Abstract
This study is aimed at characterizing medullary interstitial progenitor cells and to examine their capacity to induce tubular epithelial cell migration and proliferation. We have isolated a progenitor cell side population from a primary medullary interstitial cell line. We show that the medullary progenitor cells (MPCs) express CD24, CD44, CXCR7, CXCR4, nestin, and PAX7. MPCs are CD34 negative, which indicates that they are not bone marrow-derived stem cells. MPCs survive >50 passages, and when grown in epithelial differentiation medium develop phenotypic characteristics of epithelial cells. Inner medulla collecting duct (IMCD3) cells treated with conditioned medium from MPCs show significantly accelerated cell proliferation and migration. Conditioned medium from PGE2-treated MPCs induce tubule formation in IMCD3 cells grown in 3D Matrigel. Moreover, most of the MPCs express the pericyte marker PDGFR-b. Our study shows that the medullary interstitium harbors a side population of progenitor cells that can differentiate to epithelial cells and can stimulate tubular epithelial cell migration and proliferation. The findings of this study suggest that medullary pericyte/progenitor cells may play a critical role in collecting duct cell injury repair.
Keywords: progenitor cell, pericyte, CXCR4, CD24
the histological lesion of acute tubular injury (ATI), which is characterized by cell necrosis or apoptosis and subsequent sloughing of injured tubular epithelial cells, is the hallmark finding on kidney biopsy in patients with intrinsic acute kidney injury (AKI). AKI is a common disease in the hospital setting and contributes to significant morbidity and mortality, especially in the elderly population. Understanding the molecular mechanisms of ATI and the subsequent epithelial cell regeneration process, offers possible new therapeutic avenues for the treatment of AKI. Several studies conducted in ischemia-reperfusion tubular injury models have shown that tubular epithelial cells become mitotically active and dedifferentiate before the regeneration phase of ATI (48, 51). This process induces the reactivation of some of the developmental genes involved in nephrogenesis (48). Tubular injury repair further involves epithelial cell proliferation and migration of adjacent surviving cells to repopulate denuded basement membranes (3, 45). A variety of growth factors, cytokines, and other inflammatory mediators regulate this repair process (4, 5, 43). However, the regulatory cells and mechanisms that guide and conduct this repair process are still enigmatic.
Several recent studies have elucidated the origin of the proliferating cells involved in tubular injury repair (19, 20). Using genetic lineage analysis, Humphreys et al. (20) showed that tubular cell repair after ischemic injury does not involve replacement of injured cell by extra tubular stem cells. Moreover, they showed in a different study that the proximal tubule does not contain intratubular progenitor cells (19). A cell-therapy model in rats has shown the beneficiary effect of injected bone marrow-derived mesenchymal stem cells (MSCs) on tubular epithelial injury repair following ischemia-reperfusion injury (46). The stimulatory effect on tubular cell regeneration may be mediated through paracrine factors expressed by the injected MSCs (47). However, Duffield et al. (14) studied kidney repair in chimeric mice and showed that only a minor percentage of tubule cells were bone marrow derived after ischemic injury and repair. These findings suggest that tubular injury repair is mediated through paracrine effects that stimulate proliferation of the residual tubular epithelium and that intrinsic progenitor cells might play an important role in this process.
Renal intrinsic progenitor cells have been identified in several compartments of the kidney, including glomeruli, peritubular capillaries, and the medullary interstitium (34, 38). Several recent studies show evidence that the renal medulla harbors intrinsic progenitor cells that may be involved in tubular injury regeneration (10, 21). The isolation of pluripotent renal progenitor cells from the adult rat kidney has been described (15). These progenitor cells expressed CD44, Pax2, and there is evidence that renal progenitor cells may contribute to repair of ATI (39). However, the mechanisms of medullary progenitor cell (MPC) activation and the cytokines expressed and released by these cells during repair of ATI are still poorly understood (53).
In this study, we have isolated an MPC side population from a primary medullary interstitial cell line. We show that the MPCs strongly express CD24, CD44, CXCR7, CXCR4, nestin, and PAX7. Importantly, MPCs were CD34 negative, indicating that they are not bone marrow-derived. MPCs survived >50 passages and when grown in epithelial differentiation medium, developed phenotypic characteristics of epithelial cells. Inner medulla collecting duct (IMCD3) cells, treated with conditioned medium from progenitor cells showed significantly accelerated wound healing. Moreover, conditioned medium from progenitor cells induced tubulogenesis in IMCD3 cells. Our study shows that the medullary interstitium harbors a side population of progenitor cells that can differentiate to epithelial cells and can improve tubular epithelial cell wound closure. Moreover, most of the MPCs express PDGFR-b, suggesting that they are pericytes, which induce proliferation and migration of tubular epithelial cells. Our study suggests that medullary pericyte/progenitor cells may play a critical role in re-epithelialization of medullary tubular epithelial wounds following injury.
MATERIALS AND METHODS
Cell culture and selective growth conditions.
Mouse medullary interstitial cell (MMIC) primary cultures were generated from C57bL/6 mice as described previously (29, 30). Briefly, kidneys were removed under aseptic conditions from five of six genetically identical mice, and the medulla was dissected, minced into 1-mm fragments, and passed through a small meshed sieve by hand into 5 ml of tissue culture media. The cell suspension was then centrifuged, and the pellet was suspended in KRB buffer containing antibiotics (30). The cell suspension was then injected under anesthesia at four to six different locations intraperitoneally into the sixth mouse. Three days after injection, small intraperitoneal nodules were harvested under aseptic conditions, minced, and plated out on 100-mm cell culture dishes in DMEM (GIBCO, Carlsbad, CA). The cells were stained with oil red O after three to four passages to confirm the characteristic cytoplasmic lipid vacuoles of medullary interstitial cells. All animal experiments were approved by the Institutional Animal Care and Use Committee at Yale University. For the selective growth experiments, cells were plated in either DMEM (medium A, Table 1), in DMEM/F12 (GIBCO; medium B, Table 1), or in the above buffers supplemented with knockout serum replacement (KSR; mediums C and D; see Table 1). All buffers were held serum free since FBS contains factors that may cause unwanted cell differentiation (17). Therefore, KSR was used as a defined serum-free replacement since it has been shown in several experiments to increase proliferation as well as improve chimerism in mouse embryonic stem cell lines (11). Some cells were also cultured in the above base buffers supplemented with N-2 (1×) supplement (mediums E and F; see Table 1, Invitrogen, Carlsbad, CA). The N-2 media supplement has been shown in several studies to enhance expansion and be necessary for the maintenance and differentiation of stem/progenitor cells (13, 49). Both hyperosmotic and hypoxic conditions were tested to mimic the cell growth conditions of the renal medulla (8, 31). Hypertonic culture conditions were achieved by addition of 12.9 μl of a 5 M NaCl stock solution and adjusting the final medium osmolality to 400 mosmol/kgH2O. Hypoxic conditions were established by culturing cells in the base buffers in 1% O2, 5% CO2, and 94% N2. All other cells were incubated in 95% air, 5% CO2 humidified atmosphere at 37°C.
Table 1.
Composition of selective growth media
| Medium | Composition |
|---|---|
| A | DMEM |
| B | DMEM/F12 |
| C | DMEM+0% KSR (KO DMEM) |
| D | DMEM/F12+10% KSR (KO DMEM/F12) |
| E | KO DMEM/N-2 |
| F | KO DMEM/F12/N2 |
KSR, knockout serum replacement.
For the cell differentiation experiments, the following growth media were used: MEM Alpha (12571-063; GIBCO) with 1× insulin-transferrin-selenium (ITS; catalog 41400-045; GIBCO), containing 2% FBS. This was mixed 1:1 with either endothelium growth medium (catalog no. CC4147; LONZA; 2% FBS; 0.04% hydrocortisone; 0.4% hFGF-B; 0.1% VEGF; 0.1% R3-IGF-1; 0.1% ascorbic acid; 0.1% hEGF, 0.1% GA) or with epithelial growth medium (RenaLife medium Complete Kit, LifeLine no. LL-0025).
Immunofluorescence staining.
Cells were seeded on chamber slides (BD Falcon, Bedford, MA) at a density of 4 × 103/well in their respective cultured medium for 3 days. Cells were fixed in 4% phosphate-buffered paraformaldehyde (pH 7.4). Triton X-100 (0.1%) was used to render the cells permeable followed by blocking solution with 5% normal goat serum and 1% BSA. Cells were then incubated overnight at 4°C with antibodies directed against the following stem cell markers: CXCR4 (ab1670; Abcam, Cambridge, MA), CXCR7 (ab72100; Abcam), CD24 (ab31622; Abcam), CD34 (ab8158; Abcam), rat-CD44 (no. 18849; Santa Cruz Biotechnology, Dallas, TX), rabbit CD-44 (7946; Santa Cruz Biotechnology), nestin (no. 556309, BD Bioscience, San Jose, CA), CD133 (no. 4310; Millipore), and Pax-7 (ab34360; Abcam). Cells were then incubated with fluorescein-conjugated secondary antibodies (Vector Laboratories, Burlingame, CA) for 2 h. Cells were stained for actin filaments with rhodamine phalloidin (Invitrogen, Eugene, OR) for 15–30 min. Cell nuclei were stained with 4,6-diamidino-2-phenylindole. Slides were examined using a Zeiss Axiophot inverted fluorescence microscope. Images were obtained and processed using MagnaFire 2.1C software (Olympus, Melville, NY) and ImageJ software (National Institutes of Health, Bethesda, MD). Cells with positive expression of respective stem cell marker were quantified in five randomly picked high-power (×200 magnification) fields using the point-counting method (35). Assays were performed at least in triplicate; error bars represent SE.
Cell proliferation assay.
Cell proliferation rates were assessed by the mitochondrial-dependent reduction of MTT (M-2128, Sigma, St. Louis, MO) to purple formazan. After overnight culture in a 96-well plates, kidney fibroblasts (NRK-49F; 1 × 104 cells/well) were treated either with the original growth medium or with 50% or 100% MMICs-derived, conditioned medium. After 48 h of treatment with conditioned medium, 20 ul of 5 mg/ml MTT solution (100 μg/well) were added for an additional 2 h. The medium was then removed and resuspended with formazan (MTT metabolic product) in 200 μl isopropanol. Specimens were placed on a shaking plate at medium speed for 5 min until all crystals were dissolved. Absorbance at 550 nm was measured using a microplate reader (model 3550; Bio-Rad, Hercules, CA).
Wound healing assay.
IMCD3 cells (CRL no. 2123, ATCC) were plated (80,000 cells/well) in a 24-well Image Lock Plate (Essen Bioscience, Ann Arbor, MI). After 24 h, 100% confluent cells were wounded using a semimanual wound-maker tool, and then cells were cultured in either DMEM or progenitor cell-conditioned medium (CM), which had been collected from selectively grown progenitor cell culture. Images and measurements of the wound were taken every hour for up to 18 h after wound initiation. Changes in wound width were calculated in imaging plates using the Incucyte system (Essen Instruments), applying around-the-clock kinetic imaging.
Tubulogenesis assay.
Assays to assess tubulogenesis were performed using IMCD3 cells in three-dimensional (3D) ECM gels as described previously (40). Collagen gels were composed of 0.1 mg/ml collagen I in DMEM containing 20 mM HEPES (pH 7.2). One hundred microliters of medium supplemented with 10% FCS or conditioned medium were added to the gels after they had solidified. For quantification of the branches, cells that formed branching structures (defined as >1 branch) were counted in five randomly picked high-power fields. Image analysis was performed using a Zeiss Axiophot fluorescence microscope (Jena, Germany). The degree of branching morphogenesis was quantified by using the number of end points as a correlative measure of the number of branching events. Assays were performed at a minimum in triplicate; error bars represent SE.
Western blot analysis.
IMCD3 cells were grown in six-well plates (10 × 104 cells/well) for 48 h and then treated with either original growth medium, original growth medium+50% CM or with 100% CM from MMICs for 48 h. Cell samples were then prepared using RIPA buffer (Boston Bioproducts, Worcester, MA) containing protease inhibitors, 1 mM NaF, and 2 mM Na3VO4. The protein concentration was determined using the bicinchoninic acid method (Pierce Pharmaceuticals, Rockford, IL). Ten micrograms of cell lysates in sample loading buffer were electrophoresed on 10% SDS-PAGE minigels under denaturing conditions. After being transferred onto a nitrocellulose membrane, the membranes were incubated with blocking solution for 2 h at room temperature and hybridized overnight with rabbit polyclonal anti-proliferating cell nuclear antigen (PCNA; SC-56; Santa Cruz Biotechnology) antibody or mouse monoclonal anti-β-actin antibody (A2228; Sigma-Aldrich) in blocking buffer at 4°C. The membrane was then incubated with horseradish peroxidase-conjugated secondary antibody diluted 1:3,500 for 60 min at room temperature. The detection of specific signals was performed using the enhanced chemiluminescence method (Amersham, Buckinghamshire, UK).
Statistical analysis.
All data are presented as means ± SE. Statistical analysis used Student's t-test for pairs with the following significance levels: *P < 0.05. All figures were generated from at least three repeated experiments with similar patterns.
RESULTS
MMIC characteristics.
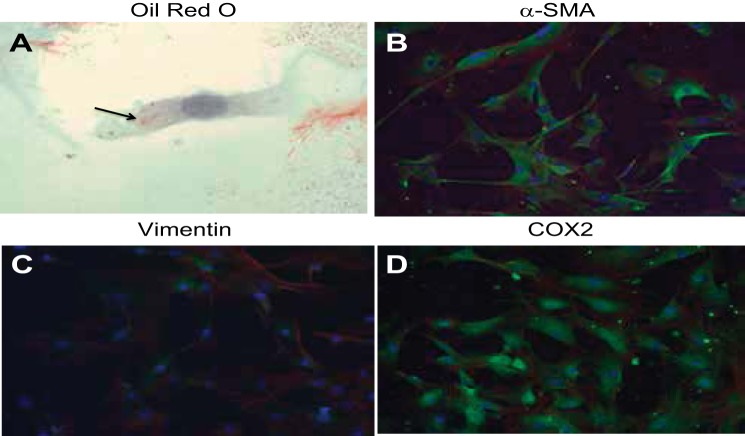
Primary cultures of MMICs showed a homogenous population of cells with >96% of cells displaying the classic medullary interstitial cell features of abundant oil red O-positive cytoplasmic lipid droplets and elongated cytoplasmic extensions (Figs. 1A and Fig. 2), which have been described previously (29). Additional immunofluorescence studies showed expression of α-smooth muscle actin (SMA; Fig. 1B), vimentin (Fig. 1C), and cyclooxygenase (COX) 2 (Fig. 1D) in nearly all of the medullary interstitial cells (MICs). Together, the above cytological features and the expressed protein patterns are consistent with a homogenous MIC population in our preparation (16, 36, 44).
Fig. 1.
Characterization of mouse medullary interstitial cell (MMIC) primary cultures. Cells show high prevalence of oil red O-positive cytoplasmic inclusions (arrow) and elongated cytoplasmic extensions (A). MMICs show strong expression of α-smooth muscle actin (SMA; B, green) and cyclooxygenase (COX) 2 (D, green). MMICs also show trace vimentin expression (C, green). Green, green fluorescent protein (GFP)-label (antibody); blue, 4,6-diamidino-2-phenylindole (DAPI; nuclear); red, phalloidin (actin).
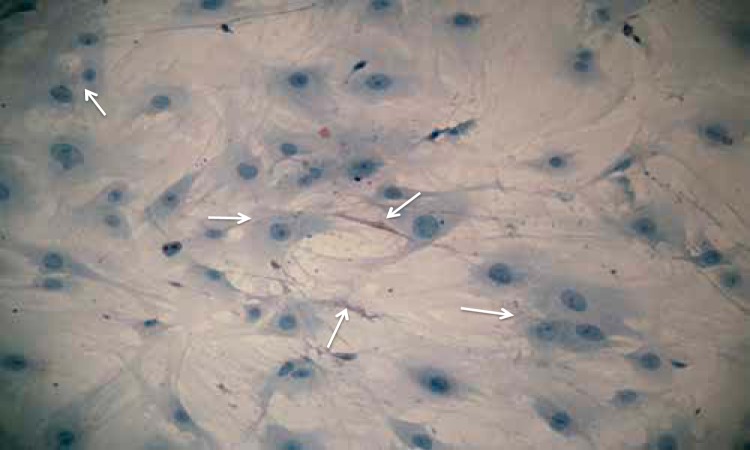
Fig. 2.
Medullary interstitial cell (MIC) primary culture. Note the cytoplasmic extensions and oil red O-positive cytoplasmic lipid droplets (white arrows; oil red O stain, ×200).
Selective growth of a kidney MPC population.
To determine the optimal culture conditions and selective growth conditions to isolate the progenitor cell subpopulation from the MIC population, primary cell cultures were plated in a 24-well plate and treated in the selective growth culture medium. Cells grown in DMEM (medium A, Table 1) typically maintained interstitial cell morphology throughout the period of observation and did not show high percentages of CXCR4+ cells after four passages (Table 2). Primary cells cultured in medium supplemented with 10% KSR (medium C or D, Table 1) showed a significant increase in the number of CXCR4+ cells with typical stem cell morphology compared with the cells cultured in medium A or B (Table 2, Fig. 3). A further increase in CXCR4+ progenitor cells was seen in cultures with selective media supplemented with 10% KSR plus N2 (mediums E and F, Table 1). Of the cells cultured in mediums E or F, 56 and 90%, respectively, showed stem cell morphology and CXCR4 positivity (Table 2). Moreover, these cells were cultured for >50 passages without significant loss of progenitor marker expression.
Table 2.
Selective growth of progenitor cells in different selective growth media
| Medium | % Viable Progenitor Cells (±SE) |
|---|---|
| A | 5 (0.1) |
| B | 25 (1.5) |
| C | 40 (2.0) |
| D | 74 (6.3) |
| E | 56 (5.2) |
| F | 90 (7.9) |
Fig. 3.

Selective growth of medullary progenitor cells (MPCs). DMEM/F12 supplemented with KSR or with KSR+N2 (see materials and methods) shows selective growth of progenitor cells characterized by globular cell shape and growth in spheroids (white arrow).
Characteristics of MPCs.
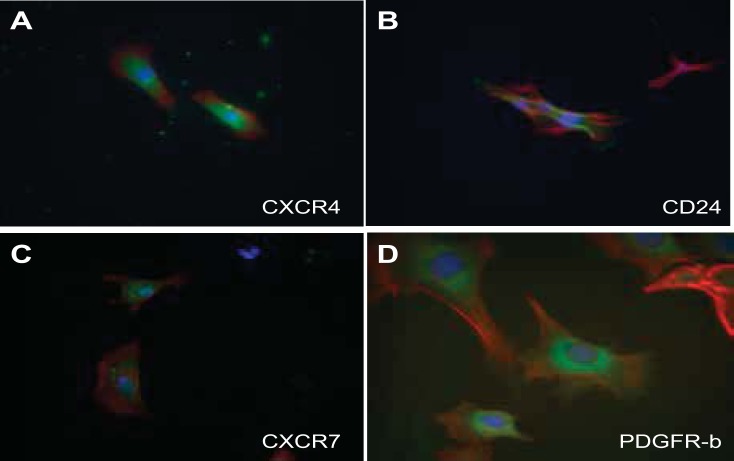
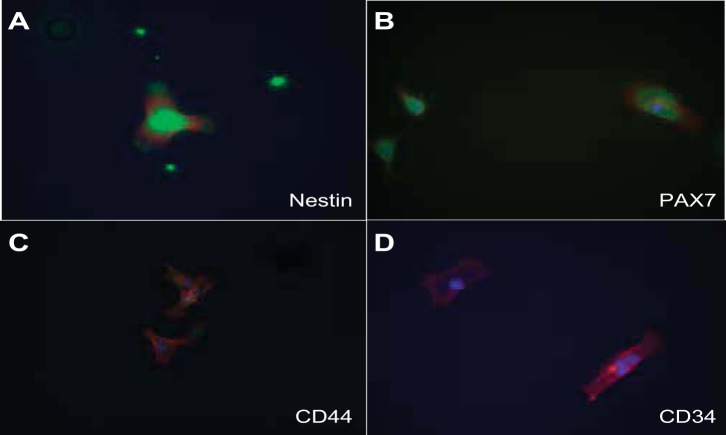
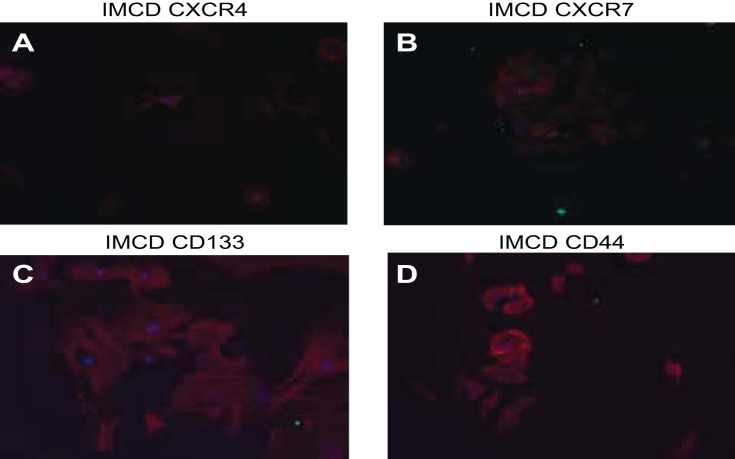
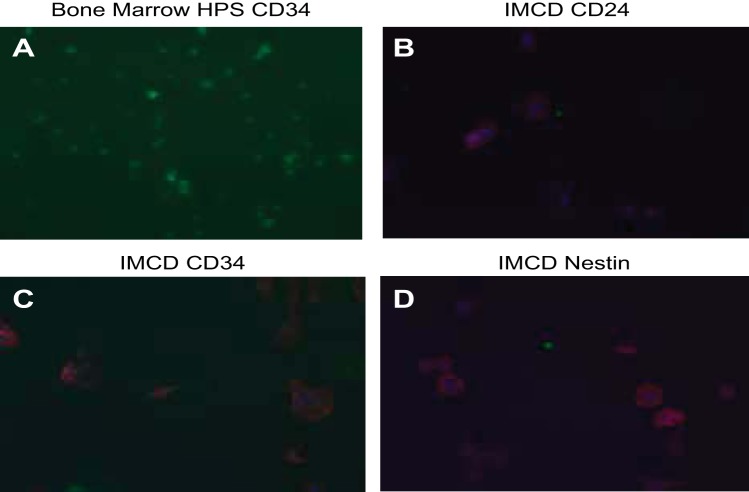
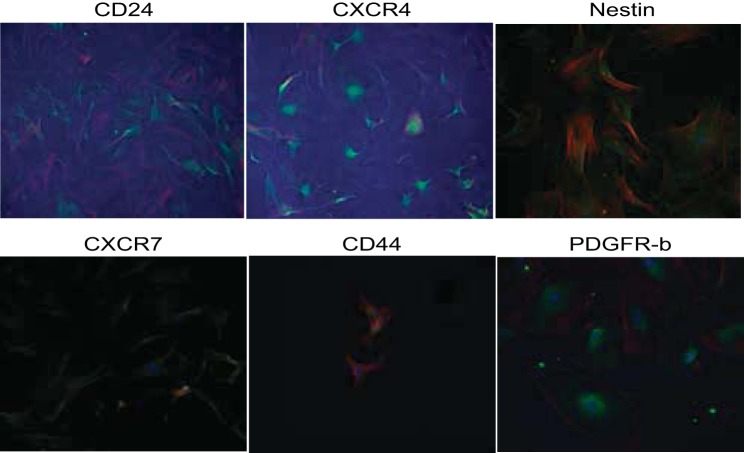
A selectively grown subpopulation of cells, which showed positive expression of six different progenitor cell markers, was generated from MMIC primary cultures. This subpopulation showed positive expression for CXCR4, CXCR7, CD24, nestin, Pax7, and CD44 (see Table 3, Figs. 4 and 5). IMCD control cells did not stain for the respective progenitor cell marker (Fig. 6 and 7). The percentage of cells expressing CXCR4 and CXCR7 increased from 11 and 10% in nonselective growth media to 90 and 56%, respectively, when cultured in DMEM supplemented with KSR and N2 (Table 3, Fig. 8). Of selectively grown progenitor cells, 69% showed positive expression for CD24, a marker associated with progenitor cell characteristics in the kidneys (1) (Figs. 4B and 8, Table 3). Moreover, 43% of selectively grown progenitor cells showed positive nestin expression, a known marker in kidney stem cells (Figs. 5A and Fig. 8, Table 3) (52). Pax7, a skeletal muscle stem cell marker (6), was positively expressed in 77% of selectively grown progenitor cells (Fig. 5B, Table 3). Interestingly, none of the selectively grown progenitor cells showed CD34 expression (Fig. 5D), which is highly expressed on hematopoietic pluripotent stem cells (26) (Fig. 4). Furthermore, selectively grown progenitor cells showed expression of the pericyte marker PDGFR-b (Figs. 4D and 8).
Table 3.
Percentage of cells with positive expression of stem cell markers
| Stem Cell Marker | % Positive after Selection in Medium F | SE |
|---|---|---|
| CD24 | 69 | 5.4 |
| CD34 | 0 | 0 |
| CXCR4 | 90 | 7.9 |
| CXCR7 | 56 | 6.9 |
| Nestin | 43 | 6.4 |
| Pax7 | 77 | 7.0 |
| CD44 (rat) | 22 | 1.2 |
| CD44 (rabbit) | 54 | 2.5 |
Fig. 4.
Characterization of MPCs by immunofluorescence staining for stem cell marker expression. A: progenitor cells show strong cytoplasm expression of CXCR4 in a speckled pattern. B: progenitor cells strongly expressed CD24 along the cell membrane. C: CXCR7 was weakly expressed in a speckled cytoplasmic pattern. D: 85% of progenitor cells express the pericyte marker PDGFR-b. Green, FITC-conjugated antibodies; red, phalloidin (actin); Blue, DAPI (nuclear).
Fig. 5.
Characterization of stem cell marker expression in MPCs by immunofluorescence stain: A: progenitor cells show strong cytoplasm expression of nestin in a speckled pattern. B: progenitor cells strongly expressed PAX7 in a speckled pattern. C: CD44 was weakly expressed in a perinuclear pattern. D: there was no CD34 expression in progenitor cells. Green, FITC-conjugated antibodies; red, phalloidin (actin); blue, DAPI (nuclear).
Fig. 6.
Negative controls for different progenitor cell markers. A–D: inner medullary collecting duct (IMCD) cells stain negative for the medullary progenitor markers CXCR4, CXCR7, CD133, and CD44.
Fig. 7.
Positive and negative controls for different progenitor cell markers. A: bone marrow hematopoietic cells (HPS) stain positive for CD34. B–D: IMCD cells stain negative for the medullary progenitor markers CD24, CD34, and nestin.
Fig. 8.
Low-power view of selected progenitor cell marker in MPCs.
Effect of MPC media on IMCD cell viability and proliferation.
IMCD cells (IMCD3) were exposed to CM from progenitor cell cultures for 48 h. Cell survival was markedly increased in CM-treated IMCD3 cells compared with control cells grown in DMEM (Fig. 9A). Western blot analysis shows that there was increased PCNA abundance with increasing concentrations of CM. There was a visible increase in the expression of PCNA in cells treated with 100% CM compared with DMEM control (Fig. 9B). These results indicate that MPCs release soluble factors that enhance cell proliferation and survival.
Fig. 9.
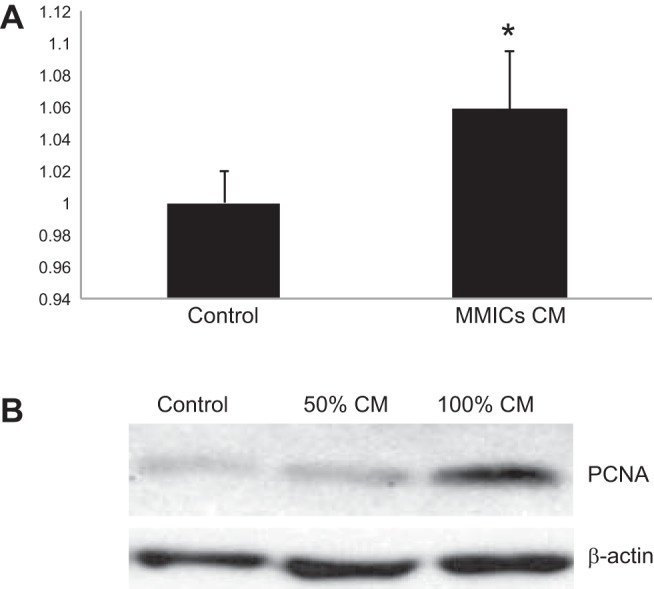
Conditioned medium from progenitor cells stimulates IMCD3 viability and proliferation. A: using an MTT assay for cell viability, the percentage of viable IMCD3 cells was calculated using absorbance of experimental group/absorbance of control group after 24 h of treatment with conditioned media. Treatment with conditioned media (CM) increased cell survival by nearly 100%. B: IMCD3 cells were treated for 48 h with progenitor cell CM. Progenitor cell nuclear antigen (PCNA) expression levels of treated IMCD3 cells were evaluated by Western blotting. PCNA levels increased in IMCD3 cells treated with 50 and 100% CM. *P < 0.005; n = 3.
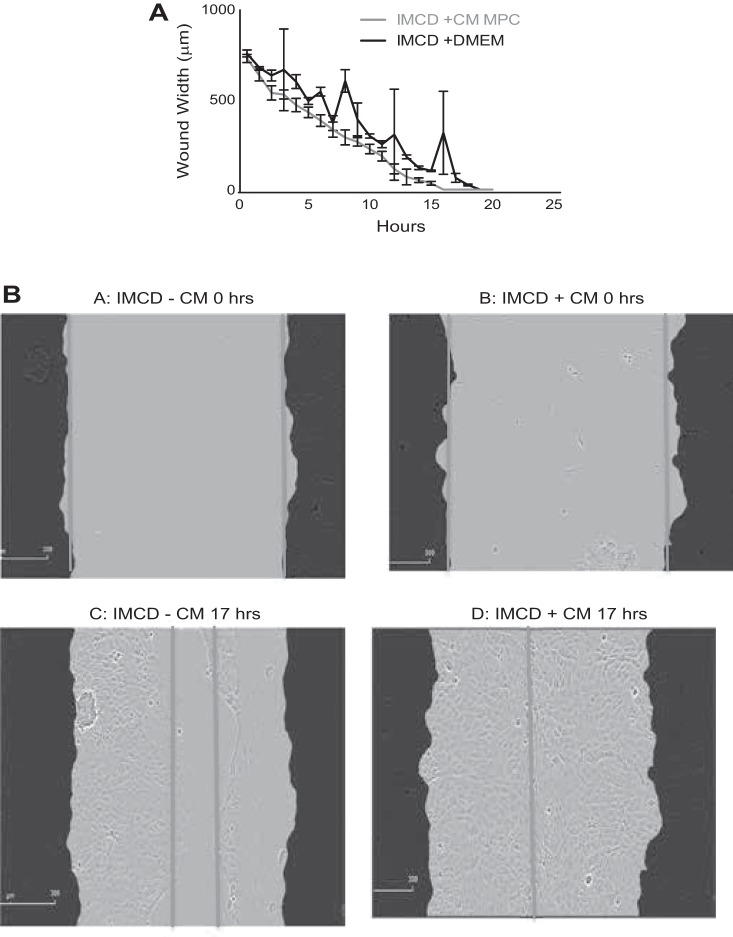
Effect of MPCs on wound repair.
IMCD3 cells treated with CM from enhanced progenitor cultures showed increased rates of wound healing (Fig. 10, A and B). During the first 6 h of IMCD growth in CM, a significant decrease in wound width was seen compared with cells in control medium. Relative to their respective 0-h wound width, IMCD3 cells grown in DMEM for 6 h showed only a 30% decrease in wound width, while the IMCD3 cells grown in CM for 6 h showed a 47% decrease in wound width. This trend in wound reduction continued further in IMCD3 cells grown in CM, which showed a 77% reduction in wound width after 10 h and 100% reduction after 17 h (Fig. 10B). Cells grown in DMEM for the same time showed reductions in wound width by only 50%.
Fig. 10.
A: CM from progenitor cells enhanced the wound closure capacity of IMCD3 cells. IMCD3 cells were grown to confluence and “wounded” as described in materials and methods. Over the time course of 21 h, the cells grown in CM, collected from progenitor cells, showed enhanced reduction in wound width (red line) compared with those cells grown in DMEM (blue line). Values are means ± SE; n = 3. B: images of wound closure after 17 h of exposure to either CM from progenitor cells (D) or DMEM (C). Treatment with CM induced complete closure of wounds, while the DMEM-treated control wounds only showed 50% closure.
Effect of MPCs on endothelial and epithelial differentiation.
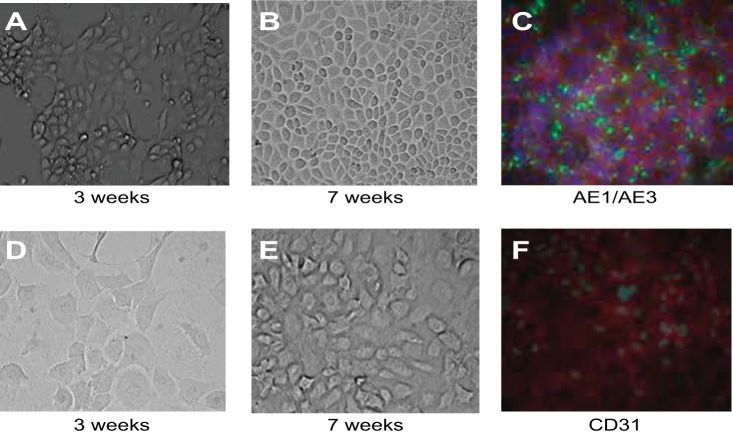
Selectively grown MPCs were cultured in renal epithelial growth medium (RenaLife Medium Complete Kit, LifeLine) for 7 wk. Throughout this growth period, these progenitor cells developed an epithelial cell-like phenotype, characterized by a dense, adherent cuboidal monolayer (Fig. 11, A and B). These differentiated cells expressed the pan-cytokeratin cell markers AE1/AE3 (Fig. 11C). MPCs were also grown in endothelial growth medium (EGM-2 MV, Lonza) for 7 wk (Fig. 11, D and E). Progenitor cells in this experiment developed a more loose arrangement of oblong and rounded cell layers without well-defined cell-cell junctions, more closely resembling an endothelial-type phenotype (Fig. 11, D and E) and showed weak expression of the endothelial marker CD31 (Fig. 11F).
Fig. 11.
Cell phenotype differentiation by MPCs. A and B: progenitor cells were grown in epithelial growth medium (RenaLife Medium Complete Kit from LifeLine and α-MEM 1:1) for 7 wk. Progression of progenitor cell differentiated toward an epithelial phenotype, shown at 3 (A)- and 7-wk (B) growth periods. At 7 wk, progenitor cells grew in a cell monolayer with cell junctions (B) and positive expression of the pan-cytokeratin markers AE1/AE3 (C). Green, GFP label (AE1/AE3); red, phalloidin (actin); blue, DAPI (nuclear). Also shown is progression of progenitor cell differentiation toward an endothelial phenotype. Cells are shown at 3 (D)- and 7 (E and F)-wk growth periods. Progenitor cells show loose, rounded cell phenotype (D and E). Immunohistochemistry studies showed staining for the endothelial marker CD31 at 7 wk of growth (F). Green, GFP (CD31); red, phalloidin (actin); blue, DAPI (nuclear).
Effect of CM from MPCs on tubulogenesis.
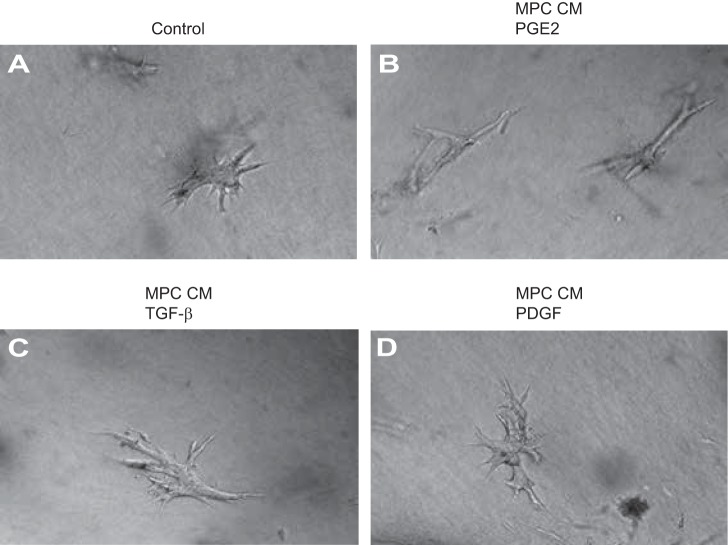
IMCD3 cells were treated with CM from PGE2-, TGF-β-, or PDGF-treated MPCs. Figure 12B shows early tubule formation in collagen I-3D gel cultures of IMCD3 cells, treated with PGE2 conditioned medium (CM-PGE2). CM from progenitor cells treated with TGF-β (CM-TGF-β, Fig. 12C) or PDGF (CM-PDGF, Fig. 12D) did not show a similar effect on tubule formation in IMCD3 cells. In comparison, IMCD3 cells treated with normal growth medium supplemented with PGE2, TGF-β, or PDGF did not show significant tubule formation (Fig. 12A). Follicular progenitor cells were used as positive controls for CD34 (Fig. 13).
Fig. 12.
Conditioned media from PGE2, transforming growth factor (TGF)-β, and PDGF-treated MPCs were used to assess tubule formation by IMCD cells grown in collagen I-3D gel cultures. A: IMCD3 cells treated with DMEM supplemented with PGE2. B: IMCD3 cells treated with CM from PGE2-treated MPCs show early tubule formation. C: IMCD3 cells treated with CM from TGF-β-treated MPCs do not show tubule formation. D: IMCD3 cells treated with CM from PDGF-treated MPCs do not show tubule formation.
Fig. 13.
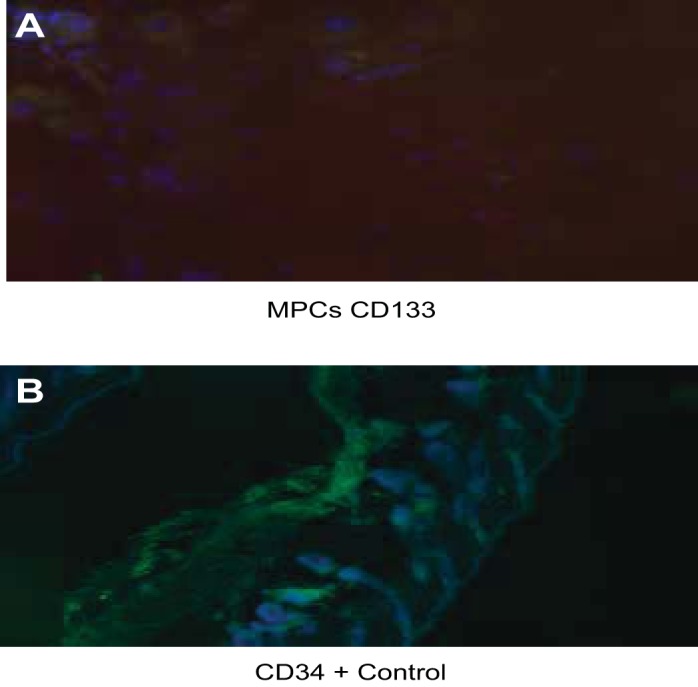
A: MPCs show weak positivity for CD133. B: positive expression of CD34 in follicular progenitor cells used as controls.
DISCUSSION
This study is aimed at characterizing a medullary interstitial progenitor cell population and assess its effect on epithelial cell wound closure. We show that the medullary interstitium harbors a side population of kidney progenitor cells that can differentiate into epithelial cells, can induce tubulogenesis in cultured medullary collecting duct cells, and can mediate tubular epithelial cell migration and proliferation. We conclude from these studies that a medullary interstitial progenitor cell population exists that can repair injured medullary collecting duct cells.
Preparation of a medullary interstitial primary cell culture generated a highly purified MIC population that showed characteristic elongated cytoplasmic extensions, oil red O-positive cytoplasmic granules, and positive α-SMA, vimentin, and COX2 expression. These characteristics have previously been observed in several studies and are consistent with MICs (16, 29, 36, 44).
When MIC primary cultures were grown for an extended period in selective knockout buffer (KSR plus N2), a cell population emerged that expressed several known progenitor/stem cell markers. Notably, nestin, PAX7, CD44, CXCR4, CXCR7, and CD24 were strongly expressed. They also expressed weak OCT4 (data not shown). These MPCs were negative for the hematopoietic stem cell marker CD34. The marker profile seen in our MPCs correlates with previously shown kidney progenitor cell profiles (32, 41, 50). It is possible that our MPCs are similar to the mouse kidney progenitor cell (MKPC) population previously isolated from Myh9-targeted mutant mice, which were also OCT4 positive and CD34 negative (25). Our MPCs also strongly expressed PDGFR-b, suggesting that they are pericytes. This is consistent with previously published data on MPCs that were injected into SCID mice and localized close to the medullary vasculature (25). These findings indicate that many of the MPCs are pericytes or have some transdifferentiation toward a pericyte phenotype.
Stromal-derived factor-1 (SDF-1) is a regulator of mobilization and migration of endothelial progenitor cells (24). SDF-1 is the ligand of important chemokine receptors CXCR4 and CXCR7 (9, 23). Both CXCR4 and CXCR7 have been shown to play vital roles in tissue regeneration and renal function improvement as well as being required for transendothelial migration (28). The SDF-1/CXCR4 axis is involved in papillary label-retaining cell (LRC) activation after AKI (33). The presence of both chemokine receptors on our MPCs indicates a possible role of SDF-1 as a local mediator in the epithelial cell trans-differentiation of MPCs. Moreover, SDF-1 and its receptors have been shown to enhance progenitor cell survival (28). This indicates that SDF-1 may mediate survival of MPCs, enhancing their possible role in tubular epithelial cell regeneration after injury.
Kidney progenitor cells repair tubular injury predominantly through paracrine effects (18). Therefore, we tested the impact of CM from MPCs on IMCD3 cell proliferation and migration. We found that CM treatment enhances proliferation and migration of IMCD3 cells. These findings are consistent with the hypothesis that MPCs stimulate proliferation of tubular epithelial cells following injury (19, 27). Paracrine actions have been implicated as predominant modality how mesenchymal stem cells mediate their effect on cell trans-differentiation and tubular injury repair in rat kidneys with AKI (47). Kidney progenitor cells have previously been isolated from rat and mouse kidneys (12, 15). Injection of these progenitor cells into injured kidneys did show a beneficial effect on renal function, even without their integration into injured nephron segments (12). However, the paracrine factors secreted by progenitor cells in AKI are still enigmatic, and the mechanisms through which these enhance tubular injury repair remain to be shown.
Progenitor cells are characteristically committed to differentiation toward a specific phenotype (2, 37). We therefore grew MPCs in epithelial and endothelial differentiation media to stimulate their development along either one of these cell lineages. We found that MPCs grown in epithelial differentiation medium showed significant expression of the pan-cytokeratin marker AE1/AE3, indicating that MPCs can differentiate to epithelial cells. However, we could not observe significant differentiation to an endothelial phenotype. Our results differ from those published by Lee et al. (25) that showed mouse kidney progenitor cell differentiation to an endothelial phenotype in vivo. A possible explanation is that they isolated a slightly different subpopulation of MPCs, which still has the potential for endothelial differentiation in vivo.
To examine whether MPCs secreted factors that enhance tubule formation, we treated IMCD3 cells that were grown in 3D Matrigel with CM from PGE2-treated MPCs, which induced growth of tubule-like structures. That was not seen when IMCD3 cells were treated with control medium or with CM from TGF-β-treated MPCs. We conclude from these results that MPCs secrete factors that stimulate tubule formation in medullary collecting duct cells. Moreover, PGE2 might play an important role as an autocrine factor inducing cytokine release from MPCs that then act as paracrine effectors to stimulate tubular epithelial cell migration and proliferation.
In summary, our results show that the renal medullary interstitium harbors a side population of cells with progenitor cell characteristics. These cells positively affect medullary collecting duct cell proliferation and migration. Previous studies have indicated that the inner medulla harbors stem cells with regenerative potential (34). Diseases associated with papillary necrosis have been shown to develop progressive chronic kidney disease and end-stage renal disease (22, 42), which may be due to the loss of MPCs. Supporting the importance of MPCs in kidney injury repair is the observation in animal models of mercuric chloride-induced AKI, showing induction of cell proliferation in the papillary region of the kidney, which likely represents a repair response by MPCs (7). MPCs may after all play an important role in the repair of tubular epithelial cell injury, likely through paracrine-mediated mechanisms.
GRANTS
This study was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant RO3 DK077700-01 to G. W. Moeckel.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.C., Z.C., Y.Z., C.P., and A.A.-O. performed experiments; D.C., Z.C., Y.Z., C.P., A.A.-O., and G.W.M. analyzed data; D.C., Y.Z., and G.W.M. interpreted results of experiments; D.C. and G.W.M. approved final version of manuscript; Y.Z. and G.W.M. provided conception and design of research; G.W.M. prepared figures; G.W.M. drafted manuscript; G.W.M. edited and revised manuscript.
REFERENCES
- 1.Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M, Rotondi M, Fogo AB, Lazzeri E, Lasagni L, Romagnani P. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 30: 1714–1725, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell 128: 445–458, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 14, Suppl 1: S55–S61, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bonventre JV. Molecular response to cytotoxic injury: role of inflammation, MAP kinases, and endoplasmic reticulum stress response. Semin Nephrol 23: 439–448, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int 66: 480–485, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Buckingham M. Skeletal muscle progenitor cells and the role of Pax genes. C R Biol 330: 530–533, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Bulger RE, Siegel FL. Alterations of the renal papilla during mercuric chloride-induced acute tubular necrosis. Lab Invest 33: 712–719, 1975 [PubMed] [Google Scholar]
- 8.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev 87: 1441–1474, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med 203: 2201–2213, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bussolati B, Moggio A, Collino F, Aghemo G, D'Armento G, Grange C, Camussi G. Hypoxia modulates the undifferentiated phenotype of human renal inner medullary CD133+ progenitors through Oct4/miR-145 balance. Am J Physiol Renal Physiol 302: F116–F128, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Cheng J, Dutra A, Takesono A, Garrett-Beal L, Schwartzberg PL. Improved generation of C57BL/6J mouse embryonic stem cells in a defined serum-free media. Genesis 39: 100–104, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Dekel B, Zangi L, Shezen E, Reich-Zeliger S, Eventov-Friedman S, Katchman H, Jacob-Hirsch J, Amariglio N, Rechavi G, Margalit R, Reisner Y. Isolation and characterization of nontubular sca-1+lin- multipotent stem/progenitor cells from adult mouse kidney. J Am Soc Nephrol 17: 3300–3314, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Dhara SK, Hasneen K, Machacek DW, Boyd NL, Rao RR, Stice SL. Human neural progenitor cells derived from embryonic stem cells in feeder-free cultures. Differentiation 76: 454–464, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 115: 1743–1755, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, Heremans Y, Lund T, Blackstad M, Jiang Y, Luttun A, Rosenberg ME. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol 17: 3028–3040, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Ann Rev Physiol 70: 357–377, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Hareendran S, Sathishkumar S, Abbas S, Mackay AM, Rajan P. A novel composition for the culture of human adipose stem cells which includes complement C3. Cytotechnology 62: 389–402, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humphreys BD, Bonventre JV. The contribution of adult stem cells to renal repair. Nephrol Ther 3: 3–10, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA 108: 9226–9231, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Kim K, Lee KM, Han DJ, Yu E, Cho YM. Adult stem cell-like tubular cells reside in the corticomedullary junction of the kidney. Int J Clin Exp Pathol 1: 232–241, 2008 [PMC free article] [PubMed] [Google Scholar]
- 22.Kincaid-Smith P. Pathogenesis of the renal lesion associated with the abuse of analgesics. Lancet 1: 859–862, 1967 [DOI] [PubMed] [Google Scholar]
- 23.Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood 105: 3793–3801, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood 106: 1901–1910, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Lee PT, Lin HH, Jiang ST, Lu PJ, Chou KJ, Fang HC, Chiou YY, Tang MJ. Mouse kidney progenitor cells accelerate renal regeneration and prolong survival after ischemic injury. Stem Cells 28: 573–584, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation 121: 2211–2220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115: 1756–1764, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML, Parente E, Mancina R, Netti GS, Becherucci F, Gacci M, Carini M, Gesualdo L, Rotondi M, Maggi E, Lasagni L, Serio M, Romagnani S, Romagnani P. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med 205: 479–490, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moeckel GW, Zhang L, Fogo AB, Hao CM, Pozzi A, Breyer MD. COX2 activity promotes organic osmolyte accumulation and adaptation of renal medullary interstitial cells to hypertonic stress. J Biol Chem 278: 19352–19357, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Muirhead EE, Brooks B, Pitcock JA, Stephenson P. Renomedullary antihypertensive function in accelerated (malignant) hypertension. Observations on renomedullary interstitial cells. J Clin Invest 51: 181–190, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuhofer W, Beck FX. Survival in hostile environments: strategies of renal medullary cells. Physiology (Bethesda) 21: 171–180, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Oliver JA, Klinakis A, Cheema FH, Friedlander J, Sampogna RV, Martens TP, Liu C, Efstratiadis A, Al-Awqati Q. Proliferation and migration of label-retaining cells of the kidney papilla. J Am Soc Nephrol 20: 2315–2327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliver JA, Maarouf O, Cheema FH, Liu C, Zhang QY, Kraus C, Zeeshan Afzal M, Firdous M, Klinakis A, Efstratiadis A, Al-Awqati Q. SDF-1 activates papillary label-retaining cells during kidney repair from injury. Am J Physiol Renal Physiol 302: F1362–F1373, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest 114: 795–804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Racusen LC, Solez K. Nephrotoxic tubular and interstitial lesions: morphology and classification. Toxicol Pathol 14: 45–57, 1986 [DOI] [PubMed] [Google Scholar]
- 36.Rodemann HP, Muller GA, Knecht A, Norman JT, Fine LG. Fibroblasts of rabbit kidney in culture. I. Characterization and identification of cell-specific markers. Am J Physiol Renal Fluid Electrolyte Physiol 261: F283–F291, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Romagnani P. From Proteus to Prometheus: learning from fish to modulate regeneration. J Am Soc Nephrol 21: 726–728, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol 17: 2443–2456, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Sakurai H, Tsukamoto T, Kjelsberg CA, Cantley LG, Nigam SK. EGF receptor ligands are a large fraction of in vitro branching morphogens secreted by embryonic kidney. Am J Physiol Renal Physiol 273: F463–F472, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Sallustio F, De Benedictis L, Castellano G, Zaza G, Loverre A, Costantino V, Grandaliano G, Schena FP. TLR2 plays a role in the activation of human resident renal stem/progenitor cells. FASEB J 24: 514–525, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Sanerkin NG. Chronic phenacetin nephropathy. (With particular reference to the relationship between renal papillary necrosis and “chronic interstitial nephritis”). Br J Urol 38: 361–370, 1966 [DOI] [PubMed] [Google Scholar]
- 43.Schena FP. Role of growth factors in acute renal failure. Kidney Int Suppl 66: S11–S15, 1998 [PubMed] [Google Scholar]
- 44.Schuttert JB, Fiedler GM, Grupp C, Blaschke S, Grunewald RW. Sorbitol transport in rat renal inner medullary interstitial cells. Kidney Int 61: 1407–1415, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med 334: 1448–1460, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289: F31–F42, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol 292: F1626–F1635, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Villanueva S, Cespedes C, Vio CP. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am J Physiol Regul Integr Comp Physiol 290: R861–R870, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Wachs FP, Couillard-Despres S, Engelhardt M, Wilhelm D, Ploetz S, Vroemen M, Kaesbauer J, Uyanik G, Klucken J, Karl C, Tebbing J, Svendsen C, Weidner N, Kuhn HG, Winkler J, Aigner L. High efficacy of clonal growth and expansion of adult neural stem cells. Lab Invest 83: 949–962, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Ward HH, Romero E, Welford A, Pickett G, Bacallao R, Gattone VH, 2nd, Ness SA, Wandinger-Ness A, Roitbak T. Adult human CD133/1(+) kidney cells isolated from papilla integrate into developing kidney tubules. Biochim Biophys Acta 1812: 1344–1357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 93: 2175–2188, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye Y, Wang B, Jiang X, Hu W, Feng J, Li H, Jin M, Ying Y, Wang W, Mao X, Jin K. Proliferative capacity of stem/progenitor-like cells in the kidney may associate with the outcome of patients with acute tubular necrosis. Hum Pathol 42: 1132–1141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeagy BA, Cherqui S. Kidney repair and stem cells: a complex and controversial process. Pediatr Nephrol 26: 1427–1434, 2011 [DOI] [PubMed] [Google Scholar]