Abstract
Ammoniagenesis and gluconeogenesis are prominent metabolic features of the renal proximal convoluted tubule that contribute to maintenance of systemic acid-base homeostasis. Molecular analysis of the mechanisms that mediate the coordinate regulation of the two pathways required development of a cell line that recapitulates these features in vitro. By adapting porcine renal epithelial LLC-PK1 cells to essentially glucose-free medium, a gluconeogenic subline, termed LLC-PK1-FBPase+ cells, was isolated. LLC-PK1-FBPase+ cells grow in the absence of hexoses and pentoses and exhibit enhanced oxidative metabolism and increased levels of phosphate-dependent glutaminase. The cells also express significant levels of the key gluconeogenic enzymes, fructose-1,6-bisphosphatase (FBPase) and phosphoenolpyruvate carboxykinase (PEPCK). Thus the altered phenotype of LLC-PK1-FBPase+ cells is pleiotropic. Most importantly, when transferred to medium that mimics a pronounced metabolic acidosis (9 mM HCO3−, pH 6.9), the LLC-PK1-FBPase+ cells exhibit a gradual increase in NH4+ ion production, accompanied by increases in glutaminase and cytosolic PEPCK mRNA levels and proteins. Therefore, the LLC-PK1-FBPase+ cells retained in culture many of the metabolic pathways and pH-responsive adaptations characteristic of renal proximal tubules. The molecular mechanisms that mediate enhanced expression of the glutaminase and PEPCK in LLC-PK1-FBPase+ cells have been extensively reviewed. The present review describes novel properties of this unique cell line and summarizes the molecular mechanisms that have been defined more recently using LLC-PK1-FBPase+ cells to model the renal proximal tubule. It also identifies future studies that could be performed using these cells.
Keywords: proximal tubule, ammoniagenesis, gluconeogenesis, pH-responsive, acid-base balance
Relationship Between Renal Ammoniagenesis and Gluconeogenesis
the onset of metabolic acidosis causes a rapid and coordinate increase in the catabolism of plasma glutamine within the renal proximal convoluted tubule (10, 99). This adaptation results in the net production of two NH4+ and two HCO3− ions per glutamine (Fig. 1). The NH4+ ions are largely excreted in the urine, while the newly synthesized HCO3− ions, along with the bicarbonate reabsorbed from the tubular lumen, are translocated across the basolateral membrane and added to the renal venous blood. In rats and in humans, the remaining carbons derived from glutamine are primarily converted to glucose (19). Thus the well-coordinated increases in ammoniagenesis, bicarbonate synthesis, and gluconeogenesis result in an enhanced excretion of acid equivalents and the partial restoration of acid-base balance. In addition, during metabolic acidosis, the kidney becomes an important gluconeogenic organ that rivals the liver in importance for sustaining glucose homeostasis (14, 93).
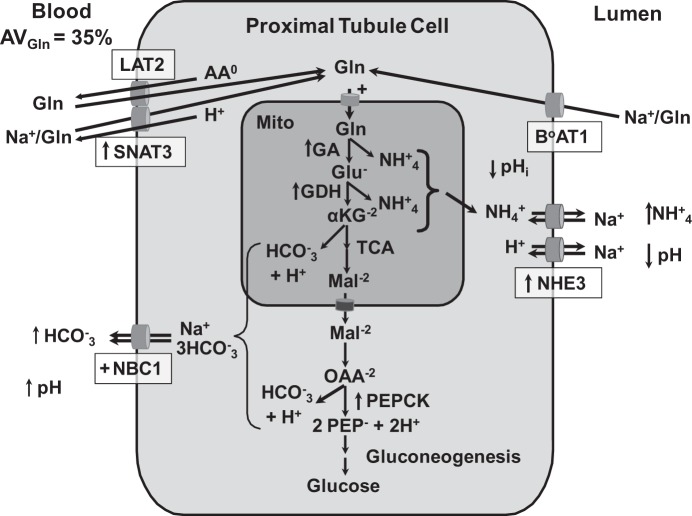
Fig. 1.
Renal proximal tubular catabolism of glutamine. During chronic acidosis, approximately one-third of the arterial glutamine is removed during a single pass through the kidney. The glutamine filtered by the glomeruli is nearly quantitatively extracted from the lumen of the proximal convoluted tubule by B0AT1, a Na+-dependent neutral amino acid cotransporter in the apical membrane. Uptake of glutamine through the basolateral membrane occurs by reversal of the neutral amino acid exchanger (LAT2) and/or through increased expression of the basolateral glutamine transporter (SNAT3). Increased renal catabolism of glutamine is facilitated by increased expression (arrows) of the genes that encode glutaminase (GA), glutamate dehydrogenase (GDH), phosphoenolpyruvate carboxykinase (PEPCK), the apical Na+/H+ exchanger (NHE3), and SNAT3. In addition, the activities of the mitochondrial glutamine transporter and basolateral Na+/3HCO3− are increased (+). Increased expression of NHE3 contributes to the transport of ammonium ions and the acidification of the luminal fluid. The combined increases in renal ammonium ion excretion and gluconeogenesis result in a net synthesis of HCO3− ions that are transported across the basolateral membrane by the Na+/3HCO3− cotransporter (NBC1). αKG, α-ketoglutarate; Mal, malate; OAA, oxaloacetate; PEP, phosphoenolpyruvate.
During metabolic acidosis, the arterial-venous difference for glutamine across the kidney increases to about one-third of the arterial glutamine (91). Approximately 20% of the plasma glutamine is filtered by the glomeruli and is nearly quantitatively reabsorbed from the lumen of the proximal convoluted tubule (88) by B0AT1 (SLC6A19), a Na+-dependent neutral amino acid cotransporter (81). Additional glutamine enters the proximal convoluted tubule through the basolateral membrane. The latter uptake is accomplished by reversal of LAT (SLC7A8)-4F2hc (SLC3A2), a heterodimeric neutral amino acid exchanger (61), and/or by the increased expression of SNAT3 (SLC38A3) that catalyzes the reversible Na+-dependent uptake of glutamine coupled to the efflux of a H+ ion (49, 90). The subsequent catabolism of glutamine requires its transport into the mitochondria, where it is initially deamidated by phosphate-dependent glutaminase and the resulting glutamate is oxidatively deaminated by glutamate dehydrogenase (GDH) (4, 12). The combined reactions convert glutamine to two NH4+ ions and an α-ketoglutarate. The α-ketoglutarate is further oxidized to phosphoenolpyruvate through reactions of the TCA cycle and the cytosolic phosphoenolpyruvate carboxykinase (PEPCK) (109). The combined reactions also generate two HCO3− ions and two H+ ions. However, the two H+ ions are consumed by conversion of phosphoenolpyruvate to glucose or its complete oxidation to CO2 (32, 89). Thus the overall pathway converts glutamine to two NH4+ ions, two HCO3− ions, and one-half molecule of glucose. Without the tight coupling of the ammoniagenic and gluconeogenic pathways, the H+ generated during the metabolism of α-ketoglutarate would combine with the HCO3− ions and form H2O and CO2. Thus the coordinate activation of the combined pathways is essential for the production of two HCO3− ions per glutamine and for the ability of the kidney to partially compensate for the systemic acidosis (10, 14).
During the onset of metabolic acidosis, the elevated renal extraction and catabolism of glutamine is accomplished by a rapid increase in the expression of the cytosolic PEPCK (5, 84) and by more gradual increases in the levels of glutaminase (13, 108) and GDH (107), which occur solely within the cells of the proximal convoluted tubule. The observed time course further supports the critical role of increased gluconeogenesis in the renal synthesis of NH4+ and HCO3− ions. The more rapid activation of gluconeogenesis may also contribute to the significant decreases in α-ketoglutarate and glutamate concentrations that occur in the renal cortex during the acute onset of acidosis (59). Such changes may contribute to the acute activation of glutamine catabolism by decreasing the product inhibition of the GDH and glutaminase reactions. The development of a cell line that models these metabolic features in vitro was essential to characterize the molecular mechanisms that regulate the expression of the GLS1 and PCK1 genes, which encode the kidney-type glutaminase and the cytosolic PEPCK, respectively, and the associated signal transduction pathways. It also provided a paradigm for understanding how renal proximal tubule cells sense changes in acid-base balance and mediate the cell-specific regulation of gene expression.
Parental Porcine Renal Epithelial LLC-PK1 Cells
The LLC-PK1 cell line (ATCC CL-101) was developed in 1958 from a mince of the whole kidney of a normal male Hampshire pig (Sus scrofa) (43). The cells retain several features of the proximal tubule (31). LLC-PK1 cells are epitheloid and nontumorigenic, form domes, and produce considerable amounts of plasminogen activator. The cells also express high activities of renal brush-border membrane marker enzymes, Na+-dependent apical transport of glucose, amino acids, and phosphate, and a basolateral, secretory transport of organic cations. Morphologically, the apical membrane domain is characterized by the presence of microvilli, which increase in number and size with time in culture (74). Cell-cell contacts are well developed. The junctional complexes are characterized by the occurrence of desmosomes and fairly shallow occluding junctions indicative of low electrical tightness of the monolayer epithelium (79, 80). When grown on microporous supports, LLC-PK1 epithelia generate a transepithelial electrical resistance of ∼150 Ω·cm2.
However, this permanent cell line has lost some features usually characteristic of proximal tubular cells. Transepithelial organic anion transport of p-aminohippurate could not be measured in LLC-PK1 epithelia. Also the hormone responsiveness of LLC-PK1 cells does not match the in vivo characteristics of the proximal tubule. LLC-PK1 cells do not respond to parathyroid hormone (PTH) (60) but express both hormone receptor subtypes for vasopressin (V1 and V2 receptor) (21) and responsiveness to calcitonin and oxytocin (37, 66). Metabolically, LLC-PK1 cells are unable to perform gluconeogenesis, i.e., to synthesize glucose from lactate or pyruvate, another prominent feature of cells of the proximal tubule. The inability of LLC-PK1 cells to perform gluconeogenesis is due to a lack of fructose-1,6-bisphosphatase (FBPase), a key enzyme in the gluconeogenic pathway (27). Low levels of the other gluconeogenic enzymes, PEPCK and glucose-6-phosphatase, have been described in LLC-PK1 cells (27, 63, 66) (Table 1). Moreover, LLC-PK1 cultures exhibit only a slight ammoniagenic response to medium that models a metabolic acidosis (25, 69, 82).
Table 1.
Expression of gluconeogenic enzyme activities in parental LLC-PK1 and gluconeogenic LLC-PK1-FBPase+ cells
| Cell Line | FBPase Activity | PEPCK Activity |
|---|---|---|
| LLC-PK1 + 5 mM glucose | undetectable | 2.3 ± 0.8 (3) |
| FBPase+ + 10 mM pyruvate | 4.23 ± 1.13 (5) | 22.6 ± 5.3 (5) |
| FBPase+ + 5 mM glucose | 4.95 ± 0.90 (3) | 38.3 ± 12.4 (7) |
Values are means ± SD with numbers of experiments in parentheses. Enzyme activities are expressed as nmoles of product produced·per minute per·milligram protein (mU/mg). FBPase, fructose-1,6-bisphosphatase; PEPCK, phosphoenolpyruvate carboxykinase. Data are taken from Gstraunthaler and Handler (21) and Holcomb et al. (39).
Isolation and Biochemical Characterization of LLC-PK1-FBPase+ Cells
In 1985, a gluconeogenic cell line was isolated from the LLC-PK1 renal epithelial cells using the selective pressure of glucose-free culture conditions (22). LLC-PK1 cells were initially adapted to a low (< 0.5 mM)-glucose medium over six to eight passages (∼10–12 wk). After this adaptation, confluent cultures were switched to essentially glucose-free culture medium (DMEM without glucose, 10% dialyzed fetal bovine serum) supplemented with 10 mM sodium pyruvate. Surviving cells were isolated and propagated in pyruvate-supplemented, glucose-free medium. This adaptation procedure has been reproduced in another laboratory (6).
Furthermore, glucose-free selection was successfully used to isolate a gluconeogenic strain of opossum kidney (OK) cells (29, 40). The OK cell line (ATCC CRL-1840) was initiated in 1975 from a female American opossum (Didelphys virginiana) (51). The cells retain a number of renal proximal tubule functions in culture. In particular, their PTH responsiveness made OK cells an attractive model for the study in vitro of the hormonal regulation of proximal tubular Na+-phosphate transport (67) and Na+/H+ exchange (68, 75). However, in contrast to LLC-PK1-FBPase+ cells, which use pyruvate as the preferred gluconeogenic substrate, the selected OK cells (OKGNG+) only grew when lactate was added to the glucose-free culture media (29) (see below).
Metabolic Features of LLC-PK1-FBPase+ Cells
The LLC-PK1 cells that survived after the glucose-free selection procedure were analyzed for FBPase activity. In contrast to the parental LLC-PK1 cells, the new strain expressed FBPase activity both under glucose-free culture conditions and when cells were maintained in glucose-containing media (Table 1). Therefore, the isolated gluconeogenic cell strain was designated LLC-PK1-FBPase+ (22). In addition, the PEPCK activity in LLC-PK1-FBPase+ cells is 10-fold higher than in the LLC-PK1 parental cells (40). PEPCK activity was increased further when the LLC-PK1-FBPase+ cells were cultured with 5 mM glucose (Table 1). The enzymatic activities are in good accord with the activities of the PEPCK promoter in these cells, determined by transient transfection with PEPCK promoter-CAT reporter constructs. The relative promoter activities were 1.4 ± 0.2 in LLC-PK1 vs. 9.0 ± 2.3 in LLC-PK1-FBPase+ cells (6).
Based upon the enzymatic analyses, it was concluded that the LLC-PK1-FBPase+ cells should be capable of conducting gluconeogenesis. However, net synthesis of glucose from precursor substrates could not be detected. The hexose phosphate intermediates synthesized via gluconeogenesis were probably utilized in the hexose monophosphate shunt for NADPH and ribonucleotide synthesis (23), preventing net release of free glucose. Therefore, metabolic flux through the gluconeogenic pathway was indirectly tested using 3-mercaptopicolinic acid (3-MPA), a specific inhibitor of PEPCK (22). When cells were incubated with substrates preceding the PEPCK step in gluconeogenesis (pyruvate, oxaloacetate, α-ketoglutarate), 3-MPA completely inhibited growth and caused lysis and death of cells. However, incubation in the presence of 3-MPA with substrates, which enter the gluconeogenic pathway above PEPCK (dihydroxyacetone, glycerol), had no effect on cell growth. Thus LLC-PK1-FBPase+ cells fully depend on metabolic flow of substrates through gluconeogenesis when incubated in the absence of sugars, and PEPCK is essential when the only substrates present are those that enter the gluconeogenic pathway below the PEPCK reaction.
Further evidence for a functional gluconeogenic pathway in LLC-PK1-FBPase+ cells was obtained in studies that model a metabolic acidosis in vitro (24, 25, 30). When LLC-PK1-FBPase+ cultures were transferred from a normal glucose-free medium (gluconeogenic conditions) to a glucose-free acidic medium (9 mM HCO3−, pH 6.9), the medium pH was not maintained but became more alkaline during a 24-h incubation period. However, when cultures were grown in the presence of 5 mM glucose (glycolytic conditions), the medium pH remained constant at acidic values. The observed alkaline shift in medium pH might be due to enhanced HCO3− production by the gluconeogenic LLC-PK1-FBPase+ cells in the acidic medium, indicative of metabolic flux from α-ketoglutarate to glucose. In renal proximal tubule cells, gluconeogenesis and ammoniagenesis are tightly coupled, especially during metabolic acidosis.
Comparison of Gluconeogenic LLC-PK1-FBPase+ and OKgng+ Cells
Another metabolic characteristic of LLC-PK1-FBPase+ cells is the fact that they are unable to appreciably utilize lactate, whether produced endogenously or exogenously added as the sodium salt (29, 40). During the isolation procedure, it was recognized that LLC-PK1-FBPase+ cells died when only 5 mM lactate was provided as the primary carbon source under glucose-free culture conditions (22). The differences in lactate and pyruvate utilization could be due to differences in the subcellular distribution of PEPCK in the two cell lines (29, 40). The PCK1 and PCK2 genes encode the cytoplasmic and mitochondrial isoforms of PEPCK, respectively. The two isoforms participate in separate pathways that differ in the reactions that are used to generate the cytosolic NADH needed to support gluconeogenesis (39). As a result, mitochondrial PEPCK is the preferred isoform to support gluconeogenesis from lactate, while the cytosolic isoform is required to convert pyruvate, glutamine, and TCA cycle intermediates to glucose. Following subcellular fractionation, the majority of PEPCK activity in LLC-PK1-FBPase+ cells was recovered in the cytosol, while only slight amounts of PEPCK activity were found in the mitochondrial fraction, indicating that the cells largely express the cytosolic isoform (40). By contrast, the OKgng+ cells express only the mitochondrial isoform of PEPCK (29), which explains their preference for lactate and their inability to grow in medium that contains only pyruvate.
The metabolic features of the two gluconeogenic cell strains were further delineated by determining the effects of adding (aminooxy)acetate (AOA), a transaminase inhibitor (40). AOA reduced lactate consumption by OKgng+ cells, whereas pyruvate consumption by LLC-PK1-FBPase+ cells was slightly stimulated. However, OKgng+ cells continued to grow on lactate in the presence of AOA. Since AOA blocks lactate conversion to glucose via the cytosolic isoform of PEPCK, it was concluded that gluconeogenesis in OKgng+ cells must proceed primarily through the mitochondrial PEPCK reaction. Various species exhibit differences in the expression of the two PEPCK isoforms and thus in the use of either “oxidized” (pyruvate, amino acids) or “reduced” (lactate) substrates for gluconeogenesis (39, 98). However, no information is available regarding the expression of PEPCK isoforms in renal proximal tubule of the marsupial from which OK cells were derived (20).
Pleiotropic Phenotype of LLC-PK1-FBPase+ Cells
Although LLC-PK1-FBPase+ cells were isolated by applying only a single selective pressure, namely, growth in glucose-free culture conditions (22), the resulting cells are not only gluconeogenic but they also exhibit other unique features that are characteristic of renal proximal tubular epithelial cells. In addition to gluconeogenic competence and pH responsiveness, LLC-PK1-FBPase+ cells exhibit apical proton secretion (24). To accomplish this, the cells express high levels of the mRNA that encodes NHE3, the apical Na+/H+ exchanger (1, 87). By contrast, NHE3 mRNA is barely detected in LLC-PK1 cells (Feifel E and Gstraunthaler G, unpublished observations). More recently, enzyme activity and mRNA expression of diaminoxidase, another proximal tubule-specific enzyme, was detected in LLC-PK1-FBPase+ cells (106). However, by contrast to the parental LLC-PK1 cells, LLC-PK1-FBPase+ cells do not express alkaline phosphatase activity (21).
When cultured on permeable supports, LLC-PK1-FBPase+ cells spontaneously generate an apical negative transepithelial potential difference (PDte) of about −1.5 mV, whereas LLC-PK1 epithelia produce an apical positive PDte. This results from different transepithelial ion permeabilities. Anion-to-cation permeability ratios were determined by dilution potentials after application of sodium or chloride gradients by replacing either sodium with N-methyl-d-glucamine, or chloride with gluconate, respectively. The results clearly showed that LLC-PK1-FBPase+ epithelia are cation selective, whereas the parental LLC-PK1 monolayers are essentially anion selective (44). The claudin family of tight junction proteins can selectively increase the paracellular permeability of cations over anions and vice versa (34, 42). In the mammalian nephron, claudin-2 is exclusively expressed in the proximal tubule and thin descending limb of the loop of Henle (50). Claudin-2 is predominantly cation pore forming. A screen for expression of various claudin proteins in the two cell lines revealed striking differences in the levels of claudin-2 in LLC-PK1-FBPase+ cells vs. LLC-PK1 cells (Feifel E and Gstraunthaler G, unpublished observations). In LLC-PK1-FBPase+ cells, strong expression of claudin-2 was observed, while in the parental LLC-PK1 cells virtually no claudin-2 could be detected. The functional significance of this difference was established by experiments, in which claudin-2 was overexpressed in LLC-PK1 cells (96). Normally, LLC-PK1 epithelia are relatively impermeable to Na+ ions. However, when claudin-2 was overexpressed, paracellular Na+ permeability increased significantly (2, 96). Karyotype analysis established that LLC-PK1-FBPase+ cells are descendants of LLC-PK1 wild-type cells, showing slight increases in tetraploidy (21). The pleiotropic phenotype of the gluconeogenic LLC-PK1-FBPase+ cells is stable, since long-term culture (>50 passages) even in medium containing 5 mM glucose does not revert the cells to a glycolytic metabolism. Thus the cumulative data, which are summarized in Table 2, demonstrate that the altered phenotype of LLC-PK1-FBPase+ cells is pleiotropic.
Table 2.
Pleiotropic phenotype of LLC-PK1-FBPase+ cells
| Growth under glucose-free culture conditions |
| Gluconeogenic capacity, metabolic flow through gluconeogenic path |
| Enhanced glutamine metabolism |
| Expression of gluconeogenic key enzymes fructose-1,6-bisphosphatase (FBPase) |
| and phosphoenolpyruvate carboxykinase (PEPCK) |
| Increased oxidative metabolism |
| Increased mitochondrial volume density |
| Increased phosphate-dependent glutaminase activity |
| Increased basal ammonia generation |
| Decreased expression of glycolytic enzymes |
| pH responsiveness |
| Adaptive responses to metabolic acidosis in vitro: |
| Adaptive increase in ammonium ion production |
| Adaptive increase in glutaminase enzyme activity and specific glutaminase |
| mRNA levels due to pH-mediated increased mRNA stability |
| Adaptive increase in PEPCK enzyme activity and mRNA levels of |
| cytosolic PEPCK isoform due to pH-induced enhanced rate of transcription |
| Apical proton secretion and |
| strong expression of apical membrane Na+/H+ exchanger NHE3 mRNA |
| Apical negative transepithelial potential difference (PDte), and high transepithelial cation permeability (PNa >> PCl) coincident with claudin-2 expression |
| Increased apical membrane surface density |
| Increased γ-glutamyltransferase activity |
| Expression of diaminoxidase enzyme activity |
| Lack of alkaline phosphatase activity |
pH Responsiveness of LLC-PK1-FBPase+ Cells
Besides the “induction” of gluconeogenesis, another prominent change observed in LLC-PK1-FBPase+ cells was a shift to enhanced oxidative metabolism. A detailed quantitative morphological (stereological) investigation revealed that LLC-PK1-FBPase+ cells exhibit an increased mitochondrial volume density (21), which may explain the increased rate of glutamine catabolism that is paralleled by increased basal activity of the mitochondrial phosphate-dependent glutaminase and the greater basal rates of NH4+ ion production (24). Most importantly, when LLC-PK1-FBPase+ cultures were adapted to acidic conditions, the cells respond with a pronounced increase in NH4+ ion production that correlates with a similar increase in assayable glutaminase activity (24). This is in contrast to the parental LLC-PK1 cells, which exhibit only a slight increase in glutamine metabolism after exposure to acidic medium (25, 30). The LLC-PK1 cells are primarily glycolytic and catabolize glutamine at much lower rates (28). Furthermore, LLC-PK1 cells lack any adaptive increase in glutaminase activity. PEPCK enzyme activity was also increased in acid-adapted LLC-PK1-FBPase+ cells (40). Thus the gluconeogenic LLC-PK1-FBPase+ strain is a pH-responsive permanent renal cell line, making it a valuable in vitro model system for studying the molecular mechanisms of renal acid-base adaptation (12, 14) (see below).
LLC-PK1-FBPase+ cells were also used to characterize how the apical catabolism of glutamine and the transport of glutamate affect proximal tubule function (103). Compared with the parental LLC-PK1 cells, LLC-PK1-FBPase+ cells exhibit an increased expression of γ-glutamyltransferase (γ-GT), which parallels the increase in apical membrane surface area (21, 26). Studies, using cultures grown on plastic dishes or on permeable filter supports (62, 64, 104), established that apical hydrolysis of glutamine by γ-GT and transport of the resulting glutamate form a functional unit that modulates intracellular catabolism of glutamine and paracellular ion permeability (101, 102). The higher γ-GT activity in LLC-PK1-FBPase+ cells resulted in higher intracellular glutamate, which alters glutamine metabolism by inhibiting phosphate-dependent glutaminase and promoting GDH and transaminase reactions (25, 100). However, overall glutamine flux is greater in the LLC-PK1-FBPase+ cells due to the higher glutaminase activity (24).
More recently, troglitazone, a high-affinity ligand for peroxisome proliferator-activated receptor-γ (PPAR-γ), was used to induce intracellular acidification in LLC-PK1-FBPase+ cells (71, 72, 95). PPAR-γ is a nuclear receptor that activates transcription of genes involved in energy homeostasis and differentiation. In LLC-PK1-FBPase+ cells, troglitazone inhibited the NHE, leading to a decrease in intracellular pH, which increased ammoniagenesis (71, 103). The primary effect was shown to be a specific activation of the ERK1/2 signaling pathway by troglitazone, resulting in mitochondrial depolarization, increased generation of acid equivalents, and inhibition of NHE-driven acid extrusion by ERK1/2 (71, 72). In this series of studies, the LLC-PK1-FBPase+ cells also served as a valuable model to delineate the signaling cascades associated with PPAR-γ-dependent and -independent effects on renal acid-base metabolism.
Molecular Biological Properties of LLC-PK1-FBPase+ Cells
The metabolic properties of LLC-PK1-FBPase+ cells were confirmed at the RNA and protein level. Using probes encoding the rat kidney cytosolic PCK1 and chicken liver mitochondrial PCK2 cDNAs, strong expression of cytosolic PEPCK mRNA was observed in LLC-PK1-FBPase+ cells, while the mitochondrial PEPCK mRNA was barely detectable (40). The unique gluconeogenic nature of the LLC-PK1-FBPase+ cells as assessed by expression of FBPase and cytosolic PEPCK mRNAs is documented in the Northern blot shown in Fig. 2. In a survey of continuous renal cell lines, only LLC-PK1-FBPase+ cells express mRNAs that encode FBPase and the cytosolic isoform of PEPCK. Total RNA isolated from the rat kidney cortex served as a control. Furthermore, when LLC-PK1-FBPase+ cells were incubated in an acidic medium for 18 h, only the cytosolic PEPCK mRNA levels increased, while the mitochondrial PEPCK mRNA levels remained unchanged (24, 40). In subsequent studies, it was shown that the adaptive increase in the cytosolic PEPCK mRNA is mediated by an increased rate of transcription (16, 41, 56), as observed in vivo in the rat kidney (45).
Fig. 2.

Expression of fructose-1,6-bisphosphatase (FBPase) and cytosolic PEPCK in various renal cell lines and in the rat kidney. Cultured cells were incubated in normal (pH 7.4) or acidic medium (pH 6.9) for 18 h. Total RNA samples (20 μg) were electrophoresed, blotted, and hybridized with cDNA probes to rat liver FBPase and rat renal cytosolic PEPCK. FBPase+, LLC-PK1-FBPase+ cells; OK, opossum kidney cells; MDCK, Madin-Darby canine kidney cells; LLC-PK1, LLC-PK1 pig kidney cells; WKPT, Wistar-Kyoto rat proximal tubular cells; HPT, primary cultures of human proximal tubular cells; CTX, rat kidney cortex; OM, outer medulla; IM, inner medulla.
The rat kidney expresses two glutaminase mRNAs, a 5.0-kb and a less abundant 3.4-kb mRNA, both of which encode the KGA variant of glutaminase. The two mRNAs, which are produced by use of alternative polyadenylation signals, are coordinately increased fivefold within 1 day after the onset of acidosis (12, 15, 76, 77). In contrast to PEPCK mRNA, this increase results primarily from an increase in the stability of the KGA mRNA (47, 52–54). Functional studies and RNA gel shift analyses of various deletion constructs were performed using LLC-PK1-FBPase+ cells. This analysis indicated that the pH-responsive stabilization is mediated by two 8-base adenylate-uridylate (AU) sequences in the portion of the 3′-untranslated region that is common to both rat KGA mRNAs. To validate these findings, a tetracycline-responsive promoter system was employed to conduct a pulse-chase analyses of the turnover of a chimeric β-globin-glutaminase (βG-GA) mRNA (85). βG-GA mRNA had a half-life of 2.9 h in cells maintained in normal medium, which was increased more than fivefold when the cells were transferred to acidic medium. When the AU elements within the βG-GA mRNA were mutated, the rate of degradation in normal medium was not affected, but the mRNA was no longer stabilized following transfer of the cells to acidic medium. By contrast, a construct containing only the AU elements (a 29-bp insert) exhibits both rapid degradation and pH-responsive stabilization (85). Thus the identified AU sequences contribute to the rapid turnover of KGA mRNA, and are both necessary and sufficient to mediate its pH-responsive stabilization.
By contrast, LLC-PK1-FBPase+ cells primarily express two glutaminase mRNAs that are 5.0 and 4.5 kb in size, which encode the KGA and GAC variants of the GLS1 gene, respectively (76, 77). The two mRNAs are produced by alternative splicing of a common transcript. They share a large stretch of identical coding sequence but encode different C-terminal domains and 3′-untranslated regions. The levels of the 4.5-kb GAC mRNA are increased threefold when LLC-PK1-FBPase+ cultures were incubated with acidic medium. This adaptation correlates with the increase in assayable glutaminase activity (see Fig. 5 in Ref. 24). The 5.0-kb KGA mRNA species is constitutively expressed and is not increased in response to treatment with acidic medium (24, 76, 77). The 3′-untranslated region of the 5.0-kb porcine and human KGA mRNAs lacks the eight-base AU sequences that function as the pH-response element in rat KGA mRNA, whereas a highly homologous sequence is present in the 3′-untranslated region of the porcine and human GAC mRNAs (38, 53, 54). The function of the homologous AU sequence was confirmed by determining the half-lives of the two mRNAs in control and acid-adapted LLC-PK1-FBPase+ cells. The apparent half-life of the 4.5-kb GAC mRNA was increased 2.3-fold when LLC-PK1-FBPase+ cells were transferred to acidic medium, while the half-life of the 5.0-kb KGA mRNA was unchanged (24, 76). Thus the pH-responsive stabilization of the 4.5-kb GAC mRNA effectively models the changes in the KGA mRNA that occur in rat renal proximal convoluted tubules (45, 46).
LLC-PK1-FBPase+ cells also recapitulate the pH-induced increase in GDH mRNA levels that occurs in rat kidney during the onset of metabolic acidosis (48, 86). GDH mRNA contains four 8-base AU-sequences in its 3′-untranslated region that are highly homologous to the pH-responsive elements identified in rat KGA mRNA. Rat renal cortex contains a cytosolic protein that binds with high affinity and specificity to the AU sequence and that may mediate the pH-responsive stabilization of KGA mRNA. LLC-PK1-FBPase+ cells also contain a protein that binds specifically to this sequence (52, 53). In subsequent studies, this protein was identified as ζ-crystallin, an NADPH:quinone reductase (92). Two of the AU sequences in GDH mRNA also bind purified recombinant ζ-crystallin with high affinity. However, overexpression or small interfering (si) RNA knockdown of ζ-crystallin had no effect on the basal half-life or the pH-responsive stabilization of the βG-GA mRNA (47). Thus, despite the fact that ζ-crystallin is the primary protein in extracts of rat kidney cortex (92) and of porcine LLC-PK1-FBPase+ proximal tubule cells that binds to the pH-response element, it appears that ζ-crystallin is not the sole or primary mediator of the rapid degradation or the selective stabilization of the GA and GDH mRNAs. However, both cells express high levels of multiple isoforms of AUF1, a destabilizing mRNA binding protein, which also binds to the pH-response element of the glutaminase mRNA with high affinity and specificity (85). Thus the binding of this protein may mediate the rapid turnover and the pH-responsive stabilization of the glutaminase mRNA during metabolic acidosis.
pH Signaling in LLC-PK1-FBPase+ Cells
Cytosolic PEPCK is encoded by the single-copy PCK1 gene whose transcription is regulated by the binding of multiple transcription factors to specific sites of the promoter. PEPCK transcription changes very rapidly in a tissue-specific manner in response to activation of various signaling pathways (7). Regulatory elements in the PEPCK promoter may best be understood as “units,” each of which is composed of distinct transcription factor-binding sites in the promoter that function together in response to hormonal or dietary stimuli or changes in acid-base status.
As detailed above, transfer of LLC-PK1-FBPase+ cells to acidic culture media (pH 6.9) caused a rapid induction of the PCK1 gene, resulting in concomitant increases in PEPCK mRNA, protein, and assayable activity (24, 65). Transcription run-off experiments indicated that increased expression of rat renal PEPCK during an acute onset of acidosis is regulated primarily at the level of transcription (45). An increase in PEPCK mRNA is observed in rat kidney within 1 h after the onset of acute acidosis. After 7 h, the increase reaches a maximum of sixfold, which is sustained as the rats become chronically acidotic (46). By contrast, confluent and well-differentiated cultures of LLC-PK1-FBPase+ cells exhibit a threefold increase in PEPCK mRNA when transferred to acidic medium for 16 h (41). The initial 490-bp of the PEPCK promoter contains at least 12 different protein-binding sites that mediate its tissue-specific expression during development and its response to various hormones (7, 73). Expression of various PEPCK promoter-chloramphenicol acetyltransferase (PCK-CAT) reporter constructs in LLC-PK1-FBPase+ cells indicated that the CRE-1, P2, and P3(II) elements are required for basal and cAMP-stimulated expression in kidney cells (57, 58). Similar experiments suggested that the pH-responsive stimulation of PEPCK expression is mediated primarily by the CRE-1 and P3(II) elements (41).
A decrease in intracellular pH must initiate a signal that mediates the increase in transcription of the PEPCK mRNA. Specific MAP kinase activators and inhibitors were used to determine the potential role of the ERK1/2, SAPK/JNK, and p38 MAPK pathways in the basal and pH-responsive expression of PEPCK mRNA (16). Anisomycin, a potent activator of p38 MAPK, increased PEPCK mRNA to levels comparable to those observed with acid stimulation. SB203580, a specific p38 MAPK inhibitor, inhibited both the acid- and anisomycin-mediated induction of PEPCK mRNA. In LLC-PK1-FBPase+ cells, only the SB-sensitive p38α isoform is strongly expressed. By contrast, the MEK1/2 inhibitors PD098059 and U0126 did not alter the basal or pH-responsive increase in PEPCK mRNA levels (16). In addition, JNK phosphorylation and JNK activity were decreased when cells were transferred to acidic medium. However, p38 MAPK is phosphorylated and thus activated when LLC-PK1-FBPase+ cells are transferred to acidic medium. One downstream substrate of p38 is ATF-2. ATF-2 is a basic-leucine zipper transcription factor that exhibits increased DNA binding and transcriptional activation following dual phosphorylation by p38 MAPK. ATF-2 was also phosphorylated when LLC-PK1-FBPase+ cells were transferred to acidic medium. This phosphorylation occurred with a slight lag compared with phosphorylation of p38 MAPK and was also blocked by addition of SB203580. The sequence of the CRE-1 element in the PCK1 promoter (TTACGTCA) is a perfect match to the consensus sequence for an ATF-2 binding site (8). Finally, gel-shift analysis (Fig. 9 in Ref. 16) confirmed that ATF-2 is contained in nuclear extracts of LLC-PK1-FBPase+ cells that binds to the CRE-1 element.
To further characterize the potential role of the p38 MAPK signaling pathway, a tetracycline-responsive promoter was used to express constitutively active (ca) and dominant negative (dn) forms of MKK3 and MKK6 in transfected LLC-PK1-FBPase+ cells (70). The two MKKs function upstream of p38 MAPK. Expression of caMKK6 produced an increase in PEPCK mRNA that closely mimicked the effect of treatment with acidic medium and also activated expression of a PEPCK-luciferase reporter construct. Expression of the dnMKKs blocked the phosphorylation of p38 MAPK and the induction of PEPCK mRNA. These experiments firmly established that the pH-responsive increase in PEPCK mRNA transcription is mediated by the p38 MAPK signaling pathway and involves the upstream activation of MKK3 and/or MKK6. Thus the SB-sensitive p38α/ATF-2 signaling pathway is the likely mediator of the pH-responsive induction of PEPCK mRNA transcription in renal LLC-PK1-FBPase+ cells.
Based upon the existing data, the following model (Fig. 3) was developed to explain the increase in transcription of PEPCK mRNA in the renal proximal convoluted tubule during metabolic acidosis (12, 93). During normal acid-base balance, HNF1 and possibly AP-1 transcription factors are bound to the P2 and P3(II) elements of the PEPCK promoter, respectively. A decrease in intracellular pH leads to activation of the α-isoform of p38 MAPK, which phosphorylates and activates ATF-2. The activated ATF-2 now binds to the CRE-1 element, where it may recruit additional transcription factors. The resulting complex recruits the appropriate coactivators and polymerase that activate transcription of the PCK1 gene (16). This proximal tubule-specific regulatory region has been recently defined as an acidosis regulatory unit (ARU) in the kidney (110).
Fig. 3.
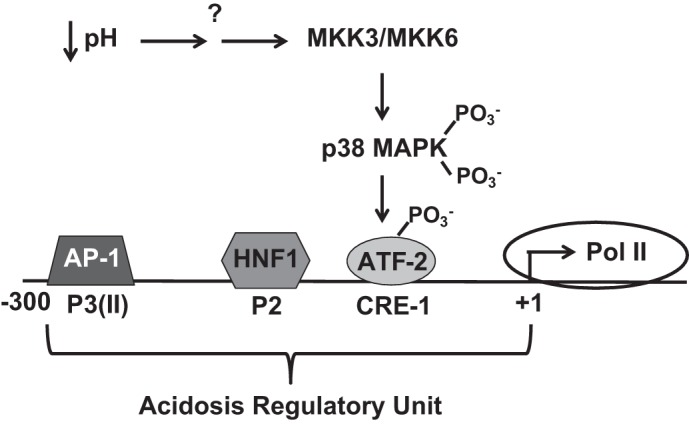
Mechanism of pH-responsive activation of PEPCK transcription in LLC-PK1-FBPase+ cells. A decrease in media pH and HCO3− leads to activation of MKK3 and MKK6, which phosphorylate and activate p38-MAPK. The activated p38-MAPK subsequently phosphorylates and activates a transcription factor (ATF-2), which binds to the CRE-1 element within the acidosis-regulatory unit of the PEPCK promoter. Activated transcription also requires the binding of hepatic nuclear factor 1 (HNF-1) to the P2 element and AP-1 to the P3(II) element.
pH-Responsive Stabilization of PEPCK mRNA
Transfer of LLC-PK1-FBPase+ cells to an acidic medium produced an increased expression of PEPCK mRNA that occurred following a pronounced delay and that reached a threefold maximum after 18 h. However, this increase in expression occurred with no evident change in the half-life of the PEPCK mRNA (41). The LLC-PK1-FBPase+ cells used in previous studies were a mixed population of cells. Thus clonal lines of LLC-PK1-FBPase+ cells were selected to identify a cell line that exhibits a greater fold-increase in cytosolic PEPCK mRNA and protein (65). When treated with acidic medium, the clonal LLC-PK1-FBPase+-9C cells exhibit a more rapid and more pronounced increase in PEPCK mRNA and protein that reached a four- to fivefold increase after 15 and 20 h, respectively. Measurement of the half-lives established that the endogenous PEPCK mRNA turns over rapidly (t1/2 = 3.2 h) in cells maintained in normal medium but is stabilized twofold when the cells are transferred to acidic medium. The pH-responsive stabilization was reproduced in a Tet-responsive expression of βG-PCK-1 mRNA, which contains the entire 3′-untranslated region of PEPCK mRNA. Therefore, the clonal line of LLC-PK1-FBPase+-9C cells effectively models both the transcriptional activation and the pH-responsive stabilization of renal PEPCK mRNA. The latter response was lost by mutation of a 17-base AU sequence in the PCK-6 segment of the 3′-untranslated region. This sequence contains a high degree of identity to the two eight-base AU sequences that mediate the pH-responsive stabilization of rat KGA mRNA (53). In addition, 11 of the 17 nucleotides (UUAAAUUAUUU) are fully conserved within the 3′-end of the 3′-untranslated region of all mammalian PCK1 genes that have been sequenced. The PCK-6 segment also binds AUF1 and is the primary element that mediates the rapid turnover of PEPCK mRNA (35). Therefore, this highly conserved sequence contributes to the rapid turnover and mediates the pH-responsive stabilization of the PEPCK mRNA.
Electrophoretic mobility shift assays established that purified recombinant HuR, a stabilizing mRNA binding protein, also binds with high affinity and specificity to two sites within the 3′-untranslated region of the PEPCK mRNA (65). These sites overlap with the AUF1 binding sites in the PCK-6 and PCK-7 segments (35). siRNA knockdown of HuR in LLC-PK1-FBPase+-9C cells caused a pronounced decrease in basal expression and reduced the pH-responsive increases in PEPCK mRNA and protein. Most importantly, the siRNA knockdown of HuR blocked the pH-responsive increase in the half-life of the endogenous PEPCK mRNA. However, treatment with acidic medium had no effect on the level or subcellular distribution of HuR or the various isoforms of AUF1 (65). Therefore, the pH-responsive stabilization of PEPCK mRNA may require covalent modifications of HuR and/or AUF1, which affects their binding to the elements that mediate the rapid turnover of PEPCK mRNA. The LLC-PK1-FBPase+-9C cells were also used to develop a recruitment assay using chimeric MS2 RNA binding proteins to establish that the concurrent binding of HuR and AUF1 is required to mediate the pH-responsive stabilization of PEPCK mRNA (33). Our current understanding of the mechanism by which the PEPCK mRNA is stabilized in response to acidosis is summarized in Fig. 4. The same mechanism is likely to regulate the expression of multiple proteins in the renal proximal convoluted tubule during the onset of acidosis (78). Given the rapid turnover and demonstrated stabilization, PEPCK mRNA expression in the clonal LLC-PK1-FBPase+-9C cells provides an excellent model system and effective paradigm to further characterize the molecular mechanism that mediates a major component of the renal response to acidosis.
Fig. 4.

Proposed mechanism for the pH-responsive stabilization of PEPCK mRNA. Interactions between the cap binding proteins (4E and 4G) and the polyA binding protein (PABP) cause mRNA to form a circular structure that enhances translation. Both a stabilizing mRNA binding protein (HuR) and destabilizing mRNA binding protein (AUF1) bind to the adenylate-uridylate (AU)-rich sequences within the 3′-untranslated region (UTR) of the PEPCK mRNA during normal acid-base balance. This complex recruits a deadenylase (Deaden) that shortens the polyA tail and causes dissociation of the polyA binding proteins (PABPs). The deadenylated mRNA is degraded in processing bodies by decapping and degradation from the 5′-end. In response to metabolic acidosis, the extent of phosphorylation of HuR is decreased while AUF1 is phosphorylated at additional sites. These changes lead to increased binding of HuR and a remodeling of the HuR/AUF1 complex that is bound to the 3′-UTR of PEPCK mRNA. The new complex is less effective at recruiting deadenylase and thereby promotes stabilization of the PEPCK mRNA.
Summary and Future Experiments
The renal proximal tubule rivals the liver in its ability to perform gluconeogenesis, i.e., the de novo synthesis of glucose from low-molecular-weight precursors (e.g., pyruvate, lactate, alanine, and glutamine). However, the mechanisms to induce and maintain the key gluconeogenic enzymes in cultured renal proximal tubular cells are largely unknown. Most established renal cell lines (Fig. 1), including RPTEC/TERT1 cells, a nontransformed, telomerase-immortalized human proximal tubular cell line (105), are not gluconeogenic. In addition, the expression of FBPase, PEPCK, and glucose-6-phosphatase is rapidly lost in primary cultures of human (9), rabbit (94), and rat (11) proximal tubular cells, while glycolytic enzyme activities (LDH, hexokinase) are dramatically increased. As a result, gluconeogenesis is not retained even in primary cultures of renal proximal tubules.
Porcine renal epithelial LLC-PK1 cells retain a number of proximal tubule-specific features (31), but they also do not express the key gluconeogenic enzymes and thus are unable to grow in the absence of glucose (27). LLC-PK1-FBPase+ cells were isolated by weaning LLC-PK1 cultures from glucose (22). The selective pressure of culturing cells under essentially glucose-free culture conditions resulted in the induction of FBPase and the increased expression of PEPCK (6). When the LLC-PK1-FBPase+ cells were recultured in the presence of glucose, the gluconeogenic phenotype was retained (Table 1). Thus the LLC-PK1-FBPase+ cells provide a unique model system for studding this important metabolic process and its role in acid-base balance.
The induction of gluconeogenic competence in LLC-PK1-FBPase+ cells may have occurred as an adaptation to the weaning process or, alternatively, the surviving cells were propagated from a preexisting, but minor pool of gluconeogenic cells in a heterogeneous population of LLC-PK1 cells. However, the selection procedure was successfully repeated using a clonal line of LLC-PK1 cells (22) and has been reproduced by others (6). Furthermore, a similar protocol was used to isolate a gluconeogenic line of OK cells (29). Thus it was concluded that LLC-PK1-FBPase+ arose from adaptation. With the present state of knowledge, we can only speculate on the underlying mechanism. As previously noted, gluconeogenesis is highly tissue specific, with maximal activity occurring in hepatocytes, renal proximal tubules, and adipocytes (7, 39). Of the genes encoding the rate-limiting gluconeogenic enzymes, the PCK1 gene has been most intensely studied (55). Expression of cytosolic PEPCK mRNA is primarily regulated by the binding of transcription factors to specific sites in the PCK1 promoter (7, 12, 110). It has been reported that binding of hepatic nuclear factor 1 (HNF-1) to the P2 element of the PCK1 promoter is essential for kidney-specific expression (6, 12, 73).
Glucose depletion (fasting) and glucose-free culture may have activated or increased expression of HNF-1 and/or additional transcription factors that account for the pleotropic phenotype of the LLC-PK1-FBase+ cells. Alternatively, chromatin remodeling or epigenetic modifications of nucleosomal histones may have produced the altered gene expression (3, 17). Changes in the methylation status of histone H3/H4 have been shown to modulate the occupancy of transcription factors on the PCK1 promoter and affect its transcription (110). Whether weaning of LLC-PK1 cells from glucose and subsequent growth in glucose-free culture conditions produced epigenetic effects (36), which resulted in increased expression of gluconeogenic enzymes in LLC-PK1-FBPase+ cells, remains to be determined.
The LLC-PK1-FBPase+ cells have been used to identify some of the RNA binding proteins that mediate the pH-responsive stabilization of glutaminase and PEPCK mRNAs (47). Recent experiments indicate that increased stability results from covalent modification and remodeling of the protein complex that associates with the functional AU element in the mRNAs (33). Future experiments using a photoactivatable crosslinker and RNA pull-downs may determine how changes in acid-base balance affect the composition of this complex and the mode and sites of covalent modifications that regulate its function. Microarray analysis of the immunoprecipitated RNAs would identify additional mRNAs that bind this complex and contribute to the renal response to acidosis or alkalosis. Recent proteomic studies have identified nearly 100 proteins that are significantly increased in the rat renal proximal tubule during acute and chronic acidosis (18, 83, 97). A similar analysis of the response of the transcriptome and proteome of LLC-PK1-FBPase+ cells would greatly expand our knowledge of how effectively this cell line models the characteristics and the responses of the renal proximal tubule. A detailed metabolomic analysis of these cells may also identify the mechanisms that mediate the rapid activation of the mitochondrial glutaminase and glutamine metabolism that occur during an acute onset of acidosis and that precede the more well-characterized increases in gene expression. Thus there are numerous ways in which this unique cell line can be studied to increase our understanding of the metabolism and function of the renal proximal tubule.
GRANTS
Research in the authors' laboratories was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK37124, DK43704, and DK75517 awarded to N. P. Curthoys and by Grants P11126, P12705, and P14981 from the Austrian Science Fund (FWF) awarded to G. Gstraunthaler.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: N.P.C. and G.G. provided conception and design of research; N.P.C. and G.G. performed experiments; N.P.C. and G.G. analyzed data; N.P.C. and G.G. interpreted results of experiments; N.P.C. and G.G. prepared figures; N.P.C. and G.G. drafted manuscript; N.P.C. and G.G. edited and revised manuscript; N.P.C. and G.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Joseph S. Handler, The Johns Hopkins University and National Institutes of Health, for continuous interest and support during the past 30 years since the isolation of the LLC-PK1-FBPase+ cell line in his laboratory. Thanks are also expressed to all of the researchers who used the LLC-PK1-FBPase+ line in studies. The enthusiasm and impact helped to establish LLC-PK1-FBPase+ cells as a valuable tool for renal biochemists and physiologists.
REFERENCES
- 1.Alexander RT, Dimke H, Cordat E. Proximal tubular NHEs: sodium, protons and calcium? Am J Physiol Renal Physiol 305: F229–F236, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol 295: F867–F876, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonasio R, Tu S, Reinberg D. Molecular signals of epigenetic states. Science 330: 612–616, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brosnan JT, Lowry M, Vinay P, Gougoux A, Halperin ML. Renal ammonia production—une vue canadienne. Can J Physiol Pharmacol 65: 489–498, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Burch HB, Narins RG, Chu C, Fagioli S, Choi S, McCarthy W, Lowry OH. Distribution along the rat nephron of three enzymes of gluconeogenesis in acidosis and starvation. Am J Physiol Renal Fluid Electrolyte Physiol 235: F246–F253, 1978 [DOI] [PubMed] [Google Scholar]
- 6.Cassuto H, Olswang Y, Livoff AF, Nechushtan H, Hanson RW, Reshef L. Involvement of HNF-1 in the regulation of phosphoenolpyruvate carboxykinase gene expression in the kidney. FEBS Lett 412: 597–602, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Chakravarty K, Cassuto H, Reshef L, Hanson RW. Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Crit Rev Biochem Mol Biol 40: 129–154, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Cheong J, Coligan JE, Shuman JD. Activating transcription factor-2 regulates phosphoenolpyruvate carboxykinase transcription through a stress-inducible mitogen-activated protein kinase pathway. J Biol Chem 273: 22714–22718, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Courjault-Gautier F, Chevalier J, Abbou CC, Chopin DK, Toutain HJ. Consecutive use of hormonally defined serum-free media to establish highly differentiated human renal proximal tubule cells in primary culture. J Am Soc Nephrol 5: 1949–1963, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Renal ammonium ion production and excretion. The Kidney: Physiology and Pathophysiology (5th ed.) edited by Alpern RJ, Moe OW, Caplan M. San Diego: Elsevier, 2012, chapt. 57, p. 1993–2018 [Google Scholar]
- 11.Curthoys NP, Bellemann P. Renal cortical cells in primary monolayer culture. Enzymatic changes and morphological observations. Exp Cell Res 121: 31–45, 1979 [DOI] [PubMed] [Google Scholar]
- 12.Curthoys NP, Gstraunthaler G. Mechanism of increased renal gene expression during metabolic acidosis. Am J Physiol Renal Physiol 281: F381–F390, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Curthoys NP, Lowry OH. The distribution of glutaminase isoenzymes in the various structures of the nephron in normal, acidotic, and alkalotic rat kidney. J Biol Chem 248: 162–168, 1973 [PubMed] [Google Scholar]
- 14.Curthoys NP, Moe OW. Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr 15: 133–159, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Feifel E, Obexer P, Andratsch M, Euler S, Taylor L, Tang A, Wei Y, Schramek H, Curthoys NP, Gstraunthaler G. p38 MAPK mediates acid-induced transcription of PEPCK in LLC-PK1-FBPase+ cells. Am J Physiol Renal Physiol 283: F678–F688, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet 13: 97–109, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Freund DM, Prenni JE, Curthoys NP. Response of the mitochondrial proteome of rat renal proximal convoluted tubules to chronic metabolic acidosis. Am J Physiol Renal Physiol 304: F145–F155, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 24: 382–391, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Graves JA, Westerman M. Marsupial genetics and genomics. Trends Genetics 18: 517–521, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Gstraunthaler G, Gersdorf E, Fischer WM, Joannidis M, Pfaller W. Morphological and biochemical changes of LLC-PK1 cells during adaptation to glucose-free culture conditions. Ren Physiol Biochem 13: 137–153, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Gstraunthaler G, Handler JS. Isolation, growth, and characterization of a gluconeogenic strain of renal cells. Am J Physiol Cell Physiol 252: C232–C238, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Gstraunthaler G, Harris HW, Jr, Handler JS. Precursors of ribose 5-phosphate suppress expression of glucose-regulated proteins in LLC-PK1 cells. Am J Physiol Cell Physiol 252: C239–C243, 1987 [DOI] [PubMed] [Google Scholar]
- 24.Gstraunthaler G, Holcomb T, Feifel E, Liu W, Spitaler N, Curthoys NP. Differential expression and acid-base regulation of glutaminase mRNAs in gluconeogenic LLC-PK1-FBPase+ cells. Am J Physiol Renal Physiol 278: F227–F237, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Gstraunthaler G, Landauer F, Pfaller W. Ammoniagenesis in LLC-PK1 cultures: role of transamination. Am J Physiol Cell Physiol 263: C47–C54, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Gstraunthaler G, Landauer F, Pfaller W. Ammoniagenesis in renal cell culture. Lack of extracellular ammoniagenesis at the apical surface of LLC-PK1 epithelia. Ren Physiol Biochem 16: 203–211, 1993 [PubMed] [Google Scholar]
- 27.Gstraunthaler G, Pfaller W, Kotanko P. Lack of fructose-1,6-bisphosphatase activity in LLC-PK1 cells. Am J Physiol Cell Physiol 248: C181–C183, 1985 [DOI] [PubMed] [Google Scholar]
- 28.Gstraunthaler G, Seppi T, Pfaller W. Impact of culture conditions, culture media volumes, and glucose content on metabolic properties of renal epithelial cell cultures. Are renal cells in tissue culture hypoxic? Cell Physiol Biochem 9: 150–172, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Gstraunthaler G, Thurner B, Weirich-Schwaiger H, Pfaller WA. A novel gluconeogenic strain of OK cells with metabolic properties different from gluconeogenic LLC-PK1 cells. Cell Physiol Biochem 3: 78–88, 1993 [Google Scholar]
- 30.Gstraunthaler GJ. Ammoniagenesis in renal cell culture. A comparative study on ammonia metabolism of renal epithelial cell lines. Contrib Nephrol 110: 88–97, 1994 [PubMed] [Google Scholar]
- 31.Gstraunthaler GJ. Epithelial cells in tissue culture. Ren Physiol Biochem 11: 1–42, 1988 [DOI] [PubMed] [Google Scholar]
- 32.Guder WG, Haussinger D, Gerok W. Renal and hepatic nitrogen metabolism in systemic acid base regulation. Zeitschrift Klin Chem Klin Biochem 25: 457–466, 1987 [DOI] [PubMed] [Google Scholar]
- 33.Gummadi L, Taylor L, Curthoys NP. Concurrent binding and modifications of AUF1 and HuR mediate the pH-responsive stabilization of phosphoenolpyruvate carboxykinase mRNA in kidney cells. Am J Physiol Renal Physiol 303: F1545–F1554, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev 93: 525–569, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hajarnis S, Schroeder JM, Curthoys NP. 3′-Untranslated region of phosphoenolpyruvate carboxykinase mRNA contains multiple instability elements that bind AUF1. J Biol Chem 280: 28272–28280, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Hall RK, Wang XL, George L, Koch SR, Granner DK. Insulin represses phosphoenolpyruvate carboxykinase gene transcription by causing the rapid disruption of an active transcription complex: a potential epigenetic effect. Mol Endocrinol 21: 550–563, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Handler JS, Perkins FM, Johnson JP. Studies of renal cell function using cell culture techniques. Am J Physiol Renal Fluid Electrolyte Physiol 238: F1–F9, 1980 [DOI] [PubMed] [Google Scholar]
- 38.Hansen WR, Barsic-Tress N, Taylor L, Curthoys NP. The 3′-nontranslated region of rat renal glutaminase mRNA contains a pH-responsive stability element. Am J Physiol Renal Fluid Electrolyte Physiol 271: F126–F131, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Hanson RW, Patel YM. Phosphoenolpyruvate carboxykinase (GTP): the gene and the enzyme. Adv Enzymol Relat Areas Mol Biol 69: 203–281, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Holcomb T, Curthoys NP, Gstraunthaler G. Subcellular localization of PEPCK and metabolism of gluconeogenic substrains of renal cell lines. Am J Physiol Cell Physiol 268: C449–C457, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Holcomb T, Liu W, Snyder R, Shapiro R, Curthoys NP. Promoter elements that mediate the pH response of PCK mRNA in LLC-PK1-F+ cells. Am J Physiol Renal Fluid Electrolyte Physiol 271: F340–F346, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Hou J, Rajagopal M, Yu AS. Claudins and the kidney. Annu Rev Physiol 75: 479–501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hull RN, Cherry WR, Weaver GW. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro 12: 670–677, 1976 [DOI] [PubMed] [Google Scholar]
- 44.Hutter E, Spitaler N, Feifel E, Gstraunthaler G. Phorbal esters differentially regulate protein kinase C and transepithelial ion permeabilities in wildtype and gluconeogeneic LLC-PK1 cells. Pflügers Arch 435: R110, 1998 [Google Scholar]
- 45.Hwang JJ, Curthoys NP. Effect of acute alterations in acid-base balance on rat renal glutaminase and phosphoenolpyruvate carboxykinase gene expression. J Biol Chem 266: 9392–9396, 1991 [PubMed] [Google Scholar]
- 46.Hwang JJ, Perera S, Shapiro RA, Curthoys NP. Mechanism of altered renal glutaminase gene expression in response to chronic acidosis. Biochemistry 30: 7522–7526, 1991 [DOI] [PubMed] [Google Scholar]
- 47.Ibrahim H, Lee YJ, Curthoys NP. Renal response to metabolic acidosis: role of mRNA stabilization. Kidney Int 73: 11–18, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaiser S, Hwang JJ, Smith H, Banner C, Welbourne TC, Curthoys NP. Effect of altered acid-base balance and of various agonists on levels of renal glutamate dehydrogenase mRNA. Am J Physiol Renal Fluid Electrolyte Physiol 262: F507–F512, 1992 [DOI] [PubMed] [Google Scholar]
- 49.Karinch AM, Lin CM, Wolfgang CL, Pan M, Souba WW. Regulation of expression of the SN1 transporter during renal adaptation to chronic metabolic acidosis in rats. Am J Physiol Renal Physiol 283: F1011–F1019, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13: 875–886, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Koyama H, Goodpasture C, Miller MM, Teplitz RL, Riggs AD. Establishment and characterization of a cell line from the American opossum (Didelphys virginiana). In Vitro 14: 239–246, 1978 [DOI] [PubMed] [Google Scholar]
- 52.Laterza OF, Curthoys NP. Effect of acidosis on the properties of the glutaminase mRNA pH-response element binding protein. J Am Soc Nephrol 11: 1583–1588, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Laterza OF, Curthoys NP. Specificity and functional analysis of the pH-responsive element within renal glutaminase mRNA. Am J Physiol Renal Physiol 278: F970–F977, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Laterza OF, Hansen WR, Taylor L, Curthoys NP. Identification of an mRNA-binding protein and the specific elements that may mediate the pH-responsive induction of renal glutaminase mRNA. J Biol Chem 272: 22481–22488, 1997 [DOI] [PubMed] [Google Scholar]
- 55.Lemaigre FP, Rousseau GG. Transcriptional control of genes that regulate glycolysis and gluconeogenesis in adult liver. Biochem J 303: 1–14, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu W, Feifel E, Holcomb T, Liu X, Spitaler N, Gstraunthaler G, Curthoys NP. PMA and staurosporine affect expression of the PCK gene in LLC-PK1-F+ cells. Am J Physiol Renal Physiol 275: F361–F369, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Liu X, Curthoys NP. cAMP activation of phosphoenolpyruvate carboxykinase transcription in renal LLC-PK1-F+ cells. Am J Physiol Renal Fluid Electrolyte Physiol 271: F347–F355, 1996 [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Wall QT, Taylor L, Curthoys NP. C/EBPβ contributes to cAMP-activated transcription of phosphoenolpyruvate carboxykinase in LLC-PK1-F+ cells. Am J Physiol Renal Physiol 281: F649–F657, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Lowry M, Ross BD. Activation of oxoglutarate dehydrogenase in the kidney in response to acute acidosis. Biochem J 190: 771–780, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malmstrom K, Murer H. Parathyroid hormone inhibits phosphate transport in OK cells but not in LLC-PK1 and JTC-12 P3 cells. Am J Physiol Cell Physiol 251: C23–C31, 1986 [DOI] [PubMed] [Google Scholar]
- 61.McGivan JD, Bungard CI. The transport of glutamine into mammalian cells. Front Biosci 12: 874–882, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Meade D, Chess C, Welbourne TC. Glutamate transport and cellular glutamine metabolism: regulation in LLC-PK1 vs. LLC-PK1-F+ cell lines. Am J Physiol Cell Physiol 274: C1616–C1624, 1998 [DOI] [PubMed] [Google Scholar]
- 63.Moran A, Turner RJ, Handler JS. Regulation of sodium-coupled glucose transport by glucose in a cultured epithelium. J Biol Chem 258: 15087–15090, 1983 [PubMed] [Google Scholar]
- 64.Mu X, Welbourne T. Response of LLC-PK1-F+ cells to metabolic acidosis. Am J Physiol Cell Physiol 270: C920–C925, 1996 [DOI] [PubMed] [Google Scholar]
- 65.Mufti J, Hajarnis S, Shepardson K, Gummadi L, Taylor L, Curthoys NP. Role of AUF1 and HuR in the pH-responsive stabilization of phosphoenolpyruvate carboxykinase mRNA in LLC-PK1-F+ cells. Am J Physiol Renal Physiol 301: F1066–F1077, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mullin JM, Cha CJ, Kleinzeller A. Metabolism of l-lactate by LLC-PK1 renal epithelia. Am J Physiol Cell Physiol 242: C41–C45, 1982 [DOI] [PubMed] [Google Scholar]
- 67.Murer H, Hernando N, Forster I, Biber J. Regulation of Na/Pi transporter in the proximal tubule. Annu Rev Physiol 65: 531–542, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Murer H, Krapf R, Helmle-Kolb C. Regulation of renal proximal tubular Na/H-exchange: a tissue culture approach. Kidney Int Suppl 44: S23–S31, 1994 [PubMed] [Google Scholar]
- 69.Nissim I, Korzets Z. Ammonia metabolism in cultured renal cells. Miner Electrolyte Metab 16: 270–276, 1990 [PubMed] [Google Scholar]
- 70.O'Hayre M, Taylor L, Andratsch M, Feifel E, Gstraunthaler G, Curthoys NP. Effects of constitutively active and dominant negative MAPK kinase (MKK) 3 and MKK6 on the pH-responsive increase in phosphoenolpyruvate carboxykinase mRNA. J Biol Chem 281: 2982–2988, 2006 [DOI] [PubMed] [Google Scholar]
- 71.Oliver R, 3rd, Friday E, Turturro F, Lacy A, Welbourne T. Troglitazone's rapid and sustained activation of ERK1/2 induces cellular acidosis in LLC-PK1-F+ cells: physiological responses. Am J Physiol Renal Physiol 288: F1257–F1266, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Oliver R, 3rd, Friday E, Turturro F, Welbourne T. Troglitazone induced cytosolic acidification via extracellular signal-response kinase activation and mitochondrial depolarization: complex I proton pumping regulates ammoniagenesis in proximal tubule-like LLC-PK1 cells. Cell Physiol Biochem 22: 475–486, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Patel YM, Yun JS, Liu J, McGrane MM, Hanson RW. An analysis of regulatory elements in the phosphoenolpyruvate carboxykinase (GTP) gene which are responsible for its tissue-specific expression and metabolic control in transgenic mice. J Biol Chem 269: 5619–5628, 1994 [PubMed] [Google Scholar]
- 74.Pfaller W, Gstraunthaler G, Loidl P. Morphology of the differentiation and maturation of LLC-PK1 epithelia. J Cell Physiol 142: 247–254, 1990 [DOI] [PubMed] [Google Scholar]
- 75.Pollock AS, Warnock DG, Strewler GJ. Parathyroid hormone inhibition of Na+-H+ antiporter activity in a cultured renal cell line. Am J Physiol Renal Fluid Electrolyte Physiol 250: F217–F225, 1986 [DOI] [PubMed] [Google Scholar]
- 76.Porter D, Hansen WR, Taylor L, Curthoys NP. Differential expression of multiple glutaminase mRNAs in LLC-PK1-F+ cells. Am J Physiol Renal Fluid Electrolyte Physiol 269: F363–F373, 1995 [DOI] [PubMed] [Google Scholar]
- 77.Porter LD, Ibrahim H, Taylor L, Curthoys NP. Complexity and species variation of the kidney-type glutaminase gene. Physiol Genomics 9: 157–166, 2002 [DOI] [PubMed] [Google Scholar]
- 78.Pullmann R, Jr, Rabb H. HuR and other turnover- and translation-regulatory RNA-binding proteins: implications for the kidney. Am J Physiol Renal Physiol 306: F569–F576, 2014 [DOI] [PubMed] [Google Scholar]
- 79.Rabito CA. Occluding junctions in a renal cell line (LLC-PK1) with characteristics of proximal tubular cells. Am J Physiol Renal Fluid Electrolyte Physiol 250: F734–F743, 1986 [DOI] [PubMed] [Google Scholar]
- 80.Rabito CA. Reassembly of the occluding junctions in a renal cell line with characteristics of proximal tubular cells. Am J Physiol Renal Fluid Electrolyte Physiol 251: F978–F987, 1986 [DOI] [PubMed] [Google Scholar]
- 81.Romeo E, Dave MH, Bacic D, Ristic Z, Camargo SM, Loffing J, Wagner CA, Verrey F. Luminal kidney and intestine SLC6 amino acid transporters of B0AT-cluster and their tissue distribution in Mus musculus. Am J Physiol Renal Physiol 290: F376–F383, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Sahai A, Cole LA, Tannen RL. Pathways and regulation of ammoniagenesis by the LLC-PK1 cells in culture. J Lab Clin Med 114: 285–293, 1989 [PubMed] [Google Scholar]
- 83.Schauer KL, Freund DM, Prenni JE, Curthoys NP. Proteomic profiling and pathway analysis of the response of rat renal proximal convoluted tubules to metabolic acidosis. Am J Physiol Renal Physiol 305: F628–F640, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schoolwerth AC, deBoer PA, Moorman AF, Lamers WH. Changes in mRNAs for enzymes of glutamine metabolism in kidney and liver during ammonium chloride acidosis. Am J Physiol Renal Fluid Electrolyte Physiol 267: F400–F406, 1994 [DOI] [PubMed] [Google Scholar]
- 85.Schroeder JM, Ibrahim H, Taylor L, Curthoys NP. Role of deadenylation and AUF1 binding in the pH-responsive stabilization of glutaminase mRNA. Am J Physiol Renal Physiol 290: F733–F740, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Schroeder JM, Liu W, Curthoys NP. pH-responsive stabilization of glutamate dehydrogenase mRNA in LLC-PK1-F+ cells. Am J Physiol Renal Physiol 285: F258–F265, 2003 [DOI] [PubMed] [Google Scholar]
- 87.Shugrue CA, Obermuller N, Bachmann S, Slayman CW, Reilly RF. Molecular cloning of NHE3 from LLC-PK1 cells and localization in pig kidney. J Am Soc Nephrol 10: 1649–1657, 1999 [DOI] [PubMed] [Google Scholar]
- 88.Silbernagl S. Tubular reabsorption of l-glutamine studied by free-flow micropuncture and microperfusion of rat kidney. Int J Biochem 12: 9–16, 1980 [DOI] [PubMed] [Google Scholar]
- 89.Silbernagl S, Scheller D. Formation and excretion of NH3↔NH4+. New aspects of an old problem. Klin Wochenschrift 64: 862–870, 1986 [DOI] [PubMed] [Google Scholar]
- 90.Solbu TT, Boulland JL, Zahid W, Lyamouri Bredahl MK, Amiry-Moghaddam M, Storm-Mathisen J, Roberg BA, Chaudhry FA. Induction and targeting of the glutamine transporter SN1 to the basolateral membranes of cortical kidney tubule cells during chronic metabolic acidosis suggest a role in pH regulation. J Am Soc Nephrol 16: 869–877, 2005 [DOI] [PubMed] [Google Scholar]
- 91.Squires EJ, Hall DE, Brosnan JT. Arteriovenous differences for amino acids and lactate across kidneys of normal and acidotic rats. Biochem J 160: 125–128, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang A, Curthoys NP. Identification of zeta-crystallin/NADPH:quinone reductase as a renal glutaminase mRNA pH response element-binding protein. J Biol Chem 276: 21375–21380, 2001 [DOI] [PubMed] [Google Scholar]
- 93.Taylor L, Curthoys NP. Glutamine metabolism: role in acid-base balance. Biochem Mol Biol Ed 32: 291–304, 2004 [DOI] [PubMed] [Google Scholar]
- 94.Toutain H, Vauclin-Jacques N, Fillastre JP, Morin JP. Biochemical, functional, and morphological characterization of a primary culture of rabbit proximal tubule cells. Exp Cell Res 194: 9–18, 1991 [DOI] [PubMed] [Google Scholar]
- 95.Turturro F, Oliver R, 3rd, Friday E, Nissim I, Welbourne T. Troglitazone and pioglitazone interactions via PPAR-gamma-independent and -dependent pathways in regulating physiological responses in renal tubule-derived cell lines. Am J Physiol Cell Physiol 292: C1137–C1146, 2007 [DOI] [PubMed] [Google Scholar]
- 96.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol 285: F1078–F1084, 2003 [DOI] [PubMed] [Google Scholar]
- 97.Walmsley SJ, Freund DM, Curthoys NP. Proteomic profiling of the effect of metabolic acidosis on the apical membrane of the proximal convoluted tubule. Am J Physiol Renal Physiol 302: F1465–F1477, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Watford M, Hod Y, Chiao YB, Utter MF, Hanson RW. The unique role of the kidney in gluconeogenesis in the chicken. The significance of a cytosolic form of phosphoenolpyruvate carboxykinase. J Biol Chem 256: 10023–10027, 1981 [PubMed] [Google Scholar]
- 99.Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol 69: 317–340, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Welbourne T, Routh R, Yudkoff M, Nissim I. The glutamine/glutamate couplet and cellular function. News Physiol Sci 16: 157–160, 2001 [DOI] [PubMed] [Google Scholar]
- 101.Welbourne TC, Chevalier D. Glutamate transport and not cellular content modulates paracellular permeability in LLC-PK1-F+ cells. Am J Physiol Endocrinol Metab 272: E367–E370, 1997 [DOI] [PubMed] [Google Scholar]
- 102.Welbourne TC, Chevalier D, Mu X. Glutamate transport modulation of paracellular permeability across LLC-PK1-F+ monolayers. Am J Physiol Endocrinol Metab 271: E889–E895, 1996 [DOI] [PubMed] [Google Scholar]
- 103.Welbourne TC, Matthews JC. Glutamate transport and renal function. Am J Physiol Renal Physiol 277: F501–F505, 1999 [DOI] [PubMed] [Google Scholar]
- 104.Welbourne TC, Mu X. Extracellular glutamate flux regulates intracellular glutaminase activity in LLC-PK1-F+ cells. Am J Physiol Cell Physiol 268: C1418–C1424, 1995 [DOI] [PubMed] [Google Scholar]
- 105.Wieser M, Stadler G, Jennings P, Streubel B, Pfaller W, Ambros P, Riedl C, Katinger H, Grillari J, Grillari-Voglauer R. hTERT alone immortalizes epithelial cells of renal proximal tubules without changing their functional characteristics. Am J Physiol Renal Physiol 295: F1365–F1375, 2008 [DOI] [PubMed] [Google Scholar]
- 106.Wilflingseder D, Gstraunthaler G, Schwelberger HG. Expression and secretion of diaminoxidase in the porcine kidney cell line LLC-PK1 (Abstract). Abstract Book of the 29th Annual Meeting of European Histamine Research Society, Rome Italy 2000. www.ehrs.org.uk [Google Scholar]
- 107.Wright PA, Knepper MA. Glutamate dehydrogenase activities in microdissected rat nephron segments: effects of acid-base loading. Am J Physiol Renal Fluid Electrolyte Physiol 259: F53–F59, 1990 [DOI] [PubMed] [Google Scholar]
- 108.Wright PA, Knepper MA. Phosphate-dependent glutaminase activity in rat renal cortical and medullary tubule segments. Am J Physiol Renal Fluid Electrolyte Physiol 259: F961–F970, 1990 [DOI] [PubMed] [Google Scholar]
- 109.Yang J, Kalhan SC, Hanson RW. What is the metabolic role of phosphoenolpyruvate carboxykinase? J Biol Chem 284: 27025–27029, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang J, Reshef L, Cassuto H, Aleman G, Hanson RW. Aspects of the control of phosphoenolpyruvate carboxykinase gene transcription. J Biol Chem 284: 27031–27035, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]