Abstract
Sestrins (Sesns) are a family of stress-sensitive genes that have been suggested to regulate lipid metabolism. Chronic ethanol feeding is known to cause lipid accumulation in hepatocytes. This study was designed to investigate the role of Sesn3 in the pathogenesis of alcohol-induced hepatic steatosis. We demonstrated that ethanol inhibited the expression of Sesn3 in VL-17A cells. Overexpression of Sesn3 ameliorated triglyceride accumulation; downregulation using short hairpin RNA significantly deteriorated triglyceride accumulation in these cells. The expression of Sesn3 was also reduced in mice fed with ethanol for 4 wk. Overexpression of Sesn3 prevented hepatic steatosis, whereas knockdown of Sesn3 worsened hepatic steatosis in ethanol-fed mice. Overexpression of Sesn3 significantly reduced the expression of genes encoding for lipid synthesis through AMPK pathway. Overexpression of Sesn3 augmented the effect of ethanol on phospho-p70 S6 kinase. The levels of hepatic light chain 3, a marker for autophagy, expression were significantly decreased in ethanol-fed mice after Sesn3 gene was knocked down. Our findings suggest that inhibitory effect of ethanol on Sesn3 may play an important role in the development of ethanol-induced fatty liver.
Keywords: Sestrin3, alcohol, steatosis
alcohol-induced hepatic steatosis is characterized by fat accumulation in the liver in response to excessive alcohol consumption (7, 22). Once considered as a benign condition, it is now known that the presence of hepatic steatosis can lead to advance clinical presentations such as steatohepatitis, advanced fibrosis, and eventually cirrhosis (21). The pathogenesis of alcoholic steatosis is complex, as multiple pathways are involved, including the synthesis, export, and oxidation of intrahepatic lipids (7).
Sestrins (Sesns) are a small family of stress-sensitive genes that are conserved across several species, including C. elegans, Drosophila melanogaster, and mammals (3, 13). Lower organisms, such as worms and fruit flies, have only one Sesns gene; however, three Sesns genes (Sesn1/2/3) have been identified in mammals. Sesns are under the regulation of p53 and involved in the control of cell growth and DNA damage secondary to external stress stimuli (2, 9). Furthermore, Sesns were shown to be negative regulators of mTOR (mechanistic target of rapamycin) signaling through the activation of AMPK (AMP-activated protein kinase) and tuberous sclerosis complex 2 phosphorylation (2, 3, 9, 13, 14).
Sesns have been shown to regulate several physiological pathways, including lipid metabolism. dSesn (Drosophila ortholog of Sestrin)-deficient fruit flies accumulate more lipid droplets in their fat bodies, equivalent to liver in mammals. The mechanism involves a negative feedback regulation of Drosophila target of target of rapamycin (dTOR) through Sestrin-induced AMPK activation (13). Feeding dSesn-null mutants with AMPK activators, such as 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranoside or metformin, or the mTOR inhibitor rapamycin reduced triglyceride (TG) accumulation (13). Additionally, the expression of the gene encoding transcription factor sterol response element binding protein and its target, such as fatty acyl coenzyme A synthase or fatty acid synthase, was significantly increased in dSesn-null mutants (13).
The role of Sesn2 and Sesn3 on metabolic homeostasis has been confirmed recently (14). Sesn2 ablation exacerbates obesity-induced mTORC1-S6K activation, glucose intolerance, insulin resistance, and hepatic steatosis, all of which are improved by AMPK activation (14). Furthermore, concomitant ablation of Sesn2 and Sesn3 provokes hepatic mTORC1-S6K activation and insulin resistance, even in the absence of nutritional overload and obesity. These results demonstrate an important homeostatic function of Sesn in the control of mammalian lipid and glucose metabolism (14).
Chronic alcohol feeding leads to the alteration in intrahepatic lipid synthesis through regulation of lipogenic genes (21), which are also under the regulation of dSesn in Drosophila (13). Because of the role of Sesn3 in lipid metabolism, the present study was designed to investigate the role of Sesn3 in the pathogenesis of alcohol-induced hepatic steatosis.
MATERIAL AND METHODS
Cell line.
The in vitro experiments were performed in the VL-17A cells (a kind gift from Dr. Dahn Clemens, University of Nebraska), the recombinant HepG2 cell line exhibiting alcohol dehydrogenase (ADH) and cytochrome P-450 2E1 specific activities (6). The VL-17A cells were cultured in Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin, and 63 μg/ml (Invitrogen).
Adenovirus transduction.
Adenovirus carrying green fluorescent protein (GFP) and Sesn3 coding sequence were generated using the pAdEasy system (Agilent). Adenovirus carrying GFP and Sesn3 short hairpin RNA sequences were generated using the BLOCK-iT system (Invitrogen), and their target sequences are the following: GFP, 5′-GCATCAAGGTGAACTTCAAGA-3′; Sesn3, 5′-GGAGAAGAACATTTGCCAACA-3′. We used a multiplicity of infection (MOI) of 50 for overexpression and a MOI of 100 for the short hairpin RNA knockdown experiments.
Animals.
Six- to eight-week-old male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were fed the Lieber-DeCarli alcohol-containing diet, as previously described (15, 16). Mice were housed individually in a room with controlled temperature (20–22°C), humidity (55–65%), and regular 12:12 light-dark cycle. Protein content was at 18% of total calories. There were two dietary groups: 1) control diet (fat comprising 10% of total calories, 6% from cocoa butter, and 4% from safflower oil, 72% of calories as carbohydrate); and 2) alcohol-containing diet [identical to the control diet but with ethanol added to account for 27.5% of total calories and the caloric equivalent of carbohydrate (maltose-dextrin) removed]. The feeding was carried out for 4 wk. Ten days before the kill date, alcohol-fed mice (n = 5 in each group) were injected with GFP (AdGFP), Sesn3-overexpressing adenoviruses (AdSesn3), shGFP, and shSesn3 through tailed vein injection. At the time of death, mice were anesthetized with isoflurane, and hepatic tissues were harvested.
The studies were approved by the Indiana University School of Medicine Animal Care and Use Committee, Indiana University Biosafety Committee, and the Roudebush Veterans Affairs Animal Use Subcommittee.
Hepatic histology, blood chemistry, and metabolic analysis.
A part of the sliced liver tissues was fixed in 10% formalin solution for hematoxylin and eosin, and the presence of intrahepatic fat was confirmed with oil red O staining. Lipids were extracted from total cell lysates (for the in vitro experiment) and hepatic tissues using a chloroform-methanol mix. TG measurements were carried out using Wako L-type TG H assay (Wako Diagnostics, Richmond, VA). Serum alanine aminotransferase (ALT) (catalog no. 752–100) was measured using the kit from BioVision (Milpitas, CA).
Protein analysis.
Hepatic tissues or cells were homogenized in the lysis buffer (50 mm HEPES, pH 7.5, 150 mm NaCl, 10% glycerol, 1% Triton X-100, 1.5 mm MgCl2, 1 mm EGTA, 10 mm sodium pyrophosphate, 100 mm sodium fluoride, and freshly added 100 μm sodium vanadate, 1 mm PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin). Protein extracts were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. Proteins were probed using the following antibodies: phosphorylated (p)-AMPK, AMPK, p-S6K1, S6K1, light chain 3 (LC3), p62, and actin (Cell Signaling Technology, Danvers, MA), Sesn1, Sesn2, and Sesn3 (Abcam). Detection of proteins was carried out by incubations with horseradish peroxidase-conjugated secondary antibodies followed by enhanced chemiluminescence detection reagents (Perkin Elmer, Waltham, MA).
Real-time RT-PCR.
Total RNA was prepared using an Absolutely RNA RT-PCR Miniprep kit (Stratagene, Cedar Creek, TX). Reverse transcription of 1-μg total RNA to cDNA was performed using the StrataScript qPCR cDNA synthesis kit (Stratagene). Real-time quantitative polymerase chain reaction amplification was performed in a Stratagene MX 3005P thermal cycler (La Jolla, CA) using RT2 SYBR Green qPCR Master Mix. Primers for SYBR Green-based real-time PCR were purchased from SuperArray Bioscience (Frederick, MD). The relative amount of target mRNA was calculated using the comparative cycle threshold (Ct) method and normalizing each target gene with Ct of housekeeping gene, GAPDH.
Statistical analysis.
All data are presented as the mean ± SD. All data in this study were analyzed with SPSS16.0 (SPSS). The significance between two groups or paired samples was analyzed by Student's t-test. The differences of quantitative variables among groups were analyzed by ANOVA. All statistical differences were two-sided, and P < 0.05 was considered statistically significant.
RESULTS
Ethanol inhibits the Sesn3 gene expression in VL-17A cells.
Recombinant HepG2 cells expressing ADH and CYP2E1 (VL-17A cells) were used in this study. Several studies have shown that VL-17A cells oxidized ethanol and generated acetaldehyde, the levels of which depended on the initial ethanol concentration (6). Thus we investigated the expression of Sesn3 in VL-17A cells in response to ethanol treatment.
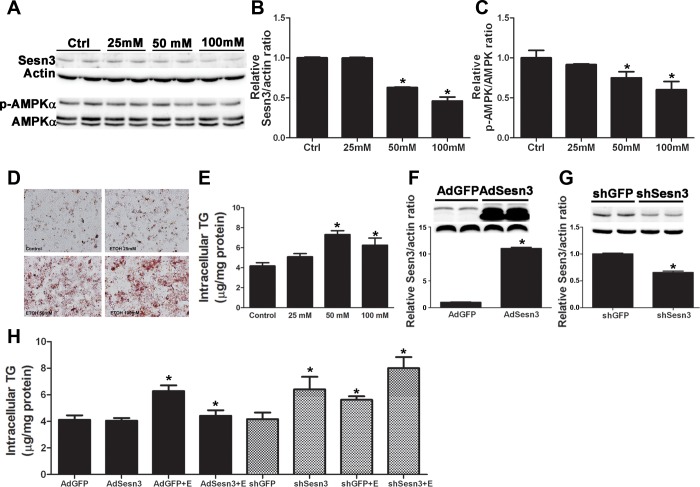
VL-17A cells were serum starved for 16 h and then exposed to ethanol at 25 mM, 50 mM, and 100 mM for 48 h. As shown in Fig. 1, A and B, ethanol inhibited the expression of Sesn3 in the dose-dependent fashion. There was a significant 40% and 60% reduction in the level of Sesn3 with the treatment of ethanol at 50 and 100 mM, respectively, compared with controls (Fig. 1, A and B). In addition, ethanol also inhibited the phosphorylation of AMPK-α on Thr172 by 30% (with ethanol 50 mM) and 40% (with 100 mM, Fig. 1, A and C). As per our laboratory's previous publications (15, 16), the ethanol concentration at 50 mM was used in the subsequent experiments.
Fig. 1.
Effect of ethanol treatment on Sestrin3 (Sesn3) and AMP-activated protein kinase (AMPK) phosphorylation in VL-17A cells. Immunoblot analysis of proteins in VL-17A cells treated with different concentrations of ethanol for 48 h is shown. The immunoblot bands (A) were quantified by densitometry analysis, and the ratio of Sesn3 to β-actin (B) and phosphorylated (p)-AMPK-α to AMPK-α (C) were calculated by setting the value of controls as one. Ethanol treatment inhibited the expression of Sesn3 and AMPK-α phosphorylation in the VL-17A cells in a dose-dependent manner. Results are means ± SD (the blot is a representative of 3 blots from 3 individual experiments). Significant differences among group were determined by one-way ANOVA. *P < 0.05, significant difference vs. control. Accumulation of triglyceride (TG) in VL-17A cells after ethanol treatment is shown. The presence of TG was confirmed by Oil Red O staining (D), as well as TG measurement (E). VL-17A cells were transduced with control vectors [AdGFP (green fluorescent protein) or shGFP], AdSesn3 (F) and shSesn3 (G). H: overexpression of Sesn3 ameliorated TG accumulation in VL-17A cells exposed to ethanol (E). Values are means ± SD. *P < 0.05, significant difference vs. respective vector controls.
Overexpression of Sesn3 reduces TG accumulation in VL-17A cells exposed to ethanol.
To determine whether the inhibition of Sesn3 by ethanol might affect the cellular TG accumulation in vitro, we first tested the ability of VL-17A cells to accumulate TG after ethanol treatment. VL-17A cells were treated with ethanol (25–100 mM) for 48 h and were harvested for TG measurement. As shown in Fig. 1, D and E, ethanol 50 and 100 mM increased the TG accumulation in VL-17A cells by 1.8- and 1.6-fold, respectively.
To assess the role of Sesn3 in ethanol-induced TG accumulation, VL-17A cells were seeded onto six-well plates at the concentration of 4 × 105 cells/well in the cultured medium described above. Cells were switched to serum-free media, infected with AdGFP and AdSesn3 adenoviruses at 50 MOIs for overexpression and 100 MOIs for shGFP and shSesn3 for knockdown, and gently rocked for 2 h. After the transduction, cells were treated with ethanol at 50 mM for 48 h, and cells were harvested for TG measurement.
To confirm the transfection efficiency, the levels of Sesn3 were determined. AdSesn3 increased the expression of Sesn3 in VL-17A by 11-fold (Fig. 1F), whereas shSesn3 decreased the expression of Sesn3 in VL-17A by 1.8-fold (Fig. 1G). Both endogenous and overexpressed Sesn3 proteins were observed between 50 and 55 kDa, indicating that GFP might be cleaved out from the recombinant protein (Fig. 1F). Intracellular TG in AdGFP cells treated with ethanol was increased by 1.6-fold, and overexpression of Sesn3 by AdSesn3 significantly reduced TG accumulation in VL-17A cells exposed to ethanol (4.4 ± 0.4 μg/mg protein vs. 6.3 ± 0.4 μg/mg protein, P < 0.05) (Fig. 1H). Knocking down Sesn3 by shSesn3 led to the increase in intracellular TG by 1.5-fold compared with controls transfected with shGFP (Fig. 1H). Likewise, ethanol treatment increased the levels of cellular TG in shGFP-treated cells by 1.4-fold. shSesn3 significantly worsened TG accumulation in VL-17A cells exposed to ethanol compared with controls (8.0 ± 0.8 vs. 4.1 ± 0.4 μg/mg protein, P < 0.05, Fig. 1H). Taken together, our results confirmed the role of Sesn3 in ethanol-induced lipid accumulation.
Ethanol suppresses hepatic Sestrins expression in mice chronically fed with ethanol.
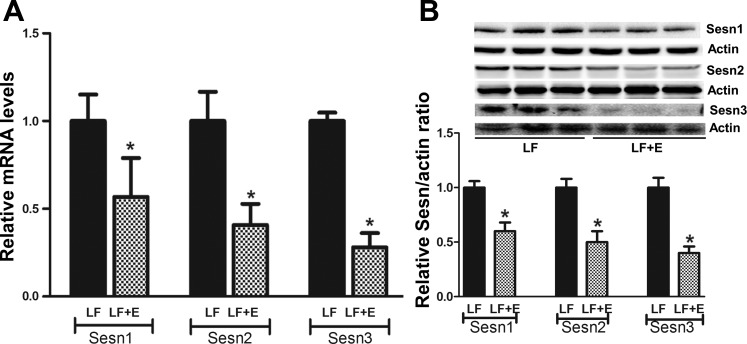
We next determined whether the effects of ethanol observed in vitro also took place in vivo using liver samples from our laboratory's previously published study from controls and pair-fed mice with Lieber Dicarli Diet (15). As shown in our previous publications, these mice developed hepatic steatosis after they were fed with ethanol-containing diet for 4 wk (15, 16). Mouse liver expressed all three Sesn family members, with Sesn3 being the most abundant Sestrin (data not shown). Ethanol feeding markedly decreased the total mRNA expression of all three Sestrins; however, its effect was most pronounced on Sesn3 (∼2-fold for Sesn1, 2.5 -fold for Sesn2, and ∼5-fold for Sesn3) (Fig. 2, A and B).
Fig. 2.
Ethanol inhibits hepatic Sesn expression. A: Sesn mRNA was analyzed from the hepatic tissues in mice fed with ethanol (E) for 4 wk compared with pair-fed controls. Ethanol's effect was most pronounced on Sesn3. B: chronic ethanol feeding significantly reduced protein expression of all three Sestrins, particularly on Sesn3. Data were analyzed from controls and ethanol-fed mice (n = 3 in each group). *Significance with P < 0.05 compared with controls. LF, low fat.
Effect of overexpression and knockdown of Sesn3 on ethanol-induced hepatic steatosis in vivo.
To further study the roles of Sesn3 and ethanol-induced hepatic steatosis in vivo, we used both gain-of-function and loss-of-function-based approaches by injecting AdSesn3 and shSesn3, respectively, into mice. Six- to eight-week-old male C57BL/6J mice were fed with the Lieber-DeCarli alcohol-containing diet for 4 wk. Ten days before death, ethanol-fed mice (n = 5 in each group) were injected with GFP (AdGFP), Sesn3-overexpressing adenoviruses (AdSesn3), shGFP, and shSesn3 through tail vein injection.
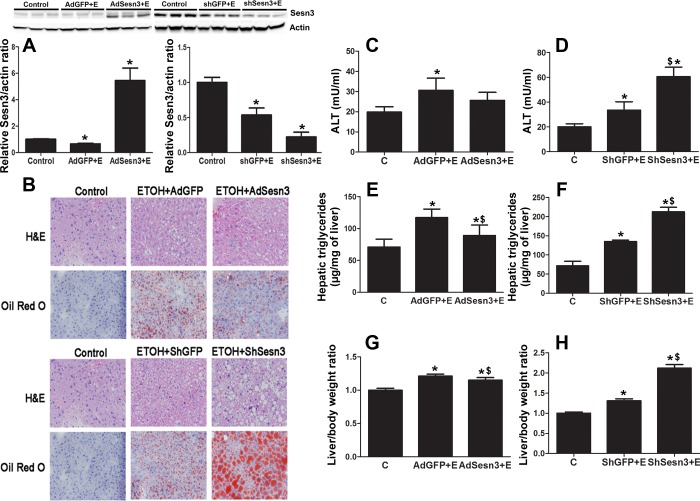
Ethanol feeding inhibited hepatic Sesn3 protein expression in mice injected with AdGFP and shGFP compared with controls by approximately twofold (Fig. 3A). AdSesn3 injection overcame the effect of ethanol on Sesn3 by increasing hepatic expression of Sesn3 by 5.5-fold compared with controls and ∼11-fold compared with ethanol-fed mice injected with AdGFP. Injection of shSesn3 in mice fed with ethanol significantly reduced hepatic Sesn3 expression by 3.3-fold (compared to controls) and 1.7-fold (compared to mice injected with shGFP) (Fig. 3A).
Fig. 3.
Effect of overexpression and knockdown of Sesn3 on ethanol (ETOH)-induced hepatic steatosis in vivo. A: hepatic Sesn3 expression from mice in each group. B: histology [hematoxylin and eosin (H&E) and Oil Red O stain of hepatic tissues in controls (C) and mice fed with ethanol]. Ethanol feeding caused hepatic steatosis. Overexpression of Sesn3 using AdSesn3 prevented ethanol-induced hepatic steatosis, and knockdown of Sesn3 with shSesn3 significantly worsened hepatic steatosis in mice fed with ethanol. C and D: serum alanine aminotransferase (ALT) levels in controls and ethanol-fed mice injected with AdGFP and AdSesn3 (C) and shGFP and shSesn3 (D). E and F: hepatic triglyceride measurements in controls and ethanol-fed mice injected with AdGFP and AdSesn3 (E) and shGFP and and shSesn3 (F). Overexpression of Sesn3 significantly reduced hepatic TG concentrations in mice fed with ethanol. G and H: liver and body weight ratio from each experimental group. Ethanol-fed mice injected with shSesn3 had the highest liver-to-body weight ratio. *P < 0.05, significant difference compared with controls. $P < 0.05 vs. respective vector controls.
Alteration of hepatic Sesn3 with AdSesn3 and shSesn3 injection affected ethanol-induced hepatic steatosis. As shown in Fig. 3B, overexpression of Sesn3 using AdSesn3 prevented hepatic steatosis, whereas knockdown of Sesn3 with shSesn3 significantly worsened hepatic steatosis in mice fed with ethanol. We observed the levels of serum ALT were elevated in ethanol-fed mice (injected with AdGFP and shGFP) compared with controls. Although hepatic steatosis was significantly improved with AdSesn3 injection, interestingly, we did not observe the differences in the levels of serum ALT compared wit that in ethanol-fed mice injected with AdGFP. On the other hand, the serum ALT levels in ethanol-fed mice injected with ShSens3 were significantly higher (60.4 ± 7.3 mU/ml) than those in controls and in ethanol-fed mice injected with shGFP (Fig. 3, C and D).
Biochemical analysis by measurement of hepatic TG confirmed that ethanol increased hepatic TG accumulation in mice injected with AdGFP and shGFP compared with controls (Fig. 3, E and F). Overexpression of Sesn3 significantly reduced hepatic TG concentrations in mice fed with ethanol compared with those injected with AdGFP by 1.4-fold. Knocking down Sesn3 by shSesn3 significantly increased hepatic TG concentrations in ethanol-fed mice by almost 4-fold (compared with pair fed controls) and 1.8-fold (compared with ethanol-fed mice injected with shGFP). Morphological examination of the hepatic tissues showed the increase in liver-to-body weight ratio in ethanol-fed mice compared with controls (Fig. 3, G and H). However, ethanol-fed mice injected with shSesn3 had the highest liver-to-body weight ratio compatible with the degree of hepatic steatosis as shown by Oil Red O staining and TG measurements (Fig. 3H).
Effects of overexpression and knockdown of Sesn3 on the AMPK signaling pathway and expression of genes involved in fatty acid synthesis and oxidation in mice fed with ethanol.
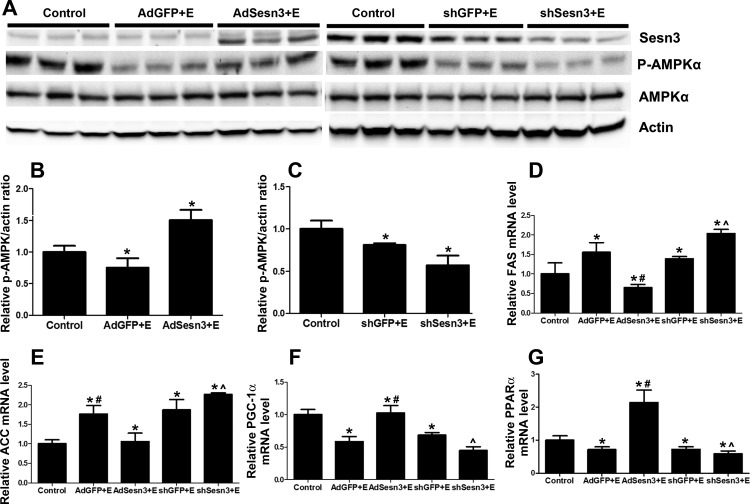
Sesn3 has been shown to activate AMPK (13, 14). We, therefore, further explored the role of Sesn3 on the AMPK signaling pathway in ethanol-fed mice. As shown in Fig. 4A, ethanol significantly inhibited the phosphorylation of AMPK-α, indicated by a reduction in hepatic p-AMPK-α-to-AMPK-α ratio in mice injected with either AdGFP or shGFP. Overexpression of Sesn3 with AdSesn3 significantly abrogated the effect of ethanol on AMPK phosphorylation, and it increased the expression of p-AMPK-α-to-AMPK-α ratio by 1.5-fold (compared with control) and 2-fold (compared with AdGFP-injected mice fed with ethanol) (Fig. 4B). The effect of ethanol on AMPK phosphorylation was augmented by knocking down Sesn3 with shSesn3 (Fig. 4, A and C). Knocking down Sesn3 led to the reduction of p-AMPK-α-to-AMPK-α ratio by 1.6-fold compared with shGFP-injected mice fed with ethanol (Fig. 4C).
Fig. 4.
Overexpression and knockdown of Sesn3 affect the AMPK signaling pathway in mice fed with ethanol. A: Western blotting of p-AMPK-α proteins in controls, AdGFP-, shGFP-, AdSesn3-, and shSesn3-injected mice. Actin was used as the loading controls. B and C: bar graphs represented the immunoblot analysis. Overexpression or knockdown Sesns3 also regulated expressions of genes encoding fatty acid synthesis (FAS) (D and E) and oxidation (F and G). ACC, acetyl-CoA carboxylase; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; PPAR-α, peroxisome proliferator-activated receptor-α. *P < 0.05, significant difference compared with controls. #,^P < 0.05 vs. respective vector controls.
We next determined the effect of overexpression and knockdown of Sesn3 on the mRNA expression of genes encoding for fatty acid oxidation or synthesis in ethanol-fed mice. As shown in Fig. 4, D and G, ethanol feeding increased mRNA expression of lipogenic enzymes (fatty acid synthase, and acetyl-CoA carboxylase) and inhibited expression of genes involved in fatty acid oxidation (peroxisome proliferator-activated receptor-γ coactivator-1α and peroxisome proliferator-activated receptor-α). Overexpression of Sesn3 reversed the ethanol effect, as it significantly reduced the expression of genes encoding for lipid synthesis and increased the levels of mRNA involved in fatty acid oxidation. Conversely, knocking down Sesn3 by shSesn3 worsened the effect of ethanol on the mRNA expression levels of these genes. Taken together, our findings suggested that Sesn3 regulated genes involved in hepatic lipid metabolism in ethanol fed mice.
Impacts of overexpression or knockdown of Sesn3 on the mTOR signaling and autophagy pathway in mice fed with ethanol.
Previous reports have showed that upregulation of Sesn3 downregulates mTOR, a major regulatory pathway suppressing the activation of autophagy (2, 3, 9, 13, 14). We thus determined the effect of overexpression and knockdown of Sesn3 on mTOR signaling and autophagy pathway in mice fed with ethanol.
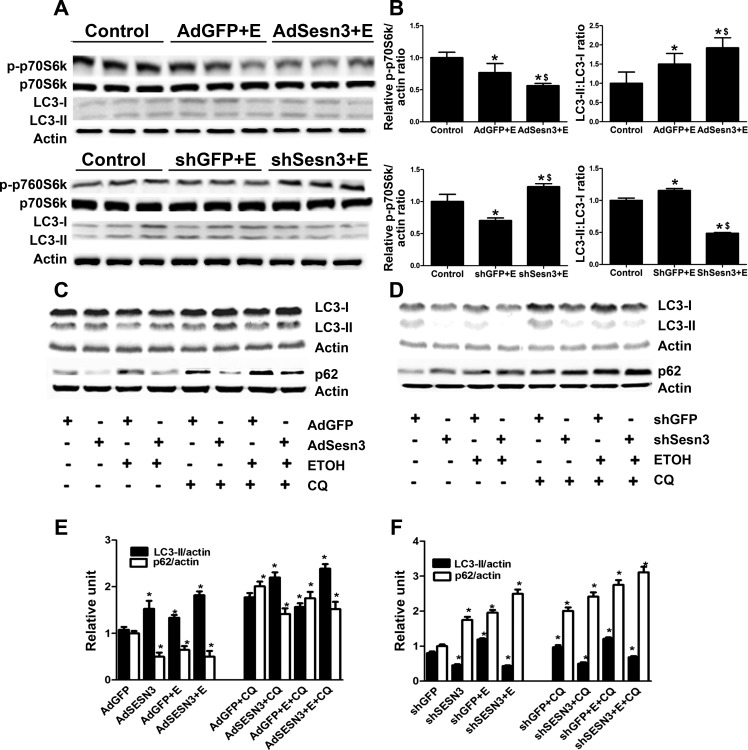
Chronic ethanol feeding for 4 wk in mice injected with either AdGFP or shGFP led to the inactivation of mTOR (∼1.3- to ∼1.4-fold compared with controls), as manifested by decreasing in phosphorylation of p70 S6 kinase, a major downstream target of mTOR. Overexpression of Sesn3 augmented the effect of ethanol on phospho-p70 S6 kinase by suppressing the expression of phospho-p70 S6K protein by 1.7- and 1.4-fold, compared with pair-fed controls and ethanol-fed mice injected with AdGFP, respectively (Fig. 5, A and B). On the other hand, knockdown of Sesn3 using shSesn3 reversed the effect of ethanol on mTOR, indicated by the increase in phospho-p70 S6K in ethanol-fed mice injected with shSesn3 by 1.2- and 1.75-fold compared with controls and ethanol-fed mice injected with shGFP, respectively (Fig. 5, A and B).
Fig. 5.
Overexpression and knockdown of Sesn3 affect the mTOR signaling and autophagy pathway in mice fed with ethanol. A and B: effect of ethanol on p70 S6K, a major downstream target of mTOR and marker of autophagy [light chain 3 (LC3)] in mice liver was analyzed by Western blotting. C and F: to confirm the findings in vivo, we performed autophagic flux assay using VL-17A cells, which were transfected with AdSesn3 and shSesn3 treated as indicated in the presence or absence of chloroquine (CQ; 25 μM). The immunoblot bands (C and D) were quantified by densitometry analysis, and the ratio of LC3-II (E) and p62 (F) to β-actin was calculated. *P < 0.05, significant difference compared with controls. $P < 0.05 vs. respective vector controls.
Next we determined the effects of overexpression and knockdown Sesn3 on the expression of microtubule-associated protein 1 LC3 in ethanol-fed mice. LC3, commonly used as a marker for autophagy, has two forms: LC3-I and LC3-II. When autophagy is activated, cytosolic LC3-I is converted to membrane-bound lapidated LC3-II. We found that the levels of LC-II were increased by ∼1.5-fold in mice chronically fed with ethanol (Fig. 5, A and B), suggesting the induction of autophagy in ethanol-fed mice. Overexpression of Sesn3 using adenovirus increased the levels of hepatic LC3-II by 1.9-fold (compared with controls) and 1.2-fold (compared with mice fed with ethanol). On the other hand, suppression of Sesn3 with shSesn3 significantly decreased the levels of hepatic LC3-II by 2-fold compared with controls and by 2.4-fold compared with ethanol-fed mice (Fig. 5, A and B).
To confirm the findings in vivo and more accurately monitor autophagic flux, we treated VL-17A cells transfected with AdSesn3 and shSesn3 in the absence or presence of chloroquine (25 μM), which modifies lysosomal pH, and thus inhibits autophagy flux through lysosomes. We performed additional analysis of autophagy using two commonly used markers, p62/Sqstm1 (sequestosome 1) and LC3-II. The p62 protein is involved in chaperone-mediated protein degradation. During autophagy, the amount of p62 is inversely correlated with cellular autophagic activity (11). Overexpression of Sesn3 by AdSens3 increased the amount of LC3-II by 1.5-fold, while the expression of p62 was reduced by almost 2-fold, suggesting the increase in autophagic activity with the overexpression of Sesn3. Ethanol increased the levels of LC3-II by 1.3-fold, and the levels of LC3-II was further increased (by 1.8-fold compared with controls and 1.3-fold compared with ethanol-treated cells) when we overexpressed Sesn3 in the VL-17A cells. In the loss of function experiments, suppression of Sesn3 led to the reduction in LC3-II expression by 1.7-fold. While ethanol treatment in VL-17A cells transfected with shGFP caused the increase in LC3-II levels by 1.2-fold, suppression of Sesn3 in the presence of ethanol decreased the expression of LC3-II by 2.8-fold. Similar observations were observed when the experiments were performed with the presence of chloroquine. Furthermore, the expression of p62 was inversely correlated with the expression of LC3-II (Fig. 5, C–F).
DISCUSSION
In this study, we observed that ethanol treatment in cultured cells and chronic ethanol feeding in mice significantly reduced hepatic Sesn3 expression. Overexpression of Sesn3 improved the effect of ethanol on TG accumulation in VL-17A cells and mouse livers. On the other hand, in the loss-of-function experiments, knockdown of Sesn3 worsened the effect of ethanol by promoting fat accumulation in VL-17A cells and the liver of mice fed with ethanol. The role of Sesn3 in ethanol-induced hepatic steatosis is likely mediated in part through AMPK signaling that leads to the alteration in the set of genes involving in fatty acid oxidation and synthesis.
Sestrins are involved in complex regulation of cell viability in response to various external stimuli causing cellular injury (3). Sestrins maintain metabolic homeostasis by suppressing mTOR activity (2, 4, 13). Sestrins can bring an upstream kinase liver kinase B1 (LKB1) and regulatory AMPK-β and AMPK-γ subunits to the catalytic AMPK-α subunit, thereby facilitating LKB1-dependent activator Thr172 phosphorylation of AMPK-α (19). Through regulation of the AMPK-mTORC1 signaling axis, Sestrins promote metabolic adaptation of cells and regulate cellular and lipid metabolism (8). In Drosophila, loss of dSesn causes moderate downregulation of AMPK and upregulation of dTORC1 in the fat body, thus leading to increased expression of mRNAs encoding lipogenic enzymes, ultimately resulting in TG accumulation. Interestingly, this excessive fat accumulation can be suppressed by pharmacological activation of AMPK and inhibition of dTORC1 (12).
In our study, we found the important role of Sesn3 in the pathogenesis of alcoholic steatosis. We found that ethanol can downregulate Sesn3 at both mRNA and protein levels. Overexpression of Sesn3 overcame the effect of ethanol on hepatic steatosis, likely through the activation of AMPK-α, which led to the inhibition of fatty acid synthesis and activation of fatty acid oxidation. By contrast, knockdown of Sesn3 led to the inhibition of AMPK-α and deteriorates the degree of hepatic steatosis in mice fed with ethanol. In our experiments, we also observed the elevation of ALT in the liver of ethanol-fed mice. In general, the ethanol feeding protocol that we used rarely caused significant hepatic inflammation, as indicated by minimal or no inflammatory cells infiltration in the hematoxylin and eosin staining. We suspected that the elevation in ALT was the surrogate for the presence of hepatic steatosis. In fact, elevation of ALT was observed in human subjects with hepatic steasosis in the absence of steatohepatitis (10, 20). Interestingly, we found significant increase in serum ALT in ethanol-fed mice injected with shSesn3, parallel with worsening hepatic steatosis in these mice.
Sestrins are a family of highly conserved stress-responsive proteins. Ethanol is known to generate intracellular oxidative stress; however, we, in fact, found the inhibition of Sesn3 by ethanol. Our results are not surprising as each Sesns gene might be regulated differently. For instance, Sesn2 can be induced by starvation, and high-fat diet (HFD) (1, 2, 14). On the other hand, Sesn3 is not induced by high-fat diet in mouse liver or by H2O2 (which generates oxidative stress) in human primary myotubes (14, 18). Clearly, the mechanism on how ethanol inhibits Sesn3 needs to be further evaluated.
Ethanol also affects autophagy via metabolic intermediates and mTOR inhibition (5). Previous studies showed that the induction of autophagy was only observed in ethanol-treated VL-17A cells (which efficiently metabolized ethanol due to overexpression of ADH and CYP2E1, similar to our findings), but not in ethanol-treated HepG2 cells, which have a very weak capability of metabolizing ethanol (5). The mTOR pathway is a major pathway suppressing the activation of autophagy. Ethanol caused a dose-dependent inhibition of mTOR, and its effect was reversed with the inhibitor of ethanol metabolism (5). Interesting, pharmacological promotion of autophagy with carbamazepine or rapamycin reduced hepatic steatosis in ethanol-fed mice (17). In our study, overexpression of Sesn3 can induce autophagy in hepatocytes, and its effect was likely through the inhibition of mTOR activity. The enhanced autophagic activity could thus result in increased degradation of lipid droplets, and thus improvement in hepatic steatosis, which was observed in mice injected with AdSesn3. We observed the opposite results with worsening hepatic steatosis when we suppressed the expression of Sesn3 with shSesn3.
It is important to point out that the previous report did not demonstrate significant hepatic steatosis in mice with concomitant loss of Sesn2 and Sesn3 (14). In our study, we found that suppression of Sesn3 alone using shSesn3 in the presence of ethanol led to significant hepatic steatosis. It is plausible that the developmental loss of Sesn2/3 might have induced a certain compensatory mechanism that overcomes or corrects the underlying metabolic derangements in Sesn2/3 double knockout mice. Perhaps such a mechanism did not take place in our model when the expression of Sesn3 was temporarily silenced with the introduction of adenovirus.
In summary, we have identified Sesn3 as a critical player in the development of ethanol-induced fatty liver. Our data also suggest that modulation of Sesn3 might be useful for the prevention and treatment of alcoholic fatty liver disease.
GRANTS
This study is supported in part by K08 AA016570 from the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism, Central Society for Clinical Research Career development award, Indiana University Research Support Fund Grant, and 1I01CX000361-01A1, Veterans Affairs Merit Review Award (all to S. Liangpunsakul), and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-091592 (to X. C. Dong).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
X.K., K.P., R.T., and X.X. performed experiments; X.K., R.T., and X.X. analyzed data; X.K. and K.P. interpreted results of experiments; X.K. and S.L. prepared figures; X.K., X.C.D., and S.L. drafted manuscript; X.C.D. conception and design of research; X.C.D. and S.L. edited and revised manuscript; S.L. approved final version of manuscript.
ACKNOWLEDGMENT
The authors thank Dr. Xiao-Ming Yin for input during manuscript preparation.
REFERENCES
- 1.Bae SH, Sung SH, Oh SY, Lim JM, Lee SK, Park YN, Lee HE, Kang D, Rhee SG. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab 17: 73–84, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134: 451–460, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budanov AV, Lee JH, Karin M. Stressin' Sestrins take an aging fight. EMBO Mol Med 2: 388–400, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CC, Jeon SM, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, Park Y, Hay N. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell 18: 592–604, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, Lu B, Stolz DB, Clemens DL, Yin XM. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology 139: 1740–1752, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donohue TM, Osna NA, Clemens DL. Recombinant Hep G2 cells that express alcohol dehydrogenase and cytochrome P450 2E1 as a model of ethanol-elicited cytotoxicity. Int J Biochem Cell Biol 38: 92–101, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141: 1572–1585, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hay N. p53 strikes mTORC1 by employing sestrins. Cell Metab 8: 184–185, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 47: 1363–1370, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Komatsu M, Kageyama S, Ichimura Y. p62/SQSTM1/A170: physiology and pathology. Pharmacol Res 66: 457–462, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Budanov AV, Karin M. Sestrins Orchestrate Cellular Metabolism to Attenuate Aging. Cell Metab 18: 792–801, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 327: 1223–1228, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Budanov AV, Talukdar S, Park EJ, Park HL, Park HW, Bandyopadhyay G, Li N, Aghajan M, Jang I, Wolfe AM, Perkins GA, Ellisman MH, Bier E, Scadeng M, Foretz M, Viollet B, Olefsky J, Karin M. Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3. Cell Metab 16: 311–321, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liangpunsakul S, Rahmini Y, Ross RA, Zhao Z, Xu Y, Crabb DW. Imipramine blocks ethanol-induced ASMase activation, ceramide generation, and PP2A activation, and ameliorates hepatic steatosis in ethanol-fed mice. Am J Physiol Gastrointest Liver Physiol 302: G515–G523, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liangpunsakul S, Sozio MS, Shin E, Zhao Z, Xu Y, Ross RA, Zeng Y, Crabb DW. Inhibitory effect of ethanol on AMPK phosphorylation is mediated in part through elevated ceramide levels. Am J Physiol Gastrointest Liver Physiol 298: G1004–G1012, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin CW, Zhang H, Li M, Xiong X, Chen X, Chen X, Dong XC, Yin XM. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J Hepatol 58: 993–999, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nascimento EB, Osler ME, Zierath JR. Sestrin 3 regulation in type 2 diabetic patients and its influence on metabolism and differentiation in skeletal muscle. Am J Physiol Endocrinol Metab 305: E1408–E1414, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanli T, Linher-Melville K, Tsakiridis T, Singh G. Sestrin2 modulates AMPK subunit expression and its response to ionizing radiation in breast cancer cells. PLos One 7: e32035, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiqui MS, Sterling RK, Luketic VA, Puri P, Stravitz RT, Bouneva I, Boyett S, Fuchs M, Sargeant C, Warnick GR, Grami S, Sanyal AJ. Association between high-normal levels of alanine aminotransferase and risk factors for atherogenesis. Gastroenterology 145: 1271–1279, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sozio MS, Liangpunsakul S, Crabb D. The role of lipid metabolism in the pathogenesis of alcoholic and nonalcoholic hepatic steatosis. Semin Liver Dis 30: 378–390, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Supakul R, Liangpunsakul S. Alcoholic-induced hepatic steatosis–role of ceramide and protein phosphatase 2A. Transl Res 158: 77–81, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]