Abstract
How vesicular dynamics parameters depend on temperature and how temperature affects the parameter change during prolonged high frequency stimulation was determined by fitting a model of vesicular storage and release to the amplitudes of the excitatory post-synaptic currents (EPSC) recorded from CA1 neurons in rat hippocampal slices. The temperature ranged from low (13 °C) to higher and more physiological temperature (34 °C). Fitting the model of vesicular storage and release to the EPSC amplitudes during a single pair of brief high–low frequency stimulation trains yields the estimates of all parameters of the vesicular dynamics, and with good precision. Both fractional release and replenishment rate decrease as the temperature rises. Change of the underlying ‘basic’ parameters (release coupling, replenishment coupling and readily releasable pool size), which the model-fitting also yields is complex. The replenishment coupling between the readily releasable pool (RRP) and resting pool increases with temperature (which renders the replenishment rate higher), but this is more than counterbalanced by greater RRP size (which renders the replenishment rate lower). Finally, during long, high frequency patterned stimulation that leads to significant synaptic depression, the replenishment rate decreases markedly and rapidly at low temperatures (<22 °C), but at high temperatures (>28 °C) the replenishment rate rises with stimulation, making synapses better able to maintain synaptic efficacy.
Keywords: Synaptic depression, Replenishment rate, Temperature, Model fitting, Excitatory synapse, Hippocampus
Introduction
Synaptic stimulation leads to a wide range of short-term and long-term changes in synaptic efficacy. They include the enhancement of the synaptic efficacy such as facilitation, augmentation, posttetanic potentiation (Magleby 1987) and long-term potentiation (Gustaffson et al. 1987), but also its lowering, and this can take the form of short-term depression (Zucker and Regehr 2002) or long-term depression (Bear and Abraham 1996). The long-term forms of plasticity are believed to be associated with learning and memory (Bliss and Collingridge 1993), whereas the short-term depression acts as a frequency-dependent filter of the presynaptic input, an action that has significant computational consequences (Abbott et al. 1997; Tsodyks and Markram 1997). In this study we focus our attention on how temperature regulates the short-term depression of excitatory synapses of rat hippocampus.
The effect of temperature on spontaneous release has been documented in a variety of cell and animal types. At the rat neuromuscular junction the rate of spontaneous quantal release rises greatly as the temperature increases (Barrett et al. 1978), but at the neuro-endocrine cells the temperature dependence is less pronounced (Pihel et al. 1996; Walker et al. 1996). Given that the regulation of vesicle fusion with the plasma membrane by the SNARE proteins is temperature dependent (Liu et al. 2009), it is not surprising that spontaneous release is also enhanced with higher temperatures. The effect of temperature on the fractional release, on the replenishment rate of the RRP of the evoked release and on their change during high frequency stimulation is far less explored. When tested by paired pulses of hypertonic solution in cultured hippocampal neurons, the synaptic output recovers faster at high temperature (Pyott and Rosenmund 2002). Moreover, the endplate potential rundown in rat diaphragm is less attenuated at higher temperatures (Moyer and van Lunteren 2001). These reports suggest that the replenishment is probably more effective at higher temperatures, but such a conclusion remains tentative. Slower endplate potential rundown at higher temperatures may indicate faster replenishment, but it also may be due to the lower fractional release. Likewise at the calyx of Held synapse, the steady-state synaptic output rises as temperature increases, suggesting that the refilling of the readily releasable pool becomes faster when the temperature increases from room to physiological temperatures (Kushmerick et al. 2006), but such a change may also be due to the changes of the fractional release (Bui and Glavinovic 2013a).
At the excitatory synapses of rat hippocampus the replenishment rate diminishes markedly and rapidly during stimulation at room temperature (Bui and Glavinovic 2013a). If this synapse is also better able to sustain the synaptic output during long high frequency stimulation at high temperatures, it is important to determine whether such a change is due to the less pronounced decrease of the replenishment rate during long stimulation at high temperatures or because the fractional release is lower at high temperatures? Moreover, if the replenishment rate decreases less with stimulation as temperature rises, does it at some temperature increase during stimulation? Finally, how does the replenishment rate depend on temperature?
In this study we evaluate the temperature dependence of all parameters that define the vesicular storage and release system from the change of synaptic output resulting from a single pair of brief high–low frequency stimulation trains. Moreover, we determine how the temperature influences the change of these parameters during synaptic depression induced by long patterned stimulation.
Methods
Solutions and recordings
All procedures were in accordance with the regulations of McGill Animal Care. Post-natal (100–125 g) male Sprague–Dawley rats were decapitated under isoflurane anaesthesia. The brain was rapidly removed and immersed in oxygenated (96 % O2, 5 % CO2) ice-cold artificial cerebrospinal fluid (ACSF) of the following composition (mM): NaCl 125, KCl 3, CaCl2 2, MgCl2 1.3, NaHCO3 26, NaH2PO4 1.25 and glucose 10, and gassed with 95 % O2 and 5 % CO2 (pH 7.2–7.4). Hippocampal slices (400–450 μm thick) were cut using a vibrating microtome (Campden, United Kingdom) and incubated at room temperature in ACSF for 1 h or more before use. The slices were subsequently transferred to a recording chamber in which they were kept fully submerged in ACSF at 20–22 °C, and with flow of 3–4 ml/min. Patch pipettes were filled with (mM): CsCl 130, NaCl 10, ATP-Mg 3, GTP 0.3, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid HEPES 10, ethyl-enebis(okonitrib) tetracetate 10; pH was adjusted to 7.2–7.3 with NaOH. Cesium was chosen as the main cation in order to block potassium conductances. This increases membrane resistance and improves space clamp. dl-2-Amino-5-phosphonovaleric acid (10 μM) was used to block NMDA-activated channels (Taniike et al. 2008), to ensure no induction of the long-term potentiation, although such an induction was unlikely to occur given comparatively low frequency of stimulation (5–10 Hz). Indeed, the difference in quantal size of miniature excitatory post-synaptic currents prior to and after long patterned stimulation was not statistically significant (Bui and Glavinovic 2013a). To suppress inhibitory (g-aminobutyric acid type A) synaptic currents bicuculline methiodide (10 μM) was added at least 10 min before recordings started.
Excitatory post-synaptic currents were recorded “blindly” at 20–22 °C (Blanton et al. 1989), in the deeper areas of the pyramidal cell layer of the CA1 region with whole-cell patch-clamp electrodes and an Axopatch-1D amplifier (Axon Instruments, USA). The holding potentials ranged from −110 to −70 mV, but did not change during an experiment. Having more negative holding potentials improves the signal-to-noise ratio, but is not expected to change vesicular dynamics or the parameters estimates because the EPSC amplitudes are simply scaled up. The recordings were anti-alias filtered with a Bessel filter whose corner frequency was set at 5 kHz. The series resistance ranged from 3 to 10 MΩ. EPSCs were digitized and recorded in real time on a PC, using a BNC 2120 interface card (National Instruments, USA), and with the sampling rate of 10 kHz. This interface card was also used to provide the timing pulses for the stimulus isolation unit and subsequent activation of the Schaffer collateral/commissural pathway. Two basic stimulation patterns were used—a pair of high-low frequency trains (5–10 and 2.5–5 Hz trains, each lasting 5 s, or as specified), long patterned stimulation (an alternating sequence of 10 and 5 Hz trains, each lasting 5 s and repeated 5–10 times). EPSC amplitude was determined as a difference between the baseline (determined as the mean value of 5–10 sample points just before an individual EPSC) and the peak value of the same EPSC (in this case the peak is calculated as the mean value of 3 points; the peak value plus 2 values, 1 on each side of the peak).
Electrical circuit model of release
We simulate the dynamics of vesicular storage using an electrical circuit model (Bielecki et al. 2008; Kruckenberg and Sandweg 1968) with two vesicular pools—the RRP (C1) and resting pool (RP; C2). The differential equations which describe the system dynamics during release (i.e. when the switch is closed) are:
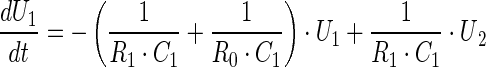 |
1 |
and
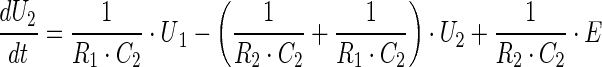 |
2 |
The variables and parameters of the electrical model and those of the vesicular storage are related as follows. The capacitor size C indicates the ability of the pool to store vesicles (it gives its storage capacity or pool size), the charge Q gives its content, and the voltage U across the capacitor the vesicular density in that pool (in terms of its content divided by its storage capacity). If we do model fitting on raw data (i.e. on EPSC amplitudes) the parameters C and R are in Farads and Ohms respectively, whereas the variables U and I are in Volts and Amps. To get the numerical values of some variables in physiologically more meaningful terms we need to determine the mean value of the mEPSCs (mmEPSC). If the values of EPSCs are divided by mmEPSC, the release is expressed in terms of the number of quanta. To express the content of individual pools, not as charge (i.e. in terms of Coulombs), but in terms of number of vesicles we have to take into account that the simulations are ‘continuous’, but the release is ‘discontinuous’, and that the release occurs only during one simulation time step dt, which is 1 ms. The unitary charge q (the charge corresponding to the presence of one vesicle) is then equal to mmEPSC * dt. The content of a chosen individual pool in terms of number of vesicles is then Q/q. Finally, all simulated vesicular fluxes (Is) can also be expressed in terms of the number of vesicles per second, if we divide the simulated current fluxes by q. Whenever the comparison of the replenishment fluxes with release (recorded or simulated) is needed it is preferable to calculate the fluxes per stimulus, and that requires an integration of the replenishment fluxes over the whole time interval (i.e. during the release induced by a stimulus as well as in between stimulation pulses, when no release occurs).
The vesicular concentration difference between two pools (RP and RRP) drives the replenishment of the RRP, which is controlled by the replenishment rate of RRP. Likewise, the vesicular synthesis (shown in Fig. 1a as the battery E) drives the replenishment of the RP, which is controlled by the replenishment rate of RP. The release from the RRP (assuming no replenishment of the pool) at any time It is governed by the equation I0 * exp −t/(R0 * C1), where I0 is the release at time zero. Note that this formalism (i.e. model of vesicular storage and release based on R and C elements) is general in nature and does not imply a specific physical process. Moreover, it is applicable not only to diffusive, but also non-diffusive processes, such as conversion of non-primed to primed vesicles (or non-docked to docked vesicles).
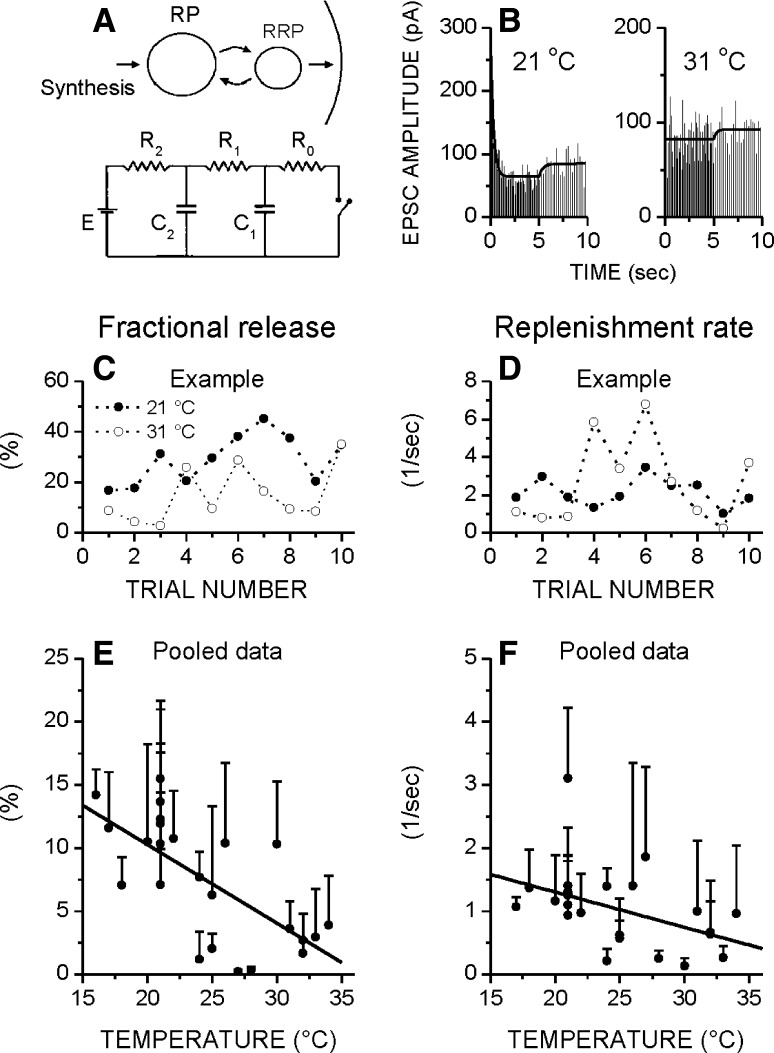
Fig. 1.
As the temperature increases, both fractional release and replenishment rate decrease. a Diagram and equivalent electrical circuit of a secretory cell with two vesicular pools, readily releasable and resting pool. b EPSC amplitudes with best model fits at 21 and 31 °C. The vertical sticks represent the amplitudes of individual EPSCs, whereas the continuous line is the model-fit of the EPSC amplitude changes. c, d Ten consecutive parameter estimates, from the same cell at both temperatures. e, f As the temperature increases, both the fractional release and replenishment rate decrease, with best-fitted lines (FitFR = −0.63 * T + 22.73; r = 0.68 and FitRR = −0.06 * T + 2.41; r = 0.47, respectively). Each circle is a different cell, and each represents the mean parameter estimate of >7 stimulation trains. Vertical bars are standard errors. Stimulation consisted of pairs of brief high (10 Hz, 5 s) and low (5 Hz, 5 s) frequency trains, repeated ≥7 times with 1 min for recovery
All parameters used to characterize vesicular storage and release by the rate-based models are also defined in the electrical model of vesicular storage and release. The fractional release is equal to dt/(R0 * C1), the replenishment rate of the readily releasable pool is 1/(R1 * C1) and the replenishment rate of the resting pool is 1/(R2 * C2). dt is the time interval during which an individual stimulus causes the vesicles to release their content and produce a post-synaptic response (1 ms). We used Simulink to simulate the circuit model (Aristizabal and Glavinovic 2004). An important advantage of this method is that its modular design provides information about the system’s topology that is not easily accessible from differential equations.
In this model of vesicular storage and release, the vesicular fluxes are driven by the difference in pool densities. However, fluxes that move against their concentration gradient or are independent of it were not neglected, but were captured as the ‘open system’ contribution. During prolonged stimulation, the vesicular parameters were estimated in 10 s windows. In a ‘closed’ type model, the estimate of the number of vesicles in each pool is taken as an initial condition for the next window until the end of stimulation, and synthesis is the only source of new quanta. In our model, however, the initial number of vesicles in each pool was estimated for every window (i.e. the initial voltages U1 and U2 across the capacitors are estimated), and the ‘open system’ contribution is assessed by comparing its release to a simulated ‘closed’ system. Finally, note that over 10 s windows used for the estimation of parameters of vesicular dynamics the ‘open system’ contribution is assumed to be constant, but this contribution may change from one window to the next. This ‘open’ vesicular model accounts also for the possibility that vesicle pools may not be in equilibrium at t = 0. If it is assumed that the vesicular pools are initially at equilibrium, the parameter estimates of the first 1–3 windows are sometimes quite inaccurate (data not shown).
Parameter estimation using optimization
The parameters of the vesicular storage and release model (R0, R1, R2, C1, C2, E, U1 and U2) were estimated by minimizing the distance, in a least squares sense, between the experimental data points (EPSC amplitudes) and the data points predicted by the Simulink model (the i0 currents, when the switch is closed). The least squares method, which minimizes the sum of the squared residuals, may be either linear or non-linear, depending on whether the residuals are linear. Whereas linear least-squares problems have closed-form solutions (i.e. there are formulas to evaluate them by a finite number of standard operations), non-linear problems have no closed-form solutions. They are solved by iterative refinement, where at each iteration the system is approximated by a linear one.
The MATLAB Optimization Toolbox function lsqnonlin was used to estimate the parameters in this study. lsqnonlin, a non-linear operator, which minimizes the sum of the squared residuals in an iterative manner, uses a subspace trust region method and is based on the interior-reflective Newton method (Coleman and Li 1996a, b). Briefly, the trust region method approximates the function to be minimized by a quadratic model within a region around the current search point. Within this region, the quadratic model is “trusted” to be correct (i.e. it approximates well the function for minimization). During the search, the size of the region is modified, based on how well the model agrees with the evaluations of the function. The steps are altered to remain within this region of trust. If the quadratic model approximates the function for minimization well, the trust region is expanded; if the approximation is poor then the region is contracted. Trust region methods are in some sense opposite of line search methods. Line search methods first choose a step direction and then a step size, whereas trust region methods first choose a step size (the size of the trust region) and then a step direction. The algorithm is said to converge when one of the following criteria is met: (a) the cost function changes less than a tolerance value between iterations, or (b) the direction of negative curvature cannot be found. The error diminishes after each iteration, until the minimal error is achieved. The parameters of the vesicular dynamics that produce minimal error—the ‘best’ parameters—are taken to be ‘true’ parameters.
Note that the contribution of storage elements (Cs) and non-storage elements (Rs) to vesicular dynamics are qualitatively different. The storage elements affect the time course, but not the steady-state values of different components of the synaptic output, whereas the non-storage elements (Rs) affect both the dynamics and the steady-state. The estimated values of storage elements (C1 and C2) provide the information about their storage capacity (i.e. how able RRP and RP are to contain quanta), whereas the values of non-storage elements (R0 and R1), give information about release coupling and the replenishment coupling between C1 and C2. In present study the replenishment coupling provides information about the speed of conversion of non-primed (or non-docked) vesicles into primed (or docked) vesicles. In secretory systems where the replenishment of RRP is associated with vesicular movement (if such exist), changes of the vesicular diffusion constant would be reflected as changes of R1, and not C1. Regardless of the type of secretory system Rs changes are thus not reflected as Cs changes.
It could be argued that in the future it may be possible to tag one way the vesicles in RRP and another way those in RP. However, even if we knew the identity and the positions of vesicles in each pool, defining their volumes would not be simple. Moreover, the vesicular pools, which are kinetically distinct, are not necessarily morphologically separate (Rizzoli and Betz 2005). We thus think that the attempts to determine the morphological correlate of pools (i.e. their volumes) are ill considered and naïve. In contrast, the storage capacity based on vesicular dynamics is well defined and can be determined accurately.
Results
Both the fractional release and the replenishment rate of the readily releasable pool decrease as temperature rises
Figure 1b shows the EPSC amplitudes at 21 and 31 °C, with best model fits. The vertical sticks or spikes represent the amplitudes of individual EPSCs, whereas the continuous line is the model-fit of the changes of the EPSC amplitude changes. To illustrate the variability of the parameters, Fig. 1c, d show ten estimates of the fractional release and the replenishment rate at 21 and 31 °C, all in the same synapse. Ten brief stimulation trains were used, with one min intervals for recovery. The fractional release [calculated as 1/(R0 * C1)] represents the fraction of the RRP that is released per impulse, and the replenishment rate [calculated as 1/(R1 * C1)] represents the rate at which the RRP refills. Figure 1e, f shows the pooled results of the temperature dependence of the fractional release and replenishment rate. Note that the fractional release estimates are overall low compared to the estimates obtained by quantal analysis (data not shown), and that they do not increase, but instead decrease with temperature. As stated in the Introduction previous studies have shown that the recovery of the synaptic efficacy becomes faster as temperature rises, arguing that the replenishment rate of the RRP is higher at higher temperatures (Pyott and Rosenmund 2002). We however find that the replenishment rate diminishes with temperature, although only modestly.
The effect of temperature on the ‘basic’ parameters
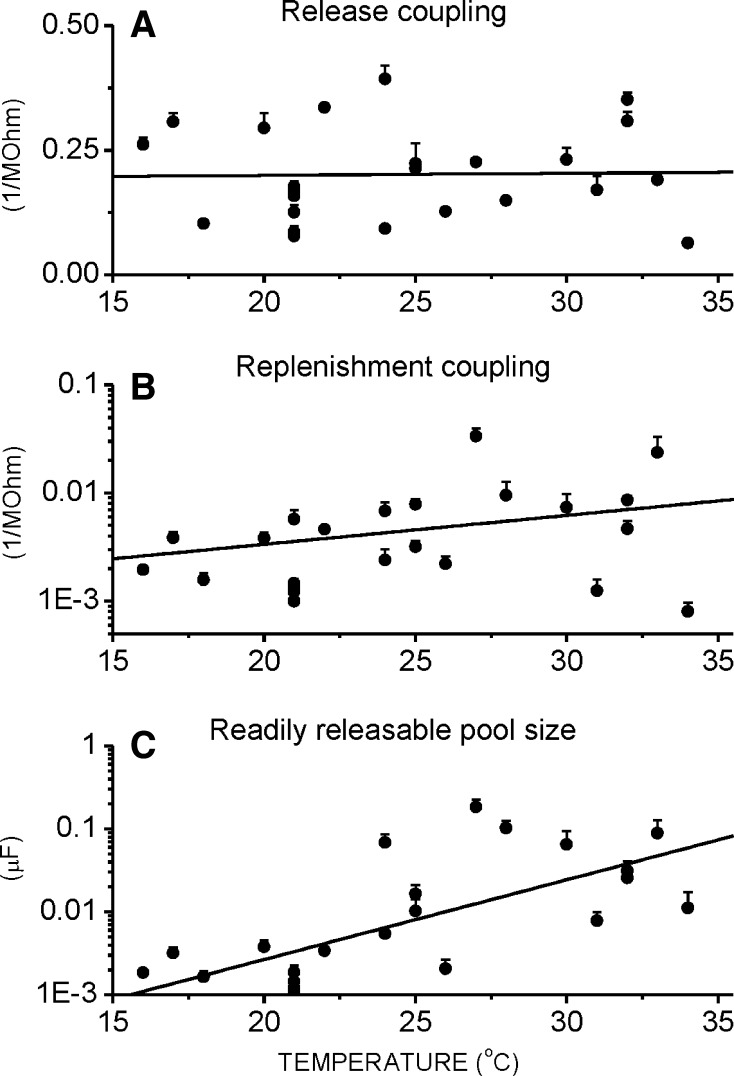
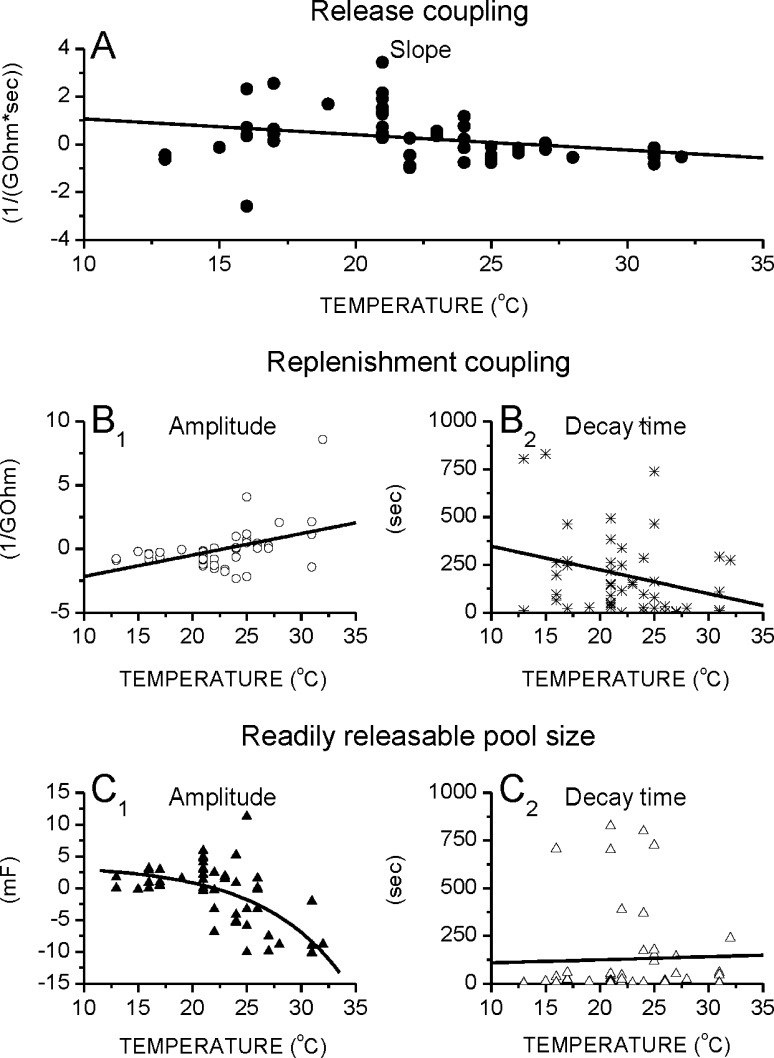
To have better insight into vesicular dynamics we also evaluated the ‘basic parameters’ (release coupling, replenishment coupling and readily releasable pool size; Bui and Glavinovic 2013a). The release coupling is independent of temperature changes (Fig. 2a), but the replenishment coupling increases with temperature (Fig. 2b; note the semi-log scale). Q10 (the rate of change as a consequence of raising the temperature by 10 °C) of the replenishment coupling is 2.0. The temperature dependence of the RRP size is significantly more pronounced (Fig. 2c; note the semi-log scale) and has Q10 value of 5.0. This clarifies why the fractional release decreases as temperature increases. The decrease is mainly due to the increase in the RRP size. Finally, note that the modestly lower replenishment rate at high temperatures (Fig. 1f) is not due to the lower replenishment coupling, which in fact increases with temperature, but is due to the greater RRP size, which is more pronounced and which makes the replenishment rate lower (see Fig. 2b, c).
Fig. 2.
Replenishment coupling and RRP size rise with temperature. a–c As the temperature increases, the release coupling (1/R0) does not change (Fit1/R0 = 0.0004 * T + 0.1908; r = 0.08), whereas the replenishment coupling (1/R1) and the readily releasable pool size rise [Fit1/R1 = 10^(0.03 * T − 3.18); r = 0.35 and FitC1 = 10^(0.10 * T − 4.50); r = 0.70, respectively]. Each circle is a different cell, and each represents the mean parameter estimate of >7 stimulation trains. Vertical bars are standard errors. Stimulation consisted of pairs of brief high (10 Hz, 5 s) and low (5 Hz, 5 s) frequency trains, repeated ≥7 times with 1 min for recovery
Effect of temperature on the change of vesicular storage and release parameters during long patterned stimulation
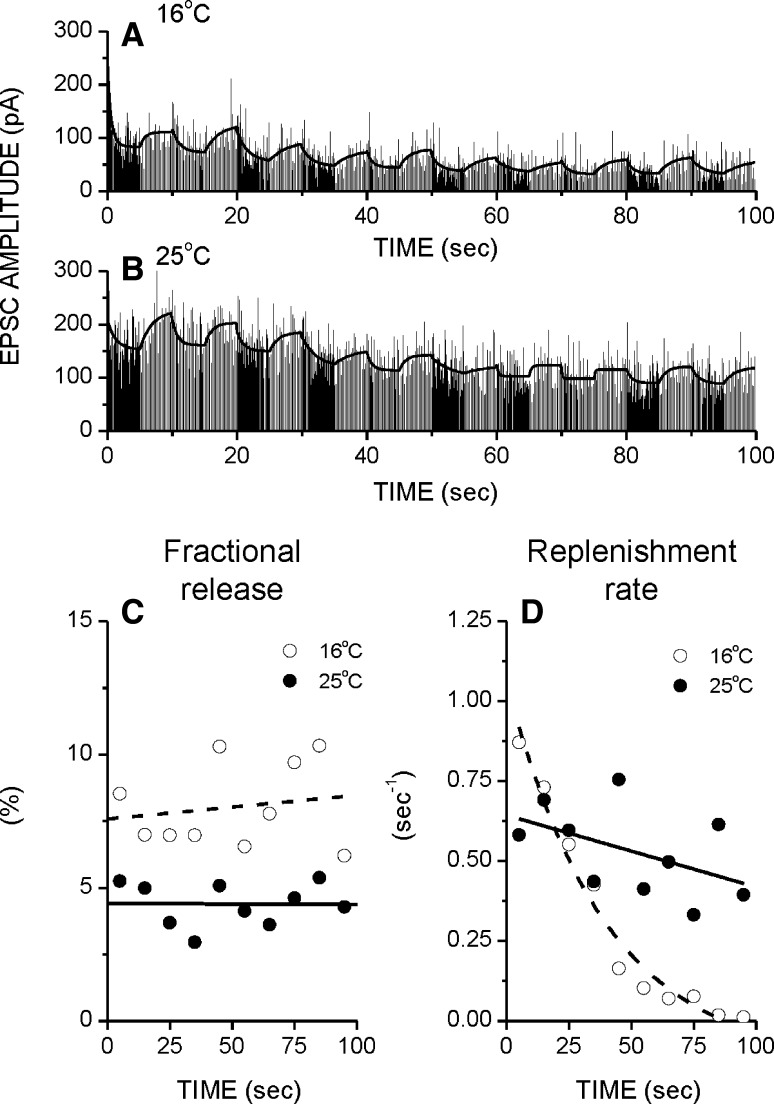
How does the temperature affect the change of parameters of a vesicular storage and release system during prolonged patterned stimulation? Figure 3a, b depict EPSC amplitudes during stimulation with the best model fits at 22 and 30 °C, in the same synapse. Figure 3c, d show the fractional release and replenishment rate estimates (taken every 10 s) of the above data. To characterize how they change with stimulation, fractional release and replenishment rate estimates were fit with lines and exponential curves, respectively. The initial fractional release value (intercept) is lower at high temperature in good agreement with the results using pairs of brief high-low frequency trains (see above). Note however that the fractional release does not change with stimulation, regardless of temperature (Fig. 3c). In contrast the replenishment rate decreases with stimulation, but this decline is much attenuated at higher temperatures (Fig. 3d). In this study we consider that the short-term synaptic depression results from the depletion of the RRP, but other factors can also contribute to it, such as lower presynaptic quantal size or lower postsynaptic quantal size due to the AMPA receptor desensitization or AMPA trafficking (Adesnik et al. 2005). However, as reported before the quantal sizes before and after stimulation were not significantly different (Bui and Glavinovic 2013a).
Fig. 3.
Vesicular storage and release parameters change during long, high frequency patterned stimulation in a temperature dependent manner. a, b EPSC amplitudes together with the model fits at 16 and 25 °C. c Fractional release is lower at high temperature, but regardless of temperature it does not change with stimulation (FitFR16 = −0.01 * T + 7.58; r = 0.17 and FitFR25 = −0.0002 * T + 4.410; r = 0.01). d The replenishment rate decreases with stimulation, but this decrease is attenuated at higher temperature [FitRR16 = −0.1 + 1.2 * exp(−T/39.7); r = 0.96 and FitRR25 = 6936.5 − 6936.8 * exp(−T/3100000); r = 0.03]. Patterned stimulation was 10 Hz for 5 s followed by 5 Hz for 5 s, repeated 10 times
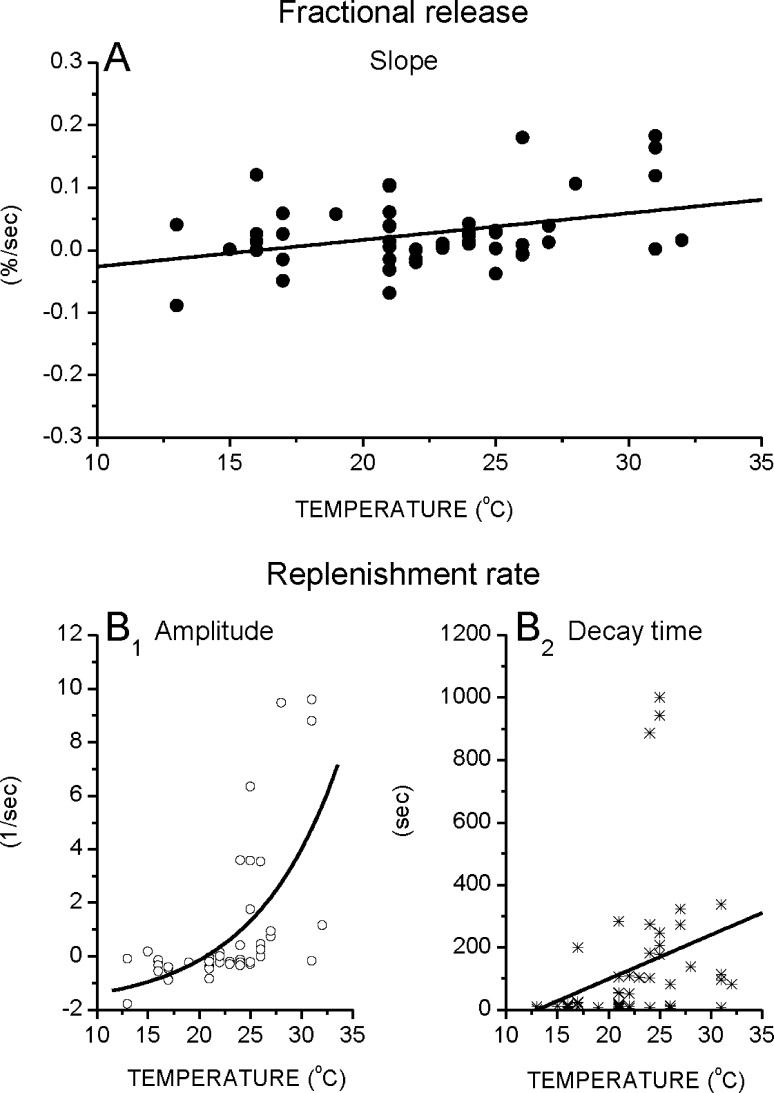
Figure 4a, b summarizes the effect of temperature on the changes of the fractional release and the replenishment rate during long, high frequency stimulation. Figure 4a shows the slope of the fractional release versus stimulation time relationship, which varies from one cell to another, but is typically near zero and only slightly increases with temperature. Slope estimates below zero (dotted line) mean that the parameter decreased with stimulation, whereas the values above zero represent an increase. Figure 4b1–2 depicts how the replenishment rate changes with stimulation, at different temperatures. At lower temperatures (<22 °C), the replenishment rate decreases with stimulation, as the amplitudes of the fitted curves are mostly below zero. However, at higher temperatures (>25 °C), the replenishment rate increases with stimulation.
Fig. 4.
The effect of temperature on the change of fractional release and replenishment rate during long, high frequency stimulation. a The slopes of the change of the fractional release versus stimulation time fits slightly increase with temperature (FitFR = 0.004 * T − 0.069; r = 0.35). b 1–2 The amplitudes, but not the time constants, of the replenishment rate versus stimulation time fits increase with temperature [FitRRAmp = −1.92 + 0.16 * exp(−T/8.30); r = 0.62 and FitRRTime = 14.1 * T − 183.4; r = 0.29, respectively]. Patterned stimulation consisted of 10 repeating trains of brief high (10 Hz, 5 s)–low (5 Hz, 5 s) frequencies. Each symbol represents a different cell
The effect of temperature on the changes of the ‘basic parameters’ during long, high frequency stimulation were also evaluated (Fig. 5a–c). Overall, the release coupling does not change with stimulation and temperature does not significantly alter this relationship (Fig. 5a). However, the replenishment coupling decreases with stimulation at 21 °C, but as the temperature rises this decrease is attenuated becoming an increase at still higher temperatures (the amplitudes of the replenishment coupling versus stimulation time fits increase with temperature; Fig. 5b1). The time constants of the replenishment coupling versus stimulation time fits in contrast are temperature independent (Fig. 5b2). Finally, the amplitude of the change of the RRP size does not significantly change with stimulation at 21 °C, but at higher temperatures the amplitude becomes greater, but more negative indicating that the RRP size decreases with stimulation time more at higher temperatures (Fig. 5c1). The time constants of the RRP size versus stimulation time fits are however temperature independent (Fig. 5c2). The replenishment rate thus increases with stimulation at higher temperatures not only because the replenishment coupling increases, but also because the RRP size decreases more at higher temperatures.
Fig. 5.
The effect of temperature on the change of ‘basic’ parameters during stimulation. a Temperature does not significantly affect the release coupling versus stimulation time slopes (Fit1/R0 = 0.06 * T + 1.73; r = 0.29). b 1–2 The amplitudes, but not the decay times, of the replenishment coupling versus stimulation time fits increase with temperature (Fit1/R1Amp = 0.17 * T − 3.85; r = 0.46 and Fit1/R1Time = −12.4 * T + 473.3; r = 0.24, respectively). c 1–2 The amplitudes, but not the time constants, of the RRP size versus stimulation time fits decrease with temperature [FitC1Amp = 3.7 − 0.2 * exp(−T/7.6); r = 0.63 and FitC1Time = 1.6 * T + 92.4; r = 0.03, respectively]. Patterned stimulation consisted of 10 repeating trains of brief high (10 Hz, 5 s)–low (5 Hz, 5 s) frequencies. Each symbol represents a different cell
‘Open system’ contribution increases with temperature
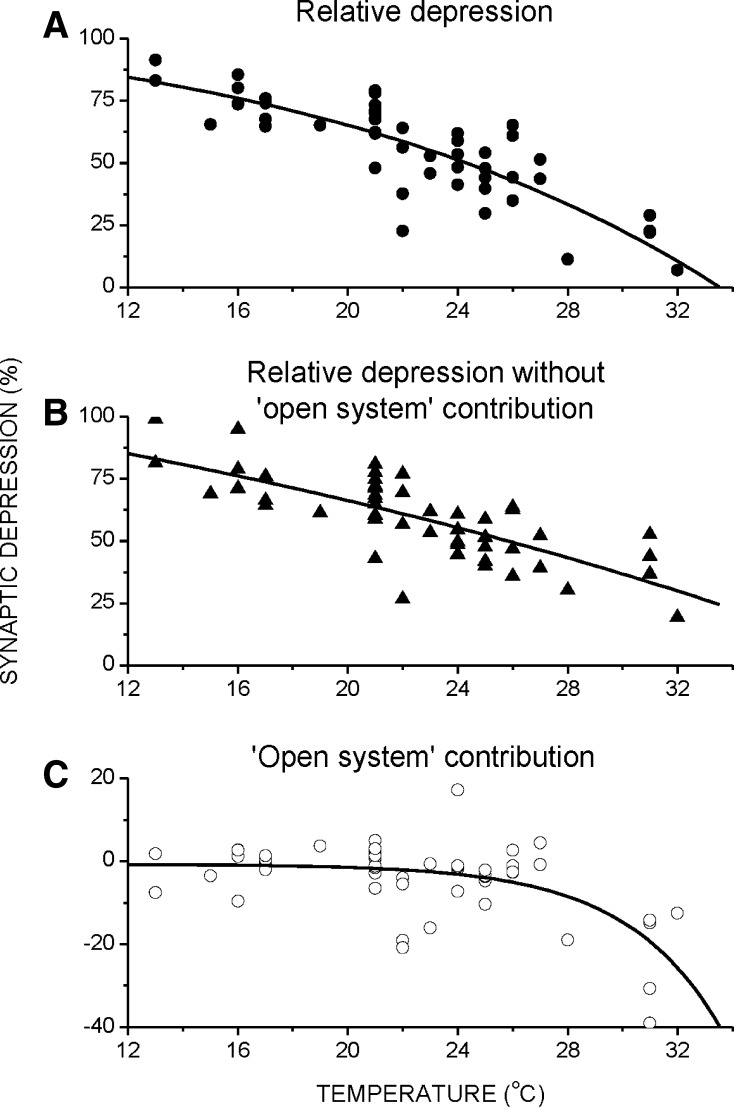
An assumption of our model is that the vesicular fluxes are driven by differences in vesicular densities. However, we do not ignore the vesicular fluxes contributing to the synaptic output that do not agree with such an assumption, such as directed movement (i.e. fluxes that move against their concentration gradient). Their contribution was captured as the ‘open system’ contribution. To study this contribution, we calculate the relative depression at 70 s [(At=0 − At=70)/At=0, where At=x is the ESPC amplitude at x sec]. Figure 6a shows the relative depression values of different cells at various temperatures, and it is clear that depression is less pronounced at higher temperatures. Figure 6b shows the relative depression of a simulated system without ‘open system’ contribution, using the parameters estimated from the ESPC amplitudes in Fig. 6a. Figure 6c depicts the contribution, calculated by the difference between Fig. 6a, b. At lower temperatures (12–26 °C), this contribution is negligible, but at higher temperatures it is not, and renders the relative depression less severe.
Fig. 6.
The temperature dependence of the ‘open system’ contribution to the synaptic depression during long, high frequency stimulation. a Relative depression [(A0 − A70)/A0] decreases with temperature [the best-fitted exponential curve is: FitRD = 114.4 − 14.2 * exp(−T/16.1); r = 0.85]. b Relative depression that would have been observed if there is no ‘open system’ contribution, with best-fitted exponential curve [FitRD = 170.3 − 63.0 * exp(−T/40.0); r = 0.77]. c Contribution of the ‘open system’ to the total depression (the difference between a and b), with best-fitted exponential curve [FitRD = −0.709 − 0.002 * exp(−T/3.425); r = 0.61]. Patterned stimulation consisted of 10 repeating trains of brief high (10 Hz, 5 s)–low (5 Hz, 5 s) frequencies. Each symbol represents a different cell
Discussion
Temperature and vesicular mobility
In this study we evaluate how temperature affects the vesicular dynamics and its changes during long high frequency stimulation, but focussing our attention on its effects of the replenishment of the RRP. Given that the vesicular replenishment has often been interpreted as indicating vesicular movement, it is important to briefly discuss the effects of temperature on vesicular mobility. Vesicle diffusion constants, which are dependent on species and preparations, are also differently affected by temperature. In the recycling pool of resting frog motor nerve terminals, vesicles are mobile at 23 °C, and their mobility is slightly reduced when temperature is decreased to 13 °C (Gaffield et al. 2006). In Drosophila, vesicles also show little sensitivity to temperature (Gaffield et al. 2006; Shakiryanova et al. 2005). In contrast, vesicles in mouse motor nerve terminals are mobile at physiological temperature, but virtually immobile at room temperature (Gaffield and Betz 2007). Finally, in hippocampus the vesicular diffusion constant decreases by a factor of 4 when the temperature is lowered by 8 °C (Shtrahman et al. 2005). The vesicular mobility thus may be temperature independent, but it also may increase and greatly as the temperature rises. Taken together it is clear that the vesicular movement is not a process of simple diffusion.
The fractional release diminishes as temperature rises
Previous studies of the temperature dependence of vesicular dynamics largely focused on the temperature sensitivity of the fractional release (or probability of release if estimated by quantal analysis). At rat neocortical synapse the probability of evoked release is low and increases modestly with temperature (Volgushev et al. 2004). Similar temperature dependence is observed at the neuromuscular junction (Barrett et al. 1978). At cultured hippocampal neurons, the fractional release, which is also low, is largely temperature independent (Granseth and Lagnado 2008). We also find that the fractional release (estimated with pairs of brief high-low frequency trains of stimulation) is low, but decreasing, though moderately as temperature rises (~10 % at 22 °C, to ~5 % at 32 °C). The fractional release is clearly not very temperature dependent, and it may increase or decrease as temperature rises depending on the secretory system. Interestingly, we observe that lower fractional release at higher temperatures, is not caused by lower release coupling, but is due to greater RRP size (i.e. its greater ability to contain quanta). Note however that the change in the storage capacity of RRP or RP pools (i.e. in their ability to contain vesicles) does not necessarily imply the change of their physical volume. If the release process of some synapses becomes inactivated, this would lead to lower RRP without change of volume, but other mechanisms may also be involved.
The effect of temperature on vesicular replenishment
How does the vesicular replenishment of the RRP depend on temperature? Whereas some studies have suggested that vesicular recruitment increases with temperature (Kushmerick et al. 2006; Pyott and Rosenmund 2002), others have argued that the recruitment is temperature-independent (Granseth and Lagnado 2008). We find that the replenishment rate (estimated with brief high-low frequency trains of stimulation) decreases with rise in temperature, but the dependence is not strong. However, the temperature dependence of the ‘basic’ parameters that underlie the replenishment rate reveals a more complex story. The replenishment coupling increases with temperature (~twofold when the temperature changes from 22 to 32 °C), but this is more than counterbalanced by the greater RRP size (RRP size rises ~fivefold as temperature increases by 10 °C). Note that this provides additional evidence supporting the notion that the replenishment of the readily releasable pool is not associated with vesicular movement (Bui and Glavinovic 2013b). Unlike vesicular mobility, which increases greatly with temperature (Shtrahman et al. 2005), the replenishment rate diminishes with temperature. More importantly the replenishment coupling, which if the replenishment of the RRP was associated with vesicular movement would be proportional to its mobility, rises with temperature, but much more modestly.
Very little has been done up to now to evaluate the size of various vesicular pools (i.e. to determine their ability to contain quanta), and how they change with temperature. Nevertheless, note that at rat hippocampal neurons the resting pool (but not the RRP) increased twofold when the temperature was raised from 23 to 37 °C (Micheva and Smith 2005). However, the RRP was defined as the synaptic vesicles that are in direct contact with the presynaptic membrane, and it is not likely that the morphological estimates of the RRP size and those obtained by kinetic analysis would agree. At the frog neuromuscular junction there is no spatial segregation between the pools, as RRP vesicles appear to be randomly distributed throughout the cluster (Rizzoli and Betz 2005). Moreover the vesicles that are docked but not primed are kinetically not in the RRP. Nevertheless, it is worth pointing out that our finding that the size of RRP rises with temperature changes in the same direction as reported for the resting pool (Micheva and Smith 2005).
Decline of the replenishment rate during long, high frequency stimulation is reversed at high temperature
This study shows that the temperature influences profoundly how the vesicular parameters change during long, high frequency stimulation, but its effect is specific. The fractional release does not change overall during long, high frequency stimulation, and this is largely true over a large range of temperatures. In contrast at room temperature the replenishment rate decreases during long, high frequency stimulation (Bui and Glavinovic 2013a), but as this study shows as the temperature rises the decrease becomes less pronounced, and at higher temperatures (>24 °C) the replenishment rate increases with stimulation, attenuating synaptic depression. Note that the stimulation induced rise of the replenishment coupling and a decrease of the RRP size both become more pronounced at higher temperatures, and both changes contribute to the fact that the replenishment rate rises during stimulation at high temperatures. It may be argued that the diffusion of neurotransmitter in the synaptic cleft, which is essentially Brownian and temperature dependent, may alter the EPSC amplitudes and distort the vesicular dynamics and its parameter estimates. This seems very unlikely. First, Brownian motion of glutamate is not very temperature dependent, as it’s Q10 value is 1.3 (Hille 1992). Second, the parameters of the vesicular dynamics obtained by model fitting of changes of EPSC amplitudes will not be different if EPSC amplitudes are proportionally greater (or smaller) due to temperature effect on the cleft or postsynaptic processes, because the parameters depend on the dynamics of EPSC amplitude change.
The ‘open system’ contribution at higher temperatures
The model of vesicular storage and release used in this study assumes that the vesicular replenishment is driven by the difference of vesicular densities (see “Methods”), although this does not imply that the replenishment is associated with vesicular movement. The vesicular fluxes may either be due to the conversion from non-primed to primed vesicles, or due to the vesicles with low [Ca2+]i in between the membrane and vesicle becoming vesicles with high [Ca2+]i. If vesicular replenishment is associated with vesicular movement, the model will describe the vesicular fluxes corresponding to the vesicles that move randomly. Nevertheless, some vesicles may move against their density gradient. An example is the flux due to the directed movement (Nofal et al. 2007), and another is the endocytotic retrieval of vesicles (Engisch and Nowycky 1998). Their contribution to the synaptic output was taken into account as the ‘open system’ contribution (see “Methods”). We find that the ‘open’ system contribution rises with temperature, rendering synaptic depression less severe during long, high frequency stimulation. Interestingly, the endocytotic retrieval of vesicles also increases with temperature in cultured rat hippocampal neurons (Fernandez-Alfonso and Ryan 2004) and mice Calyx of Held synapses (Renden and von Gersdorff 2007). Finally, note that in this study the model-fitting had two pools and synthesis, but did not include the recycling pool. Our results did not require three or more pools (instead of two). Having three (or more) pools and endocytotic input is easy to implement (Aristizabal and Glavinovic 2004). The model-fitting with endocytotic input is desirable to separate its contribution from other ‘open system’ contributions, and should be done in future studies, but combined with appropriate pharmacological tests for endocytosis.
Conclusion
The vesicular dynamics at excitatory synapses of rat hippocampus are temperature dependent, but the dependence is complex. As temperature rises, both the fractional release and replenishment rate decrease. However, this is largely due to the pronounced rise in the size of the readily releasable pool (i.e. its ability to contain vesicles), because the release coupling is not temperature dependent, and the replenishment coupling increases with temperature, albeit modestly. At high temperature the secretory system is better able to maintain the high synaptic output during long, high frequency stimulation, because at lower temperatures (<22 °C) the replenishment rate decreases with stimulation, but at higher temperatures (>24 °C) it increases, attenuating synaptic depression.
Acknowledgments
This work was supported by the grant from the Natural Sciences and Engineering Research Council of Canada and Canadian Heart and Stroke Foundation to M.I.G.
References
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. doi: 10.1016/j.neuron.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Aristizabal F, Glavinovic MI. Simulation and parameter estimation of dynamics of synaptic depression. Biol Cybern. 2004;90:3–18. doi: 10.1007/s00422-003-0432-8. [DOI] [PubMed] [Google Scholar]
- Barrett EF, Barrett JN, Botz D, Chang DB, Mahaffey D. Temperature-sensitive aspects of evoked and spontaneous transmitter release at the frog neuromuscular junction. J Physiol. 1978;279:253–273. doi: 10.1113/jphysiol.1978.sp012343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Abraham WC. Long-term depression in hippocampus. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- Bielecki A, Kalita P, Lewandowski M, Skomorowski M. Compartment model of neuropeptide synaptic transport with impulse control. Biol Cybern. 2008;99:443–458. doi: 10.1007/s00422-008-0250-0. [DOI] [PubMed] [Google Scholar]
- Blanton MG, Lo Turco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. Asynaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bui L, Glavinovic MI. Synaptic activity slows vesicular replenishment at excitatory synapses of rat hippocampus. Cogn Neurodyn. 2013;7:105–120. doi: 10.1007/s11571-012-9232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui L, Glavinovic MI. Is replenishment of the readily releasable pool associated with vesicular movement? Cogn Neurodyn. 2013 doi: 10.1007/s11571-013-9264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman TF, Li Y. A reflective Newton method for minimizing a quadratic function subject to bounds on some of the variables. SIAM J Optim. 1996;6:1040–1058. doi: 10.1137/S1052623494240456. [DOI] [Google Scholar]
- Coleman TF, Li Y. An interior, trust region approach for nonlinear minimization subject to bounds. SIAM J Optim. 1996;6:418–445. doi: 10.1137/0806023. [DOI] [Google Scholar]
- Engisch K, Nowycky M. Compensatory and excess retrieval: two types of endocytosis following single step depolarizations in bovine adrenal chromaffin cells. J Physiol. 1998;506:591–608. doi: 10.1111/j.1469-7793.1998.591bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Alfonso T, Ryan TA. The kinetics of synaptic vesicle pool depletion at CNS synaptic terminals. Neuron. 2004;41:943–953. doi: 10.1016/S0896-6273(04)00113-8. [DOI] [PubMed] [Google Scholar]
- Gaffield MA, Betz WJ. Synaptic vesicle mobility in mouse motor nerve terminals with and without synapsin. J Neurosci. 2007;27:13691–13700. doi: 10.1523/JNEUROSCI.3910-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffield MA, Rizzoli SO, Betz WJ. Mobility of synaptic vesicles in different pools in resting and stimulated frog motor nerve terminals. Neuron. 2006;51:317–325. doi: 10.1016/j.neuron.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Granseth B, Lagnado L. The role of endocytosis in regulating the strength of hippocampal synapses. J Physiol. 2008;586:5969–5982. doi: 10.1113/jphysiol.2008.159715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustaffson B, Wigstrom H, Abraham WC, Huang YY. Long-term potentiation in the hippocampus using depolarizing current pulses as the conditioning stimulus to single volley synaptic potentials. J Neurosci. 1987;7:774–780. doi: 10.1523/JNEUROSCI.07-03-00774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic channels of excitable membranes. Sunderland: Sinauer Associates Inc; 1992. [Google Scholar]
- Kruckenberg P, Sandweg R. An analog model for acetylcholine release by motor nerve endings. J Theor Biol. 1968;19:327–332. doi: 10.1016/0022-5193(68)90144-6. [DOI] [PubMed] [Google Scholar]
- Kushmerick C, Renden R, von Ruden GH. Physiological temperatures reduce the rate of vesicle pool depletion and short-term depression via an acceleration of vesicle recruitment. J Neurosci. 2006;26:1366–1377. doi: 10.1523/JNEUROSCI.3889-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Montana V, Parpura V, Mohideen U. Single molecule measurements of interaction free energies between the proteins within binary and ternary SNARE complexes. J Nanoneurosci. 2009;1:120–129. doi: 10.1166/jns.2009.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. Short-term changes in synaptic efficacy. In: Edelman GM, Gall LE, Maxwell W, Cowan WM, editors. Synaptic function. New York: Wiley; 1987. pp. 21–56. [Google Scholar]
- Micheva KD, Smith SJ. Strong effects of subphysiological temperature on the function and plasticity of mammalian presynaptic terminals. J Neurosci. 2005;25:7481–7488. doi: 10.1523/JNEUROSCI.1801-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer M, van Lunteren E. Effect of temperature on endplate potential rundown and recovery in rat diaphragm. J Neurophysiol. 2001;85:2070–2075. doi: 10.1152/jn.2001.85.5.2070. [DOI] [PubMed] [Google Scholar]
- Nofal S, Becherer U, Hof D, Matti U, Rettig J. Primed vesicles can be distinguished from docked vesicles by analyzing their mobility. J Neurosci. 2007;27:1386–1395. doi: 10.1523/JNEUROSCI.4714-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihel K, Travis ER, Borges R, Wightman MR. Exocytotic release from individual granules exhibits similar properties at mast and chromaffin cells. Biophys J. 1996;71:1633–1640. doi: 10.1016/S0006-3495(96)79368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyott SJ, Rosenmund C. The effects of temperature on vesicular supply and release in autaptic cultures of rat and mouse hippocampal neurons. J Physiol. 2002;539:523–535. doi: 10.1113/jphysiol.2001.013277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renden R, von Gersdorff H. Synaptic vesicle endocytosis at a CNS nerve terminal: faster kinetics at physiological temperatures and increased endocytotic capacity during maturation. J Neurophysiol. 2007;98:3349–3359. doi: 10.1152/jn.00898.2007. [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- Shakiryanova D, Tully A, Hewes RS, Deitcher DL, Levitan ES. Activity-dependent liberation of synaptic neuropeptide vesicles. Nat Neurosci. 2005;8:173–178. doi: 10.1038/nn1377. [DOI] [PubMed] [Google Scholar]
- Shtrahman M, Yeung C, Nauen DW, Bi GQ, Wu XL. Probing vesicle dynamics in single hippocampal synapses. Biophys J. 2005;89:3615–3627. doi: 10.1529/biophysj.105.059295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniike N, Lu YF, Tomizawa K, Matsui H. Critical differences in magnitude and duration of N-methyl-D-aspartate (NMDA) receptor activation between long-term potentiation (LTP) and long-term depression (LTD) induction. Acta Med Okayama. 2008;62:21–28. doi: 10.18926/AMO/30989. [DOI] [PubMed] [Google Scholar]
- Tsodyks MV, Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci USA. 1997;94:719–723. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgushev M, Kudryashov I, Chistiakova M, Mukovski M, Niesmann J, Eysel UT. Probability of transmitter release at neocortical synapses at different temperatures. J Neurophysiol. 2004;92:212–220. doi: 10.1152/jn.01166.2003. [DOI] [PubMed] [Google Scholar]
- Walker A, Glavinovic MI, Trifaro J. Temperature dependence of release of vesicular content in bovine chromaffin cells. Pflugers Arch. 1996;432:885–892. doi: 10.1007/s004240050212. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]