Abstract
Background
Little is known about the burden of diabetes mellitus (DM) in pregnancy in low- and middle-income countries despite high prevalence and mortality rates being observed in these countries.
Objective
To investigate the prevalence and geographical patterns of DM in pregnancy up to 1 year post-delivery in low- and middle-income countries.
Search strategy
Medline, Embase, Cochrane (Central), Cinahl and CAB databases were searched with no date restrictions.
Selection criteria
Articles assessing the prevalence of gestational diabetes mellitus (GDM), and types 1 and 2 DM were sought.
Data collection and analysis
Articles were independently screened by at least two reviewers. Forest plots were used to present prevalence rates and linear trends calculated by linear regression where appropriate.
Main results
A total of 45 articles were included. The prevalence of GDM varied. Diagnosis was made by the American Diabetes Association criteria (1.50–15.5%), the Australian Diabetes in Pregnancy Society criteria (20.8%), the Diabetes in Pregnancy Study Group India criteria (13.4%), the European Association for the Study of Diabetes criteria (1.6%), the International Association of Diabetes and Pregnancy Study Groups criteria (8.9–20.4%), the National Diabetes Data Group criteria (0.56–6.30%) and the World Health Organization criteria (0.4–24.3%). Vietnam, India and Cuba had the highest prevalence rates. Types 1 and 2 DM were less often reported. Reports of maternal mortality due to DM were not found. No geographical patterns of the prevalence of GDM could be confirmed but data from Africa is particularly limited.
Conclusion
Existing published data are insufficient to build a clear picture of the burden and distribution of DM in pregnancy in low- and middle-income countries. Consensus on a common diagnostic criterion for GDM is needed. Type 1 and 2 DM in pregnancy and postpartum DM are other neglected areas.
Keywords: diabetes mellitus, gestational diabetes mellitus, non-communicable diseases, pregnancy, prevalence
Diabetes mellitus (DM) is a metabolic disorder resulting from a defect in insulin production, impaired insulin action or both. It is one of the major non-communicable diseases on the rise worldwide, causing 4.8 million deaths and morbidity in 371 million people every year (1). In recent years, patterns of change have been observed in the age of onset of DM with younger populations now disproportionately affected. It is currently estimated that 28 million women of reproductive age suffer from DM worldwide (2). Majority of these women have type 2 DM, and 80% of the burden is found in low- and middle-income countries (2).
In pregnancy, DM can either be pre-existing (type 1 or 2) or gestational diabetes mellitus (GDM). In pre-existing DM, risk factors such as genetic predisposition, family history of type 1 DM and autoimmune disorders are crucial in the development of type 1 DM (3, 4). Factors which play a significant role in both type 2 DM and GDM include obesity, unhealthy diets, physical inactivity, family histories of type 2 DM, maternal age and ethnicity (4, 5). Other lifestyle changes such as alcohol abuse and smoking have also been implicated in the aetiology of type 2 DM (6).
A diabetic pregnant woman and her unborn child are at increased risk of pregnancy complications such as pre-eclampsia, infections, obstructed labour, postpartum haemorrhage, preterm births, stillbirths, macrosomia, miscarriage, intrauterine growth retardation, congenital anomalies, birth injuries and death in worst case scenarios (7, 8). Women are also at risk of long-term diabetic complications, including retinopathy, nephropathy and neuropathy.
Beyond the 42-day postpartum period, consequent effects of DM in pregnancy can also be seen. An estimated 30–50% of women with a previous history of GDM develop it again in subsequent pregnancies, and within 5–10 years, 50% of these women will develop type 2 DM (9–11). In addition, babies born from diabetic pregnancies have an increased risk of developing obesity in childhood, metabolic disturbances in adolescence and type 2 DM in adulthood, linked to the metabolic imbalance experienced in utero (3).
Appropriate diagnosis, care and management of DM in the pre-pregnancy, pregnancy and post-pregnancy periods are important to minimise the risk of complications, long-term effects or catastrophic death of the mother and/or baby (12). Several diagnosing criteria for GDM are used worldwide. These include the ADA (America Diabetes Association), ADIPS (Australian Diabetes in Pregnancy Society), DIPSI (Diabetes in Pregnancy Study Group India), EASD (European Association for the Study of Diabetes), IADPSG (International Association of Diabetes and Pregnancy Study Groups), NICE (National Institute of Health and Clinical Excellence), NDDG (National Diabetes Data Group), SIGN (Scottish International Guidelines Network) and WHO (World Health Organization for both pregnant and non-pregnant populations) (13, 14). These criteria differ in the group screened (universal or only high-risk women), gestational age at screening, loading dose for the oral glucose tolerance test (OGTT) and the OGTT cut-off levels of plasma glucose.
In some of the poorest areas of the world, difficulties in accessing and receiving both maternity and general medical care increase the risks pregnant women face from the complications of diabetes in pregnancy. It is estimated that women with type 1 DM face a 5–20% risk of dying in pregnancy compared to non-diabetic pregnant women if adequate care is not provided (15).
Despite the high burden of diabetes in low- and middle-income countries, little is known about the contribution of DM in pregnancy in these countries. This review aims to investigate the prevalence and geographical pattern of DM (pre-existing and gestational) in pregnancy and up to 1 year post-delivery in low- and middle-income countries. We took 1 year as the cut-off point because it is up to this period that late maternal deaths are recorded (worldwide) and is also jointly agreed by the WHO, UNFPA, UNICEF and the World Bank (16).
Methods
A priori protocol was written before undertaking the review and the PRISMA statement used to guide reporting (17).
Inclusion and exclusion criteria
Randomised, non-randomised and observational study designs of primary or secondary studies were eligible for inclusion if they reported on prevalence and/or mortality rates due to any type of DM in pregnancy up to 1 year after childbirth. Editorials, letters, commentaries and short notes were excluded. Systematic reviews were not eligible for inclusion; however, their references were screened for relevant primary or secondary studies. We also excluded studies that had modelled or extrapolated prevalence or mortality estimates.
Studies that looked at pregnant women with pre-existing DM (type 1 and 2) or GDM confirmed by any international diagnostic criteria, for example, the ADA, ADIPS, DIPSI, EASD, IADPSG, NDDG, NICE, SIGN and WHO, were included. Studies with women up to 1 year since their last delivery with confirmed diagnosis of diabetes were also eligible for inclusion. Studies regarding non-diabetic pregnant women, diabetic women who had delivered more than 1 year ago and self-reported diabetic women with no clinical and diagnostic confirmatory tests were excluded.
Prevalence of GDM and DM (type 1 and 2) in pregnancy up to 1 year post-delivery was the primary outcome measure. Mortality due to GDM and DM (type 1 and 2) in pregnancy up to 1 year post-delivery was the secondary outcome measure; screening criteria, gestational age, parity, maternal age and setting were included as explanatory outcome measures. Prevalence or mortality related to impaired glucose tolerance (IGT) and metabolic syndrome were excluded. All studies which were carried out in countries listed by the World Bank as low, lower and upper middle-income countries were considered for inclusion (18).
Electronic searches
A comprehensive search of Medline, Medline-in-process, Embase, CAB abstracts, Cochrane Central Register of Controlled Trials and Cinahl databases was conducted using appropriate MeSH terms combined by Boolean commands ‘AND’ and ‘OR’. Key words in the search strategy included (diabetes OR type 1 diabetes OR juvenile diabetes OR child diabetes OR autoimmune diabetes OR insulin-dependent diabetes OR DM OR type 2 diabetes OR adult onset diabetes OR non-insulin-dependent diabetes OR gestational diabetes) AND (maternal mortality OR maternal morbidity OR pregnancy OR pregnant women OR pregnancy complications) AND (developing countries OR low-income countries OR lower income countries OR low- and middle-income countries OR upper middle-income countries). Reference lists of included studies and review papers were screened for relevance and hand searching of relevant reports done. Although the Cochrane collaborative strongly advises against setting language restrictions to prevent effects of possible language bias by exclusion of articles (and study populations) published in non-English journals, articles in the English language were the only ones eligible for inclusion due to financial constraints tied to translation costs of the non-English papers. There were no date restrictions and all the searches ran until March 2014.
Data management and extraction
Reference Manager (version 12) was used to manage all of the citations retrieved. Two reviewers (LK, NB) initially screened titles and abstracts independently using the inclusion–exclusion criteria. Relevant articles were selected and their full texts sought. These were then screened for eligibility by all of the reviewers (LK, NB, JH and JB) independently, ensuring at least two reviewers screened each article. Where disagreements arose about inclusion of an article, discussions resolved these. A data extraction form was developed incorporating important characteristics such as study design, country, sampling frame, sample size and relevant outcomes.
Data synthesis and analysis
Table 1 and 2 were used to summarise characteristics of the included studies: one showing a general methodological description of the studies and the second showing outcome measures of interest. Prevalence of GDM and type 1 and 2 DM were computed. 95% confidence intervals of GDM prevalence were calculated and compiled (Fig. 2) using metadata viewer for epidemiological studies (version 1, March 2011). The overall prevalence results could not be pooled together by a meta-analysis due to underlying clinical heterogeneity such as differences in the gestational age for screening, maternal age and different criteria used which were all likely to influence the results and also lack of a comparator group for most studies. As part of the exploration of geographical patterns of prevalence, the (rural or urban) setting of the study was identified. Association between GDM and gross national income (GNI) per capita (19) was determined using a linear regression model and a scatter plot was used to illustrate findings (Fig. 3).
Table 1.
Description of included studies
| Author, year | Country | Study design | Setting | Sampling frame | Sample size | |
|---|---|---|---|---|---|---|
| 1. Akter et al. (1996) | Pakistan | Retrospective cohort | Unclear | Tertiary hospital | 6,830 | |
| 2. Jawad et al. (1996) | Pakistan | Prospective cohort | Unclear | Tertiary hospital | 5,559 | |
| 3. Khan et al. (1991) | Pakistan | Prospective cohort | Unclear | Tertiary hospital | 1,267 | |
| 4. Ramachandran et al. (1994) | India | Cross sectional | Unclear | 2 gynaecology centres | 950 | |
| 5. Ramachandran et al. (1998) | India | Prospective cohort | Urban | 2prenatal clinics | 1,036 | |
| 6. Grewal et al. (2012) | India | Prospective cohort | Urban | Tertiary hospital | 298 | |
| 7. Hill et al. (2005) | India | Prospective cohort | Urban | Tertiary hospital | 785 | |
| 8. Swami et al. (2008) | India | Prospective cohort | Unclear | Secondary/ tertiary hospital | 1,225 | |
| 9. Seshiah et al. (2012) | India | Prospective cohort | Unclear | Community health centres | 1,463 | |
| 10. Seshiah et al. (2004) | India | Prospective cohort | Unclear | Tertiary hospital | 1,251 | |
| 11. Tripathi et al. (2012) | India | Prospective cohort | Urban | Tertiary hospital | 687 | |
| 12. Wahi et al. (2011) | India | Prospective cohort | Unclear | Tertiary hospital | 2,025 | |
| 13. Siribaddana et al. (1998) | Sri Lanka | Prospective cohort | Unclear | Secondary/ Tertiary hospital | 721 | |
| 14. Boriboonhirunsarn et al. (2004) | Thailand | Cross sectional | Urban | Tertiary hospital | 1,200 | |
| 15. Chanprapaph et al (2004) | Thailand | Retrospective cohort | Urban | Tertiary hospital | 1,000 | |
| 16. Lueprasitsakul et al. (2008) | Thailand | Retrospective cohort | Urban | Tertiary hospital | 637 | |
| 17. Serirat, S. et al. (1991) | Thailand | Prospective cohort | Urban | Secondary/ tertiary hospital | 25,997 | Facility-based studies |
| 18. Sumeksri et al. (2006) | Thailand | Prospective cohort | Urban | Secondary/ Tertiary hospital | 1,332 | |
| 19. Fan et al. (2006) | China | Prospective cohort | Urban | Tertiary hospital | 20,512 | |
| 20. Tran et al. (2013) | Vietnam | Prospective cohort | Urban | Tertiary hospital | 2,772 | |
| 21. Hirst et al. (2012) | Vietnam | Prospective cohort | Urban | Tertiary hospitals | 2,702 | |
| 22. Baci et al. (2013) | Turkey | Prospective cohort | Urban | Tertiary hospital | 614 | |
| 23. Karcaaltincaba et al. (2009) | Turkey | Retrospective cohort | Urban | Tertiary hospital | 21,531 | |
| 24. Kosus et al. (2012) | Turkey | Retrospective cohort | Urban | Tertiary hospital | 808 | |
| 25. Erem et al. (2002) | Turkey | Cross sectional | Unclear | 7 health stations | 807 | |
| 26. Tanir et al. (2005) | Turkey | Retrospective cohort | Urban | Tertiary hospital | 3,548 | |
| 27. Hadaegh et al. (2005) | Iran | Prospective cohort | Unclear | Obstetric clinics in Bandar Abbas city | 800 | |
| 28. Hossein-Nezhad et al. (2006) | Iran | Cross sectional | Urban | 5 teaching hospitals | 2,416 | |
| 29. Keshavarz et al. (2005) | Iran | Prospective cohort | Urban | Tertiary hospital | 1,310 | |
| 30. Ranchod, H.A. et al. (1991) | South Africa | Prospective cohort | Urban | Tertiary hospital | 1,721 | |
| 31. Anzaku and Musa (2013) | Nigeria | Cross sectional | Urban | Tertiary hospital | 253 | |
| 32. Olarinoye et al. (2004) | Nigeria | Prospective cohort | Urban | Tertiary hospital | 293 | |
| 33. Ozumba et al. (2004) | Nigeria | Retrospective cohort | Urban | Tertiary hospital | 12,030 | |
| 34. Balaji et al. (2012) | India | Cross sectional | Urban, suburban & rural | Community health centres | 819 | |
| 35. Seshiah et al. (2008), (2009) | India | Cross sectional | Urban, suburban & rural | 20 health posts, 10 primary & community centres | 12,056 | |
| 36. Zargar et al. (2004) | India | Prospective cohort | Urban & rural | All ANC in 6 districts of Kashmiri valley | 2,000 | |
| 37. Dahanayaka et al. (2012) | Sri Lanka | Cross sectional | Unclear | Three MOH areas in a district | 405 | |
| 38. Sayeed et al. (2005) | Bangladesh | Prospective cohort | Rural | 10 villages with union council & local government | 172 | |
| 39. Yang et al. (2009) | China | Prospective cohort | Unclear | 26 hospitals in 18 cities | 16,286 | Population-based studies |
| 40. Yang et al. (2002) | China | Prospective cohort | Urban | All ANC units in the 6 central districts | 9,471 | |
| 41. Zhang et al. (2011) | China | Prospective cohort | Urban | All secondary/ tertiary hospitals in the 6 central districts | 105,473 | |
| 42. Seyoum et al. (1999) | Ethiopia | Cross sectional | Rural | 18 villages in East Tigray | 890 | |
| 43. McCarthy et al. (2010) | Argentina | Prospective cohort | Urban | All the 23 primary health centres in La Plata city | 1,702 | |
| 44. Schmidt et al. (2000) | Brazil | Prospective cohort | Urban | All ANC in the NHS in 6 state capitals | 5,004 | |
| 45. Davilla et al. (2011) | Cuba | Retrospective cohort | Unclear | All of Isle of Youth | 1,003 |
MOH, ministry of health; ANC, antenatal clinics; NHS, National Health Service.
Table 2.
Characteristics of included studies (gestational diabetes mellitus, type 1 and 2 diabetes mellitus, postpartum type 2 diabetes mellitus)
| Author, year | Country | Maternal age (years) | Parity (GDM only) | Gestational age at diagnosis | Diagnosing criteria | Screening criteria (GDM only) | GDM prevalence (%) | Total DM prevalence (T1, T2) | Prevalence of postpartum type 2 DM | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Akter et al. (1996) | Pakistan | Mean 26.9 | Null=19% >1=81% |
Unclear | WHO | Selective | 3.30 | 0.6% | Not reported | |
| 2. Jawad et al. (1996) | Pakistan | 20–45 | Null=21% >1=79% |
Unclear | NDDG | Universal | 3.45 | Not reported | Not reported | |
| 3. Khan et al. (1991) | Pakistan | 22–34 | Not reported | <28 weeks & 28–32 weeks | NDDG | Universal | 3.20 | Not reported | Not reported | |
| 4. Ramachandran et al. (1994) | India | <20–35+ | Not reported | Unclear | NDDG | Universal | 0.56 | 0.6% | Not reported | |
| 5. Ramachandran et al. (1998) | India | Unclear | Not reported | 24–28 weeks | NDDG | Universal | 0.86 | 0.29% | Not reported | |
| 6. Grewal et al. (2012) | India | 18–39 | Not reported | 1st trimester, & 24–28 weeks | ADA | Universal | 15.49 | Not reported | Not reported | |
| 7. Hill et al. (2005) | India | 16–40 | Not reported | 28–32 weeks | ADA | Universal | 5.80 | Not reported | Not reported | |
| 8. Swami et al. (2008) | India | 18–40 | Not reported | NR | ADA | Universal | 7.70 | Not reported | Not reported | |
| 9. Seshiah et al. (2012) | India | Mean 23.6 (±3.32) | Not reported | ~22–34 weeks | DIPSIIADPSG | Universal | 13.414.6 | Not reported | Not reported | |
| 10. Seshiah et al. (2004) | India | 19–27 | Not reported | Unclear | WHO | Universal | 17.70 | Not reported | Not reported | |
| 11. Tripathi et al. (2012) | India | 20–32 | Unclear | 24–28 weeks | ADA | Universal | 1.50 | Not reported | Not reported | |
| 12. Wahi et al. (2011) | India | 22–30 | Not reported | 24–28 weeks | WHO | Selective | 6.94 | Not reported | Not reported | |
| 13. Siribaddana et al. (1998) | Sri Lanka | 15–44 | Not reported | 24–28 weeks | WHO | Universal | 5.50 | Not reported | Not reported | |
| 14. Boriboonhirunsarn et al. (2004) | Thailand | 25–36 | Unclear | 7.6–16.6 weeks | NDDG | Selective | 5.10 | Not reported | Not reported | |
| 15. Chanprapaph et al. (2004) | Thailand | Mean 21–33 | Unclear | Unclear | NDDG | Selective | 2.90 | 0.2% | Not reported | |
| 16. Lueprasitsakul et al. (2008) | Thailand | Unclear | Not reported | Unclear | NDDG | Selective | 1.50 | Not reported | Not reported | Facility-based |
| 17. Serirat et al. (1991) | Thailand | 15–41 | Null=43% >1=57% |
Unclear | NDDG | Universal | 2.02 | Not reported | Not reported | studies |
| 18. Sumeksri et al. (2006) | Thailand | 30–34 | Not reported | 25.6–28 weeks | NDDG | Universal | 2.40 | Not reported | Not reported | |
| 19. Fan et al. (2006) | China | 26–35 | Not reported | Unclear | NDDG | Universal | 3.80 | Not reported | Not reported | |
| 20. Tran et al. (2013) | Vietnam | 16–44 | Null=36% ≥1=64% |
24–32 weeks | ADA IADPSG ADIPS WHO |
Selective | 5.9 20.4 20.8 24.3 |
Not reported | Not reported | |
| 21. Hirst et al. (2012) | Vietnam | 22–35+ | Unclear | 24–32 weeks | ADA IADPSG |
Universal | 6.1 20.3 |
Not reported | Not reported | |
| 22. Baci et al. (2013) | Turkey | 18–45 | Not reported | 24–28 weeks | ADA | Universal | 1.95 | Not reported | Not reported | |
| 23. Karcaaltincaba et al. (2009) | Turkey | 14–49 | Not reported | Unclear | ADA NDDG |
Universal | 4.48 3.17 |
Not reported | Not reported | |
| 24. Kosus et al. (2012) | Turkey | Unclear | Unclear | 24–28 weeks | ADA NDDG |
Universal | 8.1 5.6 |
Not reported | Not reported | |
| 25. Erem et al. (2002) | Turkey | <20–30+ | Unclear | 24–32 weeks | NDDG | Universal | 1.23 | Not reported | Not reported | |
| 26. Tanir et al. (2005) | Turkey | 27.3–37.9 | Unclear | Unclear | ADA | Universal | 3.10 | Not reported | Not reported | |
| 27. Hadaegh et al. (2005) | Iran | 19–30 | Not reported | Unclear | ADA NDDG |
Universal | 8.90 6.30 |
Not reported | Not reported | |
| 28. Hossein-Nezhad et al. (2006) | Iran | 15–45 | Unclear | Unclear | ADA NDDG |
Universal | 4.70 3.97 |
Not reported | Not reported | |
| 29. Keshavarz et al. (2005) | Iran | 20–35 | Unclear | 13.4–28.6 weeks | ADA | Universal | 4.80 | Not reported | Not reported | |
| 30. Ranchod et al. (1991) | South Africa | Unclear | Not reported | Unclear | EASD WHO |
Universal | 1.56 3.78 |
0.23% | Not reported | |
| 31. Anzaku and Musa (2013) | Nigeria | 21–40 | Unclear | 24–28 weeks | WHO | Universal | 1.60 | Not reported | Not reported | |
| 32. Olarinoye et al. (2004) | Nigeria | 18–41 | Mean: 1.3 | Unclear | WHO NDDG |
Universal | 6.45 2.02 |
Not reported | Not reported | |
| 33. Ozumba et al. (2004) | Nigeria | 15–54 | 0 to 4=81% >4=19% | Unclear | WHO | Universal | 1.01 | 0.65% | Not reported | |
| 34. Balaji et al. (2012) | India | Mean 23.8 (±3.48) | Not reported | 24–28 weeks | WHO | Universal | 10.5 | Not reported | Not reported | |
| 35. Seshiah et al. (2008), (2009) | India | 19–27 | Not reported | 16.9–34.3 weeks | WHO | Universal | 13.90 | Not reported | Not reported | |
| 36. Zargar et al. (2004) | India | 18–38 | Mean: 2.1 | ~24 weeks | ADA WHO |
Universal | 3.10 4.40 |
Not reported | Not reported | |
| 37. Dahanayaka et al. (2012) | Sri Lanka | 19–≥35 | 1=42.2%2 to 4=55.8%>4=2.0% | 24–28 weeks | WHO IADPSG |
Universal | 7.16 8.89 |
Not reported | Not reported | |
| 38. Sayeed et al. (2005) | Bangladesh | 18–44 | Not reported | <26 & >26 weeks | WHO | Universal | 8.20 | Not reported | Not reported | |
| 39. Yang et al. (2009) | China | 20–>30 | Not reported | Unclear | ADA | Universal | 4.35 | Not reported | Not reported | Population- |
| 40. Yang et al. (2002) | China | 26–28 | Not reported | 26–30 weeks | WHO | Universal | 1.84 | Not reported | Not reported | based studies |
| 41. Zhang et al. (2011) | China | <25–>35 | Not reported | 26–30 weeks | WHO | Universal | 4.90 | Not reported | Not reported | |
| 42. Seyoum et al. (1999) | Ethiopia | 20–35 | Unclear | Unclear | WHO | Universal | 3.70 | Not reported | Not reported | |
| 43. McCarthy et al. (2010) | Argentina | 13–45 | Unclear | 24–28 weeks | WHO | Universal | 5.80 | Not reported | Not reported | |
| 44. Schmidt et al. (2000) | Brazil | 22–33 | Unclear | 21–28 weeks | WHO | Universal | 0.40 | Not reported | Not reported | |
| 45. Davilla et al. (2011) | Cuba | Unclear | Null=14% >1=86% | <20 to>32 weeks | WHO modified | Universal | 17.25 | 0.70% | Not reported |
T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; GDM, gestational diabetes mellitus; DM, diabetes mellitus; WHO, World Health Organization; ADA, American Diabetes Association, ADIPS, Australian Diabetes in Pregnancy Society; NDDG, National Diabetes Data Group; DIPSI, Diabetes in Pregnancy Study Group India; EASD, European Association for the Study of Diabetes; IADPSG, International Association of Diabetes and Pregnancy Study Groups.
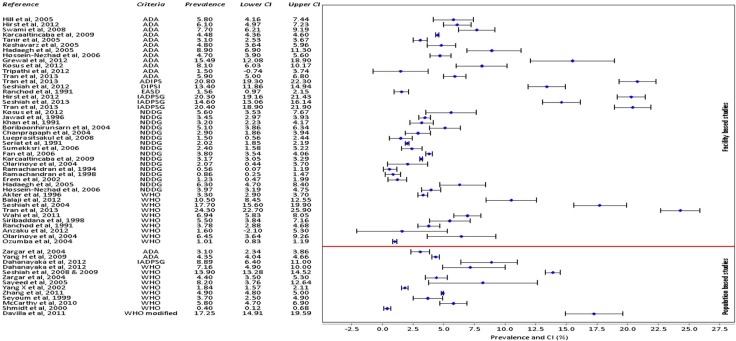
Fig. 2.
DGM prevalence and confidence intervals (CI).
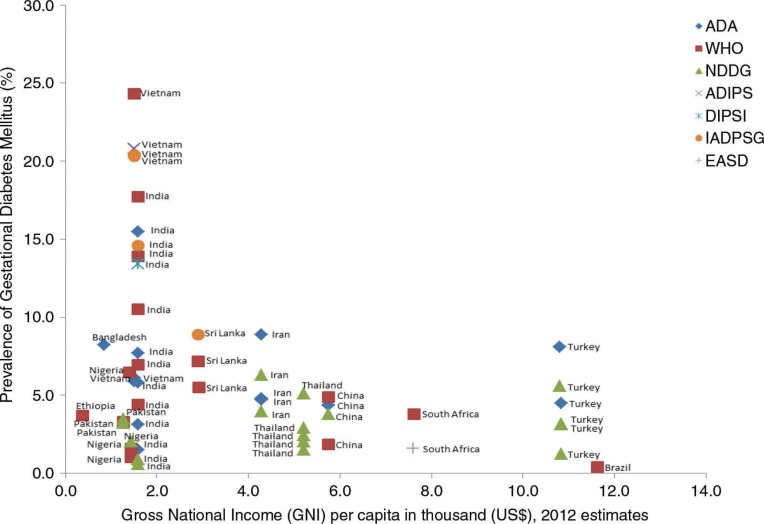
Fig. 3.
Prevalence of gestational diabetes mellitus against gross national income per capita in thousands (US$).
Risk of bias
The studies included were assessed for risk of bias by two reviewers independently (LK, NB). The validity of methodology, its appropriateness and reporting of results were assessed (20, 21). Seven criteria were used to assess three risks of biases, namely measurement bias, selection bias and attrition bias.
Results
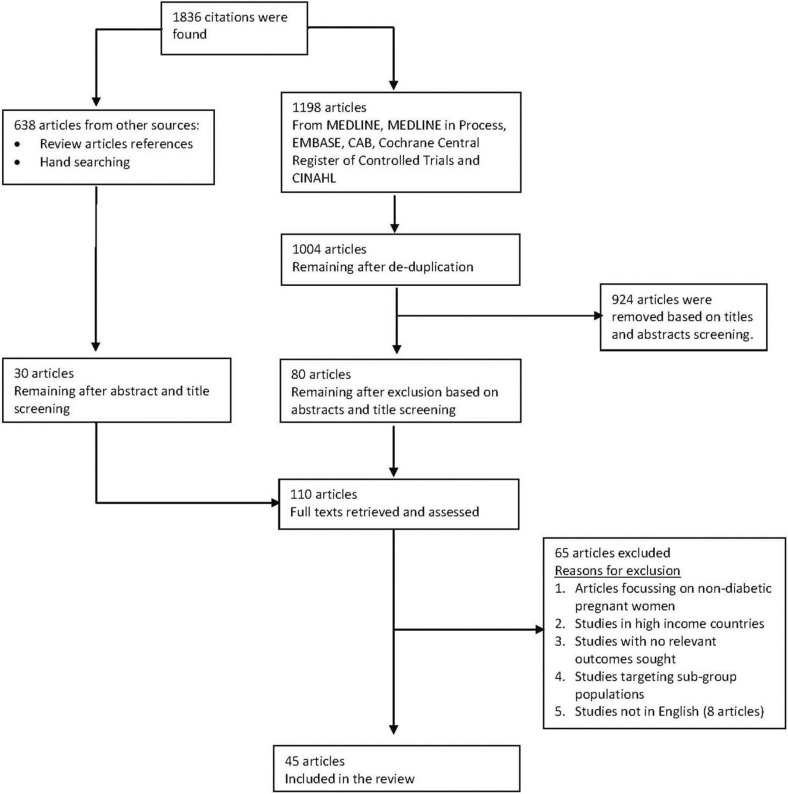
The searches conducted yielded 1,836 citations. After screening titles, abstracts and full texts, 45 studies (Fig. 1) with 281,661 participants were included. Among the excluded studies were eight non-English articles, with two each in German and French, and one each in Norwegian, Spanish, Persian and Portuguese after abstract and title screening.
Fig. 1.
Flow chart of study selection. In general, studies were excluded based on participants (if it included women who were not pregnant or those beyond 1 year in the postpartum period), study design (if these were commentaries, letters of correspondence, systematic reviews), outcome measure (if it did not include relevant outcomes sought) and was not a low- and middle-income country as defined by the World Bank.
Characteristics of included studies
The included studies were from Pakistan (22–24), India (25–37), Sri Lanka (38, 39), Bangladesh (40), Thailand (41–45), China (46–49), Vietnam (50, 51), Turkey (52–56), Iran (57–59), South Africa (60), Ethiopia (61), Nigeria (62–64), Argentina (65), Brazil (66) and Cuba (67) (Table 1). The largest number of studies (12) came from India. The studies included were either cohort or cross-sectional studies. About 60% of the studies (26 studies) were based in urban areas. Two studies from Bangladesh and Ethiopia specified a rural population base. Three other studies reported that both urban and rural areas were covered, while in 14 studies, the setting was not described. In Fig. 2, the included studies are grouped into 33 studies which were facility-based and 12 which were population-based (also seen Table 1). Facility-based studies were considered as those sampling one or a few hospitals/clinics, and were not reported to be representative of the total targeted population in the study district/region. Population-based studies included those that reported to have sampled the whole population of interest in the selected district(s)/region or those which reported systematic sampling of the population in a region/district likely to be representative of the total targeted population (Table 1, sampling frame). Sample sizes across all the studies included varied from as low as 172 to 105,472 participants.
Table 2 shows outcome measures of interest across the various studies. In general, studies varied from reporting on prevalence only to reporting on prevalence, risk factors, pregnancy outcomes and interventions (data not shown). Fifteen studies reported on prevalence only (24, 25, 28–31, 34, 35, 37, 49, 53, 60, 61, 66, 67), 10 studies on prevalence and risk factors only (38, 40, 41, 46, 47, 50, 52, 54, 57, 62), 12 studies on prevalence, risk factors and pregnancy outcomes/obstetric complications (21, 25, 26, 35, 41–44, 54, 57, 62–64), and eight studies on prevalence, pregnancy outcomes/complications and some form of intervention (22, 31, 32, 38, 47, 50, 58, 59). The interventions in the latter group included diet/medical nutrition therapy only, insulin only or combined diet and insulin therapy.
Maternal age ranged from as low as 13 years in Argentina to 54 years of age in Nigeria (Table 2). Parity was poorly reported by only 10 studies. Among these, GDM prevalence was higher in women who had given birth to one child or more, than in those giving birth for the first time (Table 2). Gestational age at diagnosis of GDM was only reported by about 60% of the studies included. The majority of these studies reported on a diagnosis being made between the 24th and 32nd gestational weeks.
The screening criteria used were reported by all the studies included. Thirty-nine studies used universal screening of participants while the remaining six studies used selective screening of pregnant women at high risk of GDM (Table 2), except those with multiple pregnancies and other predisposing medical conditions. The most common diagnosing criteria used were the WHO criteria, followed by the NDDG criteria, and then the ADA criteria. The IADPSG, ADIPS, DIPSI, EASD and modified WHO criteria were less popular diagnosing criteria. GDM was the most frequently documented type of diabetes in pregnancy reported by all the studies, while the prevalence of pre-existing type 1 or type 2 DM was only reported in seven studies.
Prevalence
There were 58 observations of GDM prevalence from the 45 studies, because more than one criterion was used by some studies (Table 2). Prevalence using the ADA criteria (15 observations) ranged from 1.50 to 15.50%. With the NDDG criteria (16 observations), prevalence ranged from 0.56 to 6.30%, the WHO criteria (19 observations) ranged from 0.4 to 24.30%, and the IADPSG criteria (four observations) ranged from 8.9 to 20.4%. EASD, ADIPS, DIPSI and WHO modified criteria each had only one observation with prevalence of 1.56, 20.8, 13.4 and 17.25% reported, respectively. GDM prevalence rates and their confidence intervals are summarised in Fig. 2.
Prevalence of type 1 and 2 DM were reported as ranging from 0.20 to 0.70%. Neither postpartum DM after 6 weeks nor maternal mortality due to any type of DM was reported.
The association between GDM and GNI per capita is shown in Fig. 3. A significant negative correlation is seen (B=−0.611; R=0.358; p=0.007). Three studies (28, 50, 51) are clear outliers on the graph, and without these the suggestion of an association is further reduced (B=−0.314; R=0.291; p=0.042).
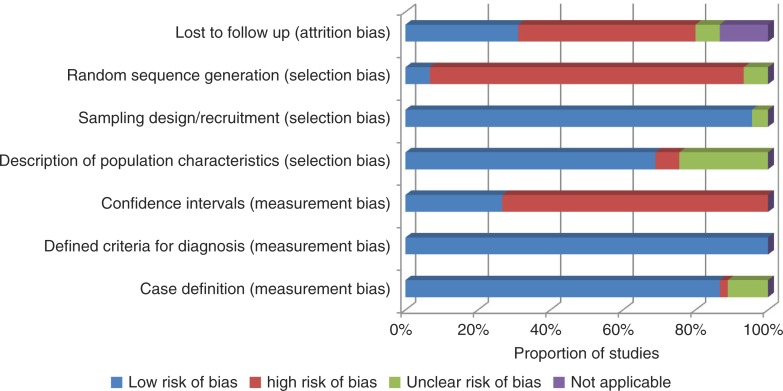
Risk of bias
A summary of the risk of bias in included studies is shown in Fig. 4. The diagnostic criteria used were well defined in all of the studies. A total of 86% (39 studies) had clear case definitions, and 95% (43 studies) reported clearly on the sampling design and recruitment processes used. However, studies were subject to a high risk of bias in a few parameters. Confidence intervals were only reported by 26% (12 studies). Only 7% (three studies) randomly sequenced the selection of participants and 31% (14 studies) reported on loss to follow-up.
Fig. 4.
Risk of bias summary (bias considerations vs. proportion of studies).
Discussion
Our review is the first to systematically summarise the published literature on prevalence of GDM and type 1 and 2 DM in pregnancy in low- and middle-income countries. We found 45 studies recording the prevalence of DM in pregnancy which passed our selection criteria. They only cover a select number of countries and large areas of Africa and Asia are not covered by existing studies. GDM prevalence ranged from 0.40 to 24.3% and pre-existing DM (type 1 and 2) ranged from 0 to 0.7%.
It is well known that a wide variation of DM is seen across countries (68). High prevalence rates are reported to occur among Asian, Latin America and Middle Eastern populations. Ethnicity (69) and geographical variation (70, 71) are important factors and are well documented across high-income countries such as Bahrain (13.5%) (72), Qatar (16.3%) (73), United Arab Emirates (14.2–23.1%) (74), Hong Kong (14.2%) (75), Ireland (9.4–12.4%) (76), Israel (6.07%) (77) and the United States (2–10%) (78). In our study, the highest prevalence of GDM was reported from Vietnam (50, 51), India (28–30, 34, 35, 37) and Cuba (67), followed closely by Bangladesh (40) and Iran (57). Although the prevalence ranges that we found were wide, most were fairly consistent with the global GDM prevalence ranging between 1 and 14% (70), except those from Vietnam which were unusually high. Although data is sparse, we also found one unexpectedly high level of GDM prevalence reported in Nigeria of 6.45%, which is comparable to the levels found in some of the Asian countries with high ethnic predisposition to DM. The comparative prevalence of DM in Africa's general population is 5.7% (19.8 million adults), which is the lowest in all the regions, and also lower than the average global prevalence estimated at 8.3% (79). Nonetheless, this finding from the study from Nigeria was not corroborated by using different diagnostic criteria (Table 2), and a comparison was not done on the same population. The study was also facility-based and participants had been randomly allocated to either WHO (75 g OGTT) or NDDG (100 g OGTT) study arms for comparison (63). In general, facility-based studies have higher GDM prevalence levels than population-based studies due to increased likelihood of patients presenting at health facilities. Hence, caution should be taken in the interpretation of this result.
One of the major limitations our review highlights is the difficulty in determining prevalence due to the lack of consistency in the use of diagnosing criteria (e.g. ADA, WHO, NDDG, IADPSG). The various diagnostic criteria use different loading doses for OGTT and different thresholds for fasting times (between 1 and 3 hours). The number of abnormal plasma glucose values considered to be adequate for a diagnosis also change when using the various criteria. For example, the ADA criteria uses a loading dose of 100 g for OGTT, and allows for two or more abnormal values for a diagnosis to be made, whereas the WHO criteria recommends 75 g for OGTT allowing only one or more abnormal values to be used for diagnosis (13, 80). This is compounded by the differences in sensitivity and specificity between the diagnosing criteria. In an ideal setting, a clinical test with the highest accuracy to identify patients with a disease (sensitivity) and those without a disease (specificity) is usually desirable. However, most clinical tests do not always satisfy this ideal. A systematic review by Donovan et al. investigated the sensitivity and specificity of the various tests for diagnosing GDM using different thresholds (81). The authors found that at the threshold of 7.8 mmol/L, the ADA criteria had the highest sensitivity of 88 (86–97)%, followed by NDDG criteria at 85 (73–92)% then the WHO criteria at 70 (43–85)%. IADPSG had very low sensitivity at 12 (7–18)%. Conversely, the IADPSG had the highest specificity at 97 (95–98)%, compared to that of the WHO, ADA and NDDG criteria at 89 (73–94)%, 84 (79–87)% and 83 (78–87)%, respectively. To date, there is still a lack of clarity as to which diagnostic criteria should be used. The various debates are centred around frequency of diagnosis by different criteria, cost effectiveness and the dilemma of undiagnosed cases who are at risk of poor maternal and perinatal outcomes if they remain undetected, and where care may be sub-optimal (82, 83).
We considered the possibility of conducting meta-analyses in this review but did not do these as there is expected to be considerable variability across ethnic groups and countries. Pooling of data even from a single country was not done due to the variation in use of diagnostic criteria and lack of a control group for most studies.
Pre-existing (type 1 and 2) DM in pregnancy was reported by very few studies. Since the highest burden of type 2 DM exists in low- and middle-income countries also affects women of reproductive age (2), it is surprising that studies did not capture these women while pregnant. A number of the studies in our review did however specifically exclude women with pre-existing diabetes. No studies reported on postpartum type 2 diabetes (after 6 weeks) indicating an acute lack of information on women followed up to 1 year after childbirth. This could be linked to poor postnatal attendance and resource constraints in health services (84, 85). There were no studies reporting on maternal deaths due to diabetes. These could be due to several reasons such as deaths being masked by misclassification or inappropriate coding or missed because they were late maternal deaths that occurred within 1 year after delivery. Undiagnosed diabetes may also be another contributing factor. Up to 50% of people living with diabetes worldwide are currently undiagnosed (1), and it is known that diabetes can lead to complications in pregnancy such as pregnancy-induced hypertension, pre-eclampsia, postpartum haemorrhage and increased risk of infections. It is possible that in undiagnosed diabetic patients who develop complications in pregnancy and succumb to it, these complications could have been attributed as the main cause of death and not diabetes which was the underlying cause of death.
A statistically significant inverse relationship was seen between the prevalence of GDM and a country's wealth as measured by the GNI. It was expected that with increasing wealth GDM would become more prevalent, and/or the functionality of the health system would improve, thus increasing the number of cases picked up through screening. Although conclusions from this rather crude analysis cannot be drawn, the trends observed would be worth investigating further to understand and plan for future health needs in countries with transitioning economies.
A number of limitations are inherent in our review design. We were unable to assess papers in languages other than the English language. Of the eight full-text papers excluded for this reason, these included papers in German (2), French (2), Norwegian (1), Spanish (1), Persian (1), and Portuguese (1) languages. The likelihood is that studies from Latin America, French-speaking African countries and the Middle East are most likely to be underrepresented if published in regional/local non-English journals. We did not restrict the dates for our search, and the majority of the studies were from the last 15 years. A few studies dated to the early 1990s and the observed demographic changes in tendency to develop DM in young adults could have affected any patterns we might have observed. Studies included in this review may have been subjected to some bias, primarily in the form of selection bias. These were in terms of how participants were selected (Fig. 4) and whether they were representative of the target population as a whole which may have affected the estimates of prevalence obtained. Commonly, selection bias occurs when the participants studied are not representative of the target population about which conclusions are to be drawn. For example, if an investigator wishes to estimate the prevalence of disease X in adult residents of a certain town/city/region, s/he may attempt to do this by selecting a random sample from all the adults enrolled with several local health facilities, and then recruit them. However, this design, which is used in some studies included in this review, would be systematically excluding participants who do not access health facilities and therefore impact on the results obtained. Eliminating selection bias in epidemiological studies is therefore critical for accurate results and should always be considered when defining a study sample. In addition, including confidence intervals which describe a range within which one can reasonably expect the true value to lie would be important. Unfortunately, this was not documented in a number of studies included in this review.
Conclusion
DM is a growing public health problem in low- and middle-income nations. This systematic review has highlighted the disparate and piecemeal data available from the published literature on prevalence of DM during pregnancy in these countries. Without such data, it will be difficult to make rational decisions for allocating precious funding within expanding health systems. A global consensus on the diagnostic criteria for DM is urgently required so that the public health burden of the condition can be assessed. Studies of prevalence should capture populations beyond those presenting in health facilities, as little is known about undetected DM in pregnancy. The current focus on GDM needs to be extended to also capture diabetes in women of reproductive age especially just before pregnancy and in the months after delivery, as these are the times when interventions can optimise the health of women and maximise the likelihood of a healthy foetus.
Acknowledgement
We thank Ann Fitzmaurice for her support and advice on statistics. We appreciate the support provided by Wendy Graham (University of Aberdeen), Bilal Avan (London School of Hygiene and Tropical Medicine) and Dineen Brendan (formerly of the University of Aberdeen) at the inception phase of the study.
Conflict of interest and funding
All the authors declare they have no conflicts of interest.
References
- 1.IDF. Diabetes atlas. 4th ed. Brussels, Belgium: International Diabetes Federation; 2013. [Google Scholar]
- 2.IDF. Reproductive, maternal and child health. Brussels, Belgium: International Diabetes Federation; 2013. [Google Scholar]
- 3.Majeed A, Hassan M. Risk factors for type 1 diabetes mellitus among children and adolescents in Basrah. Oman Med J. 2011;26:189–95. doi: 10.5001/omj.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.IDF. Risk factors. Brussels, Belgium: International Diabetes Federation; 2013. [Google Scholar]
- 5.Alberti K, Zimmet P, Shaw J. International Diabetes Federation: a consensus of type 2 diabetes prevention. Diabet Med. 2007;24:451–63. doi: 10.1111/j.1464-5491.2007.02157.x. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Global health risks. Mortality and burden of disease attributable to selected major risks. World Health Organization. 2009b. Available from: http://www.who.int/healthinfo/global_burden_disease/global_health_risks/en/ [cited 30 August 2013].
- 7.Modder J, Fitzsimons J. CMACE/RCOG joint guideline. Management of women with obesity in pregnancy. Centre for Maternal and Child Enquiries (CMACE) and Royal College of Obstetricians and gynaecologists (RCOG) 2010. Available from: http://www.rcog.org.uk/womens-health/clinical-guidance/management-women-obesity-pregnancy [cited August 2013].
- 8.Moore T, Smith C. Diabetes mellitus and pregnancy. Medscape drugs, diseases and procedures reference. 2012. Available from: http://emedicine.medscape.com/article/127547-overview [cited August 2013].
- 9.Dain K. Gestational diabetes: an invisible and serious maternal health issue. Global Campaign. 2011;56 Available from: http://www.idf.org/diabetesvoice/articles/gestational-diabetes-an-invisible-maternal-health-issue [cited August 2013]. [Google Scholar]
- 10.IDF. Global guideline. Pregnancy and diabetes. International Diabetes Federation. 2009. Available from: http://www.idf.org/guidelines/pregnancy-and-diabetes [cited August 2013].
- 11.IDF. Diabetes in pregnancy: protecting maternal health. International Diabetes Federation. Policy Briefing. 2013. Available from: http://www.idf.org/publications/diabetes-pregnancy-protecting-maternal-health [cited January 2014].
- 12.Hod M. Pregnancy outcome of diabetic women 15 years after the St. Vincent declaration: success or failure? Endocrinol Nutr. 2005;52:571–4. [Google Scholar]
- 13.Waugh N, Pearson D, Doyle P. Screening for hyperglycaemia in pregnancy: consensus and controversy. Best Pract Res Clin Endocrinol Metabol. 2010;24:553–71. doi: 10.1016/j.beem.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Santos-Ayarzagoitia M, Salinas-Martinez A, Villareal-Perez J. Gestational diabetes: validity of ADA and WHO diagnostic criteria using NDDG as the reference test. Diabet Res Pract. 2006;74:322–8. doi: 10.1016/j.diabres.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Leinonen P, Hiilesmaa V, Kaaja R, Teramo K. Maternal mortality in type 1 diabetes. Diabetes Care. 2001;24 doi: 10.2337/diacare.24.8.1501. Available from: http://care.diabetesjournals.org/content/24/8/1501.full [cited August 2013]. [DOI] [PubMed] [Google Scholar]
- 16.WHO, UNICEF, UNFPA, The World Bank estimates. Trends in maternal mortality: 1990–2010. World Health Organization. 2012. Available from: http://www.who.int/reproductivehealth/publications/monitoring/9789241503631/en/ [cited August 2013].
- 17.Moher D, Liberati A, Tetzlaff J, Altman D, The Prisma Group Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. PLOS Med. 2009;6 Available from: http://www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.1000097 [cited September 2012]. [PMC free article] [PubMed] [Google Scholar]
- 18.The World Bank. World Bank; 2013. Country and lending groups. Available from: http://data.worldbank.org/about/country-classifications/country-and-lending-groups [cited September 2012 and March 2014]. [Google Scholar]
- 19.The World Bank. World Bank; 2013. GNI per capita, atlas method (current US$) Available from: http://data.worldbank.org/indicator/NY.GNP.PCAP.CD [cited January and March 2014]. [Google Scholar]
- 20.Loney P, Chambers L, Bennet K, Roberts J, Stratford P. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can. 2000;19:170–6. [PubMed] [Google Scholar]
- 21.Zaccai J. How to assess epidemiological studies. Postgrad Med J. 2004;80:140–7. doi: 10.1136/pgmj.2003.012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akter J, Qureshi R, Rahim F, Moosvi S, Rehman A, Jabbar A, et al. Diabetes in pregnancy in Pakistani women: prevalence and complications in an indigenous South Asian community. Diabetic Med. 1996;13:189–91. doi: 10.1002/(SICI)1096-9136(199602)13:2<189::AID-DIA32>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Jawad F, Irshaduddin P. Prevalence of gestational diabetes and pregnancy outcome in Pakistan. East Mediterr Health J. 1996;2:268–73. [Google Scholar]
- 24.Khan K, Rizvi J, Qureshi R, Mazhar R. Gestational diabetes in a developing country, experience of screening at the Aga Khan University Medical Centre, Karachi. J Pak Med Assoc. 1991;41:31–3. [PubMed] [Google Scholar]
- 25.Ramachandran A, Snehalatha C, Shyamala P, Vijay V, Viswanathan M. Prevalence of diabetes in pregnant women – a study from Southern India. Diabet Res Clin Pract. 1994;25:71–4. doi: 10.1016/0168-8227(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 26.Hill J, Krishnaveni G, Annamma I, Leary S, Fall C. Glucose tolerance in pregnancy in South India: relationships to neonatal anthropometry. Acta Obstet Gynecol Scand. 2005;84:159–65. doi: 10.1111/j.0001-6349.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 27.Ramachandran A, Snehalatha C, Clementina M, Sasikala R, Vijay V. Foetal outcome in gestational diabetes in South Indians. Diabet Res Clin Pract. 1998;41:185–9. doi: 10.1016/s0168-8227(98)00081-3. [DOI] [PubMed] [Google Scholar]
- 28.Seshiah V, Balaji V, Balaji M, Sanjeevi C, Green A. Gestational diabetes mellitus in India. J Assoc Physicians India. 2004;52:707–11. [PubMed] [Google Scholar]
- 29.Seshiah V, Balaji V, Balaji M, Paneerselvam A, Arthi T, Thamizharasi M, et al. Prevalence of gestational diabetes mellitus in south India (Tamil Nadu) – a community based study. J Assoc Physicians India. 2008;56:329–33. [PubMed] [Google Scholar]
- 30.Seshiah V, Balaji V, Balaji M, Paneerselvam A, Kapur A. Pregnancy and diabetes scenario around the world: India. Int J Gynecol Obstet. 2009;104:S35–8. doi: 10.1016/j.ijgo.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 31.Swami S, Mehetre R, Shivane V, Bandgar T, Menon P, Shah N. Prevalence of carbohydrate intolerance of varying degrees in pregnant females in western India (Maharashtra) – a hospital based study. J Indian Med Assoc. 2008;106:712–14. [PubMed] [Google Scholar]
- 32.Wahi P, Dogra V, Jandial K, Bhagat R, Gupta R, Gupta S, et al. Prevalence of gestational diabetes mellitus and its outcomes in Jammu region. J Assoc Physicians India. 2011;59:227–30. [PubMed] [Google Scholar]
- 33.Zargar A, Sheikh M, Bashir M, Masoodi S, Laway B, Wani A, et al. Prevalence of gestational diabetes mellitus in Kashmiri women from the Indian subcontinent. Diabet Res Clin Pract. 2004;66:139–45. doi: 10.1016/j.diabres.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 34.Grewal A, Kansara S, Kachhawa G, Ammini A, Kriplani A, Aggarwal N, et al. Prediction of gestational diabetes mellitus at 24 to 28 weeks of gestation by using first trimester insulin sensitivity indices in Asian Indian subjects. Metab Clin Exp. 2012;61:715–20. doi: 10.1016/j.metabol.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Seshiah V, Balaji V, Shah S, Joshi S, Das A, Sahay B, et al. Diagnosis of gestational diabetes mellitus in the community. J Assoc Physicians India. 2012;60:15–17. [PubMed] [Google Scholar]
- 36.Tripathi R, Tolia N, Gupta V, Mala Y, Ramji S, Tyagi S. Screening for gestational diabetes mellitus: a prospective study in a tertiary care institution of North India. J Obstet Gynaecol Res. 2012;38:351–7. doi: 10.1111/j.1447-0756.2011.01706.x. [DOI] [PubMed] [Google Scholar]
- 37.Balaji V, Madhuri B, Paneerselvam A, Arthi T, Seshiah V. Comparison of venous plasma glucose and capillary whole blood glucose in the diagnosis of gestational diabetes mellitus: a community-based study. Diabetes Tech Therapeut. 2012;14:131–4. doi: 10.1089/dia.2011.0060. [DOI] [PubMed] [Google Scholar]
- 38.Dahanayaka N, Agampodi S, Ranasignhe O, Jayaweera P, Wickramasinghe W, Adhikari A, et al. Inadequacy of the risk factor based approach to detect gestational diabetes mellitus. Ceylon Med J. 2012;57:5–9. doi: 10.4038/cmj.v57i1.4193. [DOI] [PubMed] [Google Scholar]
- 39.Siribaddana S, Deshabandu R, Rajapakse D, Silva K, Fernando D. The prevalence of gestational diabetes in a Sri Lankan antenatal clinic. Ceylon Med J. 1998;43:88–91. [PubMed] [Google Scholar]
- 40.Sayeed M, Mahtab H, Khanam P, Begum R, Banu A, Khan A. Diabetes and hypertension in a rural community of Bangladesh: a population based study. Diabet Med. 2005;22:1267–71. doi: 10.1111/j.1464-5491.2005.01600.x. [DOI] [PubMed] [Google Scholar]
- 41.Boriboonhirunsarn D, Sunsaneevithayakul P, Nuchangrid M. Incidence of gestational diabetes mellitus diagnosed before 20 weeks of gestation. J Med Assoc Thai. 2004;87:1017–21. [PubMed] [Google Scholar]
- 42.Chanprapaph P, Sutjarit C. Prevalence of gestational diabetes mellitus (GDM) in women screened by glucose challenge test (GCT) at Maharaj Nakorn Chiang Mai hospital. J Med Assoc Thai. 2004;87:1141–6. [PubMed] [Google Scholar]
- 43.Lueprasitsakul K, Teeyapun K, Kittivarakul E, Srisupundit K, Patumanond J. Gestational diabetes in Lumphun hospital: prevalence, clinical risk factors and pregnancy outcomes. Chiang Mai Med J. 2008;47:65–73. [Google Scholar]
- 44.Seriat S, Sumnthornthepvarakul T, Deerochanawong C, Jinayon P. Gestational diabetes mellitus. J Med Assoc Thai. 1992;75:315–19. [PubMed] [Google Scholar]
- 45.Sumeksri P, Wongyai S, Aimpun P. Prevalence of gestational diabetes mellitus (GDM) in pregnant women aged 30 to 34 years old at Phramongkutklao hospital. J Med Assoc Thai. 2006;89(Suppl 4):S94–9. [PubMed] [Google Scholar]
- 46.Yang H, Wei Y, Gao X, Xu X, Fan L, He J, et al. Risk factors for gestational diabetes mellitus in Chinese women – a prospective study of 16 286 pregnant women in China. Diabet Med. 2009;26:1099–104. doi: 10.1111/j.1464-5491.2009.02845.x. [DOI] [PubMed] [Google Scholar]
- 47.Yang X, Hsu-Hage B, Zhang H, Yu L, Dong L, Li J, et al. Gestational diabetes mellitus in women of single gravidity in Tianjin city, China. Diabetes Care. 2002;25:847–51. doi: 10.2337/diacare.25.5.847. [DOI] [PubMed] [Google Scholar]
- 48.Fan Z, Yang H, Gao X, Lintu H, Sun W. Pregnancy outcome in gestational diabetes. Int J Gynecol Obstet. 2006;94:12–16. doi: 10.1016/j.ijgo.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 49.Zhang F, Dong L, Zhang C, Li B, Wen J, Gao W, et al. Increasing prevalence of gestational diabetes mellitus in Chinese women from 1999 to 2008. Diabet Med. 2011;28:652–7. doi: 10.1111/j.1464-5491.2010.03205.x. [DOI] [PubMed] [Google Scholar]
- 50.Tran T, Hirst J, Do M, Morris J, Jeffery H. Early prediction of gestational diabetes mellitus I Vietnam. Diabetes Care. 2013;36:618–24. doi: 10.2337/dc12-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirst J, Tran T, Do M, Morris J, Jeffery H. Consequences of gestational diabetes in an urban hospital in Vietnam: a prospective cohort study. PLoS Med. 9 doi: 10.1371/journal.pmed.1001272. Available from: http://www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.1001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baci Y, Ustuner I, Keskin H, Ersoy R, Avsar A. Effect of maternal obesity and weight gain on gestational diabetes mellitus. Gynecol Endocrinol. 2013;29:133–6. doi: 10.3109/09513590.2012.730571. [DOI] [PubMed] [Google Scholar]
- 53.Karcaaltincaba D, Kandermir O, Yalva S, Guvendag-Guven S, Haberal A. Prevalence of gestational diabetes mellitus and gestational impaired glucose tolerance in pregnant women evaluated by National Diabetes Data Group and Carpenter and Coustan criteria. Int J Gynecol Obstet. 2009;106:246–9. doi: 10.1016/j.ijgo.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Erem C, Cihanyurdu N, Deger O, Karahan C, Can G, Telatar M. Screening for gestational diabetes mellitus in north eastern Turkey (Trabzon city) Eur J Epidemiol. 2003;18:39–43. doi: 10.1023/a:1022585101209. [DOI] [PubMed] [Google Scholar]
- 55.Kosus A, Kosus N, Turhan N. Gestational diabetes: comparison of the Carpenter and the Coustan thresholds with the new thresholds of Turkish women and implications of variations in diagnostic criteria. J Matern Fetal Neonatal Med. 2012;25:616–22. doi: 10.3109/14767058.2011.592231. [DOI] [PubMed] [Google Scholar]
- 56.Tanir H, Sener T, Gurer H, Kaya M. A ten year gestational diabetes mellitus cohort at a University clinic of the mid-Anatolian region of Turkey. Clin Exp Obstet & Gynecol. 2005;32:241–4. [PubMed] [Google Scholar]
- 57.Hadaegh F, Tohidi M, Harati H, Kheirandish M, Rahimi S. Prevalence of gestational diabetes mellitus in Iran (Bandar Abbas city) Endocr Pract. 2005;11:313–18. doi: 10.4158/EP.11.5.313. [DOI] [PubMed] [Google Scholar]
- 58.Hossein-Nezhad A, Maghbooloi Z, Vassigh A, Larijani B. Prevalence of gestational diabetes mellitus and pregnancy outcomes in Iranian women. Taiwan J Obstet Gynecol. 2007;46:236–41. doi: 10.1016/S1028-4559(08)60026-1. [DOI] [PubMed] [Google Scholar]
- 59.Keshavarz M, Cheung N, Babaee G, Moghadam H, Ajami M, Shariati M. Gestational diabetes in Iran: incidence, risk factors and pregnancy outcomes. Diabet Res Clin Pract. 2005;69:279–86. doi: 10.1016/j.diabres.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 60.Ranchod H, Vaughan J, Jarvis P. Incidence of gestational diabetes at Northdale Hospital, Pietermaritzburg. S Afr Med J. 1991;80:14–16. [PubMed] [Google Scholar]
- 61.Seyoum B, Kiros K, Haileselase T, Leole A. Prevalence of gestational diabetes mellitus in rural pregnant women in northern Ethiopia. Diabet Res Clin Pract. 1999;46:247–51. doi: 10.1016/s0168-8227(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 62.Anzaku A, Musa J. Prevalence and associated risk factors for gestational diabetes in Jos, North-central, Nigeria. Arch Gynecol Obstetr. 2013;287:859–63. doi: 10.1007/s00404-012-2649-z. [DOI] [PubMed] [Google Scholar]
- 63.Olarinoye J, Ohwovoriole A, Ajayi G. Diagnosis of gestational diabetes mellitus in Nigerian pregnant women – comparison between 75 g and 100 g oral glucose tolerance tests. West Afr J Med. 2004;23:198–201. doi: 10.4314/wajm.v23i3.28120. [DOI] [PubMed] [Google Scholar]
- 64.Ozumba B, Obi SN, Oli J. Diabetes mellitus in pregnancy in an African population. Int J Gynecol Obstet. 2002;84:114–19. doi: 10.1016/S0020-7292(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 65.McCarthy A, Curcuarello R, Castillione N, Tayeldin M, Costa D, Arnol V, et al. Universal versus selective screening for the detection, control and prognosis of gestational diabetes mellitus in Argentina. Acta Diabetol. 2010;47:97–103. doi: 10.1007/s00592-009-0107-6. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt M, Matos M, Reicheltt A, Forti A, Lima L, Duncan B. Prevalence of gestational diabetes mellitus – do the new WHO criteria make a difference? Br Diabet Assoc. 2000;17:376–80. doi: 10.1046/j.1464-5491.2000.00257.x. [DOI] [PubMed] [Google Scholar]
- 67.Davilla H, Pena M, Matos Z. Clinical and epidemiological profile of diabetes mellitus in pregnancy, Isle of Youth, 2008. Medicc Rev. 2011;13:29–34. doi: 10.37757/MR2011V13.N1.8. [DOI] [PubMed] [Google Scholar]
- 68.Bardsley J, Resnick H. Hyattsville, MD: MedStar Research Institute; 2008. Diabetes mellitus epidemiology. [Google Scholar]
- 69.Leary J, Pettitt D, Jovanovic L. Gestational diabetes guidelines in a HAPO world. Best Pract Res Clin Endocrinol Metab. 2010;24:673–85. doi: 10.1016/j.beem.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 70.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes. Estimated for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 71.Sadikot S. The epidemiology of diabetes in women and the looming epidemic of GDM in the third world. In: Tsatsoulis A, Wyckoff J, Brown F, editors. Diabetes in women: pathophysiology and therapy. New York: Humana press; 2009. pp. 223–38. [Google Scholar]
- 72.Al Mahroos S, Nagalla D, Yousif W, Sanad H. A population based screening for gestational diabetes mellitus in non-diabetic women in Bahrain. Ann Saudi Med. 2005;25:129–33. doi: 10.5144/0256-4947.2005.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bener A, Saleh N, Al-Hamaq N. Prevalence of gestational diabetes and associated maternal and neonatal complications in a fast developing community: global comparisons. Int J Women's Health. 2011;3:367–73. doi: 10.2147/IJWH.S26094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Agarwal M, Dhatt G, Zayed R, Bali N. Gestational diabetes: relevance of diagnostic criteria and preventive strategies for type 2 diabetes mellitus. Arch Gynecol Obstet. 2007;276:237–43. doi: 10.1007/s00404-007-0334-4. [DOI] [PubMed] [Google Scholar]
- 75.Ma R, Chan J. Pregnancy and diabetes scenario around the world: China. Int J Gynecol Obstet. 2009;104:S42–5. doi: 10.1016/j.ijgo.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 76.O'Sullivan E, Avalos G, O'Reilly M, Dennedy M, Gaffney G, Dunne F, et al. Atlantic diabetes in pregnancy (DIP): the prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria. Diabetologia. 2011;54:1670–5. doi: 10.1007/s00125-011-2150-4. [DOI] [PubMed] [Google Scholar]
- 77.Chodick G, Elchalal U, Sella T, Heymann A, Porath A, Kokia E, et al. The risk of overt diabetes mellitus among women with gestational diabetes: a population based study. Diabet Med. 2010;27:779–85. doi: 10.1111/j.1464-5491.2010.02995.x. [DOI] [PubMed] [Google Scholar]
- 78.Centre for Disease Control and Prevention (CDC) Gestational diabetes in the United States. 2011 National Diabetes Fact Sheet. Centre for Disease Control and Prevention. Available from: http://www.cdc.gov/diabetes/pubs/estimates11.htm [cited January 2014].
- 79.IDF. Diabetes atlas. Regional overviews. 6th ed. 2013. Available from: http://www.idf.org/sites/default/files/EN_6E_Ch3_Regional_Overviews.pdf [cited January 2014].
- 80.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30(Suppl 1) doi: 10.2337/dc07-S042. Available from: http://care.diabetesjournals.org/content/27/suppl_1/s5.full [cited January 2014]. [DOI] [PubMed] [Google Scholar]
- 81.Donovan L, Hartling L, Muise M, Guthrie A, Vandermeer B, Dryden D. Screening tests for gestational diabetes: a systematic review for the US preventive services task force. Ann Intern Med. 2013;159:115–22. doi: 10.7326/0003-4819-159-2-201307160-00657. [DOI] [PubMed] [Google Scholar]
- 82.Vandorsten J, Dodson W, Espeland M, Grobman W, Guise J, Mercer B, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consensus State Sci Statements. 2013;29:1–31. [PubMed] [Google Scholar]
- 83.Tennant P, Glinianaia S, Bilous R, Rankin J, Bell R. Pre-existing diabetes, maternal glycated haemoglobin, and the risks of fetal and infant death: a population-based study. Diabetologia. 2014;57:285–94. doi: 10.1007/s00125-013-3108-5. [DOI] [PubMed] [Google Scholar]
- 84.Warren C, Daly P, Toure L, Mongi P. Opportunities for Africa's newborns. Geneva, Switzerland: WHO; 2006. Postnatal care. [Google Scholar]
- 85.Titaley C, Hunter C, Heywood P, Dibley M. Why don't some women attend antenatal and postnatal care services? A qualitative study of community members’ perspectives in Garut, Sukabumi and Ciamis districts of West Java province, Indonesia. BMC Pregnancy Childbirth. 2010;10:61. doi: 10.1186/1471-2393-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]