, 99% of implants; Genesis® II, 99% of implants), and pitting (NexGen®, 87% of implants; Optetrak®, 100% of implants; Genesis® II, 100% of implants). No implants in any group showed delamination. Embedded debris was found in two (1%) Optetrak® and none of the NexGen® and Genesis® II implants. Surface deformation was found on one each of the Optetrak® (1%) and Genesis® II (1%) implants with none found on the NexGen® implants. Abrasion was present on three (3%) Genesis® II and none of the either the Optetrak® or NexGen® implants.
Fig. 1.
The mean damage scores for the post and backside surfaces of the three groups of posterior stabilized TKA retrieved tibial inserts are shown. The error bars denote SDs. The backside scores for the Genesis® II inserts were significantly lower (p < 0.01) than those of the other two designs.
Fig. 2.
Backside damage scores for the three implant designs varied based on quadrant. The Genesis® II and Optetrak® inserts had significantly higher damage (p > 0.05) in the posteromedial quadrant relative to the anteromedial and anterolateral quadrants. Implants from both designs also showed significantly greater damage in the posterolateral quadrant compared with the anterolateral quadrant. In the Genesis® II group, the anteromedial quadrant also showed significantly greater damage compared with the anterolateral quadrant. There were no significant differences between quadrants in the NexGen® implant group.
No correlation was found between the location of damage on the post and location of damage on the backside of the implant.
Age, length of implantation, and BMI did not significantly affect damage score for any implant (all p > 0.05).
Discussion
Modular tibial trays frequently are used in TKAs because modularity allows surgeons to tailor the soft tissue tensioning while trying different trial inserts after final placement of the femoral and tibial components. However, backside damage between the inferior surface of the polyethylene tibial insert and the superior surface of the metallic tibial tray produces particulate debris that can lead to osteolysis. Many factors in various posterior-stabilized implants have been implicated to affect backside damage. As such, we examined if there were differences in backside damage based on surface roughness, tibial post damage location, and demographics between three contemporary posterior-stabilized implants. Our study showed that a highly polished tibial tray was associated with decreased damage to the backside of polyethylene inserts independent of post design or location, patient age or BMI, or length of time of implantation.
Our study has limitations. As with any retrieval analysis, the components may not represent well-functioning knee replacements. Nonetheless, because we had large numbers of each implant, they spanned a range of demographic and clinical data, and these data were comparable among the three implants. Additionally, subjective grading of damage as a reflection of implant performance may not directly correlate with wear. With retrieved implants, however, we and other investigators [4, 9, 12, 16, 21] have found that damage provides a useful basis for comparison among polyethylene components. Moreover, polyethylene from the NexGen® and Optetrak® implants were sterilized in a gamma-inert environment, which has a moderate amount of crosslinking, but also is more susceptible to oxidation in vivo as a result of free radicals [17]. However, the Genesis® II was sterilized in ethylene oxide, which does not induce crosslinking. As such, the intrinsic polyethylene wear resistance may be different among the three implant groups as a result of differences in sterilization method of the tibial inserts. Finally, there was a lower number of knees revised for instability in the Genesis® II group. This may be a confounding variable as an unstable knee will translate more force to the implant and potentially lead to increased wear regardless of surface roughness.
The Genesis® II implants showed the least amount of backside damage among the implants in the study, likely because it was the only one with a polished metal tray (with an estimated surface roughness approximately an order of magnitude lower than those of the other two implants). Berry et al, [3] noted similar findings when comparing roughened and polished tibial trays. They compared 94 Sigma® RP mobile-bearing retrieved tibial implants (DePuy Orthopedics, Inc, Warsaw, IN, USA) with 218 Sigma® fixed-bearing tibias (DePuy Orthopedics, Inc). The fixed-bearing series were further partitioned into 181 knees with rough (grit-blasted finish) titanium alloy trays (median implantation time, 81 months) and 37 with polished cobalt chromium alloy trays (median implantation time, 17 months). Wear penetration was calculated by subtracting the measured thickness from the manufacturer’s stated dimension for the implant. Inserts with polished cobalt-chromium trays were found to have less penetration (ie, better damage performance). Other in vitro simulation studies showed that smooth metallic tibial trays can produce 20 times less backside polyethylene wear than more roughened surfaces [3, 4, 10].
Interestingly, backside damage was independent of post design or location. Previous studies analyzed the relationship between location of the tibial post and the subsequent damage patterns in a subset of retrieved tibial inserts from the same three implants as were used in the current study [8, 9]. In these previous investigations, the NexGen® tibial post showed predominantly anterior damage, indicating impingement in extension. The Genesis® II tibial posts had predominantly posterior damage, indicating damage consistent with the contact between the femoral cam and post that is the intended constraint provided by posterior stabilized designs. Finally, the Optetrak® tibial posts showed more global tibial post damage. In the current study, we hypothesized that the forces from the femoral cam on the tibial post would cause different patterns of micromotion between the polyethylene insert and the metallic tray among the three implants and thus cause unique patterns of backside damage. Instead, our results showed that the different interactions among the femoral box, cam, and tibial post among these implants had no influence on the pattern; all three implants showed similar backside damage modes of burnishing, scratching, and pitting.
Although the backside damage modes were similar among the three posterior stabilized implants, preferential differences in damage were found on the posterior ½ of the polyethylene inserts between the Optetrak® and Genesis® II. Harman et al. [12] also found that damage patterns were concentrated on the posterior ½ of retrieved polyethylene inserts. While all of these retrievals were from a different design than that in our study, it highlights that posterior damage is a dominant finding of many systems.
Other differences among the implants in our study include tibial insert geometries and the polyethylene manufacturing technique. Differences in post damage caused by differences among posterior stabilized implants (primarily shapes and locations of the posts) did not translate into differences in backside damage. This is interesting information for orthopaedic surgeons and manufacturers when considering posterior stabilized implants, because it suggests that the posterior stabilized constraint can be designed without concern for negatively affecting backside damage. However, an effective locking mechanism that limits micromotion is imperative; all three implants had a similarly effective peripheral capture locking mechanism.
Backside damage is an important potential source of damage debris in all modular TKA implants. A highly polished tibial tray may decrease damage to the backside of polyethylene inserts and thus backside damage independent of post design and location. Understanding design features that contribute to backside damage will help improve future TKA implants.
Footnotes
One author certifies that he (SBH), or a member of his immediate family, has or may receive payments or benefits, during the study period, an amount in excess of USD 1,000,000 from Smith & Nephew, Inc (Memphis, TN, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Hospital for Special Surgery, New York, NY, USA.
References
- 1.Barrack RL. Modularity of Prosthetic Implants. J Am Acad Orthop Surg. 1994;2:16–25. doi: 10.5435/00124635-199401000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DL, Burstein AH, Santavicca EA, Insall JN. Performance of the tibial component in total knee replacement. J Bone Joint Surg Am. 1982;64:1026–1033. [PubMed] [Google Scholar]
- 3.Berry DJ, Currier JH, Mayor MB, Collier JP. Knee wear measured in retrievals: a polished tray reduces insert wear. Clin Orthop Relat Res. 2012;470:1860–1868. doi: 10.1007/s11999-012-2248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billi F, Sangiorgio SN, Aust S, Ebramzadeh E. Material and surface factors influencing backside fretting wear in total knee replacement tibial components. J Biomech. 2010;43:1310–1315. doi: 10.1016/j.jbiomech.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Conditt MA, Ismaily SK, Alexander JW, Noble PC. Backside wear of modular ultra-high molecular weight polyethylene tibial inserts. J Bone Joint Surg Am. 2004;86:1031–1037. doi: 10.2106/00004623-200405000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Conditt MA, Thompson MT, Usrey MM, Ismaily SK, Noble PC. Backside wear of polyethylene tibial inserts: mechanism and magnitude of material loss. J Bone Joint Surg Am. 2005;87:326–331. doi: 10.2106/JBJS.C.01308. [DOI] [PubMed] [Google Scholar]
- 7.Cuckler JM, Lemons J, Tamarapalli JR, Beck P. Polyethylene damage on the nonarticular surface of modular total knee prostheses. Clin Orthop Relat Res. 2003;410:248–253. doi: 10.1097/01.blo.0000063794.32430.05. [DOI] [PubMed] [Google Scholar]
- 8.Dolan MM, Kelly NH, Nguyen JT, Wright TM, Haas SB. Implant design influences tibial post wear damage in posterior-stabilized knees. Clin Orthop Relat Res. 2011;469:160–167. doi: 10.1007/s11999-010-1515-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furman BD, Lipman J, Kligman M, Wright TM, Haas SB. Tibial post wear in posterior-stabilized knee replacements is design-dependent. Clin Orthop Relat Res. 2008;466:2650–2655. doi: 10.1007/s11999-008-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galvin A, Jennings LM, McEwen HM, Fisher J. The influence of tibial tray design on the wear of fixed-bearing total knee replacements. Proc Inst Mech Eng H. 2008;222:1289–1293. doi: 10.1243/09544119JEIM434. [DOI] [PubMed] [Google Scholar]
- 11.Gupta SK, Chu A, Ranawat AS, Slamin J, Ranawat CS. Osteolysis after total knee arthroplasty. J Arthroplasty. 2007;22:787–799. doi: 10.1016/j.arth.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 12.Harman MK, Banks SA, Hodge WA. Backside damage corresponding to articular damage in retrieved tibial polyethylene inserts. Clin Orthop Relat Res. 2007;458:137–144. doi: 10.1097/BLO.0b013e3180320b01. [DOI] [PubMed] [Google Scholar]
- 13.Hood RW, Wright TM, Burstein AH. Retrieval analysis of total knee prostheses: a method and its application to 48 total condylar prostheses. J Biomed Mater Res. 1983;17:829–842. doi: 10.1002/jbm.820170510. [DOI] [PubMed] [Google Scholar]
- 14.Jayabalan P, Furman BD, Cottrell JM, Wright TM. Backside wear in modern total knee designs. HSS J. 2007;3:30–34. doi: 10.1007/s11420-006-9033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Scuderi G, Furman BD, Bhattacharyya S, Schmieg JJ, Insall JN. Assessment of backside wear from the analysis of 55 retrieved tibial inserts. Clin Orthop Relat Res. 2002;404:75–82. doi: 10.1097/00003086-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Lombardi AV, Jr, Ellison BS, Berend KR. Polyethylene wear is influenced by manufacturing technique in modular TKA. Clin Orthop Relat Res. 2008;466:2798–2805. doi: 10.1007/s11999-008-0470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Effect of sterilization method and other modifications on the wear resistance of acetabular cups made of ultra-high molecular weight polyethylene: a hip-simulator study. J Bone Joint Surg Am. 2000;82:1708–1725. doi: 10.2106/00004623-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Parks NL, Engh GA, Topoleski LD, Emperado J. The Coventry Award: Modular tibial insert micromotion. A concern with contemporary knee implants. Clin Orthop Relat Res. 1998;356:10–15. doi: 10.1097/00003086-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Rao AR, Engh GA, Collier MB, Lounici S. Tibial interface wear in retrieved total knee components and correlations with modular insert motion. J Bone Joint Surg Am. 2002;84:1849–1855. doi: 10.2106/00004623-200210000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Small SR, Berend ME, Ritter MA, Buckley CA. A comparison in proximal tibial strain between metal-backed and all-polyethylene anatomic graduated component total knee arthroplasty tibial components. J Arthroplasty. 2010;25:820–825. doi: 10.1016/j.arth.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Taki N, Goldberg VM, Kraay MJ, Rimnac CM. Backside wear of Miller-Galante I and Insall-Burstein II tibial inserts. Clin Orthop Relat Res. 2004;428:198–206. doi: 10.1097/01.blo.0000148571.34835.ce. [DOI] [PubMed] [Google Scholar]
- 22.Wasielewski RC. The causes of insert backside wear in total knee arthroplasty. Clin Orthop Relat Res. 2002;404:232–246. doi: 10.1097/00003086-200211000-00037. [DOI] [PubMed] [Google Scholar]
- 23.Wasielewski RC, Parks N, Williams I, Surprenant H, Collier JP, Engh G. Tibial insert undersurface as a contributing source of polyethylene wear debris. Clin Orthop Relat Res. 1997;345:53–59. doi: 10.1097/00003086-199712000-00009. [DOI] [PubMed] [Google Scholar]