Abstract
Synaptic vesicles (SVs) are the repositories of neurotransmitters. They are locally recycled at nerve terminals following exocytosis. A unique feature of these vesicles is the uniformity of their morphology, which is well maintained even after rounds of exocytosis and endocytosis. Several studies suggest that disruption of clathrin adaptor proteins leads to defects in sorting cargoes and alterations in SV morphology. Here, we review the links between adaptor proteins and SV size, and highlight how protein sorting may impact SV architecture. Molecular players such as clathrin, adaptor proteins, accessory proteins, SV cargoes and lipid composition may act together to establish a stable regulatory network to maintain SV morphology.
Introduction
Synaptic vesicles (SVs) are tiny membrane-enclosed organelles that store neurotransmitters at presynaptic terminals. These vesicles undergo Ca2+-dependent fusion with plasma membrane and, consequently, neurotransmitters are released to propagate chemical signaling between neurons [1,2]. Following fusion, the membrane and protein components of SVs are incorporated into the plasma membrane and must be retrieved into newly formed vesicles to sustain rounds of synaptic transmission [3,4]. Deciphering the molecular mechanisms that govern SVs dynamics is crucial to understanding how synaptic transmission is precisely tuned to support neural network activity.
A unique feature of SVs is their uniform size. In a typical synapse, all SVs are round spheres with ~30–50 nm in diameter (Figure 1a) [4]. The uniform morphology indicates that SVs store similar amount of neurotransmitters, which tend to increase the reliability of synaptic transmission. Several models have been proposed to explain how SVs maintain their shape despite undergoing rounds of exocytosis and endocytosis cycles. A “kiss-and run” model suggests that SVs discharge their intravascular content through transient fusion pores, which allow SVs to keep their gross shape and to preserve molecular machinery. This model has been extensively discussed in several reviews [5,6]. Here, we focus on SV recycling after full fusion. In these events, SVs completely collapse into plasma membranes. We will discuss recent progress toward an understanding of how molecular machinery in the clathrin-mediated endocytosis pathway contributes to sort SV components and to maintain vesicle morphology.
Figure 1.
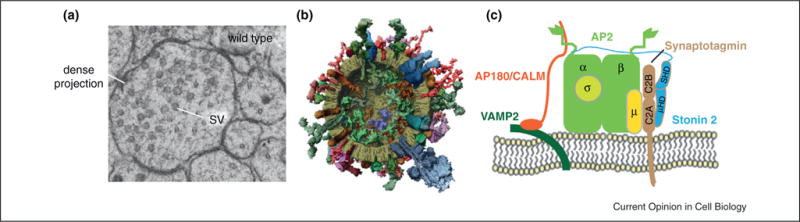
Architecture of SVs. Synaptic vesicles with uniform morphology are enriched at acetylcholine neuromuscular junctions in C. elegans (a). Adapted with permission from [10••]. A molecular model of a SV cross-section is shown in (b). Adapted with permission from [7••]. Schematic diagram of clathrin adaptor complexes that sort SV proteins are shown in (c).
SVs are complex organelles
SVs exhibit an extremely high protein/lipid ratio [7••]. Proteins account for more than 60% of the total mass of vesicles, suggesting that the vesicle surface is fully covered by proteins (Figure 1b). Each SV has approximately 600 transmembrane domains, roughly representing a quarter of vesicle surface. These results suggest that sorting of SV proteins is a major task for vesicle recycling, and protein composition is likely to play an important role in setting up the vesicle morphology. Furthermore, heterogeneity in SV protein abundance adds more challenges to the recycling machinery. For example, only one or two copies of the vacuolar ATPase reside on a SV. In contrast, each vesicle has roughly seventy copies of the vesicle SNARE (soluble NSF attachment protein receptor) protein synaptobrevin. Because full fusion of SV leads to dispersion of SV proteins into the plasma membrane [8,9], these SV proteins must be recaptured and enriched to the correct abundance to produce functional SVs. Several studies on endocytic machinery such as adaptor proteins, suggest a potential link between SV protein sorting and the SV morphology [10••,11••,12,13,14].
AP2 in synaptic vesicle endocytosis
The clathrin adaptor protein complex AP2 is a major protein–protein interaction hub that coordinates cargo sorting, accessory protein binding, and membrane internalization during endocytosis (Figure 1c) [15–18]. It internalizes transmembrane proteins by recognizing two sorting signals, that is, tyrosine-based YxxF (F bulky hydrophobic residue) and dileucine-based [DE]XXXL [LI] motifs. Disruption of AP2 complexes impairs the retrieval of SV proteins [19••] and alters the vesicle morphology [10••,20,21], which demonstrates the importance of AP2 in SV endocytosis.
The AP2 complex is thought to function as an obligate tetramer that is composed of four subunits — two large subunits α and β2, a medium subunit μ2, and a small subunit σ2 [18,22]. In cultured hippocampal neurons, removal of the μ2 subunits by shRNA leads to a ~96% loss of the AP2 complexes, suggesting that the only stable form is the tetrameric AP2 complex [19•]. However, studies in Caenorhabditis elegans suggest an alternative view, in which the AP2 hemicomplexes, α-σ2 and β2-μ2 both exist to sort cargoes for SV endocytosis [10••,20]. Further analyses showed that, in animals that lack either α-adaptin/APA-2 or μ2/APM-2, the number of SVs decreases and the vesicle size increases. These defects become more severe in the apa-2, apm-2 double mutants, suggesting that the AP2 hemicomplexes play genetically distinct functions in SV endocytosis. One difference between the two hemicomplexes resides in their ability to recognize cargoes, for example, the α-σ2 hemicomplexes bind di-leucine motifs [23], while the β2-μ2 hemicomplexes interact with tyrosine-based YxxF motifs [24]. Therefore, it is likely that the hemicomplexes internalize different sets of cargo proteins onto SVs in C. elegans. It is currently unclear whether AP2 hemicomplexes function in mammalian neurons. While μ2 knockdown leads to degradation of AP2, it remains possible that both hemicomplexes and tetrameric AP2 complexes exist and act in concert in wild type neurons to sort SV proteins.
In the absence of AP2, synaptic vesicle endocytosis persists at some level, suggesting the existence of compensatory mechanisms. In hippocampal neurons, removal of AP2 slows down but does not prevent the retrieval of SV proteins from plasma membranes [19•]. In C. elegans, 30% of SVs remain in the mutant animals that lack AP2 [10••]. Several endocytic proteins, including members of the heterotetrameric adaptors (e.g., AP1 and AP3) and other monomeric endocytic proteins (e.g., AP180 and stonin2) may contribute to the compensatory mechanisms [19•,25,26].
Cargo recognition by AP180/CALM
AP180 (neuronal clathrin assembly protein) and CALM (Clathrin-assembly lymphoid myeloid leukaemia protein) are two monomeric adaptor proteins that play important roles in clathrin-mediated endocytosis [4]. Their function is thought to recruit clathrin to the membrane of endocytic vesicles because of their ability to simultaneously bind membrane and clathrin (Figure 1c). However, clathrin-mediated endocytosis persists in the absence of yeast AP180 homologues Yap1801 and Yap1802 [28]. Instead of recruiting clathrin, Yap1801/Yap1802 appear to play a role in sorting and recycling vesicle-associated membrane proteins (VAMPs) [29]. Several studies further suggest that, in neurons, AP180 and CALM selectively sort cargoes such as SNARE proteins [13,30•,31,32], and contribute to the maintenance of vesicle morphology.
AP180 is mainly expressed in neuronal tissues. Mutant flies, worms, and cultured hippocampal neurons that lack AP180 proteins exhibit SV endocytosis defects, that is, enlarged SVs and mislocalized vesicle SNARE protein VAMP2/synaptobrevin2 on axonal membranes [13,14,33,34]. The sorting capability of neuronal AP180 seems to be less specific than its close homologues in yeast. Several proteins including synaptotagmin, synaptophysin, intersectin, and postsynaptic glutamate receptors show altered distribution in the absence of AP180 [33,35–37]. Biophysical analyses have shown that the AP180 N-terminal homology (ANTH) domain weakly binds the N-terminal half of the SNARE motif of VAMP2 ([30•] but also see [38•]), which provides a potential mechanism for the AP180 recognition of VAMP2/synaptobrevin2.
CALM is ubiquitously expressed in all mammalian cells. Knockdown of CALM expression in neurons causes selective increases in surface accumulation of VAMP2/synaptobrevin2, which is similar to the defects in AP180 deficient cells. SVs also become larger in the absence of CALM [33]. The defects in SV morphology appear to vary with the developmental stage of neurons, as the shRNA transfection in 6–8 div young neurons does not change SV size [33]. Structural analyses demonstrate that the CALM ANTH domain directly associates with a number of SNAREs, including VAMP8, VAMP3, and VAMP2, which are required for endosomal trafficking [38•]. These data suggest that CALM and AP180 may act in concert to regulate protein composition and membrane morphology of SVs. Because the synaptic defects associated with CALM disruption are relatively mild [38•,39], it is likely that CALM function can be partially compensated by AP180 in mammalian neurons.
Stonin 2 — a specific adaptor protein for synaptotagmin
Stonin 2 and its homologues are conserved endocytic adaptor proteins that specifically sort synaptotagmin 1 (syt1) to recycling vesicles [27,40–43]. Syt1 is a SV protein that plays critical roles in both Ca2+-dependent exocytosis and endocytosis. The μ-homology domain (μHD) of stonin 2 directly binds the C2 domains of syt1 (Figure 1c) [40,44,45]. The specificity in sorting syt1 has been demonstrated in worms, flies, and mammals. Disruption of stonin 2 leads to accumulation of surface syt1, but the loss of stonin 2 does not impair the recycling of other SV proteins, such as synaptophysin, VAMP2/synaptobrevin2, and synaptogyrin [11••].
While there is general agreement about the syt1 sorting function of stonin 2, several issues remain. First, it is not clear how stonin 2 is targeted to presynaptic sites. It has been proposed that syt1 acts as a recruiter for stonin 2. In support of this model, mutant stonin 2 that lacks the syt1 binding sites does not localize to synapses in C. elegans [45]. However, removal of syt1 does not alter the synaptic distribution of stonin 2 in worms, arguing there are alternative means for stonin 2 recruitment exists [12]. Second, the role of stonin 2 in synaptic endocytosis appears to vary among animals. In worms and flies, loss of stonin 2 homologs severely impairs SV endocytosis, resulting in diminished SV pool size, enlarged vesicles, and reduced synaptic transmission. These data indicate an important role of stonin 2 in promoting SV endocytosis. In contrast, stonin 2 knockout mice have more SVs present at synapses. Instead of impaired endocytosis, the rate of SV retrieval is accelerated in the absence of stonin 2 [11••]. Similar changes in SV endocytosis can be recapitulated in wild type neurons by increasing surface syt1, suggesting that mouse stonin 2 plays a more significant role in sorting syt1 than in regulating SV endocytosis per se. The reasons for such difference in stonin 2 functions among animals are currently unknown.
Potential links between SV morphology and protein sorting
SVs are small membrane organelles with extremely high protein density (Figure 1b) [7••]. Protein composition not only defines the SV’s function, but also provides regulatory means to stabilize these otherwise fusogenic and unstable vesicles. In fact, alterations in adaptor proteins often lead to defects in both cargo composition and vesicle morphology, suggesting that SV proteins contribute to the robustness of the SV architecture. Multiple complimentary mechanisms may act in concert to ensure the robustness and uniformity of SVs. Potential molecular players include clathrin coat, accessory proteins, and lipids, all of which have links to adaptor protein and their cargoes. Below we discuss the potential involvement of these players in setting up SV morphology [46,47].
Adaptor proteins are capable of adjusting the size of clathrin cages to determine vesicle morphology, at least during the early phases of endocytosis. Because clathrin and AP2 participate in the formation of various vesicles, the unique morphology of SVs cannot be simply explained by the intrinsic properties of these proteins. SV specific adaptors such as AP180 and stonin 2 may further constrain the clathrin cages for SV production. Differences in SV protein abundance are likely to impact the adaptor composition involved in clathrin cage formation, which ultimately defines SV morphology (Figure 2). Indeed, when synaptobrevin, synaptogyrin, or synaptotagmin is removed from synapses, SVs become enlarged [48–50].
Figure 2.
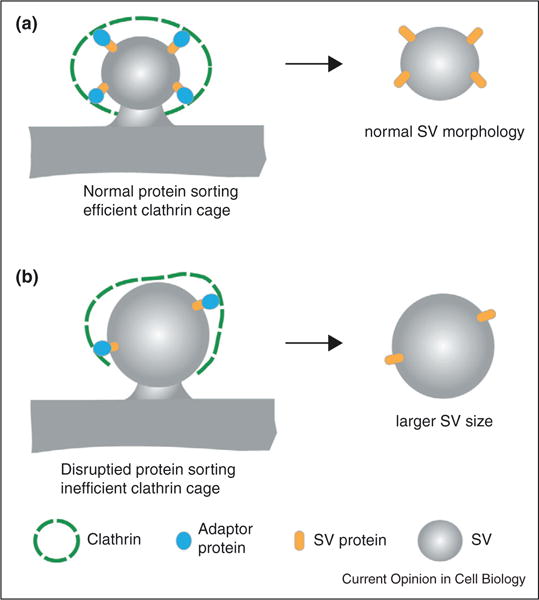
Adaptor proteins contribute to SV size. SV proteins recruit adaptor proteins, which leads to correctly formed clathrin cage and subsequently SV with normal size (a). Disruption of the abundance and the composition of SV proteins disrupts clathrin cage formation and ultimately affects SV size (b). Number of copies of SV protein and adaptor proteins shown are not to scale.
SV cargoes and clathrin adaptors interact with other accessory proteins to control membrane shape. Several accessory proteins are capable of deforming and curving membranes by their membrane-interaction domains, such as the BAR (Bin–Amphiphysin–Rvs) and ENTH (Epsin N-Terminal Homology) domains [4]. Membrane deformation often starts with insertion of amphipathic helixes into the cytoplasmic leaflet of the membranes [51] and further progresses by building up bending pressure through molecular crowding effects [52,53]. The timing of arrival and the intrinsic curvature of these proteins are likely to define their role in SV endocytosis. For example, syndapin arrives early and enforces a shallow curvature [54]. These properties make syndapin a good candidate for membrane deformation at the early stage of endocytosis. Disruption of adaptor protein recruitment and cargo sorting may interfere with how accessory proteins are recruited and where they function, consequently changing the morphology of SVs.
Finally, intrinsic properties of SV proteins and lipids may also contribute to the formation and stability of highly curved SV membranes. For example, most transmembrane proteins on SVs have larger cytoplasmic domain and less significant luminal domain (Figure 1b) [7••]. The transmembrane domains of several SV proteins such as VAMP2/synaptobrevin, synaptotagmin and synaptophysin form oligomeric structures [55–57]. As a consequence, SV proteins may be held as clusters that favor positively curved membranes. In addition, SVs have unusually high amounts of cholesterol [7••]. The effects of cholesterol on membrane bending are at least two fold; (1) cholesterol rapidly flips between membrane leaflets, which stabilizes membrane curvature by changing membrane surface areas [58–60], that is, decreasing the surface area of the compressed leaflet while increasing the surface area of the expanded leaflet; and (2) cholesterol makes membranes more rigid and therefore harder to bend [61]. How exactly these two opposite features contribute to SV biogenesis remains elusive. Nonetheless, it has been demonstrated that cholesterol interacts with SV protein synaptophysin [62]; this may explain the defects in SV morphology upon removal of synaptophysin [62,63].
Conclusions and perspectives
Brain function relies on efficient communication between neurons. Changes in SV morphology alter the quantal size of synaptic transmission, which consequently impairs signal propagation in neural circuitries. Biochemical and cell biological studies have identified several molecules involved in shaping SVs. It has become clear that adaptor proteins and protein sorting pathways play an important role in setting SV morphology, but how exactly these molecules act during SV endocytosis is still unclear. It is almost certain that SV endocytosis requires actions from a network of proteins. Further progress in cell biology will be necessary for us to understand when and where these proteins act and how endocytic network is established and modulated to support SV architecture.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
-
•
of special interest
-
••
of outstanding interest
- 1.Almers W. Exocytosis. Annu Rev Physiol. 1990;52:607–624. doi: 10.1146/annurev.ph.52.030190.003135. [DOI] [PubMed] [Google Scholar]
- 2.Jahn R, Südhof TC. Synaptic vesicles and exocytosis. Annu Rev Neurosci. 1994;17:219–246. doi: 10.1146/annurev.ne.17.030194.001251. [DOI] [PubMed] [Google Scholar]
- 3.Saheki Y, De Camilli P. Synaptic vesicle endocytosis. Cold Spring Harb Perspect Biol. 2012;4:a005645. doi: 10.1101/cshperspect.a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dittman J, Ryan TA. Molecular circuitry of endocytosis at nerve terminals. Annu Rev Cell Dev Biol. 2009;25:133–160. doi: 10.1146/annurev.cellbio.042308.113302. [DOI] [PubMed] [Google Scholar]
- 5.Alabi AA, Tsien RW. Perspectives on kiss-and-run: role in exocytosis, endocytosis, and neurotransmission. Annu Rev Physiol. 2013;75:393–422. doi: 10.1146/annurev-physiol-020911-153305. [DOI] [PubMed] [Google Scholar]
- 6.He L, Wu LG. The debate on the kiss-and-run fusion at synapses. Trends Neurosci. 2007;30:447–455. doi: 10.1016/j.tins.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 7••.Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. This paper provides the first comprehensive picture of the proteins and lipids that make up a synaptic vesicle. It demonstrates that these vesicles have high protein/lipid ratio, and are enriched with cholesterol. [DOI] [PubMed] [Google Scholar]
- 8.Fernández-Alfonso T, Kwan R, Ryan TA. Synaptic vesicles interchange their membrane proteins with a large surface reservoir during recycling. Neuron. 2006;51:179–186. doi: 10.1016/j.neuron.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Wienisch M, Klingauf J. Vesicular proteins exocytosed and subsequently retrieved by compensatory endocytosis are nonidentical. Nat Neurosci. 2006;9:1019–1027. doi: 10.1038/nn1739. [DOI] [PubMed] [Google Scholar]
- 10••.Gu M, Liu Q, Watanabe S, Sun L, Hollopeter G, Grant BD, Jorgensen EM. AP2 hemicomplexes contribute independently to synaptic vesicle endocytosis. Elife. 2013;2:e00190. doi: 10.7554/eLife.00190. This study shows that two AP2 hemicomplexes are active and play non-overlapping role in synaptic vesicle endocytosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Kononenko NL, Diril MK, Puchkov D, Kintscher M, Koo SJ, Pfuhl G, Winter Y, Wienisch M, Klingauf J, Breustedt J, et al. Compromised fidelity of endocytic synaptic vesicle protein sorting in the absence of stonin 2. Proc Natl Acad Sci USA. 2013;110:E526–E535. doi: 10.1073/pnas.1218432110. This study shows that Stonin 2 is required for sorting synaptotagmin 1 in mammalian neurons, but is dispensable for maintaining the speed of SV endocytosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullen GP, Grundahl KM, Gu M, Watanabe S, Hobson RJ, Crowell JA, McManus JR, Mathews EA, Jorgensen EM, Rand JB. UNC-41/Stonin functions with AP2 to recycle synaptic vesicles in Caenorhabditis elegans. PLoS ONE. 2012;7:e40095. doi: 10.1371/journal.pone.0040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nonet ML, Holgado AM, Brewer F, Serpe CJ, Norbeck BA, Holleran J, Wei L, Hartwieg E, Jorgensen EM, Alfonso A. UNC-11, a Caenorhabditis elegans AP180 homologue, regulates the size and protein composition of synaptic vesicles. Mol Biol Cell. 1999;10:2343–2360. doi: 10.1091/mbc.10.7.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Koh YH, Beckstead RB, Budnik V, Ganetzky B, Bellen HJ. Synaptic vesicle size and number are regulated by a clathrin adaptor protein required for endocytosis. Neuron. 1998;21:1465–1475. doi: 10.1016/s0896-6273(00)80664-9. [DOI] [PubMed] [Google Scholar]
- 15.Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2003;163:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Schmid EM, McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448:883–888. doi: 10.1038/nature06031. [DOI] [PubMed] [Google Scholar]
- 18.Slepnev VI, De Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat Rev Neurosci. 2000;1:161–172. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- 19•.Kim SH, Ryan TA. Synaptic vesicle recycling at CNS synapses without AP-2. J Neurosci. 2009;29:3865–3874. doi: 10.1523/JNEUROSCI.5639-08.2009. This paper shows that synaptic vesicle proteins can be recycled when the main clathrin endocytic adaptor AP-2 is downregulated. One of the compensatory pathways is through the AP1 adaptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu M, Schuske K, Watanabe S, Liu Q, Baum P, Garriga G, Jorgensen EM. 2 adaptin facilitates but is not essential for synaptic vesicle recycling in Caenorhabditis elegans. J Cell Biol. 2008;183:881–892. doi: 10.1083/jcb.200806088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González-Gaitán M, Jäckle H. Role of Drosophila α-adaptin in presynaptic vesicle recycling. Cell. 1997;88:767–776. doi: 10.1016/s0092-8674(00)81923-6. [DOI] [PubMed] [Google Scholar]
- 22.Matsui W, Kirchhausen T. Stabilization of clathrin coats by the core of the clathrin-associated protein complex AP-2. Biochemistry. 1990;29:10791–10798. doi: 10.1021/bi00500a011. [DOI] [PubMed] [Google Scholar]
- 23.Doray B, Lee I, Knisely J, Bu G, Kornfeld S. 2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol Biol Cell. 2007 doi: 10.1091/mbc.E07-01-0012. http://dx.doi.org/10.1091/mbc.E07. [DOI] [PMC free article] [PubMed]
- 24.Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282:1327–1332. doi: 10.1126/science.282.5392.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voglmaier SM, Kam K, Yang H, Fortin DL, Hua Z, Nicoll RA, Edwards RH. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron. 2006;51:71–84. doi: 10.1016/j.neuron.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Glyvuk N, Tsytsyura Y, Geumann C, D’Hooge R, Hüve J, Kratzke M, Baltes J, Böning D, Klingauf J, Schu P. AP-1/σ1B-adaptin mediates endosomal synaptic vesicle recycling, learning and memory. EMBO J. 2010;29:1318–1330. doi: 10.1038/emboj.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maritzen T, Podufall J, Haucke V. Stonins-specialized adaptors for synaptic vesicle recycling and beyond? Traffic. 2009;11:8–15. doi: 10.1111/j.1600-0854.2009.00971.x. [DOI] [PubMed] [Google Scholar]
- 28.Huang KM, D’Hondt K, Riezman H, Lemmon SK. Clathrin functions in the absence of heterotetrameric adaptors and AP180-related proteins in yeast. EMBO J. 1999;18:3897–3908. doi: 10.1093/emboj/18.14.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burston HE, Maldonado-Baez L, Davey M, Montpetit B, Schluter C, Wendland B, Conibear E. Regulators of yeast endocytosis identified by systematic quantitative analysis. J Cell Biol. 2009;185:1097–1110. doi: 10.1083/jcb.200811116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Koo SJ, Markovic S, Puchkov D, Mahrenholz CC, Beceren-Braun F, Maritzen T, Dernedde J, Volkmer R, Oschkinat H, Haucke V. SNARE motif-mediated sorting of synaptobrevin by the endocytic adaptors clathrin assembly lymphoid myeloid leukemia (CALM) and AP180 at synapses. Proc Natl Acad Sci USA. 2011;108:13540–13545. doi: 10.1073/pnas.1107067108. This paper provides mechanistic insight into endocytic sorting of the synaptic vesicle protein-synaptobrevin 2. The results show that the ANTH domains of AP180 and CALM specifically recognize synaptobrevin 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tebar F, Bohlander SK, Sorkin A. Clathrin assembly lymphoid myeloid leukemia (CALM) protein: localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic. Mol Biol Cell. 1999;10:2687–2702. doi: 10.1091/mbc.10.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyerholz A, Hinrichsen L, Groos S, Esk P-C, Brandes G, Ungewickell EJ. Effect of clathrin assembly lymphoid myeloid leukemia protein depletion on clathrin coat formation. Traffic. 2005;6:1225–1234. doi: 10.1111/j.1600-0854.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- 33.Petralia RS, Wang Y-X, Indig FE, Bushlin I, Wu F, Mattson MP, Yao PJ. Reduction of AP180 and CALM produces defects in synaptic vesicle size and density. Neuromol Med. 2012;15:49–60. doi: 10.1007/s12017-012-8194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dittman JS, Kaplan JM. Factors regulating the abundance and localization of synaptobrevin in the plasma membrane. Proc Natl Acad Sci USA. 2006;103:11399–11404. doi: 10.1073/pnas.0600784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burbea M, Dreier L, Dittman JS, Grunwald ME, Kaplan JM. Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in. C elegans Neuron. 2002;35:107–120. doi: 10.1016/s0896-6273(02)00749-3. [DOI] [PubMed] [Google Scholar]
- 36.Bao H, Daniels RW, MacLeod GT, Charlton MP, Atwood HL, Zhang B. AP180 maintains the distribution of synaptic and vesicle proteins in the nerve terminal and indirectly regulates the efficacy of Ca2+-triggered exocytosis. J Neurophysiol. 2005;94:1888–1903. doi: 10.1152/jn.00080.2005. [DOI] [PubMed] [Google Scholar]
- 37.Ch’ng Q, Sieburth D, Kaplan JM. Profiling synaptic proteins identifies regulators of insulin secretion and lifespan. PLoS Genet. 2008;4:e1000283. doi: 10.1371/journal.pgen.1000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Miller SE, Sahlender DA, Graham SC, Höning S. The molecular basis for the endocytosis of small R-SNAREs by the clathrin adaptor CALM. Cell. 2011;147:1118–1131. doi: 10.1016/j.cell.2011.10.038. This study demonstrates that CALM sorts a subset of v-SNAREs via direct interactions with the N-terminal half of the SNARE domain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki M, Tanaka H, Tanimura A, Tanabe K, Oe N, Rai S, Kon S, Fukumoto M, Takei K, Abe T, et al. The clathrin assembly protein PICALM is required for erythroid maturation and transferrin internalization in mice. PLoS ONE. 2012;7:e31854. doi: 10.1371/journal.pone.0031854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martina JA, Bonangelino CJ, Aguilar RC, Bonifacino JS. Stonin 2: an adaptor-like protein that interacts with components of the endocytic machinery. J Cell Biol. 2001;153:1111–1120. doi: 10.1083/jcb.153.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willox AK, Royle SJ. Stonin 2 is a major adaptor protein for clathrin-mediated synaptic vesicle retrieval. Curr Biol. 2012;22:1435–1439. doi: 10.1016/j.cub.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diril MK, Wienisch M, Jung N, Klingauf J, Haucke V. Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev Cell. 2006;10:233–244. doi: 10.1016/j.devcel.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Robinson MS, Bonifacino JS. Adaptor-related proteins. Curr Opin Cell Biol. 2001;13:444–453. doi: 10.1016/s0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- 44.Fergestad T, Broadie K. Interaction of stoned and synaptotagmin in synaptic vesicle endocytosis. J Neurosci. 2001;21:1218–1227. doi: 10.1523/JNEUROSCI.21-04-01218.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung N, Wienisch M, Gu M, Rand JB, Muller SL, Krause G, Jorgensen EM, Klingauf J, Haucke V. Molecular basis of synaptic vesicle cargo recognition by the endocytic sorting adaptor stonin 2. J Cell Biol. 2007;179:1497–1510. doi: 10.1083/jcb.200708107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye W, Lafer EM. Clathrin binding and assembly activities of expressed domains of the synapse-specific clathrin assembly protein AP-3. J Biol Chem. 1995;270:10933–10939. doi: 10.1074/jbc.270.18.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaremba S, Keen JH. Assembly polypeptides from coated vesicles mediate reassembly of unique clathrin coats. J Cell Biol. 1983;97:1339–1347. doi: 10.1083/jcb.97.5.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deák F, Schoch S, Liu X, Südhof TC, Kavalali ET. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat Cell Biol. 2004;6:1102–1108. doi: 10.1038/ncb1185. [DOI] [PubMed] [Google Scholar]
- 49.Stevens RJ, Akbergenova Y, Jorquera RA, Littleton JT. Abnormal synaptic vesicle biogenesis in Drosophila synaptogyrin mutants. J Neurosci. 2012;32:18054–18067. doi: 10.1523/JNEUROSCI.2668-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reist NE, Buchanan J, Li J, DiAntonio A, Buxton EM, Schwarz TL. Morphologically docked synaptic vesicles are reduced in synaptotagmin mutants of Drosophila. J Neurosci. 1998;18:7662–7673. doi: 10.1523/JNEUROSCI.18-19-07662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 52.Stachowiak JC, Schmid EM, Ryan CJ, Ann HS, Sasaki DY, Sherman MB, Geissler PL, Fletcher DA, Hayden CC. Membrane bending by protein–protein crowding. Nat Cell Biol. 2012;14:944–949. doi: 10.1038/ncb2561. [DOI] [PubMed] [Google Scholar]
- 53.Zhu C, Das SL, Baumgart T. Nonlinear sorting, curvature generation, and crowding of endophilin N-BAR on tubular membranes. Biophys J. 2012;102:1837–1845. doi: 10.1016/j.bpj.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quan A, Xue J, Wielens J, Smillie KJ, Anggono V, Parker MW, Cousin MA, Graham ME, Robinson PJ. Phosphorylation of syndapin I F-BAR domain at two helix-capping motifs regulates membrane tubulation. Proc Natl Acad Sci USA. 2012;109:3760–3765. doi: 10.1073/pnas.1108294109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bai J, Earles CA, Lewis JL, Chapman ER. Membrane-embedded synaptotagmin penetrates cis or trans target membranes and clusters via a novel mechanism. J Biol Chem. 2000;275:25427–25435. doi: 10.1074/jbc.M906729199. [DOI] [PubMed] [Google Scholar]
- 56.Bowen ME, Engelman DM, Brunger AT. Mutational analysis of synaptobrevin transmembrane domain oligomerization. Biochemistry. 2002;41:15861–15866. doi: 10.1021/bi0269411. [DOI] [PubMed] [Google Scholar]
- 57.Johnston PA, Südhof TC. The multisubunit structure of synaptophysin. Relationship between disulfide bonding and homo-oligomerization. J Biol Chem. 1990;265:8869–8873. [PubMed] [Google Scholar]
- 58.Hamilton JA. Fast flip-flop of cholesterol and fatty acids in membranes: implications for membrane transport proteins. Curr Opin Lipidol. 2003;14:263. doi: 10.1097/00041433-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Choubey A, Kalia RK, Malmstadt N, Nakano A. Cholesterol translocation in a phospholipid membrane. Biophys J. 2013;104:2429–2436. doi: 10.1016/j.bpj.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruckner RJ, Mansy SS, Ricardo A, Mahadevan L, Szostak JW. Flip-flop-induced relaxation of bending energy: implications for membrane remodeling. Biophys J. 2009;97:3113–3122. doi: 10.1016/j.bpj.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henriksen J, Rowat AC, Ipsen JH. Vesicle fluctuation analysis of the effects of sterols on membrane bending rigidity — Springer. Eur Biophys J. 2004;33:732–741. doi: 10.1007/s00249-004-0420-5. [DOI] [PubMed] [Google Scholar]
- 62.Thiele C, Huttner WB, Hannah MJ, Fahrenholz F. Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles. Nat Cell Biol. 1999;2:42–49. doi: 10.1038/71366. [DOI] [PubMed] [Google Scholar]
- 63.Kwon SE, Chapman ER. Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron. 2011;70:847–854. doi: 10.1016/j.neuron.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


