Abstract
Refeeding syndrome describes the metabolic and clinical changes attributed to aggressive rehabilitation of malnourished subjects. The metabolic changes of refeeding are related to hypophosphatemia, hypokalemia, hypomagnesemia, sodium retention and hyperglycemia, and these are believed to be mainly the result of increased insulin secretion following high carbohydrate intake. In the past few decades, increased consumption of processed food (refined cereals, oils, sugar and sweeteners, and so on) lowered the intake of several macrominerals (mainly phosphorus, potassium and magnesium). This seems to have compromised the postprandial status of these macrominerals, in a manner that mimics low grade refeeding syndrome status. At the pathophysiological level, this condition favored the development of the different components of the metabolic syndrome. Thus, it is reasonable to postulate that metabolic syndrome is the result of long term exposure to a mild refeeding syndrome.
Refeeding syndrome represents a group of metabolic and clinical changes that occur in severely malnourished patients undergoing aggressive nutritional support.1 Metabolic changes include: hypophosphatemia, hypokalemia, hypomagnesemia, sodium retention and hyperglycemia.2 Although clinical changes cover most organ systems, including cardiovascular, gastrointestinal, musculoskeletal, respiratory, neurological and hematological abnormalities, these changes are the outcome of the metabolic changes in a scale that is synergistically related to the degree of the metabolic changes, in which under severe conditions multiple organ failure may occur leading to death.1 On the other hand, metabolic syndrome is a name for a group of risk factors that occur together, increasing the risk for coronary artery disease, stroke and type 2 diabetes. These factors are: central obesity, high triglycerides, low high-density lipoprotein cholesterol, elevated blood pressure and raised blood glucose.3 Classification according to the US National Cholesterol Education Program Adult Treatment Panel III requires the presence of at least three of the above factors.3
The pathophysiology of refeeding syndrome is related to the fact that under conditions of starvation, the body shifts from carbohydrate to fat and protein utilization (state of catabolism) to produce glucose and energy.2 Therefore malnutrition, which usually exists in different disease states including cancer, Marasmus/Kwashiorkor, neurological problems, respiratory diseases, gastrointestinal and liver diseases, and so on,2, 4 is the major risk factor for refeeding syndrome.2, 4 Upon refeeding, especially with carbohydrate, the body shifts back instantaneously to carbohydrate metabolism (state of anabolism).2 Concomitantly, insulin secretion is increased leading to an increase in the cellular uptake of glucose and macrominerals (in particular phosphorus, potassium and magnesium) mainly occurring in the liver and muscles, and thus resulting in hypophosphatemia, hypomagnesaemia and hypokalemia.2 Simultaneously, insulin resistance prevails as indicated by the coexistence of hyperglycemiam and hyperinsulinemia,1, 2, 4 which reduces sodium clearance leading to sodium retention and thus resulting in fluid retention and expansion of the extracellular fluid volume.1, 2 Thus, the clinical manifestations of these macromineral abnormalities have serious deleterious effects, some of which are hypotension, bradycardia, weakness, heart failure and arrhythmias.4 In brief, refeeding syndrome is the consequence of the ingestion of a high carbodydrate–low macrominerals diet following prolonged fasting.
In normal subjects and under normal conditions, energy metabolism is known to fluctuate diurnally, as meal ingestion causes a shift to carbohydrate metabolism and an increase in both energy expenditure and carbohydrate oxidation.5 Meal ingestion ensues an increase in cellular uptake and utilization of glucose and macrominerals (predominantly phosphorus, potassium and magnesium), as a result of increased insulin secretion and demand for metabolic processes (for example, phosphorylation and so on). Therefore, plasma status of these macrominerals depends on insulin secretion (that is highly dependent on carbohydrate intake) and their meal content of marominerals. Ingestion of pure glucose is known to be associated with a reduction in plasma concentration of these macrominerals and their inclusion in a meal was reported to improve their status.6 Thus, it is reasonable to postulate that under normal conditions the postprandial metabolic changes following the ingestion of high carbohydrate–low macrominerals diet resemble those of the refeeding syndrome but to a lower extent. Hence, what remains to be elucidated is whether the dietary changes that have occurred in the past few decades favored the consumption of high carbohydrate–low macrominerals diets and thus have exacerbated these metabolic changes.
Evidence from epidemiological studies reveal that the increased prevalence of the ‘Western diet' is implicated in the increased prevalence of obesity, diabetes and hypertension, observed in Africa, Asia, South America, Australia/New Zealand and Oceania.7, 8, 9, 10 Western diet is characterized by the consumption of refined (cereals) carbohydrates, sugars, sweeteners (especially high fructose corn syrup), oils and fats.7, 8 The implication of the Western diet has further been proposed to promote the incidence of insulin resistance11 and metabolic syndrome.12 Furthermore, the increased intake of fructose-based sweeteners has been also reported to be associated with the development of metabolic syndrome and obesity.13 This association was proposed to be related to its capacity to “sequester phosphate”,14 stimulate triglyceride synthesis13, 15, 16 and promote insulin resistance.13, 17
During the past few decades, the major changes in dietary habits, as have been discussed earlier in this paper, are mainly related to a dramatic increase in the intake of: (1) macromineral (P, K and Mg)-free commodities, such as oils, sugar and sweeteners, which contains negligible amounts of the above macrominerals) and (2) refined cereals commodities, where refinement is known to reduce the content of these macrominerals by about 70%. Cereals are known to contribute to more than 50% of the total energy intake in most countries;18 therefore, the shift from whole grain cereals (whole wheat, brown rice) to refined cereals would be expected to result in substantial reduction in the intake of these macrominerals. A further reduction would be expected from the increased consumption of macrominerals-free commodities. In developed and transitional countries, the consumption of these (above) commodities is known to be inversely related to socioeconomic status, mainly because of their high energy density (kcal g−1 food) and low energy cost (US$ per 1000 kcal). This has also been proposed to be an important factor behind the high prevalence of obesity and metabolic syndrome among urban people of low socioeconomic status.19
It is well known that in the past few decades, urbanization has increased in most countries. The changes in the consumption of the different food groups based on the change in gross national product per capita of the country and change in urbanization was studied by Popkin and Gordon–Larsen9 and Drewnowski and Popkin.20 Increased urbanization worldwide was found to be associated with increased consumption of vegetable fats and sugars. At the same time, a direct relationship was reported to be present between urbanization and gross national product per capita and the increase in the consumption of fats and sweeteners. High gross national product per capita was associated with higher consumption of vegetable, animal fats and sugars with a sharp decrease in the consumption of complex carbohydrates.20 Rapid urbanization worldwide has a major influence on accelerating the nutrition transition. It was also reported that an increased production and consumption of sweeteners derived from starch has been observed in the last several decades.21 For example, in the year 2000, the caloric consumption of sweeteners increased by one-third more than in the year 1962. Similarly, in the United States, the daily caloric intake was reported to increase, mainly from energy-dense and nutritiously poor food choices,8, 22, 23, 24, 25, 26, 27 such as fast food, salty snacks8, 22, 23, 24 and added caloric sweeteners.8, 21, 28 In addition to fast food choices lacking essential nutrients,8, 29, 30 fruit and vegetable consumption was observed to be far lower than the recommended levels.8, 21, 22
It can therefore be deduced that the high intake of refined carbohydrates, fats and sweeteners accompanied with the low intake of fruits and vegetables, leads to a diet that is deficient or suboptimal in vitamins and minerals (including potassium, phosphorus and magnesium). Thus, the increased prevalence of metabolic syndrome among people consuming high quantities of commodities containing low levels of these macrominerals may implicate these macrominerals in the development of metabolic syndrome, as is the case in its implication in refeeding syndrome. Thus, decreased intake of these macrominerals would be expected to undermine their postprandial concentration, and it is yet to be determined whether such undermining would have any health implications. Such a question can be clarified by looking at the relationship between these macrominerals and the different components of the metabolic syndrome.
Phosphorus and metabolic syndrome
Phosphorus is an essential mineral and is known to be involved in several metabolic reactions especially that of glucose and energy metabolism. Hypophosphatemia is known to be associated with insulin resistance and impaired glucose tolerance, and plasma phosphate level was reported to be synergistically related to glucose tolerance and insulin sensitivity.31, 32, 33 In the postprandial status, low serum phosphate levels were reported to be associated with elevated blood glucose levels and reduced insulin sensitivity.34 We have recently found that the inclusion of phosphorus in oral glucose load was able to improve insulin sensitivity6 and this may probably relate to its capacity to trap glucose intracellularly as a result of its phosphorylation. In addition, phosphorus seems to be involved in the control of both energy intake and expenditure.35, 36, 37, 38, 39 Food intake control is believed to be partially governed by signals related to hepatic postprandial ATP production, which is dependent on adequate sources of phosphorus.35 In line with that, human studies reported an inverse relation between hepatic ATP status and body mass index.36 We have previously reported that the addition of 500 mg phosphorus to different preloads resulted in a substantial reduction in subsequent energy intake.37 The relation between phosphorus and obesity has been reviewed recently by Obeid,40 and low phosphorus status was hypothesized to be involved in the development of obesity. Moreover, ingestion of phosphorus was reported to increase resting metabolic rate38 and postprandial thermogenesis.39 Thus, it is of no surprise that several authors have found an inverse relation between plasma phosphorus status and the different components of the metabolic syndrome.31, 32, 33 Table 1 summarizes the relationship between phosphorus status and the different components of metabolic syndrome as reported by several studies. In brief, the majority of the studies showed an inverse relationship between phosphorus status and adiposity,31, 41, 42, 43, 44, 45, 46, 47, 48, 49 glycemia,31, 32, 44, 45, 46, 47, 48, 50, 51 lipid profile32, 47, 51 and blood pressure.43, 45, 46, 47, 48, 49, 51, 52, 53, 54, 55, 56, 57, 58, 59
Table 1. Results of human studies that investigated the association between serum/dietary phosphorous and components of the metabolic syndrome.
| Authors | Country | Study design | Biomarkers for phosphorus |
Outcomes |
||||
|---|---|---|---|---|---|---|---|---|
| Obesity | BP | Lipid profile | Blood glucose | Metabolic syndrome | ||||
| Çelik and Andiran, 201141 | Turkey | Cross-sectional (n=298). Age: 6–12 and 12–16 years | Serum | (↓) Obese in 6–12 age group (∼) Obese in 12–16 age group | ND | ND | (↓) IR in 6–12-year old obese children | ND |
| Farhangi et al., 201150 | Iran | Cross-sectional (n=82). 100% F Age: 17–50 years | Serum | (∼) BMI | ND | (∼) TC (∼) TG (∼) LDL (∼) HDL | (↓) FBG | ND |
| Holecki et al., 201142 | Poland | Cross-sectional (n=77). 100% F. Age: 46–57 years | Serum | (↓) BMI | ND | ND | ND | ND |
| Alonso et al., 201043 | United States | Cohort (n=13 444). Age: 45–64 and 45–84 years | Dietary (P from dairy products) | (↓) BMI | (↓) SBP and DBP (↓) risk for HTN | ND | ND | ND |
| Vyssoulis et al., 201052 | Greece | Cohort (n=2600). 100% M. Age: 55±11 years | Serum | ND | (↓) HTN | ND | ND | (↓) MS |
| Foley et al., 200944 | United Kingdom | Cohort (n=3015). Age: 18–30 years. 42.7% M and 57.3% F | Serum | (↓) BMI | (↓) SBP (↑) DBP | (↑) TG (↑) HDL (↓) LDL | (↓) FBG | ND |
| Lippi et al., 200932 | Italy | Retrospective study (n=11 228). Age: >20 years. 42.7% M and 57.3% F | Serum | ND | ND | (↓)TG (↓) TC (↓) LDL (↑) HDL (↓) TC/HDL-C | (↓) FBG | ND |
| Park et al., 200945 | South Korea | Cross-sectional (n=46 798). 64.6% M, 35.4% F. Age: above 20 years | Serum | (↓) BMI(↓) WC | (↓) SBP and DBP | (↑) TC (↑) LDL (↑) HDL (↑) Lp a (↓) TG | (↓) FBG (↓) Insulin (↓) HOMA-IR | (↓) MS |
| Elliott et al., 200853 | Japan, China, UK and US | Cross-sectional (n=4680). Age: 40–59 years. 50.4% M and 49.6% F | Dietary | ND | (↓) BP | ND | ND | ND |
| Gudmundsdottir et al., 200851 | Norway | Cohort (n=56). Age: ≈42 years. 100% M | Serum | (∼) BMI (∼) WC | (↓) BP (↓) HTN | (↑) HDL (↓) TG | (↓) HOMA-IR in men (↓) Insulin in men (↓) BG in men | (↓) MS |
| Dhingra et al., 200746 | United States; Framingham Study | Prospective study (n=3368). Age: 44 years. 51% F and 49% M | Serum | (↓) BMI | (↓) SBP (↓) DBP (↓) HTN | (∼) TC/HDL-C ratio (∼) TG | (↓) Diabetes | ND |
| Haap et al., 200633 | Germany | Cross-sectional (n=881) 61% F, 39% M Age: 19–73 years | Serum | (↓) BMI | ND | ND | (↓) 2-h blood glucose (↑) IS | ND |
| Kalaitzidis et al., 200547 | Greece | Cross-sectional (n=255). 54.5% M, 45.5% F. Age: 48.8±10.5 years | Serum | (↓) WC | (↓) SBP and DBP | (↑) HDL (↓) TG | (↓) FBG (↓) HOMA and Insulin | (↓) MS (dietary and serum phosphorus) |
| Hajjar et al., 200354 | United States | Analysis of data obtained from NHANES-III (n=17 752). Age: ⩾18 years. 47% M and 53% F | Dietary | ND | (↓) BP | ND | ND | ND |
| Haglin et al., 200148 | Sweden | Cross-sectional (n=2752). 43.8% M, 56.2% F. Age: 50.1±10 years | Serum | (↓) BMI in F (∼) BMI in M | (↓) SBP and DBP in M (∼) SBP and DBP iin F | (↑) TC in F (∼) TC in M (∼) HDL (∼) TG | (↓) FBG in M (∼) FBG in F | ND |
| Paula et al., 199886 | Brazil | Case–control (n=19). 47% M, 53% F. Age: 25–50 years | Serum | ND | ND | ND | (↓) hyperinsulinemia (↑) IS | ND |
| Kesteloot et al., 198855 | Belgium | Cross-sectional (n=8058). Age: 49±13 years. 51.7% M and 48.3% F | Serum | ND | (↓) SBP in M and F (∼) DBP | ND | ND | ND |
| Kjeldsen et al., 198856 | Oslo | Cross sectional (n=79). Age: 40 years. 100% M | Serum | ND | (↓) BP | ND | ND | ND |
| Harlan et al., 198457 | United States | Longitudinal data obtained from NHANES-I (n=20 749). Age: 1–74 years | Dietary and Serum | ND | (↓) BP | ND | ND | ND |
| Daniels et al., 198358 | Albany | Retrospective (n=120). Age: 55±2 years. 30% M and 70% F | Serum | ND | (↓) BP | ND | ND | ND |
| Havlik et al., 198059 | United States; Framingham | Cohort (n=5430) Age: 20–56 years. 53% F and 47% M | Serum | ND | (↓) BP | ND | ND | ND |
| Ljunghall et al., 197649 | Sweden | Cross sectional (n=1768). Age: 49–50 years. 100% M | Serum | (↓) Body weight | (↓) BP | ND | ND | ND |
Abbreviations: BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; F, female; FBG, fasting blood glucose; HDL, high-density lipoprotein; HTN, hypertension; IR, insulin resistance; IS, insulin sensitivity; LDL, low-density lipoprotein; Lp a, lipoprotein a; M, male; ND, not determined; SBP, systolic blood pressure; TG, triglycerides; TC/HDL-C, total cholesterol to HDL cholesterol ratio; WC, waist circumference; (↑) positive relation; (↓) inverse relation; (∼) no relation.
Magnesium and metabolic syndrome
The role of serum or dietary magnesium, the resultant altered magnesium status and the effect on the development of metabolic syndrome has been reviewed by Belin and He.39 Low magnesium status was found to be associated with the development of hypertension, insulin resistance, impaired glucose tolerance, dyslipidemia and central obesity. A meta-analysis conducted by Kass et al.60 concluded that despite the need for further large randomized trials to support such an association, magnesium supplementation was found to significantly reduce blood pressure. Another clinical review by Gums61 states that hypertension, congestive heart failure, arrhythmias, myocardial infarction and diabetes mellitus are all conditions that might be associated with magnesium deficiency; thus concluding that clinical studies found benefits when magnesium was supplemented in these cases. Similarly, magnesium was reported in another review by Guerrera et al.62 to be possibly effective in lowering the risk of metabolic syndrome and improving the metabolism of both glucose and insulin. In a prospective study by He et al.,63 an inverse association was determined between magnesium intake and the risk of metabolic syndrome and its components in healthy young adults. In a large cohort study on middle-aged and older US female women from the Women's Health Study,64 a significant inverse association was found between magnesium intake and the prevalence of metabolic syndrome and its components. Similarly, in a nationally representative sample of US adults, the dietary intake of magnesium was found to be inversely related to the prevalence of metabolic syndrome concluding that a diet rich in magnesium may be essential in maintaining a good cardiometabolic health.65 Lima et al.66 reported that serum and intracellular magnesium deficiency is common in obese patients with metabolic syndrome and is common in non-white patients suffering from insulin resistance. Similarly, Corica et al.67 found that hypomagnesemia is highly prevalent in a sample of diabetic patients from Italy. Serum magnesium levels were also found to be low in patients with low high-density lipoprotein-C, high triglyceride values, elevated blood pressure and elevated waist circumference in this study population.67 Serum magnesium levels have been also reported to be inversely related to glycated hemoglobin levels and be directly related to glucose disposal in diabetic subjects upon glucose injection.68 Another study by Zofkova et al.69 reports further that acute hypermagnesemia reduces glucose tolerance during oral glucose tolerance test. Magnesium supplementation of a meal was also reported to increase postprandial magnesium levels and to improve hyperlipidemia in healthy subjects.70 Table 2 summarizes the relationship between magnesium status and the different components of metabolic syndrome as reported by several studies. In brief, the majority of the studies showed an inverse relationship between magnesium status and adiposity,64, 65, 66, 67 glycemia,63, 64, 65, 66, 67, 68 lipid profile63, 65, 67, 70 and blood pressure.63, 64, 66, 67, 71
Table 2. Results of human studies that investigated the association between serum/dietary magnesium and components of the metabolic syndrome.
| Authors | Country | Study design | Biomarkers for magnesium |
Outcomes |
||||
|---|---|---|---|---|---|---|---|---|
| Obesity | BP | Lipid profile | Blood glucose | MS | ||||
| Lima et al., 200966 | Brazil | Cross-sectional (n=72). Age: 18–54 years | Serum | (↓) BMI (↓) WC in F | (↓) SBP | (↑) HDL (∼) TG | (↓) HOMA-IR | (↓) MS |
| Kishimoto et al., 201070 | Tokyo | Case–control (n=16) 100% M. Age: 41.7±2.6 years | Dietary, Supplementation | ND | ND | (↓) TG (↓) Postprandial lipidemia | ND | ND |
| Corica et al., 200667 | Italy | Cross-sectional (n=290). 51.7% M and 48.3% F. Age: 63.1± 10.4 years | Serum | (↓) WC | (↓) BP | (↑) HDL (↓) TG | (↓) HbA1C (↓) Diabetes | ND |
| Ford et al., 200765 | United States | Cross-sectional (NHANES-III) (n=7669). Age:⩾20 years | Dietary | (↓) BMI(↓) WC | (∼) BP | (↓) LDL (∼) TG | (↓) Insulin (↓) BG | (↓) MS |
| He et al., 200663 | United States | Prospective (n=4637). 46.2% M and 53.8% F. Age: 18–30 years | Dietary | (↓) WC in White M&F (∼) WC in Black M&F (∼) BMI in Blacks and Whites | (↓) SBP and DBP in White M&F (∼) SBP and DBP in Black M&F | (↓) HDL in Blacks and Whites (↓) TG in White M&F (∼) TG in Black M&F | (↓) FBG in Black M&F (∼) FBG in White M&F | (↓) MS |
| Song et al., 200564 | Boston | Cross-sectional (n=11 686)100% F. Age: above 45 years | Dietary | (↓) WC (↓) BMI | (↓) BP | (↓) HDL (∼) TG | (↓) BG | (↓) MS |
| Witteman et al., 199471 | Belgium | Case–control (n=91)100% F. Age: ≈57 years | Magnesium supplementation | (∼) Body weight | (↓) SBP(↓) DBP | (∼) TC (∼) HDL | ND | ND |
| Yajnik, et al., 198468 | Britain | Cross-sectional (n=117). 78% M and 22% F. Age≈50 years | Serum | ND | ND | ND | (↓) HbA1c (↑) Glucose disposal | ND |
| Zofkova et al., 198069 | Prague | Case–control (n=14). 93% F and 7% M. Age: 20–38 years | Serum | ND | ND | ND | (↓) Glucose tolerance | ND |
Abbreviations: BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; F, female; FBG, fasting blood glucose; HDL, high-density lipoprotein; HTN, hypertension; IR, insulin resistance; LDL, low-density lipoprotein; M, male; MS, metabolic syndrome; ND, not determined; SBP, systolic blood pressure; WC, waist circumference; TG, triglycerides; (↑) positive relation; (↓) inverse relation; (∼) no relation.
Potassium and metabolic syndrome
One of the most pronounced effects of consuming increased potassium intake is its inverse relationship with blood pressure and cardiovascular diseases as reviewed by He and MacGregor.72 In line with that, a strong relationship was also determined between thiazide-induced hypokalemia and glucose intolerance in thiazide-treated subjects. One other effect is related to the involvement of potassium in glucose metabolism, in which potassium depletion resulting from a low potassium diet impairs insulin secretion, which in turn induces glucose intolerance.73 Similarly, another study by Dluhy et al.74 found that the potassium ion is involved in regulating or augmenting the secretion of insulin in humans. Resnick et al.75 further confirmed that potassium deficiency is a common feature involved in essential hypertension as well as in type 2 diabetes. Low potassium intake was also reported to be significantly associated with elevated systolic blood pressure and diastolic blood pressure in hypertensive Japanese patients and with the prevalence of metabolic syndrome in Japanese women.76 Potassium supplementation was found to attenuate salt-induced high blood pressure77, 78 and to improve salt-induced insulin resistance79 in hypertensive patients and animals.80 In parallel with these findings, the DASH diet has been considered to lower lipid-induced oxidative stress in obese individuals and to decrease blood pressure and fasting blood glucose in hypertensive patients80, 81 related to the diet's composition of fruits and vegetables, which are rich sources of potassium.80 On the basis of these findings, Fujita80 concluded that salt restriction along with a diet rich in potassium food sources (fruits and vegetables) is the first-line therapy for treating patients with metabolic syndrome; when combined together with physical activity, further improvement in insulin resistance and salt-sensitive hypertension can be achieved. Lee et al.82 further explained that higher dietary potassium intake was found to be associated with a reduced risk of insulin resistance and metabolic syndrome among Korean women. Table 3 summarizes the relationship between potassium status and the different components of metabolic syndrome as reported by several studies. In brief, the majority of the studies showed an inverse relationship between phosphorus status and adiposity,82 glycemia,73, 75, 82 lipid profile82 and blood pressure.75, 76, 82
Table 3. Results of human studies that investigated the association between serum/dietary potassium and components of the metabolic syndrome.
| Authors | Country | Study design | Biomarkers for potassium | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| |
|
|
|
Obesity |
BP |
Lipid profile |
Blood glucose |
MS |
| Lee et al., 201382 | Korea | Data obtained from the Korean National health and Nutritional Examination (n=16 637). 40.5% M and 59.5% F. Age: 44±0.25 years | Dietary | (↓) BMI in F | (↓) HTN in F | (↓) TG in F (↓) HDL in F | (↓) IR in F (↓) glucose in F (↓) diabetes in F | (↓) MS in F |
| Teramoto et al., 201176 | Japan | Prospective, large-scale observational study (n= 9585). 48.6% M and 51.4% F. Age: 50–79 years | Dietary | ND | (↓) SBP (↓) DBP | ND | ND | (↓) MS in F (∼) MS in M |
| Resnick et al., 200175 | New York | Cross-sectional (n=42). 38% M and 62% F. Age 54–68 years | Serum | ND | (↓) SBP and DBP (↓) HTN | ND | (↓) Diabetes | ND |
| Rowe et al., 198073 | United States, Boston | Case–control (n=7) 100% M. Age 20–31 years | Dietary | ND | ND | ND | (↓) Glucose intolerance (↓) impaired insulin secretion | ND |
| Dluhy et al., 197274 | United States, Boston | Case–control (n=10). 80% M and 20% F. Age | Dietary and serum | ND | ND | ND | (↓) Insulin secretion | ND |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; F, female; HDL, high-density lipoprtotein; HTN, hypertension; IR, insulin resistance; M, male; MS, metabolic syndrome; ND, not determined; SBP, systolic blood pressure; TG, triglycerides(↑) positive relation; (↓) inverse relation; (∼) no relation.
In summary, the above macrominerals have the metabolic and the mechanistic bases to be involved in the different components of metabolic syndrome, especially that of insulin sensitivity. Such metabolic bases are supported by evidence that show a link between the status of these macrominerals and the different metabolic abnormalities associated with metabolic syndrome. In addition, these associations may contribute to the understanding of several experimental observations. For example, their high content in dairy products may have contributed to the observed inverse association between dairy product intake and metabolic syndrome,12 especially given that calcium failed to explain such association.83 Moreover, the inverse relationship between increased intake of whole grains and the risk of the different components of metabolic syndrome84 may be partially explained by their richness in these macrominerals, as added cereal fiber failed to induce such an effect and was proposed to be a marker of other components of whole grains that impart health advantages.85 Thus, it is plausible to assume that the benefits of whole grains were highly ascribed to their contents of these macrominerals (the other components) rather than to their fiber content.
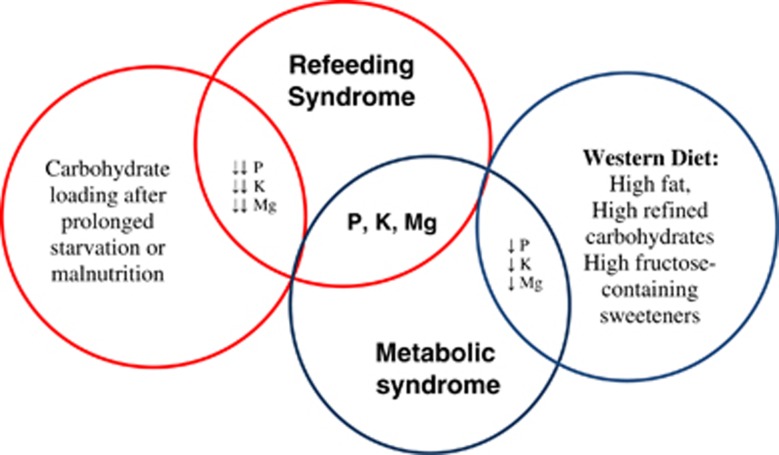
In conclusion, the proper treatment or management for the increasing epidemic of obesity and its health-related diseases has been studied extensively in the literature, through proposing and testing different dietary interventions that focused mainly on macronutrients. However, these types of interventions seem to have a limited rate of success, especially with the global change in dietary habits, favoring the Western diet, and the increasing prevalence of obesity in developing as well as in developed countries. For this reason, attention should be given to the role of macrominerals that are involved in carbohydrate metabolism and thus call for research to further clarify the link or the role of these macrominerals in metabolic syndrome and its components, especially given evidence suggesting a protective effect of protein and dairy (rich in phosphorus)12 and fruit and vegetable (rich in magnesium and potassium)62, 80 consumption on metabolic syndrome and its abnormalities. Moreover, evidence suggests that the global increase in intake of fats, processed food and sugars that are calorically dense and nutritiously poor, lacking phosphorus, potassium and magnesium, appear to compromise the postprandial status of these macrominerals in blood. Knowing that macrominerals are essential for the metabolism of macronutrients (mainly carbohydrates) renders the importance of these minerals' involvement in the development of metabolic syndrome. Metabolically, such a compromise seems to induce several metabolic conditions that favor the development of the different components of the metabolic syndrome, starting with the state of insulin resistance observed in both syndromes (Figure 1). The pathophysiology of these metabolic conditions appears to stem from an alteration in insulin status and thereby, resemble that of refeeding syndrome. The resemblance in the metabolic basis between metabolic and refeeding syndromes demands the use of a common approach for their management, necessitating boosting the status of these macrominerals in the diet, especially stable food. This may perhaps be accomplished by the restoration or fortification of white flour and/or the establishment of a carbohydrate to macromineral ratio comparable to that of carbohydrate to thiamin. Therefore, the significance of this paper is its role in understanding the drawbacks of the global dietary changes on macromineral imbalances, and further explains the common link between refeeding syndrome and metabolic syndrome. This supports our hypothesis that metabolic syndrome seems to manifest from the sustenance of a mild refeeding syndrome status.
Figure 1.
Common link between metabolic syndrome and refeeding syndrome.
The authors declare no conflict of interest.
References
- Boateng AA, Sriram K, Meguid MM, Crook M. Refeeding syndrome: treatment considerations based on collective analysis of literature case reports. Nutrition. 2010;26:156–167. doi: 10.1016/j.nut.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Fuentebella J, Kerner JA. Refeeding syndrome. Pediatr Clin N Am. 2009;56:1201–1210. doi: 10.1016/j.pcl.2009.06.006. [DOI] [PubMed] [Google Scholar]
- National Heart Lung and Blood Institute. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- Khan IR, Ahmed J, Khan S, MacFle J.Refeeding syndrome: a literature review Gastroenterol Res and Pract 20112011doi: 10.1155/2011/410971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC. Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur J Clin Nutr. 2012;66:201–208. doi: 10.1038/ejcn.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattab M, dit El-Khoury D, Azar S, Mattar M, Obeid O. Effect of phosphorus on the oral glucose tolerance test. Proc Nutr Soc. 2011;70:E60. [Google Scholar]
- Popkin BM. Global nutrition dynamics: the world is shifting rapidly toward a diet linked with noncommunicable diseases. Am J Clin Nutr. 2006;84:289–298. doi: 10.1093/ajcn/84.1.289. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes. 2004;28:S2–S9. doi: 10.1038/sj.ijo.0802804. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Titte S, Cade JR, Rideout BA, Oliver W. Uric acid, evolution, and primitive cultures. Sem Nephrol. 2005;25:3–8. doi: 10.1016/j.semnephrol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Reddy S, Ounouu S, Anand S. Global burden of cardiovascular diseases. Part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- Esmaillzadeh A, Kimiager M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am J Clin Nutr. 2007;85:910–918. doi: 10.1093/ajcn/85.3.910. [DOI] [PubMed] [Google Scholar]
- Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the atherosclerosis risk in communities study. Circulation. 2008;117:754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract. 2005;1:80–87. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- Karczmar GS, Kurtz T, Tavares NJ, Weiner MW. Regulation of hepatic inorganic phosphate and ATP in response to fructose loading: an in vivo 31P-NMR study. Biochem Biophys Acta. 1989;1012:121–127. doi: 10.1016/0167-4889(89)90084-0. [DOI] [PubMed] [Google Scholar]
- Lingelbach LB, McDonald RB. Description of the long-term lipogenic effects of dietary carbohydrates in male Fischer 344 rats. J Nutr. 2004;130:3077–3084. doi: 10.1093/jn/130.12.3077. [DOI] [PubMed] [Google Scholar]
- Teff KL, Elliott SS, Tschop M, Kieffer TJ, Raeder D, Heiman M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–2972. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- Zammit VA, Waterman IJ, Topping D, McKay G. Insulin stimulation of hepatic triacylglycerol secretion and the etiology of insulin resistance. J Nutr. 2001;131:2074–2077. doi: 10.1093/jn/131.8.2074. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations/ FAOSTAT.PC. Food balanced sheets 2009. Accessed on 10 January 2014.
- Drewnowski A. Obesity, diets, and social inequalities. Nutr Rev. 2009;67 (Suppl 1:S36–S39. doi: 10.1111/j.1753-4887.2009.00157.x. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Popkin BM. The nutrition transition: new trends in the global diet. Nutr Rev. 1997;55:31–43. doi: 10.1111/j.1753-4887.1997.tb01593.x. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Nielsen SJ. The sweetening of the world's diet. Obes Res. 2003;11:1325–1332. doi: 10.1038/oby.2003.179. [DOI] [PubMed] [Google Scholar]
- French S, Story M, Neumark-Sztainer D, Fulkerson JA, Hannan P. Fast food restaurant use among adolescents: associations with nutrient intake, food choice, and behavioral and psychosocial variables. Int J Obes Relat Metab. 2001;25:1823–1833. doi: 10.1038/sj.ijo.0801820. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Siega-Riz A, Popkin BM. Trends in energy intake in the US between 1977 and 1996: similar shifts seen across age groups. Obes Res. 2002;10:370–378. doi: 10.1038/oby.2002.51. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Siega-Riz A, Popkin BM. Trends in food locations and sources among adolescents and young adults. Prev Med. 2002;35:107–113. doi: 10.1006/pmed.2002.1037. [DOI] [PubMed] [Google Scholar]
- Haines P, Hama M, Guilkey DK, Popkin BM. Weekend eating in the United States is linked with greater energy, fat and alcohol intake. Obes Res. 2003;11:945–949. doi: 10.1038/oby.2003.130. [DOI] [PubMed] [Google Scholar]
- Jeffery R, Utter J. The changing environment and population obesity in the United States. Obes Res. 2003;11:12S–22S. doi: 10.1038/oby.2003.221. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Popkin BM. Patterns and trends in portion sizes, 1977–1998. JAMA. 2003;289:450–453. doi: 10.1001/jama.289.4.450. [DOI] [PubMed] [Google Scholar]
- Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- Guthrie J, Lin B, Frazao E. Role of food prepared away from home in the American diet, 1977–78 versus 1994–96: changes and consequences. J Nutr Educ Behav. 2002;34:140–150. doi: 10.1016/s1499-4046(06)60083-3. [DOI] [PubMed] [Google Scholar]
- Paeratakul S, Ferdinand DP, Champagne CM, Ryan DH, Bray GA. Fast-food consumption among US adults and children: dietary and nutrient intake profile. J Am Diet Assoc. 2003;103:1332–1338. doi: 10.1016/s0002-8223(03)01086-1. [DOI] [PubMed] [Google Scholar]
- Håglin L. Hypophosphataemia: cause of the disturbed metabolism in the metabolic syndrome. Med Hypotheses. 2001;56:657–663. doi: 10.1054/mehy.2000.1272. [DOI] [PubMed] [Google Scholar]
- Lippi G, Montagnana M, Salvagno GL, Targher G, Guidi GC. Relationship between serum phosphate and cardiovascular risk factors in a large cohort of adult outpatients. Diabetes Res Clin Pract. 2009;84:e3–e5. doi: 10.1016/j.diabres.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Haap M, Heller E, Thamer C, Tschritter O, Stefan N, Fritsche A. Association of serum phosphate levels with glucose tolerance, insulin sensitivity and insulin secretion in non-diabetic subjects. Eur J Clin Nutr. 2006;60:734–739. doi: 10.1038/sj.ejcn.1602375. [DOI] [PubMed] [Google Scholar]
- Friedman MI. Obesity and the hepatic control of feeding behavior. Drug News Perspect. 2007;20:9. doi: 10.1358/dnp.2007.20.9.1162243. [DOI] [PubMed] [Google Scholar]
- Nair S, Chacko VP, Arnold C, Diehl M. Hepatic ATP reserve and efficiency of replenishing: comparison between obese and nonobese normal individuals. Am J Gastroenterol. 2003;98:466–470. doi: 10.1111/j.1572-0241.2003.07221.x. [DOI] [PubMed] [Google Scholar]
- Obeid OA, Dimachkie S, Hlais S. Increased phosphorus content of preload suppresses ad libitum energy intake at subsequent meal. Int J Obes. 2010;34:1446–1448. doi: 10.1038/ijo.2010.74. [DOI] [PubMed] [Google Scholar]
- Nazar K, Kaciuba-Uściłko H, Szczepanik J, Zemba AW, Kruk B, Chwalbińska-Moneta J, et al. Phosphate supplementation prevents a decrease of triiodothyronine and increases resting metabolic rate during low energy diet. J Physiol Pharmacol. 1996;47:373–383. [PubMed] [Google Scholar]
- Jaedig S, Lindgärde F, Arborelius M. Increased postprandial energy expenditure in obese women after peroral K- and Mg-phosphate. Miner Electrolyte Metab. 1994;20:147–152. [PubMed] [Google Scholar]
- Belin RJ, He K. Magnesium physiology and pathogenic mechanisms that contribute to the development of the metabolic syndrome. Magnes Res. 2007;20:107–129. [PubMed] [Google Scholar]
- Obeid O. Low phosphorus status might contribute to the onset of obesity. Obes Rev. 2013;14:659–664. doi: 10.1111/obr.12039. [DOI] [PubMed] [Google Scholar]
- Celik N, Andiran N. The relationship between serum phosphate levels with childhood obesity and insulin resistance. J Pediatr Endocrinol Metab. 2011;24:81–83. doi: 10.1515/jpem.2011.116. [DOI] [PubMed] [Google Scholar]
- Holecki M, Chudek J, Wiecek A, Titz-Bober M, Dulawa J. The serum level of fibroblast growth factor-23 and calcium-phosphate homeostasis in obese perimenopausal women. Int J Endocrinol. 2011;2011:707126. doi: 10.1155/2011/707126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Nettleton JA, Ix JH, de Boer IH, Folsom AR, Bidulescu A, et al. Dietary phosphorus, blood pressure, and incidence of hypertension in the atherosclerosis risk in communities study and the multi-ethnic study of atherosclerosis. Hypertension. 2010;55:776–784. doi: 10.1161/HYPERTENSIONAHA.109.143461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol. 2009;20:397–404. doi: 10.1681/ASN.2008020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W, Kim BS, Lee JE, Huh JK, Kim BJ, Sung KC, et al. Serum phosphate levels and the risk of cardiovascular disease and metabolic syndrome: a double-edged sword. Diabetes Res Clin Pract. 2009;83:119–125. doi: 10.1016/j.diabres.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Dhingra R, Sullivan LM, Fox SC, Wang TJ, D'Agostino RB, Gaziano JM, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- Kalaitzidis R, Tsimihodimos V, Bairaktari E, Siamopoulos KC, Elisaf M. Disturbances of phosphate metabolism: another feature of metabolic syndrome. Am J Kidney Dis. 2005;45:851–858. doi: 10.1053/j.ajkd.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Haglin L, Lindbland A, Bygren LO. Hypophosphatemia in the metabolic syndrome. Gender differences in body weight and blood glucose. Eur J Clin Nutr. 2001;55:493–498. doi: 10.1038/sj.ejcn.1601209. [DOI] [PubMed] [Google Scholar]
- Ljunghall S, Hvarfner A, Lind L. Clinical studies of calcium metabolism in essential hypertension. Eur Heart J. 1987;8 (Suppl B:37–44. doi: 10.1093/eurheartj/8.suppl_b.37. [DOI] [PubMed] [Google Scholar]
- Farhangi MA, Ostadrahimi A, Mahdoob S. Serum calcium, magnesium, phosphorus and lipid profile in healthy Iranian premenopausal women. Biochemia Medica. 2011;21:312–320. doi: 10.11613/bm.2011.042. [DOI] [PubMed] [Google Scholar]
- Gudmundsdottir H, Starnd AH, Kjeldsen SE, Hoieggen A, Os I. Serum phosphate, blood pressure, and the metabolic syndrome-20-year follow up of middle-aged men. J Clin Hypertens (Greenwich) 2008;10:814–821. doi: 10.1111/j.1751-7176.2008.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyssoulis G, Karpanou E, Tzamou V, Kyvelou SM, Michaelidis A, Stefanadis C. Serum phosphate in white-coat hypertensive patients: focus on dipping status and metabolic syndrome. Hypertens Res. 2010;33:825–830. doi: 10.1038/hr.2010.86. [DOI] [PubMed] [Google Scholar]
- Elliott P, Kesteloot H, Appel LJ, Duer AR, Ueshima H, Chan Q. Dietary phosphorus and blood pressure: intesnational study of macro- and micro-nutrients and blood pressure. Hypertension. 2008;51:669–675. doi: 10.1161/HYPERTENSIONAHA.107.103747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar I, Kotchen T. Regional variations of blood pressure in the united states are associated with regional variations in dietary intake: the NHANES-III Data. J Nutr. 2003;133:211–214. doi: 10.1093/jn/133.1.211. [DOI] [PubMed] [Google Scholar]
- Kesteloot H, Joossens JV. Relationship of serum sodium, potassium calcium, and phosphorus with blood pressure. Belgian Interunivesrity Research on Nutrition and Health. Hypertension. 1988;12:589–593. doi: 10.1161/01.hyp.12.6.589. [DOI] [PubMed] [Google Scholar]
- Kjeldesen SE, Os I, Westheim A, Frederichsen P, Hjermann I, Eide IK. Decreased serum phosphate in essential hypertension. Related to increased sympathetic tone. Am J Hypertens. 1988;1:403–409. doi: 10.1093/ajh/1.4.403. [DOI] [PubMed] [Google Scholar]
- Harlan WR, Hull AL, Schmouder RL, Landis JR, Thompson FE, Larkin FA. Blood pressure and nutrition in adults. The National Health and Nutrition Examination Survey. Am J Epidemiol. 1984;120:17–28. doi: 10.1093/oxfordjournals.aje.a113870. [DOI] [PubMed] [Google Scholar]
- Daniels J, Goodman DA. Hypertension and Hyperparathyroidism. Inverse relation of serum phosphate level and blood pressure. Am J Med. 1983;75:17–23. doi: 10.1016/0002-9343(83)91162-2. [DOI] [PubMed] [Google Scholar]
- Havlik RJ, Garrison RJ, Feinleib M, Padgett S, Castelli WP, McNamara PM. Evidence for additional blood pressure correlates in adults 20-56 years old. Circulation. 1980;61:710–715. doi: 10.1161/01.cir.61.4.710. [DOI] [PubMed] [Google Scholar]
- Kass L, Weekes J, Carpenter L. Effect of magnesium supplementation on blood pressure: a meta-analysis. Eur J Clin Nutr. 2012;66:411–418. doi: 10.1038/ejcn.2012.4. [DOI] [PubMed] [Google Scholar]
- Gums JG. Magnesium in cardiovascular and other disorders. Am J Health-Syst Pharm. 2004;61:1569–1576. doi: 10.1093/ajhp/61.15.1569. [DOI] [PubMed] [Google Scholar]
- Guerrera MP, Volpe SL, Mao JJ. Therapeutic uses of magnesium. Am Fam Physician. 2009;80:157–162. [PubMed] [Google Scholar]
- He K, Liu K, Daviglus ML, Morris SJ, Loria CM, Van Horn L, et al. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation. 2006;113:1675–1682. doi: 10.1161/CIRCULATIONAHA.105.588327. [DOI] [PubMed] [Google Scholar]
- Song Y, Ridker PM, Manson JE, Cook NR, Burning JE, Liu S. Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older US women. Diabetes Care. 2005;28:1438–1444. doi: 10.2337/diacare.28.6.1438. [DOI] [PubMed] [Google Scholar]
- Ford ES, Li C, McGuire LC, Mokdad AH, Liu S. Intake of dietary magnesium and the prevalence of the metabolic syndrome among US adults. Obesity. 2007;15:1139–1146. doi: 10.1038/oby.2007.628. [DOI] [PubMed] [Google Scholar]
- Lima ML, Cruz T, Rodrigues LE, Bomfim O, Melo J, Correia R, et al. Serum and intracellular magnesium deficiency in patients with metabolic syndrome- Evidence for its relation to insulin resistance. Diabetes Res Clin Pract. 2009;83:257–262. doi: 10.1016/j.diabres.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Corica F, Corsonello A, Ientile R, Cucinotta D, Di Bendetto A, Perticone F. Serum ionized magnesium levels in relation to metabolic syndrome in type 2 diabetic patients. J Am Coll Nutr. 2006;25:210–215. doi: 10.1080/07315724.2006.10719534. [DOI] [PubMed] [Google Scholar]
- Yajnik CS, Smith RF, Hockaday TDR, Ward NI. Fasting plasma magnesium concentrations and glucose disposal in diabetes. Br Med J. 1984;288:1032–1035. doi: 10.1136/bmj.288.6423.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zofkova I, Nedvidkove J, Zamrazil V, Simeckova A. Influence of magnesium on glucose tolerance acute hypomagnesemia reduces the glucose tolerance independently on hormonal indicators. Horm Metabol Res. 1987;19:228–229. doi: 10.1055/s-2007-1011785. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Tani M, Uto-Kondo H, Saita E, Iizuka M, Sone H. Effects of magnesium on postprandial serum lipid responses in healthy human subjects. Br J Nutr. 2010;103:469–472. doi: 10.1017/S0007114509992716. [DOI] [PubMed] [Google Scholar]
- Witteman JC, Grobee DE, Derkx FH, Bouillon R, de Bruijn AM, Hofman A. Reduction of blood pressure with oral magnesium supplementation in women with mild to moderate hypertension. J Clin Nutr. 1994;60:129–135. doi: 10.1093/ajcn/60.1.129. [DOI] [PubMed] [Google Scholar]
- He FJ, MacGregor GA. Beneficial effects of potassium on human health. Physiol Plant. 2008;133:725–735. doi: 10.1111/j.1399-3054.2007.01033.x. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Tobin JD, Rosa RM, Andres R. Effect of experimental potassium deficienc on glucose and insulin metabolism. Metabolism. 1980;29:498–505. doi: 10.1016/0026-0495(80)90074-8. [DOI] [PubMed] [Google Scholar]
- Dluhy RG, Axelrod L, Williams GH. Serum immunoreactive insulin and growth hormone response to potassium infusion in normal man. J Appl Physiol. 1972;33:22–27. doi: 10.1152/jappl.1972.33.1.22. [DOI] [PubMed] [Google Scholar]
- Resnick LM, Barbagallo M, Dominguez LJ, Veniero JM, Nicholson JP, Gupta RK. Relation of cellular potassium to other mineral ions in hypertension and diabetes. Hypertension. 2001;38:709–712. doi: 10.1161/01.hyp.38.3.709. [DOI] [PubMed] [Google Scholar]
- Teramoto T, Kawamori R, Miyazaki S, Teramukai S. Sodium intake in men and potassium intake in women determine the prevalence of metabolic syndrome in Japanese hypertensive patients: OMEGA Study. Hypertens Res. 2011;34:957–962. doi: 10.1038/hr.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Sato Y. Natriuretic and antihypertensive effects of potassium in DOCA-salt hypertensive rats. Kidney Int. 1983;24:731–739. doi: 10.1038/ki.1983.220. [DOI] [PubMed] [Google Scholar]
- Fujita T, Ando K. Hemodynamic and endocrine changes associated with potassium supplementation in sodium-loaded hypertensives. Hypertension. 1984;6:184–192. [PubMed] [Google Scholar]
- Ogihara T, Asano T, Ando K, et al. High-salt diet enhances insulin signaling and induces insulin resistance in Dahl saltsensitive rats. Hypertension. 2002;40:83–89. doi: 10.1161/01.hyp.0000022880.45113.c9. [DOI] [PubMed] [Google Scholar]
- Fujita T. Insulin resistance and salt-sensitive hypertension in metabolic syndrome. Nephrol Dial Transplant. 2007;22:3102–3107. doi: 10.1093/ndt/gfm409. [DOI] [PubMed] [Google Scholar]
- Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi T, Azizi F. Beneficial effects of a dietary approaches to stop hypertension eating plan on features of the metabolic syndrome. Diabetes Care. 2005;28:2823–2831. doi: 10.2337/diacare.28.12.2823. [DOI] [PubMed] [Google Scholar]
- Lee H, Lee J, Hwang S, Kim S, Chin HJ, Han JS. Potassium intake and the prevalence of metabolic syndrome: the Korea National Health and Nutrition Examintaion Survey 2008-2010. PLoS One. 2013;8:1–9. doi: 10.1371/journal.pone.0055106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovski JA, Parikh SJ, Yanoff LB, Denkinger BI, Calis KA, Reynolds JC. Effects of calcium supplementation on body weight and adiposity in overweight and obese adults: a randomized trial. Ann Intern Med. 2009;150:W145–W146. doi: 10.7326/0003-4819-150-12-200906160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr. 2012;142:1304–1313. doi: 10.3945/jn.111.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins DJ, Kendall CW, Augustin LS, Martini MC, Axelsen M, Faulkner D. Effect of wheat bran on glycemic control and risk factors for cardiovascular disease in type 2 diabetes. Diabetes Care. 2002;25:1522–1528. doi: 10.2337/diacare.25.9.1522. [DOI] [PubMed] [Google Scholar]
- Paula FJ, Plens AE, Foss MC. Effects of hypophosphatemia on glucose tolerance and insulin secretion. Horm Metab Res. 1998;30:281–284. doi: 10.1055/s-2007-978884. [DOI] [PubMed] [Google Scholar]