Abstract
Low vitamin B-6 nutritional status is associated with increased risk for cardiovascular disease and certain cancers. Pyridoxal 5′-phosphate (PLP) serves as a coenzyme in many cellular processes, including several reactions in one-carbon (1C) metabolism and the transsulfuration pathway of homocysteine catabolism. To assess the effect of vitamin B-6 deficiency on these processes and associated pathways, we conducted quantitative analysis of 1C metabolites including tetrahydrofolate species in HepG2 cells cultured in various concentrations of pyridoxal. These results were compared with predictions of a mathematical model of 1C metabolism simulating effects of vitamin B-6 deficiency. In cells cultured in vitamin B-6-deficient medium (25 or 35 nmol/l pyridoxal), we observed >200% higher concentrations of betaine (P < 0.05) and creatinine (P < 0.05) and >60% lower concentrations of creatine (P < 0.05) and 5,10-methenyltetrahydrofolate (P < 0.05) compared with cells cultured in medium containing intermediate (65 nmol/l) or the supraphysiological 2,015 nmol/l pyridoxal. Cystathionine, cysteine, glutathione, and cysteinylglycine, which are components of the transsulfuration pathway and subsequent reactions, exhibited greater concentrations at the two lower vitamin B-6 concentrations. Partial least squares discriminant analysis showed differences in overall profiles between cells cultured in 25 and 35 nmol/l pyridoxal vs. those in 65 and 2,015 nmol/l pyridoxal. Mathematical model predictions aligned with analytically derived results. These data reveal pronounced effects of vitamin B-6 deficiency on 1C-related metabolites, including previously unexpected secondary effects on creatine. These results complement metabolomic studies in humans demonstrating extended metabolic effects of vitamin B-6 insufficiency.
Keywords: one-carbon, metabolite profile, vitamin B-6, folate
vitamin b6, as pyridoxal 5′-phosphate (PLP), is an essential cofactor in cellular homeostasis through its role in 1C metabolism. Five reactions of the 1C pathways require PLP as a coenzyme, including: cytoplasmic and mitochondrial forms of serine hydroxymethyltransferase (SHMT), glycine decarboxylase of the glycine cleavage system (GCS), and cystathionine β-synthase (CBS) and cystathionine γ-lyase (CGL) which comprise the transsulfuration pathway. Multiple epidemiological studies have shown an association between inadequate vitamin B-6 nutritional status and risk of cardiovascular disease (6, 35, 41, 42). Suboptimal vitamin B-6 status, as indicated in humans by plasma PLP concentrations of 20–30 nmol/l (24, 26), is not uncommon in the American population (30, 55), whereas vitamin B-6 deficiency (indicated by plasma PLP < 20 nmol/l) occurs in ∼10% of the population. The associations between vitamin B-6 status and chronic disease have been largely observational, and mechanistic connections between vitamin B-6 and impaired cardiovascular risk are unclear.
The sensitivity of PLP-dependent enzymes in 1C metabolic processes to vitamin B-6 insufficiency has been directly measured in animal studies. Both isozymes of SHMT show inactivation in proportion to the degree of B-6 inadequacy over a wide range of dietary vitamin B-6 in rats, with cytoplasmic and mitochondrial forms equally susceptible (27, 44). CSE and SHMT isozymes are similarly sensitive to inactivation by PLP depletion (15), whereas CBS is much less sensitive to loss of activity from inadequate vitamin B-6 status (25, 44). Other PLP-dependent pathways can also be affected. For example, severe vitamin B-6 deficiency in rodents has been shown to perturb glucose homeostasis (1, 18, 20).
Even a marginally deficient vitamin B-6 status (plasma PLP 20–30 nmol/l) leads to biochemical alterations in the 1C pathway as detected in targeted quantitative metabolite analysis (9, 11, 12, 21–23) and global metabolomic analysis (16). After a controlled dietary vitamin B-6 restriction in healthy men and women as a tool to achieve plasma PLP less than 30 nmol/l, consistent elevation of plasma glycine and cystathionine have been observed (9, 11, 23, 29), while plasma concentrations of serine, methionine, and total homocysteine were not affected (11, 12, 23). Moderate to severe vitamin B-6 deficiency in rats causes increased liver glutathione (25). The concentration of cysteine, the limiting precursor of glutathione, undergoes little change of concentration in a marginal vitamin B-6 deficiency in humans (11, 23) and rats (25). Whether vitamin B-6 deficiency affects the intracellular distribution of tetrahydrofolate species is unknown, but we hypothesize that such changes would occur in view of the impact of nutritional or genetic factors affecting 1C metabolism, such as was shown for vitamin B-12 (46). Because of the role of tetrahydrofolates in mediating the biochemical processing of 1C units, the profile of folate species would provide functional information about possible perturbations of these metabolic cycles.
Mathematical models have been developed to describe many complex biological systems. Such models allow variables to be controlled and modified in order to simulate the consequences on the system. In this manner, effects of nutritional deficiencies or genetic mutations can be simulated in silico, and the effect on metabolic pathways can be predicted. A mathematical model of 1C metabolism was developed and later expanded to include the methionine cycle and cytoplasmic and mitochondrial compartmentalization (33, 34, 38, 39). The model has been used to predict the effects of vitamin B-6 deficiency on 1C metabolism (31). The present study employed the same mathematical model to study vitamin B-6 deficiency and extends the number of metabolites quantified to encompass several of the 1C-related compounds targeted in our metabolic profile.
We report here the results of experiments conducted using targeted metabolomic analysis to characterize the profile of 1C metabolites and related compounds under controlled vitamin B-6 concentrations in mammalian cells cultured with various degrees of vitamin B-6 restriction shown previously (59) to give a range of reduced intracellular PLP concentrations. These multifaceted investigations involved analysis of 1) 1C metabolite profiles in cultured liver tumor-derived HepG2 cells maintained in media containing various adequate through deficient concentrations of pyridoxal, and 2) in silico simulations to predict the effect of vitamin B-6 deficiency on metabolite concentrations.
MATERIALS AND METHODS
Materials.
All standards were purchased from Sigma-Aldrich except symmetric dimethylarginine and asymmetric dimethylarginine (ADMA), which were purchased from Axxora (San Diego, CA; formerly Alexis Biochemicals). Internal standards used in LC-MS-MS analyses {[13C2]glycine, [13C5]methionine, [15N2]arginine, [2H3]serine, [2H6]dimethylglycine (DMG), [2H9]choline, [2H11]betaine, [2H3]creatine, [2H3]creatinine, [2H2]threonine, and [2H4]cystathionine} were purchased from Cambridge Isotopes (Cambridge, MA). HepG2 cells were obtained from ATCC (HB-8065). Modified essential Eagles medium with Earle's Balanced Salts (MEM) was purchased as the standard formulation containing ∼2,000 nmol/l pyridoxal and in custom form from HyClone (RR 10986.01) having no added vitamin B-6.
Cell culture in vitamin B-6-deficient media.
HepG2 human hepatoma cells were cultured at 37°C in a 5% CO2 atmosphere using standard MEM containing 2,000 nmol/l pyridoxal or custom pyridoxal-free MEM to which we added 25, 35, and 65 nmol/l pyridoxal-HCl. All media were supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1 mmol/l sodium pyruvate, and 0.1 mmol/l nonessential amino acids. Cells were initially cultured in standard MEM and, after reaching 80% confluence, were transferred to 75-cm2 flasks and cultured in MEM that contained one of four different concentrations of pyridoxal. Cells were passaged every 3–5 days. Intracellular PLP was measured weekly, with a steady state attained by 4 wk. The analyses reported here were conducted after 4 wk of culturing at each extracellular concentration of pyridoxal.
The 2,015 nmol/l level of total PL (i.e., the standard MEM formulation) is a nonphysiological concentration that cannot be achieved by nutritional supplement usage. This level is included as a reference only. Although PLP bound to albumin is the major form of vitamin B-6 in plasma, PLP must undergo dissociation from albumin followed by enzymatic dephosphorylation before cellular uptake in the form of PL (19, 28), Consequently, we chose to use PL for physiological relevance.
The FBS employed at the level of 10% (vol/vol) addition to the basal MEM medium provided 0.81 nmol/l PLP and 15 nmol/l PL. We considered this low PLP concentration to be negligible. Accounting for the PL content provided by FBS, the media used in cell culturing contained 2,015, 65, 35, and 25 total nmol/l total PL. The concentration of one-carbon metabolites from FBS was not measured and was equivalent for all PL treatments.
Analytical methods.
Intracellular PLP was determined by reverse-phase HPLC with fluorescence detection (49). Total intracellular cysteine, homocysteine, glutathione, and cysteinylglycine (i.e., the sums of oxidized and reduced species of each) were measured as ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate derivatives by reverse-phase HPLC with fluorescence detection (37). Intracellular folates were extracted, enzymatically converted to monoglutamyl forms, purified by affinity chromatography, and then quantified by HPLC with electrochemical detection (2, 13). The acidic mobile phases used in our HPLC analysis causes complete conversion of 10-formyl-THF (tetrahydrofolate) to 5,10-methenyl-THF; thus, the 5,10-methenyl-THF peak included both 10-formyl-THF and any preexisting 5,10-methenyl-THF. Similarly, the assay conditions cause conversion of 5,10-methylene-THF to THF, so that the THF peak comprises both THF and preexisting 5,10-methylene-THF.
A panel of 12 1C metabolites and related compounds was quantified by liquid chromatography-tandem mass spectrometry (LC-MS-MS) at the Biomedical Mass Spectrometry Laboratory, Clinical and Translational Sciences Institute, University of Florida. Quantification was performed by isotope dilution with the internal standards (9), which was similar to the approach described previously (53). DMG and sarcosine were below detection limits in these cell samples. Data were normalized by protein concentration determined by the Bradford assay (4) and by cell count determined with a TC10 automated cell counter (Bio-Rad). Trends were similar between these normalization approaches; the latter are reported here.
Mathematical modeling.
Complementary to the analytical data, the effects of reduced vitamin B-6 status on 1C metabolism were further evaluated in mathematical simulations of adequate and moderately vitamin B-6-deficient states by using a previously reported model to predict metabolite concentrations based on precursor inputs, enzyme and transport kinetics, and calculated fluxes (31, 38). Vitamin B-6 deficiency was simulated by reducing the Vmax of CBS by 20% and the Vmax values of serine hydroxymethyltransferase (SHMT), CGL, and the glycine decarboxylation by the glycine cleavage system (GCS) by 60%. These percentages were based on observations of lower sensitivity of CBS activity to vitamin B-6 deficiency and the greater reduction of the other enzymes by vitamin B-6 deficiency (31). In silico simulation also evaluated the concurrent effects of a vitamin B-6 deficiency and associated oxidative stress, because oxidative stress may occur during vitamin B-6 deficiency (48) and affects enzymes of 1C metabolism (40). In this mathematical model, the increase in oxidative stress associated with vitamin B-6 deficiency was represented by an increase in H2O2 concentration as conducted previously, which inhibits methionine synthase and betaine homocysteine methyltransferase (BHMT), and stimulates CBS (40).
Statistical analysis.
All data are presented as means ± SD. Differences in intracellular concentrations of PLP, folate forms, and transsulfuration metabolites were analyzed by one-way analysis of variance (ANOVA). Using the Bonferroni correction, statistical significance was determined at the 0.0125 (0.05/4) level for each folate form and at the 0.0125 (0.05/4) level for each transsulfuration metabolite. Pairwise comparisons were made for significant metabolites with the Holm-Sidak method at their aforementioned respective level of significance. When an overall difference is detected but none of the pairwise comparison supports significance, differences can come from beyond paired groups, such as average between the 25 and 35 nmol/l groups vs. average between the 65 and 2,015 nmol/l groups.
For the 12 1C metabolites, the analysis was done in two stages: 1) using repeated-measures ANOVA (7) to test overall difference across the 12 metabolites for each of the six pairs of B-6 concentration groups; 2) performing step-down t-tests for comparing the pair(s) with a significant overall difference. Statistical significance for the repeated-measures ANOVA and t-tests was determined at the 0.0083 (0.05/6) level after the Bonferroni correction. The two-stage hierarchical approach provides protection to the Type I error rate inflation at the step-down tests.
All data analyses were performed using SigmaPlot 11.0 (SPSS) or SAS 9.3 software. Partial least squares discriminant analysis (3, 58) was conducted on the pooled data for intracellular 1C metabolites, folates, and aminothiols in HepG2 cells by use of Simca 13 software (MKS Umetrics) (54). Score plots were assessed visually to evaluate groupings according to vitamin B-6 concentration in culture media. The role of each variable in discriminating differences among vitamin B-6 levels was determined using VIP (variable importance in the projection) plots in histogram format with confidence intervals for each VIP value determined by jackknifing at the 95% level of significance (57, 58). As stated previously (9), the VIP values in these plots are weighted sums of squares of the PLS weights of the various metabolites and take into account the proportion of Y-variance in each dimension (54). In the VIP plots, each variable is provided with a VIP value and a 95% confidence interval (CI) derived from jackknifing (14, 54, 57). Variables for which the VIP ± 95% CI exceeds 1 are designated as significant biomarkers in this analysis.
RESULTS
Targeted metabolic profiling in HepG2 hepatoma cells cultured in medium containing different concentrations of vitamin B-6.
HepG2 cells cultured in medium containing total (added and naturally occurring) pyridoxal of 25, 35, 65, or 2,015 nmol/l yielded steady-state concentrations for intracellular PLP after ∼4 wk of culture in each vitamin B-6 condition. Intracellular PLP concentrations at steady state in all four groups were significantly different from one another (P < 0.001; Table 1) and showed that the range of PL treatments used caused a physiologically relevant range of intracellular PLP similar to that occurring in rat liver due to dietary vitamin B-6 restriction (17). There were no observed changes in morphology or growth rate.
Table 1.
Intracellular PLP concentrations in HepG2 cells after 4 wk cultured in media containing different vitamin B-6 concentrations
| Media Vitamin B-6, nmol/l | Intracellular PLP, pmol/mg protein |
|---|---|
| 25 | 26.6 ± 0.66a |
| 35 | 33.9 ± 1.75b |
| 65 | 48.5 ± 3.46c |
| 2,015 | 90.9 ± 3.53d |
Values are means ± SD; n = 3.
PLP, pyridoxal 5′-phosphate.
Different superscripts indicate significant difference between groups. P < 0.001 (one-way ANOVA).
The cell culture medium contained a high concentration of folic acid (2.27 μmol/l), but all intracellular folates detected were reduced folates [i.e., no dihydrofolate (DHF) or folic acid was detected]. The analysis of intracellular folates revealed an effect of vitamin B-6 on the distribution of folate species and total intracellular folate concentration (Table 2). THF constituted the most abundant intracellular folate form (>70% of total folate). In this analysis, the value for THF represents actual THF plus 5,10-methylene-THF that dissociates to yield THF and formaldehyde during extraction and analysis. Concentrations of THF were significantly higher in cells cultured in medium containing 25 nmol/l vitamin B-6 compared with THF concentrations in cells cultured in media containing the higher vitamin B-6 concentrations (P < 0.05). The concentration of 5-methyl-THF was not affected by vitamin B-6 concentration (Table 2). The 5,10-methenyl-THF concentrations in cells cultured in 25 and 35 nmol/l vitamin B-6 were significantly lower than in the higher vitamin B-6-treated cells (P < 0.05; Table 2). Due to the acidic conditions of this folate analysis, both 10-formyl-THF and actual 5,10-methenyl-THF are detected as 5,10-methenyl-THF. Folic acid, DHF, and 5-formyl-THF were either below analytical limits of quantitation or not detected in every sample.
Table 2.
Folate concentration in HepG2 cells cultured in media containing different concentrations of pyridoxal
| Media Vitamin B-6 |
||||
|---|---|---|---|---|
| pmol/106 cells | 25 | 35 | 65 | 2,015 |
| THF | 12.7 ± 2.73a | 7.87 ± 0.99b | 8.10 ± 1.56b | 7.88 ± 0.6b |
| 5-Methyl-THF | 3.27 ± 0.67 | 2.62 ± 0.50 | 3.57 ± 0.56 | 3.48 ± 0.12 |
| 5,10-Methenyl-THF | 0.60 ± 0.31a | 0.32 ± 0.13a | 1.91 ± 0.54b | 2.44 ± 0.39b |
| Total folate | 16.6 ± 3.32a | 10.8 ± 1.20b | 13.6 ± 2.17a,b | 13.8 ± 0.49a,b |
Values are means + SD (nmol/l); n = 5. Different superscripts indicate significant difference between groups. In this analysis, both existing 5,10-methylene-THF and THF are quantified as THF (tetrahydrofolate), while existing cellular 5,10-methenyl-THF and 10-formyl-THF are measured as 5,10-methenyl-THF. P < 0.05 (one-way ANOVA). Within each row, values with a common superscript letter were not significantly different.
Total glutathione and homocysteine concentrations were more than two times higher in vitamin B-6-deficient cells cultured in media containing 25 and 35 nmol/l pyridoxal, while cysteine concentrations also were higher (Table 3). Cysteinylglycine concentration, reflecting glutathione turnover, was the most sensitive to vitamin B-6 availability, and was an order of magnitude higher in the cells cultured in low vitamin B-6 conditions. The production of glucose was not affected by vitamin B-6 status in the HepG2 cells (data not shown). Targeted LC-MS-MS profiling of 1C-related metabolites conducted on HepG2 cells cultured in different vitamin B-6 conditions allowed the quantification of 12 metabolites. Statistical analysis revealed an overall effect of extracellular pyridoxal concentration on the intracellular concentration of this panel of metabolites. In this analysis, the pyridoxal concentration in the culture media was associated with changes in intracellular concentrations of betaine, glycine, cystathionine, creatine, and creatinine (Table 4).
Table 3.
Intracellular transsulfuration metabolite concentrations in HepG2 cells cultured in media containing different vitamin B-6 concentrations
| Media Vitamin B-6 |
||||
|---|---|---|---|---|
| nmol/106 cells | 25 | 35 | 65 | 2,015 |
| Homocysteine | 0.012 ± 0.002a | 0.009 ± 0.002b | 0.003 ± 0.001c | 0.005 ± 0.001b,c |
| Glutathione | 3.76 ± 0.57a | 3.83 ± 1.09a | 1.26 ± 0.35b | 1.60 ± 0.28b |
| Cysteine | 1.18 ± 0.15a,c | 1.30 ± 0.23a | 0.88 ± 0.08b | 0.91 ± 0.14b,c |
| Cysteinylglycine | 0.31 ± 0.10a | 0.32 ± 0.07a | 0.03 ± 0.01b | 0.04 ± 0.01b |
Values are means ± SD (nmol/l); n = 5. Significance indicated at P < 0.05. Data were analyzed by one-way ANOVA. Within each row, values with a common superscript letter were not significantly different.
Table 4.
Intracellular concentrations of 1C-related metabolites in HepG2 cells cultured in media containing different concentrations of vitamin B-6
| Media Vitamin B-6 |
|||||
|---|---|---|---|---|---|
| pmol/106 cells | 25a | 35a,b | 65b | 2015a,b | t-Test P* |
| Betaine | 12.9 ± 0.4b | 14.1 ± 0.6b | 4.7 ± 1.3a | 5.1 ± 3.3a | 0.0033‡ |
| Serine | 260 ± 50 | 290 ± 120 | 250 ± 30 | 230 ± 140 | 0.6869 |
| Glycine | 610 ± 130b | 650 ± 27b | 370 ± 6a | 360 ± 210a | 0.0054* |
| Threonine | 120 ± 40 | 140 ± 80 | 70 ± 20 | 70 ± 50 | 0.0317 |
| Choline | 100 ± 20a | 170 ± 80a,b | 240 ± 2b | 270 ± 190b | <0.0001* |
| Cystathionine | 80 ± 40b | 150 ± 90c | 60 ± 20b | 30 ± 20a | 0.1826 |
| Creatine | 155 ± 43a | 152 ± 56a | 680 ± 160b | 650 ± 46b | 0.0029* |
| Creatinine | 20 ± 10b | 20 ± 10b | 8.3 ± 3.0a | 6.5 ± 5.4a | 0.0097 |
| Methionine | 40 ± 10 | 40 ± 20 | 40 ± 5 | 40 ± 30 | 0.0.9446 |
| Arginine | 100 ± 20 | 100 ± 40 | 90 ± 10 | 90 ± 60 | 0.7753 |
| Asymmetric dimethyl arginine | 6.2 ± 1.4 | 7.5 ± 0.3 | 6.6 ± 1.0 | 6.8 ± 5.0 | 0.5320 |
| Symmetric dimethyl arginine | 0.3 ± 0.07 | 0.4 ± 0.2 | 0.4 ± 0.07 | 0.4 ± 0.03 | 0.2494 |
Values are means ± SD (nmol/l); n = 5.
1C, one-carbon.
Different superscripts on column headings indicate an overall difference between the 25 and 65 nmol/l groups, at the 0.0083 level after Bonferroni correction for the 6 pairwise group comparisons. Different subscripts on individual metabolite values indicate a difference in pairwise comparisons at the 0.0083 level.
P values for t-tests comparing the 25 and 65 nmol/l groups; significance was determined at the 0.0083 level.
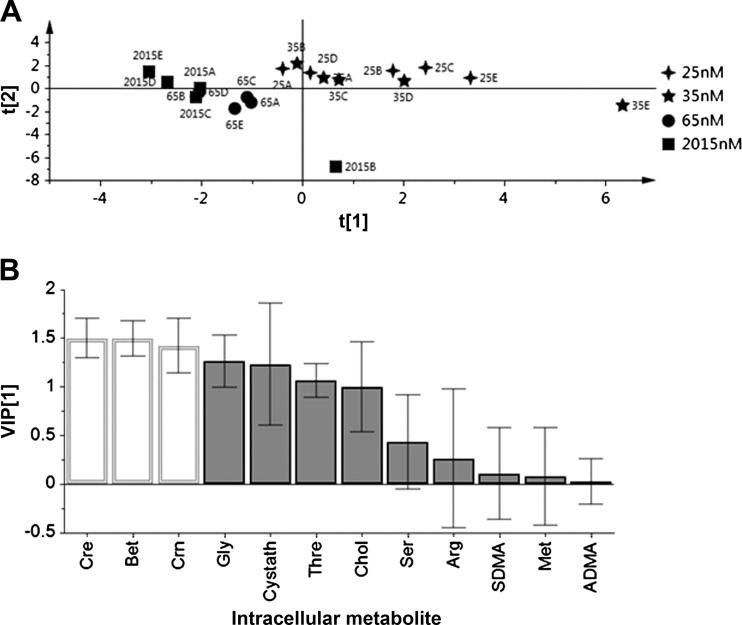
PLS-DA allowed an alternate evaluation of overall patterns of variables and allowed detection of the most important discriminating variables (58). PLS-DA on the set of 12 1C metabolites determined in LC-MS-MS analysis showed grouping on the score plot according to vitamin B-6 status, with one cluster comprising cells cultured at 25 and 35 nmol/l pyridoxal and the other cluster comprised of the higher (65 and 2,015 nmol/l) vitamin B-6 treatments (Fig. 1A). This analysis based solely on LC-MS-MS analysis of these 1C constituents shows clear overall compositional differences in the metabolic profile of the measured 1C metabolites in HepG2 cells cultured in media containing 25–35 nmol/l vitamin B-6 compared with 65–2,015 nmol/l. The VIP plot (Fig. 1B) identified creatine, betaine, and creatinine as significant discriminating variables at the 95% level, with glycine closely approaching significance.
Fig. 1.
Partial least squares discriminant analysis of HepG2 intracellular concentrations of 12 one-carbon (1C) metabolism-related compounds according to different vitamin B-6 concentrations in cell culture media (n = 5 per treatment). A: scores plot showing separation of the 2 deficient conditions (25 and 35 nmol/l vitamin B-6) from each other and from the 2 higher vitamin B-6 treatments; component 1 (t[1]) = 31.8% of variation, component 2 (t[2]) = 54.8% of variation. B: variable importance for the projection (VIP) plot. Metabolites for which the VIP criterion ± its 95% confidence interval exceeded a value of 1 constitute significant discriminating metabolites (open bars).
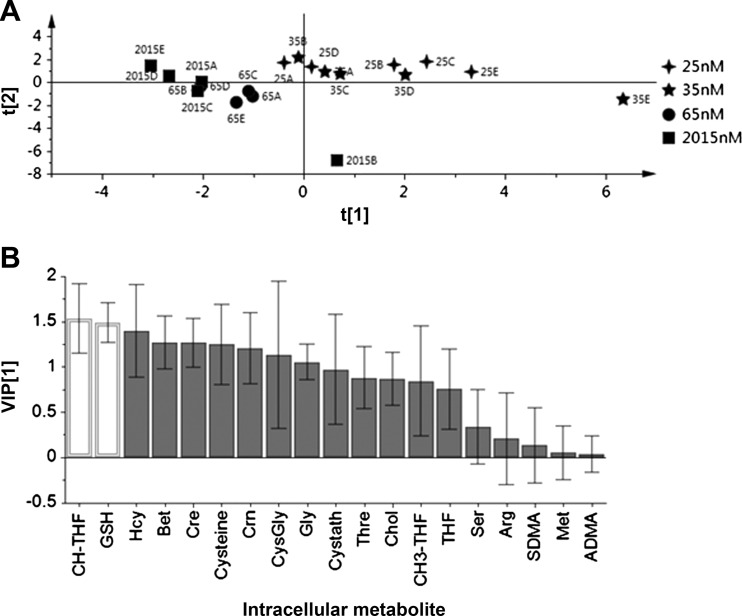
PLS-DA of the full datasets (including 1C metabolites, folates, and aminothiols) yielded a score plot that showed greater separation according to the level of vitamin B-6 than obtained for the analysis of 1C metabolites alone (Fig. 2A). In this analysis of the full dataset, the score plot showed separation between the clusters derived from cells cultured at the 25 and 35 nmol/l vitamin B-6 levels, and both of these were separated from the cluster from the 65 and 2,015 nmol/l levels. Glutathione and 5,10-methenyl-THF (comprising existing 5,10-methenyl-THF plus 10-formyl-THF) were significant discriminating variables in the VIP plot for this analysis, while betaine and creatine very closely approached significance (Fig. 2B).
Fig. 2.
Partial least squares discriminant analysis of HepG2 intracellular concentrations of combined dataset of 12 1C metabolism-related compounds plus folates and aminothiols (19 total variables) according to different vitamin B-6 concentrations in cell culture media (n = 5 per treatment). A: scores plot showing separation of deficient conditions (25–35 nmol/l vitamin B-6) from other treatments; component 1 (t[1]) = 47.0% of variation, component 2 (t[2]) = 28.9% of variation. B: VIP plot. Metabolites for which the VIP criterion ± its 95% confidence interval exceeded a value of 1 constitute significant discriminating metabolites (open bars).
Mathematical model predictions of 1C-related metabolites in vitamin B-6 deficiency.
The predicted intracellular concentrations of folate forms and 1C-related compounds in a vitamin B-6 deficiency are presented in Table 5. In a simulated moderate vitamin B-6 deficiency, a redistribution of folate forms was observed, with a 31% increase in THF and comparable but slightly lesser decreases of DHF,5,10-methylene-THF, 5,10-methenyl-THF, 10-formyl-THF, and 5-methyl-THF. Introducing oxidative stress into the model simulation did not change these trends in the distribution of folate species compared with vitamin B-6 deficiency alone in the model. The notable exception was 5-methyl-THF, for which oxidative stress caused a small increase (9%) compared with adequate vitamin B-6 status. The model assumes that no change occurs in total folate concentrations.
Table 5.
Concentration of 1C-related metabolites in vitamin B-6 adequate cells and cells in a vitamin B-6 deficiency with or without concurrent oxidative stress predicted using a mathematical model of 1C metabolism (31)
| Vitamin B-6 Deficiency | Vitamin B-6 Deficiency with Oxidative Stress | Vitamin B6 Adequate | |
|---|---|---|---|
| Folate forms, total | |||
| THF | 11.4 | 10.7 | 8.7 |
| 5,10-Methylene-THF | 0.6 | 0.6 | 0.8 |
| Sum, THF + 5,10-methylene-THF | 12 | 11.3 | 9.5 |
| 5,10-Methenyl-THF | 0.5 | 0.5 | 0.6 |
| 10-Formyl-THF | 4.8 | 4.7 | 6.5 |
| Sum, 10-formyl + 5,10-methenyl-THF | 5.3 | 5.2 | 7.1 |
| 5-Methyl-THF | 2.7 | 3.7 | 3.4 |
| DHF | 0.023 | 0.021 | 0.029 |
| 1C metabolites, total | |||
| Serine | 1,080 | 1,100 | 951 |
| Glycine | 2,620 | 2,720 | 1,200 |
| Sarcosine | 8.96 | 7.13 | 9.16 |
| Dimethylglycine | 0.69 | 0.55 | 0.71 |
| Cystathionine | 102 | 105 | 36.9 |
| Cysteine | 193 | 155 | 195 |
| Formate | 8.3 | 8.77 | 13.1 |
| Glutathione (reduced) | 6,550 | 7,330 | 6,591 |
| Glutathione (oxidized) | 61.1 | 108 | 61.3 |
| Glutathione (total) | 6,610 | 7,440 | 6,650 |
| Methionine | 50.9 | 48.4 | 41.2 |
| S-adenosylmethionine | 77.8 | 66.9 | 81.2 |
| S-adenosylhomocysteine | 22.4 | 17.8 | 19.1 |
| Homocysteine | 1.31 | 1.26 | 1.12 |
Values are in μmol/l. DHF, dihydrofolate.
Vitamin B-6 deficiency yielded large increases in glycine concentration, although there was little or no effect of oxidative stress on the glycine and serine concentrations. Major increases in cystathionine concentrations (175%) occurred in low vitamin B-6 status, and homocysteine concentrations increased modestly (17%), whereas methionine concentration was not greatly changed (3%). CBS activity increased in oxidative conditions, but this did not greatly affect predicted cystathionine concentrations (184% increase vs. 175% increase in vitamin B-6 deficiency alone). Oxidative stress had a clear effect on the concentrations of sarcosine and DMG, which decreased slightly in vitamin B-6 deficiency (∼3%) but decreased further when oxidative stress was simulated (−20 vs. vitamin B-6 deficiency alone). Total glutathione concentration was unchanged in vitamin B-6 deficiency alone but increased 12% when effects of oxidative stress were introduced in modeling.
DISCUSSION
The effect of vitamin B-6 restriction on fluxes through the 1C pathway and on circulating concentrations of 1C-associated metabolites has been examined (9–12, 21–23). Overall, these findings support the conclusion that the in vivo fluxes of many processes associated with 1C metabolism are subject to strong homeostasis and resist nutritional alterations such as the vitamin B-6 restriction examined here and in our human studies (9–12, 21–23). Coordinate allosteric regulation of methylation and transsulfuration processes in 1C metabolism (45) and long-range allosteric effects within methionine and folate cycles (32) strongly contribute to this homeostasis. However, subtle changes in enzyme activities can alter the concentrations of precursors and products, which may or may not cause significant changes of fluxes. The results of targeted metabolite profiling analysis (9) and metabolomic analysis (16) in recent human studies form the basis of our contention that the effects of vitamin B-6 inadequacy can affect metabolite concentrations in 1C metabolism directly or indirectly, whereas numerous secondary consequences can occur on many other aspects of metabolism. The previous results were confirmed and extended through the cell culture/metabolite profiling studies reported here. The use of the mathematical model that incorporates such homeostatic regulation and cellular compartmentalization complemented and extended the analytical data of this study.
We have reported previously that the increase in cystathionine concentration is due to partial loss of CSE activity arising from insufficiency of PLP (11, 25). To compensate at least partially for loss of CSE activity due to B-6 insufficiency, the increase in substrate concentration (i.e., cystathionine) would yield a nearly proportional increase in v/Vmax due to the high Km (25), This allows cysteine concentrations, cysteine flux, and net transsulfuration flux to be maintained in mild to moderate vitamin B-6 deficiency. We also propose that the more subtle increases in glycine and serine concentrations observed here and modeled previously (31) tend to exert similar kinetic effects on the glycine cleavage system, cytoplasmic and mitochondrial SHMT, thus tending to maintain fluxes of those reactions. Our use of the HepG2 model allowed an in-depth investigation into the metabolic effect of vitamin B-6 deficiency in a metabolically active cell type with precise nutritional control. The profiling of intracellular 1C metabolism reported here included the quantification of folate species, constituents of transsulfuration and glutathione metabolism, and a selection of other 1C-related metabolites.
The 1C pathways rely on THF as the major acceptor and carrier of single carbon units. The 1C unit acquired by THF to form 5,10-methylene-THF comes primarily from serine or glycine, via SHMT or the GCS, respectively. Both enzymes are PLP dependent and have been shown to be sensitive to vitamin B-6 restriction (12, 27, 43, 44). We hypothesized that inadequate vitamin B-6 status may affect the entry of 1C units to the 1C pathways and the distribution of THF species, and we observed marked differences among the various vitamin B-6 cultured conditions. Intracellular 5,10-methenyl-THF in culture conditions of 25 and 35 nmol/l total pyridoxal was decreased relative to cells cultured at higher vitamin B-6 concentrations, while THF was increased. 5-Methyl-THF remained relatively constant over a broad range of vitamin B-6 culture conditions, as did methionine. The higher THF concentrations at the lowest vitamin B-6 treatment and lower 5,10-methenyl-THF may suggest that flux through the methionine cycle is maintained by directing 1C units to remethylation processes rather than purine synthesis. However, such hypotheses require further testing using alternative methods of folate analysis and additional tracer studies. Studies of flux through the remethylation pathway in healthy human subjects have shown that dietary vitamin B-6 restriction does not alter rates of homocysteine remethylation (8, 12).
An unexpected finding was the response of betaine as a discriminating metabolite affected by vitamin B-6 status. The interchangeability of 5-methyl-THF and betaine as possible methyl donors for the remethylation of homocysteine tends to maintain the supply of 1C units for the formation of S-adenosylmethionine. In the kidney and liver, betaine donates a methyl group to homocysteine via the enzyme BHMT and is converted to DMG (52). DMG was below the LC-MS-MS detection limits in the HepG2 analyses; however, in humans plasma DMG decreased from vitamin B-6 restriction (9). Experimental assessment of the fluxes of betaine appearance and disappearance are needed for confirmation. Additionally, betaine was among the subset of metabolites responsible for the observed statistical effect of vitamin B-6 availability on the overall metabolite profile. Intracellular concentrations of choline were directly related to the vitamin B-6 in the culture media. Overall, low vitamin B-6 led to a redistribution of folate forms in HepG2 cells and lower choline and higher betaine concentrations. The relationship between folate and vitamin B-12 deficiencies and choline metabolism has been extensively examined (51), but the interaction with vitamin B-6 status is a novel observation of unknown mechanism.
Vitamin B-6 deficiency is associated with elevated oxidative stress (48), which may increase flux through the transsulfuration pathway (56) and, presumably, promote glutathione synthesis. The transsulfuration pathway relies on two PLP-dependent enzymes, CBS and CGL. The sensitivity of CGL to vitamin B-6 deficiency has been widely reported (11, 12, 23, 25, 29, 36, 50). In our HepG2 cells, intracellular cystathionine concentrations were lower in cells cultured in 25 nmol/l vitamin B-6 compared with 35 nmol/l (P < 0.05), which might indicate greater depression of CBS activity at the lower vitamin B-6 concentration. Intracellular cysteine concentrations were modestly higher in HepG2 cells cultured in low vitamin B-6 conditions (25 and 35 nmol/l PL) compared with cells cultured in adequate vitamin B-6 conditions. The greater concentrations of total glutathionine and its catabolic product cysteinylglycine were greater in cells cultured at 25 and 35 nmol/l compared with cells cultured at 65 and 2,015 nmol/l. Although the cause is unclear, the results are consistent with observations that rat liver glutathione increases dramatically in direct proportion to the extent of vitamin B-6 deficiency (25).
The non-monotone behavior of cystathionine demonstrates an important property of these metabolic systems. The behavior of one variable (intracellular cystathionine) may be a complicated function of another variable (external B-6 availability) because the system is highly nonlinear. This means that one must be careful in interpreting correlations because they may be context dependent, i.e., may depend on the current values of all the variables, not just the two being compared. One of the advantages of the full mathematical model, which includes the nonlinearities, is that it allows one to explore the reasons for particular non-monotone behavior if it is physiologically important.
The observed effect of vitamin B-6 supply on creatine and creatinine concentrations was unexpected. In our study, HepG2 concentrations of creatinine were more than doubled in cells cultured in media containing 25 or 35 nmol/l vitamin B-6 compared with 65 or 2,015 nmol/l vitamin B-6, whereas creatine concentrations were sharply decreased. In healthy men and women participating in our vitamin B-6 restriction studies, plasma creatine declined ∼40%, whereas creatinine did not change significantly (9). Protein intake differences might have a role in the decreased plasma concentrations of creatine and creatinine after a 28-day controlled dietary vitamin B-6 restriction, but that difference was nonexistent in our cell media and does not explain the effect observed in our cultured cells. The influence of vitamin B-6 availability on creatine metabolism may be a selective effect on creatine synthesis, a major methyl demand (5). Because creatinine is formed nonenzymatically from creatine (and creatine phosphate), one would expect general parallel concentrations if transport and turnover kinetics were similar for creatine and creatinine. Although ADMA and SDMA (which originate from the methylation of protein arginyl residues) did not respond to altered vitamin B-6 supply, these are sensitive biomarkers of methylation status.
The mathematical model predictions of the effect of vitamin B-6 deficiency on 1C metabolism provided qualitative support of most experimental observations in our study. The results extend those initially reported (31), which examined the influence of vitamin B-6 deficiency on the PLP-dependent enzymes in 1C metabolism. For this study, we were interested in the predictions the mathematical model provided on metabolite concentrations. The model's sensitivity of glycine and cystathionine, and to a lesser extent serine and homocysteine, to vitamin B-6 status had been previously reported (31). The mathematical model also predicted the redistribution of folate forms in lower vitamin B-6 availability, as observed. Similarly, the near constancy of methionine concentrations and an increase in homocysteine at low vitamin B-6 were predicted by the model. The mathematical model currently does not include prediction of betaine or choline concentrations.
Vitamin B-6 is essential to amino acid metabolism and to the proper functioning of the one-carbon cycle. This study aimed to improve our understanding of the functional interactions of vitamin B-6 and one-carbon metabolism and possibly identify changes indirectly affected by a decreased availability of vitamin B-6. HepG2 cells cultured in media containing 25 or 35 nmol/l vitamin B-6 showed clear differences in metabolic profiles compared with cells cultured in media containing 65 or 2,015 nmol/l vitamin B-6. For most compounds measured, the metabolic effect of a severe vitamin B-6 deficiency (25 nmol/l) was not different from that of marginally deficient cells (35 nmol/l). A closer examination of the interaction of vitamin B-6 status and the folate-independent homocysteine remethylation pathway is warranted. In addition, the effect of low vitamin B-6 availability on the processes involved in creatine and creatinine turnover should be further explored. Metabolic profiling such as reported here can aid in identifying markers indicative of a nutrient deficiency and offer insight into the mechanistic connections between vitamin B-6 and impaired health.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-072398 and by National Center for Research Resources (NCRR) CTSA Grant 1UL1 RR-029890.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.R.d.S., H.F.N., M.C.R., and J.F.G. conception and design of research; V.R.d.S., M.A.R., E.P.Q., B.N.D., T.J.G., H.F.N., and M.C.R. performed experiments; V.R.d.S., M.A.R., E.P.Q., B.N.D., T.J.G., Y.-Y.C., H.F.N., M.C.R., and J.F.G. analyzed data; V.R.d.S., Y.-Y.C., H.F.N., M.C.R., and J.F.G. interpreted results of experiments; V.R.d.S., Y.-Y.C., H.F.N., M.C.R., and J.F.G. drafted manuscript; V.R.d.S., M.A.R., E.P.Q., T.J.G., Y.-Y.C., H.F.N., M.C.R., and J.F.G. edited and revised manuscript; V.R.d.S., M.A.R., E.P.Q., B.N.D., T.J.G., Y.-Y.C., H.F.N., M.C.R., and J.F.G. approved final version of manuscript; J.F.G. prepared figures.
REFERENCES
- 1.Angel JF. Gluconeogenesis in meal-fed, vitamin B-6-deficient rats. J Nutr 110: 262–269, 1980 [DOI] [PubMed] [Google Scholar]
- 2.Bagley PJ, Selhub J. A common mutation in the methylenetetrahydrofolate reductase gene is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proc Nat Acad Sci USA 95: 13217–13220, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker M, Rayens W. Partial least squares for discrimination. J Chemometrics 17: 166–173 [Google Scholar]
- 4.Bradford MM. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 5.Brosnan JT, da Silva RP, Brosnan ME. The metabolic burden of creatine synthesis. Amino Acids 40: 1325–1331, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Cheng CH, Lin PT, Liaw YP, Ho CC, Tsai TP, Chou MC, Huang YC. Plasma pyridoxal 5′-phosphate and high-sensitivity C-reactive protein are independently associated with an increased risk of coronary artery disease. Nutrition 24: 239–244, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Chi YY, Gribbin M, Lamers Y, Gregory JF, Muller KE. Global hypothesis testing for high-dimensional repeated measures/outcomes. Stat Med 31: 724–742, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuskelly GJ, Stacpoole PW, Williamson J, Baumgartner TG, Gregory JF. Deficiencies of folate and vitamin B-6 exert distinct effects on homocysteine, serine, and methionine kinetics. Am J Physiol Endocrinol Metab 281: E1182–E1190, 2001 [DOI] [PubMed] [Google Scholar]
- 9.da Silva VR, Rios-Avila L, Lamers Y, Ralat MA, Midttun O, Quinlivan EP, Garrett TJ, Coats B, Shankar MN, Percival SS, Chi YY, Muller KE, Ueland PM, Stacpoole PW, Gregory JF., 3rd. Metabolite profile analysis reveals functional effects of 28-day vitamin B-6 restriction on one-carbon metabolism and tryptophan catabolic pathways in healthy men and women. J Nutr 143: 1719–1727, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis SR, Coats BS, Stacpoole PW, Gregory JF. Effects of dietary vitamin B-6 restriction on the kinetics of homocysteine and cysteine metabolism in humans. FASEB J 18: A174–A175, 2004 [Google Scholar]
- 11.Davis SR, Quinlivan EP, Stacpoole PW, Gregory JF., 3rd. Plasma glutathione and cystathionine concentrations are elevated but cysteine flux is unchanged by dietary vitamin B-6 restriction in young men and women. J Nutr 136: 373–378, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Davis SR, Scheer JB, Quinlivan EP, Coats BS, Stacpoole PW, Gregory JF., 3rd. Dietary vitamin B-6 restriction does not alter rates of homocysteine remethylation or synthesis in healthy young women and men. Am J Clin Nutr 81: 648–655, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Díaz de la Garza R, Quinlivan E, Klaus S, Basset G, Gregory JF, Hanson A. Folate biofortification in tomatoes by engineering the pteridine branch of folate synthesis. Proc Natl Acad Sci USA 101: 13720–13725, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Efron B, Gong G. A leisurely look at the bootstrap, the jackknife, and cross-validation. Am Statist 37: 36–48, 1983 [Google Scholar]
- 15.Finkelstein JD, Chalmers FT. Pyridoxine effects on cystathionine synthase in rat liver. J Nutr 100: 467–469, 1970 [DOI] [PubMed] [Google Scholar]
- 16.Gregory JF, 3rd, Park Y, Lamers Y, Bandyopadhyay N, Chi YY, Lee K, Kim S, da Silva V, Hove N, Ranka S, Kahveci T, Muller KE, Stevens RD, Newgard CB, Stacpoole PW, Jones DP. Metabolomic analysis reveals extended metabolic consequences of marginal vitamin B-6 deficiency in healthy human subjects. PLoS One 8: e63544, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory JF. Determination of pyridoxal 5′-phosphate as the semicarbazone derivative using high-performance liquid chromatography. Anal Biochem 102: 374–379, 1980 [DOI] [PubMed] [Google Scholar]
- 18.Guggenheim K, Diamant EJ. Carbohydrate metabolism in pyridoxine-deficient rats. J Biol Chem 224: 861–869, 1957 [PubMed] [Google Scholar]
- 19.Ink SL, Henderson LM. Vitamin B-6 metabolism. Annu Rev Nutr 4: 455–470, 1984 [DOI] [PubMed] [Google Scholar]
- 20.Inubushi T, Katsunuma N. Effects of vitamin B-6 on glucose utilization and serum insulin level in mice. Vitamins 76: 403–411, 2002 [Google Scholar]
- 21.Lamers Y, Coats B, Ralat M, Quinlivan EP, Stacpoole PW, Gregory JF., 3rd. Moderate vitamin B-6 restriction does not alter postprandial methionine cycle rates of remethylation, transmethylation, and total transsulfuration but increases the fractional synthesis rate of cystathionine in healthy young men and women. J Nutr 141: 835–842, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamers Y, O'Rourke B, Gilbert LR, Keeling C, Matthews DE, Stacpoole PW, Gregory JF., 3rd. Vitamin B-6 restriction tends to reduce the red blood cell glutathione synthesis rate without affecting red blood cell or plasma glutathione concentrations in healthy men and women. Am J Clin Nutr 90: 336–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamers Y, Williamson J, Ralat M, Quinlivan EP, Gilbert LR, Keeling C, Stevens RD, Newgard CB, Ueland PM, Meyer K, Fredriksen A, Stacpoole PW, Gregory JF., 3rd. Moderate dietary vitamin B-6 restriction raises plasma glycine and cystathionine concentrations while minimally affecting the rates of glycine turnover and glycine cleavage in healthy men and women. J Nutr 139: 452–460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leklem JE. Vitamin B-6: a status report. J Nutr 120, Suppl 11: 1503–1507, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Lima CP, Davis SR, Mackey AD, Scheer JB, Williamson J, Gregory JF., 3rd. Vitamin B-6 deficiency suppresses the hepatic transsulfuration pathway but increases glutathione concentration in rats fed AIN-76A or AIN-93G diets. J Nutr 136: 2141–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Mackey A, Davis S, Gregory J. Vitamin B-6. In: Modern Nutrition in Health and Disease (9th Ed.), edited by Shils M. Baltimore, MD: Wilkins and Wilkins, 2005, p. 452–461 [Google Scholar]
- 27.Martinez M, Cuskelly GJ, Williamson J, Toth JP, Gregory JF. Vitamin B-6 deficiency in rats reduces hepatic serine hydroxymethyltransferase and cystathionine beta-synthase activities and rates of in vivo protein turnover, homocysteine remethylation and transsulfuration. J Nutr 130: 1115–1123, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Merrill A, Burnham F. Vitamin B-6. In: Present Knowledge in Nutrition, edited by Brown M. Washington, DC: International Life Sciences Inst., 1990, p. 155–162 [Google Scholar]
- 29.Midttun O, Hustad S, Schneede J, Vollset SE, Ueland PM. Plasma vitamin B-6 forms and their relation to transsulfuration metabolites in a large, population-based study. Am J Clin Nutr 86: 131–138, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Morris MS, Picciano MF, Jacques PF, Selhub J. Plasma pyridoxal 5′-phosphate in the US population: the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr 87: 1446–1454, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Nijhout HF, Gregory JF, Fitzpatrick C, Cho E, Lamers KY, Ulrich CM, Reed MC. A mathematical model gives insights into the effects of vitamin B-6 deficiency on 1-carbon and glutathione Metabolism. J Nutr 139: 784–791, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nijhout HF, Reed MC, Anderson DF, Mattingly JC, James SJ, Ulrich CM. Long-range allosteric interactions between the folate and methionine cycles stabilize DNA methylation reaction rate. Epigenetics 1: 81–87, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Nijhout HF, Reed MC, Budu P, Ulrich CM. A mathematical model of the folate cycle: new insights into folate homeostasis. J Biol Chem 279: 55008–55016, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Nijhout HF, Reed MC, Lam SL, Shane B, Gregory JF, 3rd, Ulrich CM. In silico experimentation with a model of hepatic mitochondrial folate metabolism. Theor Biol Med Model 3: 40, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Page JH, Ma J, Chiuve SE, Stampfer MJ, Selhub J, Manson JE, Rimm EB. Plasma vitamin B-6 and risk of myocardial infarction in women. Circulation 120: 649–655, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park YK, Linkswiler H. Effect of vitamin B-6 depletion in adult man on the excretion of cystathionine and other methionine metabolites. J Nutr 100: 110–116, 1970 [DOI] [PubMed] [Google Scholar]
- 37.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem 45: 290–292, 1999 [PubMed] [Google Scholar]
- 38.Reed MC, Nijhout HF, Neuhouser ML, Gregory JF, 3rd, Shane B, James SJ, Boynton A, Ulrich CM. A mathematical model gives insights into nutritional and genetic aspects of folate-mediated one-carbon metabolism. J Nutr 136: 2653–2661, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Reed MC, Nijhout HF, Sparks R, Ulrich CM. A mathematical model of the methionine cycle. J Theor Biol 226: 33–43, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Reed MC, Thomas RL, Pavisic J, James SJ, Ulrich CM, Nijhout HF. A mathematical model of glutathione metabolism. Theor Biol Med Modelling 5: 8, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rimm EB, Willett WC, Hu FB, Sampson L, Colditz GA, Manson JE, Hennekens C, Stampfer MJ. Folate and vitamin B-6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA 279: 359–364, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Robinson K, Arheart K, Refsum H, Brattstrom L, Boers G, Ueland PM, Rubba P, Palma-Reis R, Meleady R, Daly L, Witteman J, Graham I, Grp EC. Low circulating folate and vitamin B-6 concentrations—risk factors for stroke, peripheral vascular disease, and coronary artery disease. Circulation 97: 437–443, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Runyan TJ, Gershoff SN. Glycine metabolism in vitamin B-6-deficient and deoxypyridoxine-treated rats. J Nutr 98: 113–118, 1969 [DOI] [PubMed] [Google Scholar]
- 44.Scheer JB, Mackey AD, Gregory JF. Activities of hepatic cytosolic and mitochondrial forms of serine hydroxymethyltransferase and hepatic glycine concentration are affected by vitamin B-6 intake in rats. J Nutr 135: 233–238, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Selhub J, Miller JW. The pathogenesis of homocysteinemia: interruption of the coordinate regulation by S-adenosylmethionine of the remethylation and transsulfuration of homocysteine. Am J Clin Nutr 55: 131–138, 1992 [DOI] [PubMed] [Google Scholar]
- 46.Smulders YM, Smith DEC, Kok RM, Teerlink T, Swinkels DW, Stehouwer CDA, Jakobs C. Cellular folate vitamer distribution during and after correction of vitamin B-12 deficiency: a case for the methylfolate trap. Br J Haematol 132: 623–629, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Spear N, Aust SD. Thiol-mediated NTA-Fe(III) reduction and lipid peroxidation. Arch Biochem Biophys 312: 198–202, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Taysi S. Oxidant/antioxidant status in liver tissue of vitamin B-6 deficient rats. Clin Nutr 24: 385–389, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Ubbink JB, Serfontein WJ, Devilliers LS. Stability of pyridoxal-5-phosphate semicarbazone—applications in plasma vitamin-B-6 analysis and population surveys of vitamin-B-6 nutritional-status. J Chromatogr 342: 277–284, 1985 [DOI] [PubMed] [Google Scholar]
- 50.Ubbink JB, vanderMerwe A, Delport R, Allen RH, Stabler SP, Riezler R, Vermaak WJH. The effect of a subnormal vitamin B-6 status on homocysteine metabolism. J Clin Invest 98: 177–184, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueland PM. Choline and betaine in health and disease. J Inherit Metab Dis 34: 3–15, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Ueland PM, Holm PlI, Hustad S. Betaine: a key modulator of one-carbon metabolism and homocysteine status. Clin Chem Lab Med 43: 1069–1075, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Ueland PM, Midttun O, Windelberg A, Svardal A, Skalevik R, Hustad S. Quantitative profiling of folate and one-carbon metabolism in large-scale epidemiological studies by mass spectrometry. Clin Chem Lab Med 45: 1737–1745, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Umetrics M. User Guide to SIMCA. Malmö, Sweden: MKS Umetrics, 2013, p. 645 [Google Scholar]
- 55.US Centers for Disease Control and Prevention. Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population. Atlanta, GA: National Center for Environmental Health, 2012, p. 1–484 [Google Scholar]
- 56.Vitvitsky V, Mosharov E, Tritt M, Ataullakhanov F, Banerjee R. Redox regulation of homocysteine-dependent glutathione synthesis. Redox Rep 8: 57–63, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Wiklund S, Johansson E, Sjostrom L, Mellerowicz EJ, Edlund U, Shockcor JP, Gottfries J, Moritz T, Trygg J. Visualization of GC/TOF-MS-based metabolomics data for identification of. Anal Chem 80: 115–122, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Wold S, Sjostrom M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemometr Intell Lab Syst 58: 109–130, 2001 [Google Scholar]
- 59.Zhao M, Ralat MA, da Silva VR, Garrett TJ, Melnyk S, James SJ, Gregory JF. Vitamin B-6 restriction impairs fatty acid synthesis in cultured human hepatoma (HepG2) cells. Am J Physiol Endocrinol Metab 304: E342–E351, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]