Abstract
The amount of Na+ and K+ in the diet promotes significant changes in endothelial cell function. In the present study, a series of in vitro and in vivo experiments determined the role of Na+ and K+ in the regulation of two pleckstrin homology domain-containing intracellular signaling molecules, phospholipase C (PLC)-γ1 and epithelial and endothelial tyrosine kinase/bone marrow tyrosine kinase on chromosome X (Bmx), and agonist-generated Ca2+ signaling in the endothelium. Extracellular K+ concentration regulated the levels of activated PLC-γ1, Bmx, and carbachol-stimulated intracellular Ca2+ mobilization in human endothelial cells. Additional experiments confirmed that high-conductance Ca2+-activated K+ channels and phosphatidylinositol 3-kinase mediated these effects. The content of Na+ and K+ in the diet also regulated Bmx levels in endothelial cells and activated PLC-γ1 levels in rats in vivo. The effects of dietary K+ on Bmx were more pronounced in rats fed a high-salt diet compared with rats fed a low-salt diet. These experiments elucidated an endothelial cell signaling mechanism regulated by electrolytes, further demonstrating an integral relationship between endothelial cell function and dietary Na+ and K+ content.
Keywords: dietary salt, potassium, endothelium, phospholipase c-γ, Tec kinase, bone marrow tyrosine kinase
epithelial and endothelial tyrosine kinase/bone marrow tyrosine kinase on chromosome X (termed Bmx in this report), which is a member of the Tec kinase family, and phospholipase C (PLC)-γ1 are expressed in hematopoietic and endothelial cells (ECs) and are important constituents of multiple signaling cascades (16, 21, 35). Among other functions, Bmx and PLC-γ1 are involved in the mobilization of intracellular Ca+ stores (5, 23, 27, 30, 31). Targeting PLC-γ to the plasma membrane through the production of phosphatidylinositol 3,4,5-trisphosphate (PIP3) is sufficient to catalyze the production of inositol trisphosphate (IP3) (1). The production of IP3 induces the release of Ca+ from intracellular stores (30). Evidence from multiple laboratories has also demonstrated a critical role for Tec kinases in catalyzing the activation of PLC-γ and the generation of PLC-γ-dependent intracellular Ca+ signaling pathways (5, 27, 31). Tec kinases serve as important cofactors that facilitate PLC-γ activation through multiple mechanisms that include functions as a scaffolding molecule as well as tyrosine phosphorylation of linker proteins and PLC-γ (23, 27). The level of protein expression of Bmx is an important determinant of cytoprotection and cell proliferation (3, 11, 13).
Phosphatidylinositol 3-kinase (PI3K) and phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a lipid phosphatase that directly antagonizes 3-kinase activity of the class I PI3K family, regulate intracellular levels of PIP3 (6, 18, 28, 33, 34). PIP3 promotes the recruitment to the plasma membrane of pleckstrin homology (PH) domain-containing intracellular molecules (22, 29), permitting their activation and initiation of cell signaling pathways. PI3K and PTEN therefore regulate the function of PH-containing proteins that bind to PIP3, which include PKB (Akt) (18, 28, 29, 33, 34), isoforms of PLC-γ, including PLC-γ1 (1, 36), and Bmx (22, 25, 27).
Previous studies have demonstrated the involvement of PI3K and PTEN in determining EC function during changes in dietary salt intake (40, 43). While increased dietary salt intake, but not extracellular Na+ concentration per se, increased endothelial PIP3 through activation of PI3K (40), PTEN levels decreased in a dose-dependent fashion with decreasing extracellular K+ concentration (43). The latter mechanism was mediated through transforming growth factor-β and activation of the Smad signaling pathway, which regulate intracellular PTEN levels in the endothelium (43). Based on these previous studies, the hypothesis of the present study was that Na+ and K+ also regulated PLC-γ1 and Bmx and agonist-generated Ca+ signaling in the endothelium.
METHODS
Human umbilical vein EC incubation experiments.
Primary cultures of macrovascular ECs [human umbilical vein ECs (HUVECs)] were obtained commercially (Life Technologies, Grand Island, NY) and grown at 37°C in medium 200 (Life Technologies) and 5% CO2-95% air. Medium was exchanged at 48-h intervals, and cells were not used beyond 25–30 passages. Monolayers of HUVECs in 96- or 6-well plates were incubated in medium 200 that was produced without K+ by the manufacturer. This medium permitted the addition of KCl to final concentrations between 0 and 5 meq/l; choline chloride (Sigma-Aldrich, St. Louis, MO) at 0–5 meq/l replaced the KCl. Replacing KCl with choline chloride permitted no changes in extracellular osmolality among the groups. During these experiments, the medium was also supplemented with Low Serum Growth Supplement (Life Technologies), which resulted in a final concentration of 2% (vol/vol) FBS. Plates were incubated in these conditions for 24 h at 37°C before experiments. Some experiments also included the addition of iberiotoxin (Sigma-Aldrich) at 100 nM or vehicle as the medium K+ concentration was changed. Iberiotoxin served as a selective and reversible inhibitor of large-conductance Ca2+-activated K+ (BKCa) channels (7). In additional experiments, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY-294002) at 10 μM was used to inhibit PI3K (37). Cell lysates were obtained for analysis of Bmx, phospho-PLC-γ1 (Y783), which indicated activation of PLC-γ1 (14), and GAPDH.
Intracellular Ca2+ assay.
Changes in cytoplasmic Ca+ levels were determined using a kit (Molecular Probes Fluo-4 NW Calcium Assay, Life Technologies). On the day of the assay, the growth medium was removed from HUVECs, and 100 μl of the dye-loading solution containing the fluorescent Ca2+ indicator (Fluo-4 NW) were added into each well. Cells were incubated at 37°C for 30 min and then at room temperature for an additional 30 min. To demonstrate an effect on intracellular Ca2+ mobilization, HUVECs were then treated with carbachol (200 nM), which is an acetylcholinesterase-resistant acetylcholine analog that activates the M3 muscarinic acetylcholine receptor and increases intracellular Ca+ through PLC-dependent production of IP3 (17). Fluorescence was determined between 0 and 5 min after addition of carbachol using an excitation wavelength of 494 nm and emission wavelength of 516 nm (Spectramax M2e microplate reader, Molecular Devices, Sunnyvale, CA).
Animal and tissue preparation.
This study was carried out in accordance with recommendations in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee of the University of Alabama at Birmingham approved the project. Experiments were conducted using 32 male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) that were 28 days of age at the start of the study. Rats were housed under standard conditions and permitted free access to water. In the first series of experiments, rats were fed formulated diets (AIN-76A, Dyets, Bethlehem, PA) that contained a standard amount [0.95% (wt/wt)] of K+ but differed in the content of NaCl, which was either 0.3% or 8.0% (wt/wt) NaCl. In the second series of experiments, rats were divided into four groups and given one of four formulated diets (AIN-76A, Dyets) that contained different amounts of NaCl and K+. Two of the diets contained 0.3% (wt/wt) NaCl and either 0% or 1.99% (wt/wt) K+; the other two diets contained 8.0% (wt/wt) NaCl and either 0% or 1.99% (wt/wt) K+. Diets were prepared specifically to be identical in protein composition and differed only in Na+, K+, and sucrose content. By replacing the electrolytes, the sucrose content was therefore the highest in the 0.3% NaCl-0% K+ diet and lowest in the 8.0% NaCl-1.99% K+ diet. On the final day of the study, rats were anesthetized with 2% isoflurane. Aortae were harvested under sterile conditions, and aortic EC lysates were obtained as previously described (41, 44). Sera were harvested at the termination of the study for the determination of concentrations of Na+ and K+ (Nova 16 Clinical Analyzer, Nova Biomedical, Waltham, MA).
Western blot analysis.
Cell pellets were suspended in 300 μl of modified radioimmunoprecipitation assay buffer that contained the following: 10 mmol/l Tris·HCl (pH 7.4), 100 mM NaCl, 1 mmol/l of EDTA, 1 mmol/l EGTA, 0.5% sodium deoxycholate, 1% Triton X-100, 10% glycerol, 0.1% SDS, 20 mmol/l sodium pyrophosphate, 2 mmol/l Na3VO4, 1 mM NaF, 1 mmol/l PMSF, and protease inhibitor mixture. The total protein concentration of the lysates was determined using a kit (BCA Protein Assay Reagent kit, Thermo Scientific Pierce Protein Biology Products, Rockford, IL). Samples containing 25 μg total protein were processed for Western blot analysis, as we have previously described (39, 40, 42). The primary antibodies, diluted 1:1,000, specifically recognized Bmx (BD Biosciences, BD Transduction Laboratories, San Jose, CA), phospho-PLC-γ1 (Y783; Abcam, Cambridge, MA), and GAPDH (Abcam,), which served as a loading control. Membranes were developed in standard fashion (SuperSignal West Dura Chemiluminescent Substrate, Thermo Scientific Pierce Protein Biology Products). Density of the bands was quantified using Quantity One software (Bio-Rad Laboratories, Hercules, CA).
Statistical analysis.
Data are expressed as means ± SE. Significant differences were determined by ANOVA with post hoc testing, as appropriate. Data that were not normally distributed were analyzed using nonparametric ANOVA (Kruskal-Wallis test). For some experiments, data were modeled using factorial ANOVA, and mean differences were determined with Tukey's post hoc testing (Proc GLM). P values of <0.05 were assigned statistical significance.
RESULTS
Extracellular K+ concentration determined levels of activated PLC-γ1 and Bmx in HUVECs, and the effect was mediated through BKCa channels.
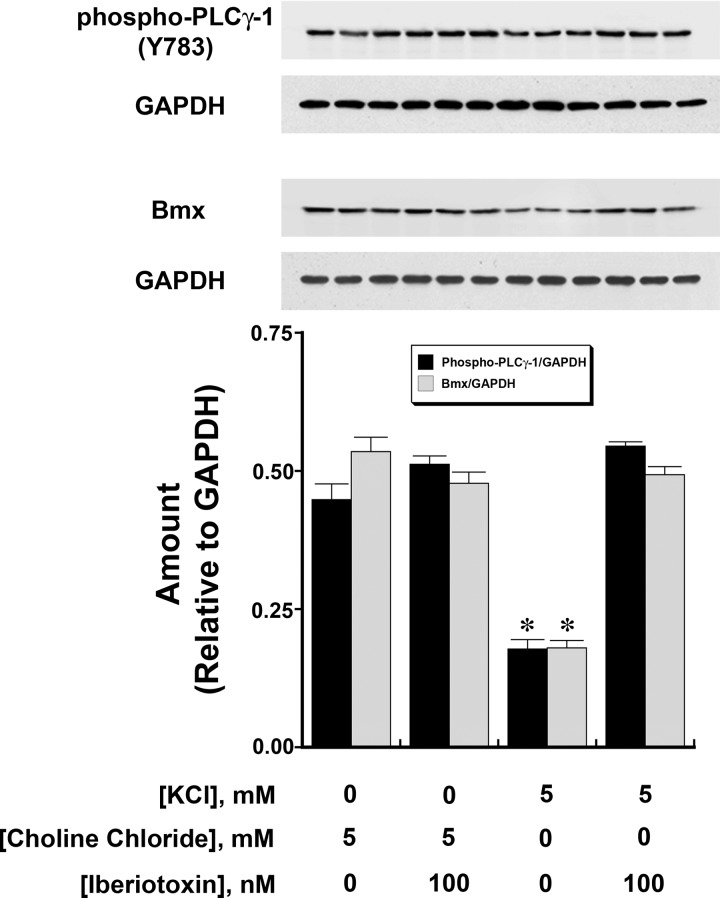
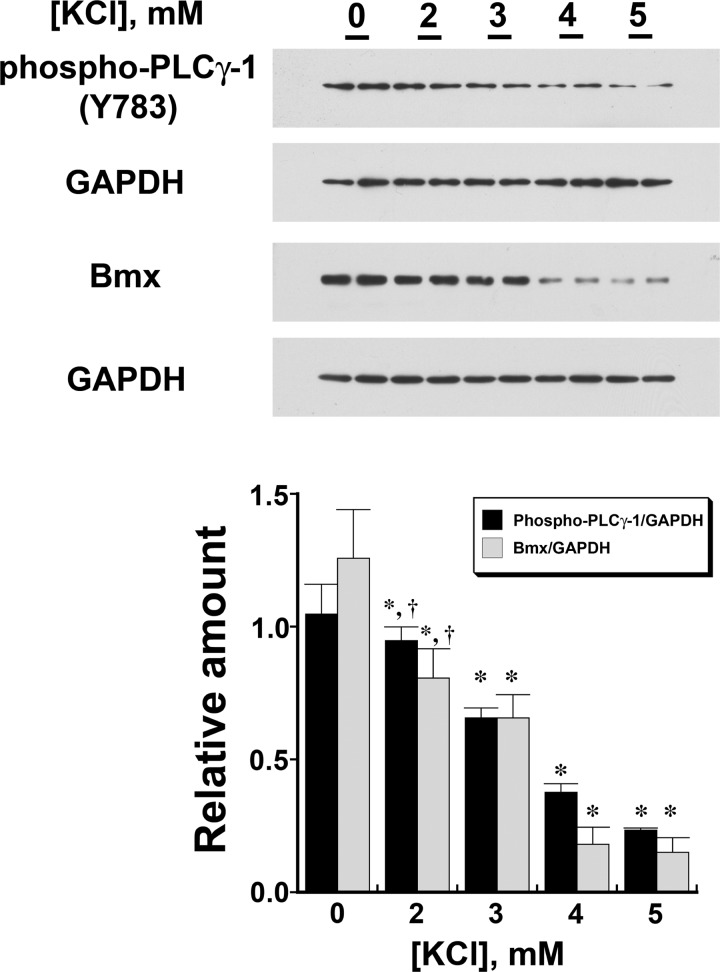
Tyrosine phosphorylation of PLC-γ1 at Y783 has been shown to indicate activation of this enzyme (14). Compared with HUVECs incubated overnight in medium containing 0 mM KCl, HUVECs in medium containing 5 mM KCl demonstrated reductions in phospho-PLC-γ1 (Y783) and Bmx; this effect was lost with the addition of iberiotoxin (100 nM) to cells in medium containing 5 mM KCl (Fig. 1). A dose-dependent effect of extracellular K+ concentration on phospho-PLC-γ1 (Y783) and Bmx was demonstrated (Fig. 2). In these experiments, choline chloride was added to maintain constant extracellular osmolality and Cl− concentration.
Fig. 1.
The amount of activated phospholipase C (PLC)-γ1 [phospho-PLC-γ1 (Y783); top] and endothelial tyrosine kinase/bone marrow tyrosine kinase on chromosome X (Bmx; middle) in human umbilical vein endothelial cells (HUVECs) decreased when the extracellular K+ concentration was increased from 0 to 5 meq/l. Choline chloride was added to maintain constant osmolality. The findings (n = 6 experiments/group) were summarized in the graph (bottom), which was aligned with Western blots that contained three samples of each of the groups. The graph represents the expression of the two different dependent variables relative to GAPDH as determined by densitometry. The inhibitory effect of K+ concentration (5 mM) was prevented by the addition of iberiotoxin (100 nM), a specific inhibitor of large-conductance Ca2+-activated K+ (BKCa) channels, but iberiotoxin did not alter these findings when the extracellular K+ concentration was 0 mM. *P < 0.001 compared with the other three groups.
Fig. 2.
Increasing extracellular K+ concentration from 0 to 5 mM produced a dose-dependent (P < 0.001) decrease in both phospho-PLC-γ1 (Y783; top) and Bmx (middle) in HUVECs. The two different dependent variables (n = 3 different experiments) were summarized in the graph (bottom), which was aligned with the Western blots to match the groups. *P < 0.05 compared with the corresponding data obtained from cells incubated in 0 mM KCl; †P < 0.05 compared with the corresponding data obtained from cells incubated in 4 and 5 mM KCl.
Extracellular K+ concentration determined the level of intracellular Ca2+ mobilization in response to carbachol.
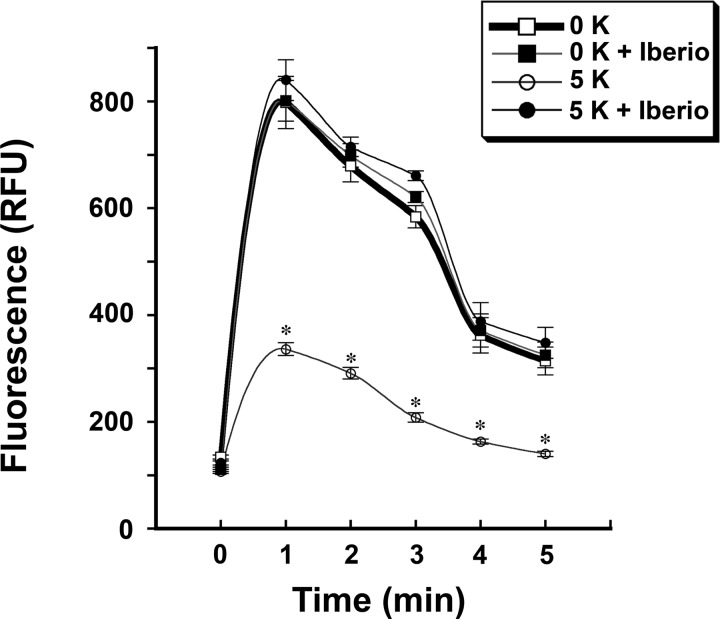
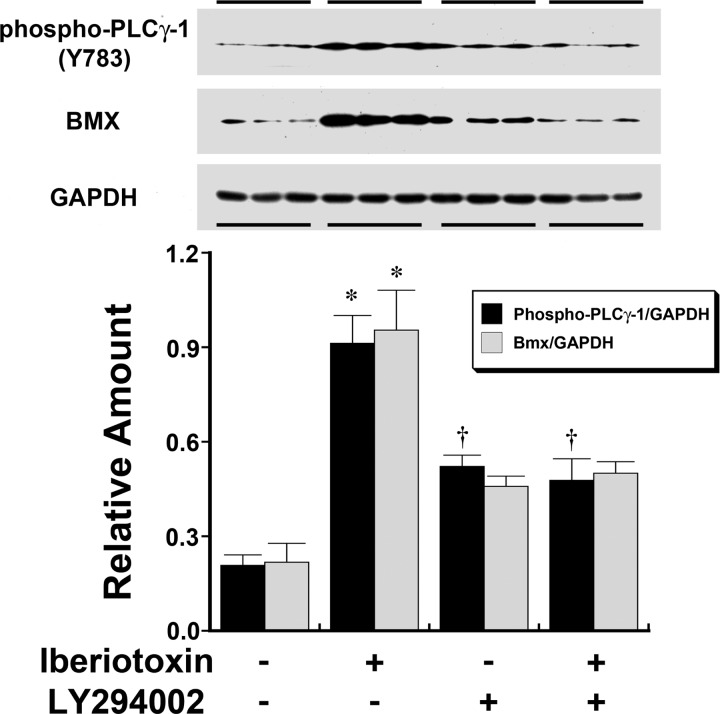
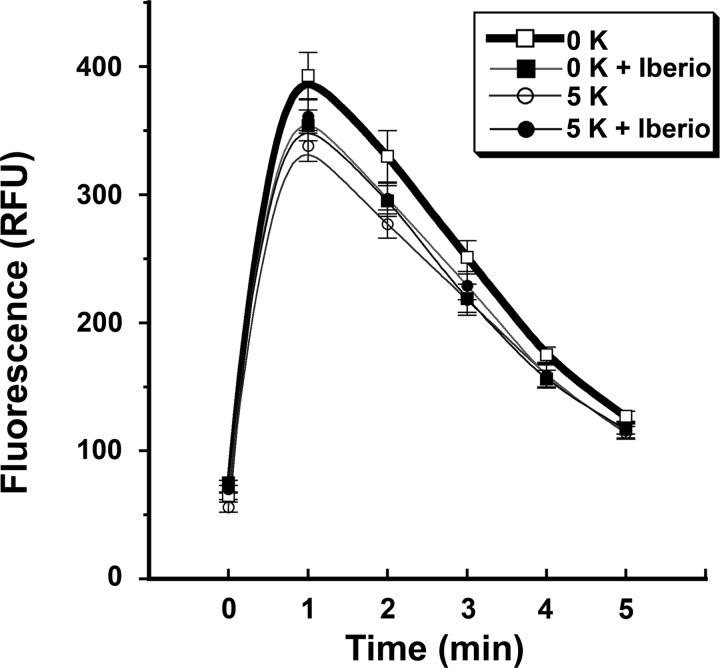
Changes in cytoplasmic Ca+ levels were determined after an overnight incubation of HUVECs in medium containing either 0 or 5 mM KCl. On the day of the assay, carbachol-induced mobilization of intracellular Ca2+ was reduced in cells incubated in the medium containing 5 mM KCl compared with cells incubated in the medium containing 0 mM KCl (Fig. 3). In these experiments, the concomitant addition of iberiotoxin abrogated the differences in intracellular Ca2+ mobilization generated by extracellular K+ concentration. To determine the involvement of PI3K, cells were incubated overnight in medium containing 5 mM KCl with or without iberiotoxin. Experiments were then performed 4 h after the addition of LY-294002 or vehicle. The observed effects of iberiotoxin on the increase in intracellular phospho-PLC-γ1 (Y783) and Bmx were inhibited by the addition of LY-294002 (Fig. 4). Ca2+ mobilization experiments using cells preincubated in medium containing LY-294002 demonstrated the loss of the differences in intracellular Ca2+ mobilization generated by extracellular K+ concentration (Fig. 5).
Fig. 3.
The degree of carbachol-induced intracellular Ca2+ mobilization was dependent upon extracellular K+ concentration. Compared with cells exposed to medium containing 0 mM K+ concentration, HUVECs incubated in medium containing 5 mM K+ concentration demonstrated reduced intracellular Ca2+ mobilization after carbachol stimulation. RFU, relative fluorescence units. n = 28 experiments in each group at each time point. *P < 0.05 compared with the other three groups.
Fig. 4.
Western analyses demonstrating the effect of iberiotoxin, an inhibitor of the BKCa channel (7), and LY-294002, a phosphatidylinositol 3-kinase inhibitor (37), on phospho-PLC-γ1 (Y783; top) and Bmx (middle) in HUVECs incubated in medium containing 5 mM K+ concentration. The two different dependent variables (n = 6 experiments total/group) were summarized in the accompanying graph (bottom), which was aligned with the Western blots to match the groups. The addition of iberiotoxin increased phospho-PLC-γ1 (Y783) and Bmx. LY-294002 (10 μM) pretreatment had an independent effect on endothelial phospho-PLC-γ1 but not Bmx. However, pretreatment of HUVECs with LY-294002 prevented the iberiotoxin-induced increase in both proteins. *P < 0.05 compared with the other three groups; †P < 0.05 compared with vehicle-treated groups (first columns).
Fig. 5.
Pretreatment of HUVECs with LY-294002 resulted in similar degrees of carbachol-induced intracellular Ca2+ mobilization. The previously identified reduction in carbachol-induced intracellular Ca2+ mobilization observed in HUVECs incubated in medium containing 5 mM K+ concentration was lost with pretreatment with LY-294002. n = 16 experiments/group.
Increased dietary salt intake increased endothelial Bmx and activated PLC-γ1 levels, whereas increased dietary K+ intake mitigated these findings in vivo.
In initial experiments, rats (n = 4 rats/group) on either 0.3% or 8.0% NaCl diets for 4 days were compared. In these experiments, the K+ content [0.95% (wt/wt)] of both diets was identical. EC lysates from rats on the 0.3% NaCl diet contained less Bmx/GAPDH (0.32 ± 0.02 vs. 0.65 ± 0.01, P < 0.001) than lysates from rats on the 8.0% NaCl diet. Lysates from rats on the 0.3% NaCl diet also contained less phospho-PLC-γ1 (Y783)/GAPDH (0.22 ± 0.03 vs. 0.53 ± 0.03, P < 0.001) than lysates from rats on the 8.0% NaCl diet.
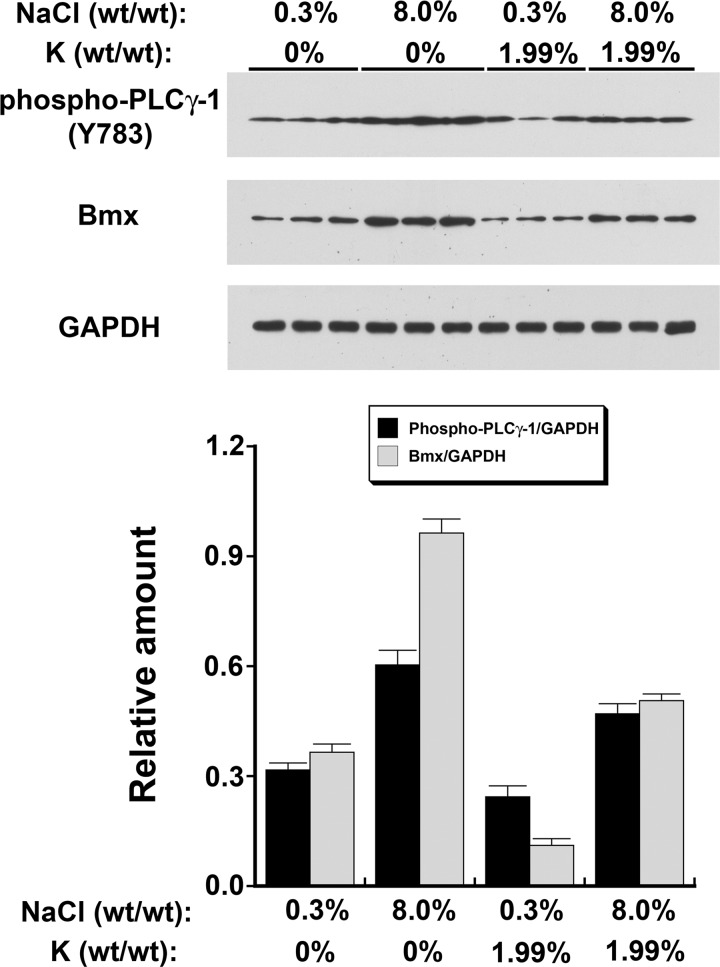
In the second series of experiments, the effect of dietary K+ was examined (Fig. 6). In these experiments, serum K+ concentration, but not serum Na+ concentration, differed among the four groups (Table 1). Endothelial Bmx levels differed (P < 0.0001) among the four dietary groups. In post hoc analyses, the intake of dietary salt and K+, as well as their interaction, predicted Bmx levels (P < 0.001 for all analyses; Table 2). For endothelial phospho-PLC-γ1 (Y783) levels, the model main effects for dietary salt and K+, but not their interaction, were statistically significant (P < 0.005). Post hoc testing demonstrated that the dietary salt and K+ content predicted phospho-PLC-γ1 (Y783) levels (P < 0.01; Table 2).
Fig. 6.
Dietary Na+ and K+ regulated levels of phospho-PLC-γ1 (Y783; top) and Bmx (middle) in the endothelium in vivo. The findings of the experiments (n = 6 animals/group) were summarized in the graph (bottom), which was aligned with the Western blots to match the groups. See text for details.
Table 1.
Comparison of serum electrolytes of rats on the four diets
| Dietary Electrolyte Composition |
Serum Electrolytes |
||
|---|---|---|---|
| Na+, % (wt/wt) | K+, % (wt/wt) | Na+ concentration, meq/l | K+ concentration, meq/l |
| 0.3 | 0 | 137 ± 5 | 3.9 ± 0.3* |
| 0.3 | 1.99 | 140 ± 5 | 5.5 ± 0.3 |
| 8.0 | 0 | 144 ± 3 | 3.2 ± 0.2* |
| 8.0 | 1.99 | 135 ± 2 | 4.6 ± 0.2 |
Significantly different (P <0.05) than the means of the groups on diets containing the corresponding identical salt content and increased (1.99%) K+.
Table 2.
Results of two-way ANOVA of the effects of dietary NaCl and dietary K+ on endothelial Bmx and PLC-γ1 in vivo
| Bmx |
PLC-γ1 |
|||
|---|---|---|---|---|
| Least square mean | P value | Least square mean | P value | |
| Main effect of dietary NaCl | ||||
| Low dietary NaCl | 0.24 | <0.001 | 0.28 | <0.001 |
| High dietary NaCl | 0.73 | 0.54 | ||
| Main effect of dietary K+ | ||||
| No K+ | 0.66 | <0.001 | 0.46 | 0.002 |
| 2× K+ | 0.31 | 0.36 | ||
| Dietary NaCl × K+ interaction | ||||
| Low NaCl × no K+ | 0.37 | <0.001 | 0.31 | 0.34 |
| Low NaCl × 2× K+ | 0.11 | 0.24 | ||
| High NaCl × no K+ | 0.96 | 0.60 | ||
| High NaCl × 2× K+ | 0.51 | 0.47 | ||
Bmx, endothelial tyrosine kinase/bone marrow tyrosine kinase on chromosome X; PLC, phospholipase C.
DISCUSSION
The dietary content of Na+ and K+ modulates EC function through a series of signaling events that center primarily on PI3K, PTEN, and the generation of PIP3 (40–43). Previous studies have demonstrated specifically that extracellular K+ concentration directly regulated cellular function (42, 43), but similar increases in Na+ concentration had no effect and instead dietary salt altered endothelial cell function in vivo through a mechanism(s) reminiscent of shear forces (39, 40, 42, 43, 46). The novel findings of the present series of in vitro and in vivo experiments build on these studies and include 1) extracellular K+ concentration regulated the levels of activated PLC-γ1, Bmx, and agonist-stimulated intracellular Ca2+ mobilization in human ECs and these effects were mediated through BKCa channels; 2) the dietary content of Na+ and K+ regulated EC PLC-γ1 and Bmx in rats in vivo; and 3) the effects of reduced dietary K+ intake on Bmx were more pronounced in rats fed a high-salt diet compared with rats fed a low-salt diet. These documented increases in Bmx and active PLC-γ1 after an increase in dietary salt intake, as well as through modulation by extracellular K+ concentration, were anticipated by previous studies that demonstrated the involvement of a PI3K/PTEN interaction, which regulated the activity of Akt, another PH-containing enzyme (43). In those experiments, extracellular K+ concentration but not Na+ concentration promoted the effects on this pathway.
Bmx, a member of the Tec kinase family, is expressed in ECs (16, 21, 35). While mice lacking the Bmx gene grow normally and lack a phenotype (21), Bmx is critically important in endothelial and vascular remodeling in pathological states (11). Current evidence supports an integral role of Tec kinases in signal transduction mechanisms that involve PLC-γ (5, 27, 31). PI3K products activate Bmx through the PH domain, which permits localization at sites in the plasma membrane where this protein can catalyze the activation of PLC-γ (25). Consistent with the literature, the findings in the present study demonstrated that the amount of dietary salt, as well as K+, intake regulated Bmx protein expression in ECs.
The two isoforms of PLC-γ have traditionally been associated with signal transduction involving receptor and nonreceptor tyrosine kinases. However, recent studies have shown that phosphoinositide products of PI3K (1) as well as G protein-coupled receptors (10, 24, 35, 36) may also activate PLC-γ1. Intracellular Ca+ mobilization in response to agonists, such as acetylcholine, is a critically important feature of EC function (15). In the present study, lowering extracellular K+ concentration, which has been shown to increase PI3K activity in HUVECs (43), increased Bmx and activated PLC-γ1 and promoted Ca2+ mobilization after muscarinic receptor stimulation. A role for PI3K was demonstrated since inhibition of PI3K abrogated these findings (Figs. 4 and 5). These observations are also consistent with other studies that demonstrated synergistic interactions between G protein-coupled receptors and PI3K (19, 32). In response to increased salt intake, levels of Bmx and active PLC-γ1 in ECs increased; the involvement of these molecules in intracellular Ca+ mobilization should facilitate, for example, endothelial nitric oxide production, which has previously been documented to occur in young rats in response to increased salt intake (4, 39, 40, 43, 45).
The findings of the present study suggest that dietary Na+ and K+ intake interacted to promote an interesting coordinated effect on the signaling molecules Bmx and PLC-γ1 in ECs. Although not directly confirmed, the inhibitory effect of dietary K+ on dietary salt-induced Bmx levels is likely mitigated by the concomitant inhibition of transforming growth factor-β production (42, 43, 47). However, isolated inhibition of Bmx might promote hypertension. A recently developed Bruton tyrosine kinase (Btk) inhibitor is also a highly effective inhibitor of other Tec kinases, including Bmx (12). In a recent trial involving the use of this synthetic irreversible Btk inhibitor in chronic lymphocytic leukemia, 18% of patients developed hypertension as an adverse event (2). The potential role of Bmx in the development of hypertension or, more specifically, the blood pressure response to increased dietary salt intake, especially with concomitantly low K+ intake, bears additional scrutiny in future studies.
The present study also demonstrated that the BKCa channel was a prominent part of the mechanism that increased PLC-γ1 and Bmx. Mice that lack the α-subunit gene (KCNMA1) or β1-subunit gene (KCNMB1) of the BKCa channel are hypertensive (20, 26). A recent report (38) showed that mRNA and protein expression of KCNMB1 as well as the associated whole cell current and Ca2+ sensitivity of the BKCa channel were reduced in smooth muscle obtained from Chinese patients with primary hypertension. While reduced expression of the BKCa channel would have effects on cell types that include vascular smooth muscle and the connecting tubule epithelium (8, 9), the present experiments, combined with previous studies (42, 43), further support an integral function of BKCa channels in determining EC responses to the effects of dietary salt and K+ and associated changes in arterial function. Targeted genetic deletion studies would be required to determine how expression of BKCa subunits on these cell types affects blood pressure and the response to salt and K+ intake.
GRANTS
This work was supported by Office of Research and Development, Medical Research Service, Department of Veterans Affairs Grant 1 IP1 BX001595 and by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-046199 and P30-DK-079337 (George M. O'Brien Kidney and Urological Research Centers Program).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.-Z.Y. and K.J.A. performed experiments; W.-Z.Y., K.J.A., and P.W.S. analyzed data; W.-Z.Y. and P.W.S. interpreted results of experiments; W.-Z.Y. and P.W.S. prepared figures; W.-Z.Y., K.J.A., and P.W.S. edited and revised manuscript; W.-Z.Y., K.J.A., and P.W.S. approved final version of manuscript; P.W.S. conception and design of research; P.W.S. drafted manuscript.
REFERENCES
- 1.Bae YS, Cantley LG, Chen CS, Kim SR, Kwon KS, Rhee SG. Activation of phospholipase C-γ by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273: 4465–4469, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Sukbuntherng J, Chang BY, Clow F, Hedrick E, Buggy JJ, James DF, O'Brien S. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 369: 32–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chau CH, Clavijo CA, Deng HT, Zhang Q, Kim KJ, Qiu Y, Le AD, Ann DK. Etk/Bmx mediates expression of stress-induced adaptive genes VEGF, PAI-1, and iNOS via multiple signaling cascades in different cell systems. Am J Physiol Cell Physiol 289: C444–C454, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Chen PY, Sanders PW. l-Arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest 88: 1559–1567, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fluckiger AC, Li Z, Kato RM, Wahl MI, Ochs HD, Longnecker R, Kinet JP, Witte ON, Scharenberg AM, Rawlings DJ. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J 17: 1973–1985, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem 67: 481–507, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem 265: 11083–11090, 1990 [PubMed] [Google Scholar]
- 8.Grimm PR, Irsik DL, Settles DC, Holtzclaw JD, Sansom SC. Hypertension of Kcnmb1−/− is linked to deficient K secretion and aldosteronism. Proc Natl Acad Sci USA 106: 11800–11805, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimm PR, Sansom SC. BK channels and a new form of hypertension. Kidney Int 78: 956–962, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haendeler J, Yin G, Hojo Y, Saito Y, Melaragno M, Yan C, Sharma VK, Heller M, Aebersold R, Berk BC. GIT1 mediates Src-dependent activation of phospholipase Cγ by angiotensin II and epidermal growth factor. J Biol Chem 278: 49936–49944, 2003 [DOI] [PubMed] [Google Scholar]
- 11.He Y, Luo Y, Tang S, Rajantie I, Salven P, Heil M, Zhang R, Luo D, Li X, Chi H, Yu J, Carmeliet P, Schaper W, Sinusas AJ, Sessa WC, Alitalo K, Min W. Critical function of Bmx/Etk in ischemia-mediated arteriogenesis and angiogenesis. J Clin Invest 116: 2344–2355, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, Buggy JJ. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA 107: 13075–13080, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang X, Borgesi RA, McKnight NC, Kaur R, Carpenter CL, Balk SP. Activation of nonreceptor tyrosine kinase Bmx/Etk mediated by phosphoinositide 3-kinase, epidermal growth factor receptor, and ErbB3 in prostate cancer cells. J Biol Chem 282: 32689–32698, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Kim HK, Kim JW, Zilberstein A, Margolis B, Kim JG, Schlessinger J, Rhee SG. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-γ1 phosphorylation on tyrosine residues 783 and 1254. Cell 65: 435–441, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res 112: 1171–1188, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao HJ, Kume T, McKay C, Xu MJ, Ihle JN, Carpenter G. Absence of erythrogenesis and vasculogenesis in Plcg1-deficient mice. J Biol Chem 277: 9335–9341, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Luo J, Busillo JM, Benovic JL. M3 muscarinic acetylcholine receptor-mediated signaling is regulated by distinct mechanisms. Mol Pharmacol 74: 338–347, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellor P, Furber LA, Nyarko JN, Anderson DH. Multiple roles for the p85α isoform in the regulation and function of PI3K signalling and receptor trafficking. Biochem J 441: 23–37, 2012 [DOI] [PubMed] [Google Scholar]
- 19.New DC, Wong YH. Molecular mechanisms mediating the G protein-coupled receptor regulation of cell cycle progression. J Mol Signal 2: 2, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pluger S, Faulhaber J, Furstenau M, Lohn M, Waldschutz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel β1 subunit gene feature abnormal Ca2+ spark/STOC coupling and elevated blood pressure. Circ Res 87: E53–E60, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Rajantie I, Ekman N, Iljin K, Arighi E, Gunji Y, Kaukonen J, Palotie A, Dewerchin M, Carmeliet P, Alitalo K. Bmx tyrosine kinase has a redundant function downstream of angiopoietin and vascular endothelial growth factor receptors in arterial endothelium. Mol Cell Biol 21: 4647–4655, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rameh LE, Arvidsson A, Carraway KL, 3rd, Couvillon AD, Rathbun G, Crompton A, VanRenterghem B, Czech MP, Ravichandran KS, Burakoff SJ, Wang DS, Chen CS, Cantley LC. A comparative analysis of the phosphoinositide binding specificity of pleckstrin homology domains. J Biol Chem 272: 22059–22066, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Readinger JA, Mueller KL, Venegas AM, Horai R, Schwartzberg PL. Tec kinases regulate T-lymphocyte development and function: new insights into the roles of Itk and Rlk/Txk. Immunol Rev 228: 93–114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev 80: 1291–1335, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Saito K, Scharenberg AM, Kinet JP. Interaction between the Btk PH domain and phosphatidylinositol-3,4,5-trisphosphate directly regulates Btk. J Biol Chem 276: 16201–16206, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Sausbier M, Arntz C, Bucurenciu I, Zhao H, Zhou XB, Sausbier U, Feil S, Kamm S, Essin K, Sailer CA, Abdullah U, Krippeit-Drews P, Feil R, Hofmann F, Knaus HG, Kenyon C, Shipston MJ, Storm JF, Neuhuber W, Korth M, Schubert R, Gollasch M, Ruth P. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation 112: 60–68, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Scharenberg AM, Kinet JP. PtdIns-3,4,5-P3: a regulatory nexus between tyrosine kinases and sustained calcium signals. Cell 94: 5–8, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95: 29–39, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Stocker H, Andjelkovic M, Oldham S, Laffargue M, Wymann MP, Hemmings BA, Hafen E. Living with lethal PIP3 levels: viability of flies lacking PTEN restored by a PH domain mutation in Akt/PKB. Science 295: 2088–2091, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306: 67–69, 1983 [DOI] [PubMed] [Google Scholar]
- 31.Takata M, Kurosaki T. A role for Bruton's tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-γ2. J Exp Med 184: 31–40, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uruno A, Sugawara A, Kanatsuka H, Kagechika H, Saito A, Sato K, Kudo M, Takeuchi K, Ito S. Upregulation of nitric oxide production in vascular endothelial cells by all-trans retinoic acid through the phosphoinositide 3-kinase/Akt pathway. Circulation 112: 727–736, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem 70: 535–602, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol 13: 195–203, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Venema VJ, Ju H, Sun J, Eaton DC, Marrero MB, Venema RC. Bradykinin stimulates the tyrosine phosphorylation and bradykinin B2 receptor association of phospholipase Cγ1 in vascular endothelial cells. Biochem Biophys Res Commun 246: 70–75, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Vines CM. Phospholipase C. Adv Exp Med Biol 740: 235–254, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 269: 5241–5248, 1994 [PubMed] [Google Scholar]
- 38.Yang Y, Li PY, Cheng J, Mao L, Wen J, Tan XQ, Liu ZF, Zeng XR. Function of BKCa channels is reduced in human vascular smooth muscle cells from Han Chinese patients with hypertension. Hypertension 61: 519–525, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Ying WZ, Sanders PW. Increased dietary salt activates rat aortic endothelium. Hypertension 39: 239–244, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Ying WZ, Aaron K, Sanders PW. Dietary salt activates an endothelial proline-rich tyrosine kinase 2/c-Src/phosphatidylinositol 3-kinase complex to promote endothelial nitric oxide synthase phosphorylation. Hypertension 52: 1134–1141, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ying WZ, Aaron K, Sanders PW. Mechanism of dietary salt-mediated increase in intravascular production of TGF-β1. Am J Physiol Renal Physiol 295: F406–F414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ying WZ, Aaron K, Wang PX, Sanders PW. Potassium inhibits dietary salt-induced transforming growth factor-β production. Hypertension 54: 1159–1163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ying WZ, Aaron KJ, Sanders PW. Effect of aging and dietary salt and potassium intake on endothelial PTEN (phosphatase and tensin homolog on chromosome 10) function. PLOS ONE 7: e48715, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ying WZ, Aaron KJ, Sanders PW. Transforming growth factor-β regulates endothelial function during high salt intake in rats. Hypertension 62: 951–956, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ying WZ, Sanders PW. Dietary salt increases endothelial nitric oxide synthase and TGF-β1 in rat aortic endothelium. Am J Physiol Heart Circ Physiol 277: H1293–H1298, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Ying WZ, Sanders PW. Dietary salt modulates renal production of transforming growth factor-β in rats. Am J Physiol Renal Physiol 274: F635–F641, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Ying WZ, Sanders PW. The interrelationship between TGF-β1 and nitric oxide is altered in salt-sensitive hypertension. Am J Physiol Renal Physiol 285: F902–F908, 2003 [DOI] [PubMed] [Google Scholar]