Abstract
The human intestinal tract harbors a complex ecosystem of commensal bacteria that play a fundamental role in the well-being of their host. There is a general consensus that diet rich in plant-based foods has many advantages in relation to the health and well-being of an individual. In adults, diets that have a high proportion of fruit and vegetables and a low consumption of meat are associated with a highly diverse microbiota and are defined by a greater abundance of Prevotella compared with Bacteroides, whereas the reverse is associated with a diet that contains a low proportion of plant-based foods. In a philosophical term, our consumption of processed foods, widespread use of antibiotics and disinfectants, and our modern lifestyle may have forever altered our ancient gut microbiome. We may never be able to identify or restore our microbiomes to their ancestral state, but dietary modulation to manipulate specific gut microbial species or groups of species may offer new therapeutic approaches to conditions that are prevalent in modern society, such as functional gastrointestinal disorders, obesity, and age-related nutritional deficiency. We believe that this will become an increasingly important area of health research.
Keywords: colon cancer, dysbiosis, gastrointestinal tract, microbiota, natural products
“The doctor of the future will no longer treat the human frame with drugs, but rather will cure and prevent disease with nutrition.”
—Thomas Edison
Natural Products and Human Health
A large number of diverse natural products or secondary metabolites produced from plants are integral to normal cell growth, development, or the reproduction of organisms. In some cases, these plants have been utilized as key food sources or herbal remedies by human societies for thousands of years. Many of the metabolites of these plants have important biological properties, which make them desirable candidates for optimization of drug discovery and developmental processes.
Consumption of natural products and vegetables can reduce risk of a series of life-threatening diseases, including cardiovascular disorders, diabetes, and cancer. Population-based studies, including case-control and cohort studies, indicate that five or more servings of fruits and vegetables a day reduces the risk of developing cancer by half, especially for cancers of the digestive and respiratory systems. The National Cancer Institute has identified plant-based foods that contain cancer-preventive properties, including soybeans, garlic, onion, ginger, turmeric, tomatoes, and cruciferous vegetables (135). The antiproliferative activities of many fruit and vegetable extracts derive from a combination of phytochemicals inside, leading to potential anti-inflammatory and anticancer usage (26, 134). Currently, most immunomodulatory agents that are also anticarcinogenic belong to two classes: 1) blocking agents that inhibit tumor initiation and 2) suppressing agents that attenuate tumor progression and frank malignancy (154). Because of safety concerns related to potent chemotherapeutic drugs, phytochemicals derived from the diet are considered potential alternatives to chemoprevention (119).
The phytochemicals in many fruits and vegetables have complementary and overlapping modes of action, such as free radical scavenging and antioxidant activity, gene regulation in cell proliferation and apoptosis, etc. Dietary chemopreventive compounds functioning as detoxifying enzyme inducers primarily include phenolic and sulfur-containing compounds. Phenolic compounds may be classified into polyphenols and flavonoids including epigallocatechin-3-gallate (EGCG) from green tea, curcumin from turmeric, and resveratrol from grapes. Quercetin from citrus fruits and genistein from soy are examples of flavonoids. Sulfur-containing compounds similarly may be classified into isothiocyanates and organosulfur compounds. Isothiocyanates include, among others, sulforaphane from broccoli, phenethyl isothiocyanate from turnips and watercress, and allyl isothiocyanate from Brussels sprouts. Organosulfur compounds chiefly include diallyl sulfides from garlic oil. Dietary isothiocyanates are derived in vivo from the hydrolysis of glucosinolates present in cruciferous vegetables.
Given the fact that phytochemicals are natural, comparatively safe, and low cost, researchers around the world have begun to focus on their characteristics. The key question, however, is whether a purified phytochemical has the same health benefits as the same constituent when being present in the whole food or a mixture of dietary components. Epidemiological studies have suggested that herbal medicines or fruit extracts play a major role in the prevention and treatment of many types of cancer, including that of the colon (98, 107, 147). Despite these studies, a report published in 2007 found severe skepticism regarding the protective nature of fruits and vegetables against cancer (66). However, it is important to keep in mind that a plethora of factors, including the amount of phytochemicals consumed, have to be considered before a pessimistic conclusion can be drawn. In general, appropriate concentrations of dietary phytochemicals along with mechanistic studies are essential to generate conclusive results that are physiologically relevant. Hence, it is imperative to unveil the exact physiological (or nutritional) dose of the phytochemicals or herbal extracts that can be used to improve and maintain optimal health. Since the development of many cancers, in particular colorectal cancer, involves multiple signaling pathways and numerous molecular targets, the use of a single phytochemical molecule may not suffice in isolation. Nonetheless, it has been proven that a mixture of bioactive herbal compounds or phytochemicals, possibly with synergistic effects from constituents, can effectively prevent mutations, modulate inflammation and immune responses, and prevent tumor cell proliferation (75). Despite enormous strides in the effort to search for novel drugs and treatments, cancer continues to be a major public health problem. Moreover, the emergence of resistance to cancer chemotherapy often prevents complete remission. Researchers have thus begun to turn to natural products from plant origin to circumvent resistance. A summary of dietary plant extracts or bioactive compounds thought to be effective in the treatment of cancers are described below under individual headings.
Pectin.
Henri Braconnot, a French chemist and pharmacist in 1825, was the first to discover a heteropolysaccharide with gelling properties, which he named “pectic acid” (74). Pectins are a family of covalently linked galacturonic acid-rich polymers that are found in high amounts in the cell wall of the plants. The main role of the cell wall components is to give mechanical strength to plants, to maintain an extracellular water phase by imbibition, and to provide a barrier from external environment. Pectin and pH- or heat-modified pectin have demonstrated chemopreventive and antitumoral activities against some aggressive and recurrent cancers. In addition to being used as a gelling agent in the food industry, pectin displays properties useful in medicine (72). In humans, pectin, as a dietary fiber, is not enzymatically digested in the small intestine but is degraded by the microbiota in the colon. It keeps its gelling action in the digestive tract, so that it slows down digestion. This is especially beneficial to patients with dumping syndrome who exhibit rapid gastric emptying, a condition in which ingested foods bypass the stomach too rapidly and enter the small intestine largely undigested (73). Pectin diminishes blood cholesterol level and stimulates lipid excretion. However, the exact mechanisms underlying these effects are not known (16). PectaSol, a modified form of pectin, when eaten during several days, allows a better clearance via the urinary tract of toxic elements like arsenic or cadmium, which seem to be chelated by modified pectin and then eliminated in the urine (35). In addition, several studies have shown that orally taken pectin decreases the risk of intestinal infection and of diarrhea in children by favoring the growth of Bifidobacteria and Lactobacillus bacterial species in the colon (95).
We have shown in an in vivo model of Citrobacter rodentium (CR) that dietary pectin modulates colonic crypt hyperproliferation and hyperplasia (20, 21, 143). In susceptible hosts, when CR-infected mice were placed on 6% pectin diet for 9 days starting 2 days post-CR infection, it 1) reduced the morbidity and mortality of CR-infected C3H mice, 2) restored the junctional labeling of β-catenin/E-cadherin and ZO-2, and 3) restored mucosal barrier function. Additionally, we found that mice treated with dietary pectin and subjected to 14 Gy irradiation (IR) had significant crypt stem cell survival following microcolony assay (unpublished observation). Furthermore, IR-subjected mice treated with pectin had improvement in overall survival compared with mice treated with normal diet. Thus pectin administration following severe IR can mitigate radiation-induced deletion of gut stem and/or progenitor cells, facilitate crypt regeneration, enhance mucosal barrier function, and ultimately promote survival. This important work may begin to justify the use of prebiotics as a preventive or chemoprotectant.
As a dietary fiber, pectin also plays a role in preventing colon cancer. In 1979, Watanabe et al. (153) showed that rats treated with azoxymethane or methylnitrosourea developed less colon tumors if their diet was enriched in pectin. Heitman et al. (47) similarly demonstrated fewer colon tumors in rats treated with 1,2-dimethylhydrazine if they were given pectin. Ohkami et al. (94) evidenced that citrus and apple pectin in the diet of rats exposed to azoxymethane decreased carcinogenesis. Both types of pectin decreased the number of tumors, and apple pectin decreased the activity of β-glucuronidase, an enzyme from fecal bacteria whose activity has been linked to colon cancer development.
Colon carcinogenesis is a multistep process that results from disruption of the balance between proliferation of colonocytes at the base of the colonic crypts and loss of colonocytes at the luminal surface due to apoptosis. Most colon cancer cells become resistant to apoptosis, hence promoting tumor growth. A chemoprotection strategy that restores luminal colonocyte sensitivity to apoptosis would have tremendous potential. Indeed, in a study by Schwartz et al. (118), a pectin-rich diet, compared with a standard diet, facilitated the expression of caspase-1 in luminal colonocytes from colon crypts and increased cleaved PARP level in basal and luminal colonocytes of rats. Similarly in an elegant study, Avivi-Green et al. (9) demonstrated that activation of apoptosis due to a pectin-rich diet had protective effects and diminished the number and the size of tumors in rats treated with 1,2-dimethylhydrazine. Colonocytes of rats receiving dietary pectin exhibited a high activity of caspase-1 with a higher level of cleaved PARP. Pectin per se may induce apoptosis since the viability of cells exposed in culture to different pectin-derived oligosaccharides is decreased. In similar studies in either colon cancer or pituitary tumor cells, pectin has been shown to induce various levels of DNA fragmentation and apoptosis in a concentration-dependent manner (7, 96). In combination with n-3 polyunsaturated fatty acid-rich fish oil, pectin intervention was associated with a decrease in Bcl-2 expression due to promoter methylation (25) as well as to changes in the expression profile of miRNA targeting canonical oncogenic signaling pathway (24, 31, 121).
Interestingly, pectin's ability to modulate apoptotic machinery is predominantly mediated by butyrate, a metabolite generated following bacteria-induced fermentation of pectin in the colon (8, 9). Indeed, intracolonic instillation of butyrate recapitulates the effect of orally administered pectin (8). Butyrate is also able to induce apoptosis in colonocytes in vitro in a p53-independent manner (64) and by inducing mitochondrial Ca2+ overload (63). In parallel, both in vitro in rat intestinal epithelial cells exposed to butyrate and in mice fed with a diet supplemented with 20% pectin, TGF-β signaling is enhanced, leading to colonocyte growth inhibition and apoptosis. Apoptosis seems to be induced via an increased expression of inhibitor of differentiation 2, probably via inhibition of selective isoforms of HDACs (17). In recent studies, our laboratory has used the CR infection model to demonstrate significant inhibitory effect of pectin on Wnt/β-catenin signaling as well as immunomodulatory effect on epithelial-stromal cross talk via NF-κB signaling (21, 143).
In summary, pectin has been shown to exert significant anti-inflammatory and antitumor activities through different cell lines and in a variety of mouse models. As a dietary fiber, pectin is not digested in the upper digestive tract but is fermented in the colon by bacteria into butyrate, which inhibits colon inflammation and prevents carcinogenesis. pH-modified pectin as well as galactan-rich pectin are capable of interacting with galectin-3, thus inhibiting cell-cell interactions and cancer cell metastasis. Furthermore, homogalacturonan-rich pectin with a high degree of esterification competes with LPS for TLR4 binding, hence preventing inflammatory cell activation. Finally, heat-modified pectin initiates apoptosis in cancer cells, in a galectin-3-independent manner. The exact structure and the mechanism of action of modified pectin molecules is not yet known. But modified pectin may be a promising antimetastatic drug in humans, especially if used in combination with more conventional molecules.
Curcumin.
Turmeric is derived from the rhizomes of the plant Curcuma longa, a member of the ginger family. Curcumin, which has potent antioxidant and anti-inflammatory properties, is the most active ingredient of turmeric (62, 112). Curcumin has been shown to inhibit both constitutive and inducible NF-κB activation and to promote TNF-induced apoptosis. In an in vivo model of colonic crypt hyperproliferation/hyperplasia and/or inflammation associated with significant epithelial and stromal compartmentalization of NF-κB activity, we showed recently that curcumin significantly blocked NF-κB activity in the stroma. It also controlled NF-κB activation in the crypts to allow crypt regeneration following an inflammatory insult (21), suggesting a potent immunomodulatory role. We believe that curcumin is similar to a pectin diet and provides a potent immune modulator that maintains homeostasis in the colon by regulating NF-κB-dependent epithelial-stromal cross talk in vivo (21).
We and other investigators have also demonstrated that curcumin inhibits several important signaling pathways including Notch and Wnt signaling (4, 132). Interestingly, it was found that curcumin could inhibit mammosphere formation and could also decrease the amount of aldehyde dehydrogenase-positive cells in normal and malignant breast cancer cells through the inhibition of Wnt signaling (23), suggesting the inhibitory effects of curcumin on breast cancer stem cells (23). Curcumin has also been found to inhibit the expression of several molecules in Wnt/β-catenin pathway including disheveled, β-catenin, cyclin D1, and slug in both MCF-7 and MDA-MB-231 breast cancer cells (23). Immunofluorescence staining showed that curcumin significantly reduced the nuclear expression of disheveled and β-catenin proteins. The expression levels of GSK-3β and E-cadherin were also altered by curcumin treatment (103). These findings suggest that curcumin likely inhibits cell proliferation and induces apoptosis through regulation of β-catenin signaling in human breast cancer cells.
In colon cancer, curcumin treatment has been shown to promote p53- and p21-independent G2/M arrest and apoptosis in HCT-116 cells (53). In addition, curcumin has also been implicated in caspase-3-mediated modulation of Wnt/β-catenin signaling, decreased promoter DNA-binding activity of the β-catenin/TCF/LEF complex, and decreased levels of c-Myc protein (53), suggesting a protective effect. Curcumin delays the progression of osteosarcoma by suppressing intrinsic and activated β-catenin/TCF transcriptional activities. Overexpression of the wild-type β-catenin in osteosarcoma cells enhanced cell invasiveness whereas curcumin significantly blocked cancer cell invasion by targeting β-catenin and its downstream targets including matrix metalloproteinase (MMP)-9, cyclin D1, c-Myc, and survivin (76), suggesting that curcumin has therapeutic potential for the treatment of osteosarcoma. During microarray profiling, the expression of Wnt pathway components, AXIN2 and FRA1 (FOS-like antigen 1) decreased following curcumin treatment (122), providing additional evidence in support of the inhibitory effects of curcumin on Wnt signaling. Ryu et al. (113) reported that novel curcumin analogs, demethoxycurcumin and bisdemethoxycurcumin, can efficiently attenuate the Wnt/β-catenin pathway through downregulation of the transcriptional coactivator p300, which is a positive regulator of the Wnt/β-catenin pathway. In addition, curcumin also inhibited inflammation-induced obesity through the suppression of Wnt signaling (3), suggesting that Wnt signaling pathway is involved in curcumin-induced suppression of adipogenesis (5).
Studies have also shown that curcumin suppresses medulloblastoma cell proliferation by modulating the cross talk between Hedgehog and Wnt signaling pathways. More importantly, curcumin could enhance the efficiency of nontoxic doses of cisplatin and γ-rays through the downregulation of Bcl-2 (34), which is also a downstream gene of Hedgehog signaling. These results indicate that curcumin, a natural nontoxic compound, could be a promising agent in Shh-targeted therapy for the treatment of medulloblastomas. Although curcumin's poor absorption and low systemic bioavailability limit the access of adequate concentrations for pharmacological effects in certain tissues, active levels in the gastrointestinal tract have been found in animal and human pharmacokinetic studies. Curcumin has been used clinically in humans to treat various cancers, inflammatory disorders, skin diseases, and neurodegenerative diseases (129). Currently, sufficient data have been shown to advocate phase II and phase III clinical trials of curcumin for a variety of cancer conditions including multiple myeloma, pancreatic, and colon cancer.
Isoflavones.
Flavonoids in general are subdivided into subclasses including flavonols, flavones, flavanones, flavan-3-ols, anthocyanidins, and isoflavones. Isoflavones are found predominantly in members of the Leguminosae family. Soy, lentil, bean, and chickpea are sources of isoflavones. Genistein, daidzein, and glycitein are the three main isoflavones found in soybeans and most soy-protein products. Epidemiological studies suggest that dietary intake of flavonoids may reduce the risk of tumors of the breast, colon, lung, prostate, and pancreas (1, 2, 46, 52). Isoflavones, particularly genistein and daidzein, exert their antioxidant effects on human cells by scavenging free radicals and reducing the expression of stress-response related genes. Genistein is also well known as a tyrosine kinase inhibitor, and most studies suggest isoflavones to have a pleiotropic effect that targets multiple signaling pathways (115).
Studies have found that isoflavone mixtures significantly inhibit the activation of Wnt signaling (79), aberrant regulation of which represents the hallmark of numerous cancers including colon cancer (101). Isoflavone specifically attenuated Wnt signaling in prostate cancer cells by promoting GSK-3β-dependent phosphorylation and degradation of β-catenin (79). Isoflavone genistein has also been shown to upregulate E-cadherin expression and block Wnt-1-induced cell proliferation by targeting cyclin D1 and c-Myc (131). Isoflavone genistein also inhibited the expression of Wnt-5a (130), suggesting that genistein can also modulate Wnt signaling. More recently, genistein was found to inhibit Dkk-1, an antagonist of the Wnt/β-catenin signaling, by restoring membrane localization of E-cadherin and β-catenin in vitro (68). Finally, genistein has also been shown to be effective in the regulation of Hedgehog pathway (123). Importantly, genistein was found to reduce or delay prostate cancer growth in TRAMP mice (123). Thus isoflavones can provide significant preventive or therapeutic options with minimal side effects to block hyperactive Wnt or Hedgehog pathways. Various studies have tested isoflavones for the treatment of postmenopausal hot flashes and for prevention of prostate cancer and other proliferative disorders (114). However, more exhaustive mechanistic preclinical and clinical studies are required to assess the health benefit of isoflavones in cancer patients.
Polyphenols.
Consumption of green tea has been shown to be associated with human health including prevention of cancer and heart disease. Green tea contains several catechins including epicatechin, epigallocatechin, epicatechin-3-gallate, and EGCG. Of all the catechins, EGCG has been shown to be the most potent for the inhibition of tumorigenesis in different animal models, including those for cancers of the skin, lung, oral cavity, esophagus, stomach, small intestine, colon, bladder, liver, pancreas, prostate, and mammary glands. Epidemiological studies have shown lower incidence of prostate cancer among Asian men with a high dietary intake of green tea, suggesting that green tea might be a preventive agent against cancers (55). Indeed, high consumption of green tea has been associated with a decreased risk of advanced prostate cancer (71). On the other hand polyphenols have been tested clinically to prevent skin aging, promote weight loss, prevent cancers, reduce cardiovascular disease, and slow neurological decline (93).
The inhibitory effects of EGCG on Wnt and Hedgehog signaling have been reported in various cancers (30, 42, 97). Moreover, a combination of EGCG with fish oil during intestinal tumorigenesis in ApcMin/+ mice fed a high-fat diet for 9 wk significantly reduced the tumor burden through inhibition of Wnt/β-catenin signaling (14). In breast cancer cells, EGCG treatment induced HBP1 transcriptional repressor levels through an increase in HBP1 mRNA stability (59). However, knockdown of HBP1 reduced sensitivity to EGCG in the suppression of Wnt signaling and its target gene c-Myc expression (59). Moreover, EGCG also reduced both breast cancer cell proliferation and invasiveness in an HBP1-dependent manner, suggesting that EGCG blocks Wnt signaling and inhibits invasion of breast cancer through the induction of HBP1 transcriptional repressor (59). EGCG can also reduce or delay prostate cancer growth in TRAMP mice (123). Taken together, these results indicate that the Hedgehog signaling could be a target of EGCG, leading to the inhibition of prostate cancer cell growth in vitro and in vivo. Finally, EGCG has also been shown to effectively inhibit cellular proliferation and induce apoptosis of SW1353 and CRL-7891 human chondrosarcoma cells by targeting the Indian Hedgehog pathway, suggesting that EGCG can have therapeutic relevance in the treatment of patients with chondrosarcoma (138). Yet the molecular mechanisms of inhibition of carcinogenesis in animals and humans remain to be further investigated.
Resveratrol.
Resveratrol, a naturally occurring stilbene found in the skin of red grapes and peanuts, has been shown to exhibit a number of beneficial effects, including anticancer, antioxidative, anti-inflammatory, and antimicrobial activity. Resveratrol suppresses multiple signaling pathways including IGF-1R, Akt and Wnt signaling (146). When combined with dexamethasone, fludarabine, and bortezomib (109), resveratrol exhibited significant inhibitory effect on Waldenstrom's macroglobulinemia cells (109), suggesting resveratrol's therapeutic effects. Interestingly, resveratrol promotes differentiation of osteoblasts by positively regulating Wnt/β-catenin signaling within mesenchymal cells (164). Resveratrol has also been shown to inhibit tumor-induced neovascularization in lung metastasis model, block binding of vascular endothelial growth factor to human umbilical vein endothelial cells (HUVEC) and inhibit the formation of capillary-like tube from HUVEC, suggesting that the antimetastatic activities of resveratrol might be due to the inhibition of neovascularization and tube formation (angiogenesis) (60). Resveratrol, similar to tea polyphenols, also regulates Hedgehog signaling by inhibiting Gli1 mRNA expression and downregulating Gli reporter activity, suggesting that resveratrol could be positively associated with either preventing or lowering the risk for prostate cancer. Finally, resveratrol was also shown to reduce metastasis of CT-26 mouse adenocarcinoma cells to the lungs and increase survival (156). And although tested as a cancer preventative, resveratrol has garnered significant interest as a cardiovascular disease modifier in humans (100).
Lycopene.
Lycopene is the pigment principally responsible for the deep-red color of tomatoes and its products including ketchup, tomato juice, and pizza sauce, which are the richest sources of lycopene in the US diet. Lycopene is a potent antioxidant. Frequent consumption of tomato products is associated with a lower risk of breast, prostate, and endometrial cancer cells. Human epidemiological and clinical studies have suggested a potential role for lycopene in cardiovascular and prostate cancer risk reduction (36). An in vivo animal study showed that lycopene had antitumor effects that could be potentiated by vitamin E, an antioxidant that is also present in tomatoes (81), confirming the anticancer activity of lycopene. A phase II clinical trial has shown that lycopene supplements reduced tumor size and prostate-specific antigen level in localized prostate cancer (69, 70), suggesting its promising effects on prostate cancer prevention and/or treatment. Studies have further shown that lycopene could reduce inflammatory signals, prevent oxidative DNA damage, and regulate the expression or activity of IGF/Akt, Wnt/β-catenin, and androgen receptor (AR) signaling (157). Lycopene reduced AR and β-catenin nuclear localization and inhibited IGF-1-stimulated prostate cancer growth, perhaps by attenuating the effects of IGF-1 on phosphorylation of Akt and GSK-3β. Both lycopene and β-carotene have been shown to inhibit lung metastasis in an experimental setting, whereas lycopene also decreases the level of vascular endothelial growth factor and metalloproteinases (51).
Indole-3-carbinol and 3,3′-diindolylmethane.
3,3′-diindolylmethane (DIM) is the dimeric product of indole-3-carbinol (I3C), which is produced from naturally occurring glucosinolates contained in a wide variety of plants including members of the family Cruciferae. In the stomach, I3C undergoes extensive and rapid self-condensation reactions to form several derivatives including DIM, which is the major derivative and condensation product of I3C. Epidemiological studies indicate that human exposure to indoles through consumption of cruciferous vegetables can decrease cancer risk (50). Clinical studies have assessed these agents for treatment of obesity, hormonal imbalance, and various cancers (89). DIM has been shown to reduce oxidative stress and stimulate the expression of antioxidant response element-driven gene, suggesting the antioxidant function of indole compounds (65, 92). In vivo, DIM is not toxic and is in fact preventive against the development of prostate cancer in a mouse model (38). DIM is also found to regulate Wnt signaling through the regulation of Akt/GSK-3β signaling leading to the inhibition of prostate cancer cell growth and induction of apoptosis (79). Sulforaphane is another natural compound derived from broccoli/broccoli sprouts that has been shown to target cancer stem cells, thereby suggesting a potent preventive or antimetastatic role (80).
Miscellaneous agents.
Selenium is an essential micronutrient found in grains, fish, meat, poultry, or eggs and is available in over-the-counter supplements and multivitamins. Epidemiological studies have shown that selenium could be a protective agent against the development of prostate and colorectal cancers. A study conducted to test the level of selenium in serum and prostate of 52 men after selenium supplementation found significantly higher levels in the prostatic tissue (44), a testament of its bioavailability. Although more studies are needed, selenium seems to target Wnt, AR, and Notch pathways to affect the disease process (61). The plant flavonoid fisetin can induce apoptosis and suppress the growth of colon cancer cells by inhibition of Wnt and COX-2 signaling. It was found that the treatment of colon cancer cells with fisetin inhibited the activity of Wnt signaling through downregulation of β-catenin and TCF-4, resulting in decreased expression of Wnt signaling target genes such as cyclin D1 and MMP-7 (133). Another novel natural product, dammarane-type triterpene sapogenin (PPD25) isolated from the leaves of Panax notoginseng, showed anticancer activity in colon and lung cancer cells by targeting components of the Wnt/β-catenin pathway (11). In addition, a naturally derived agent deguelin was found to inhibit Wnt/β-catenin signaling in breast cancer cell lines (91). Finally, it was reported that dietary fish oil could play a protective role against colorectal cancer through the downregulation of COX and Wnt/β-catenin pathways (145), suggesting the added beneficial effects of fish oil.
In conclusion, the failure of conventional chemotherapy to reduce mortality makes natural products ideal candidates for exerting synergism and reducing side effect burden in combination with conventional anticancer drugs.
Microbial Ecosystem and Human Health
During our lifetime, we consume on average 30–45 tons of food that passes through our gastrointestinal tract. Although food is indispensable, its passage also poses a considerable health risk since it provides exposure to dietary antigens, viable microorganisms, and bacterial products. In the intestinal tract, the mucosa is the first line of defense to protect against pathogenic products (e.g., endotoxins, hydrogen sulfide, phenols, ammonia, and indoles) or microbiota. At the same time, the intestinal tract absorbs essential nutrients and acts as an ideal fermenter for microbiota (45). Indeed, the human gastrointestinal tract is home to an extremely numerous and diverse collection of microbes, collectively termed the “intestinal microbiota.” This microbiota is considered to play a number of key roles in the maintenance of host health, such as aiding digestion of otherwise indigestible dietary compounds, synthesis of vitamins and other beneficial metabolites, immune system regulation, and enhanced resistance against colonization by pathogenic microorganisms. The human microbiota is a diverse and dynamic ecosystem, which has evolved in a mutualistic relationship with its host. Recent findings have demonstrated that the gut microbiome complements our human genome with at least 100-fold more genes. In contrast to our Homo sapiens-derived genes, the microbiome is much more plastic, and its composition changes with age and diet, among other factors. Elucidating the mechanisms behind host-microbe interaction involved in health and disease is a prerequisite for understanding processes and the subsequent development of therapies, drugs, and probiotics. To accomplish these goals, researchers require tractable model ecosystems such as ex vivo gastrointestinal model systems to recapitulate and investigate host-microbe interaction in real time (110).
“Microbiota” is the term used to describe the community of microorganisms (bacteria, viruses, and fungi) that normally live in or on a given organ in the body. There's a unique microbiota that inhabits the mouth, for example; another that lives on the skin; and still another that resides in the intestine. Given an intestinal surface area of about 2,700 square feet, the microbiota inhabiting the gut is the largest and most diverse in the body. How large and diverse? The gut microbiota contains roughly one quadrillion cells, at least ten times as many cells as does the human body itself. More than 1,000 bacterial species having been identified to date, with unknown numbers yet to be discovered. How do all those bacteria get there? The fetal intestine, in the absence of congenital infection, is sterile in utero. The bacteria that come to colonize the bowel are acquired during birth and shortly afterward, a process that is very much influenced by how a baby is born.
In vaginally born babies, the bacteria destined for the gut microbiota originate primarily in the maternal birth canal and rectum. Once these bacteria are swallowed by the newborn, they travel through the stomach and colonize the upper and lower intestine, a complicated process that evolves rapidly. Infants born by cesarean section (C-section), particularly cesareans performed before labor begins, don't encounter the bacteria of the birth canal and maternal rectum (if a cesarean is performed during labor the infant may be exposed to these bacteria, but to a lesser degree than in vaginal birth). Instead, bacteria from the skin and hospital environment quickly populate the bowel. As a result, the bacteria inhabiting the lower intestine following a cesarean birth can differ significantly from those found in the vaginally born babies. Whatever the mode of delivery, a core gut microbiota is well established within a few weeks of life and persists largely intact into adulthood. A less stable peripheral microbiota, one that is more sensitive to changes in diet and environmental factors, like antibiotics, is created as well. Between 1 and 2 years of age, when weaning from breast milk typically leads to a diet lower in fat and higher in carbohydrates, the gut microbiota takes on its final, mature profile. The dramatic first steps in immune system development take place at the same time the core microbiota is being formed, and the gut bacteria play a key role in that process. In the hours and days following birth, the newly arrived gut bacteria stimulate the newborn to produce white blood cells and other immune system components, including antibodies directed at unwelcome, disease-causing microorganisms. The bacteria of the microbiota also “teach” the newborn's immune system to tolerate their own presence: to differentiate bacterial friend from foe, in other words. In babies born via C-section, the young immune system is confronted with unfamiliar, often hostile bacteria, including Clostridium difficile, a species particularly common in hospitals. In addition, the healthy probiotic bacteria associated with vaginal birth (e.g., lactobacillus) arrive later and in lower numbers. These changes in the composition of the normal gut microbiota occur during a critical time in immune system development.
The gastrointestinal tract microbiota (Fig. 1) contributes to the development and differentiation of the mammalian immune system. The composition of the microbiota affects immune responses and susceptibility to infection by intestinal pathogens and development of allergic and inflammatory bowel diseases (IBDs). Antibiotic administration, while facilitating clearance of targeted infections, also perturbs commensal microbial communities and decreases host resistance to antibiotic-resistant microbes. Hundreds of bacterial species make up the mammalian intestinal microbiota. Following perturbations by antibiotics, diet, immune deficiency, or infection, this ecosystem can shift to a state of dysbiosis.
Fig. 1.
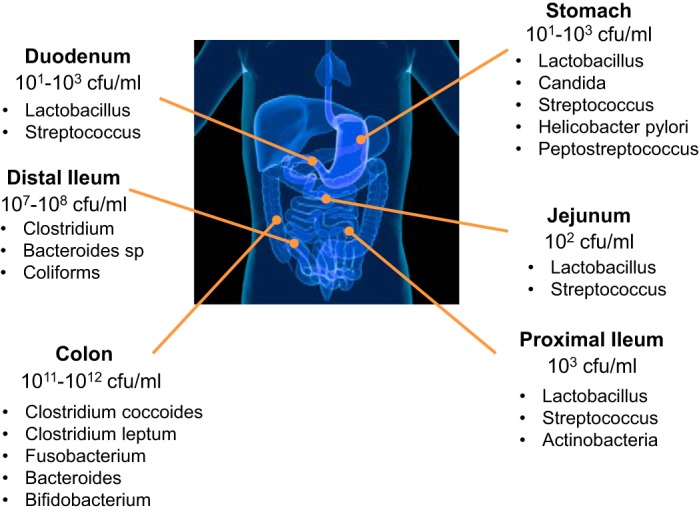
A gradient defining the spatial arrangement of the intestinal microbiota. Please note increasing bacterial density and diversity, in the jejunum/ileum from the stomach and duodenum, and in the large intestine. Bacteria that reside in the colon represent the highest cell densities recorded for any ecosystem.
Dysbiosis: Is It Causatively Linked to Human Diseases?
There is a growing interest in research on the importance of gut microbiome in energy homeostasis, inflammation, and its role in pathogenesis of obesity-related disorders. Recent evidence suggests that there is a link between the human gut microbiome and the development of obesity, cardiovascular disease, and metabolic syndromes, such as Type 2 diabetes. Similarly, there is increasing evidence for dysbiotic microbiota in carcinogenesis (117). Although perturbations in the composition or functions of the microbiota are linked to inflammatory and metabolic disorders [e.g., IBDs, irritable bowel syndrome (IBS), and obesity], it is unclear at this point whether these changes are a symptom of the disease or a contributing factor. A better knowledge of the mechanisms through which changes in microbiota composition (dysbiosis) promote disease states is needed to improve our understanding of the causal relationship between the gut microbiota and disease.
“Dysbiosis” describes a situation in which the composition/activities of the microbiota are altered to a state that may be deleterious to the host (41, 136). However, the underlying mechanisms behind the development of clinical dysbioses are complicated because of the fact that no single species, or collection of species, is universally accepted as a definitive biomarker of dysbiosis (13). It is clear that interindividual variation in microbiota composition is as common a feature in dysbiosis as it is in health (40, 88, 149, 158). But there is no doubt that the intestinal microbiota structure and composition is altered in numerous disorders, and mounting evidence suggests that these alterations may play roles in disease progression and maintenance (33). The evidence for intestinal microbiota involvement is strongest for diseases of the gastrointestinal tract. However, it should be noted that there is also evidence that links the intestinal microbiota to disorders such as diabetes, obesity, metabolic syndrome, asthma, cardiovascular disease, and atopy (49, 78, 148, 150, 152, 155). Despite interindividual variation, there are a number of recurring features that typically characterize the intestinal microbiota in disease states. It appears, for example, that a reduction in overall bacterial diversity is common in dysbiosis (22, 77, 86, 88). Furthermore, regardless of the disease under investigation, reductions in the obligatory anaerobic members of the Firmicutes phylum are commonly reported (22, 40, 126, 159). The evidence is not as consistent for members of the Bacteroidetes phylum, although they have been shown to be severely reduced during antibiotic-associated diarrhea and C. difficile disease (22, 141) and in some, but not all, IBD patients (40, 88). Very often reductions in the obligatory anaerobic lineages occur in tandem with increased presence of facultative anaerobes, including members of the Enterobacteriaceae family of the Proteobacteria phylum (43, 83, 85, 127). Of note, this family contains a number of strict and opportunistic pathogens such as Salmonella spp., Shigella spp., Klebsiella spp., Proteus spp., and Escherichia coli. Broad-scale changes in composition, and reductions in overall diversity, in dysbiosis can also have major impacts on the range and volume of metabolites that are produced by microbes in the colon. For example, reduced or perturbed SFCA production is a hallmark of intestinal disturbance caused by factors such as antibiotic treatment or diarrhea (27, 106).
Changes in intestinal microbiota composition often correlate with chronic inflammation and disruptions in the epithelial/mucosal barrier. Such correlations do not of course necessarily equate to causality (41), but there are a number of ways in which altered microbiota might impact or prolong disease and dysbiosis. A reduction in overall short-chain fatty acid (SCFA) levels, for example, may contribute to diarrhea by reducing sodium and water absorption (106). This would act to inhibit the regrowth of beneficial microbes, thus delaying the reestablishment of a healthy microbiota profile. Furthermore, given the postulated anti-inflammatory effects of butyrate, it is possible that depletion in butyrate-producing bacteria in dysbiosis (for example Faecalibacterium prausnitzii in IBD) may be a contributory factor toward excessive inflammation (126).
Inflammation per se may also be a key driver of the failure to resolve dysbiosis. Specific bacteria, including members of the Enterobacteriaceae family, appear to survive better under the prevailing conditions in the inflamed gut than the anaerobic commensals that are dominant in a healthy gut (18, 39, 43, 83, 127). Salmonella enterica enterica, serovar Typhimurium, for example, has diminished susceptibility to host-derived antimicrobials and has evolved the capacity to incorporate host compounds generated during inflammation for growth (82, 105, 128, 140, 162). In this model, opportunistic species, for example adherent-invasive E. coli, interact with the mucosal surface to weaken the barrier function and initiate host immune responses against other commensals. This enhances their own survival in the gut but also prolongs dysbiosis. Inflammation-induced depletion of potentially anti-inflammatory species such as butyrate-producing Firmicutes, and/or anti-inflammatory microbial factors like polysaccharide A of B. fragilis may exacerbate the cycle of inflammation and so further favor the growth of resistant proinflammatory species (160). If this theory is correct, then it is possible that these proinflammatory cycles will need to be broken to disrupt dysbiosis and restore homeostasis. Finally, dysbiosis may also impact the host's exposure to deleterious compounds. Ammonia, phenols, indoles, sulfide, and amines are known to be toxic and are produced in varying amounts by the gut microbiota (124). If there is a predominance of microbes that produce these compounds in dysbiosis, regardless of whether they are the direct cause of disease, they may still impact long-term host health. Given the far reaching influence of the intestinal microbiota on human health, a clear future goal must be to develop reliable means to alter the composition of the microbiota and restore a healthy balance of microbial species. Although it is clear that much fundamental research remains to be done, potentially important therapeutic options include narrow-spectrum antibiotics, novel probiotics, dietary interventions, and more radical techniques such as fecal transplantation, all of which aim to suppress clinical dysbiosis, restore intestinal microbiota diversity, and improve host health.
Microbes and Specific Diseases
One of the most important modern discoveries linking a specific human disease condition to the microbiome or a microbe was the discovery of Helicobacter pylori's association with peptic ulcer disease (PUD). Much of this work was done by investigators Warren and Marshall in 1983 (152a). Helicobacter pylori has also been linked to gastric cancer and mucosa-associated lymphoid tissue (MALT) gastric lymphomas. Although much of the world's population has a gastrointestinal tract colonized by Helicobacter pylori and suffers little or no sequelae, Warren and Marshall established that in some individuals the organism leads to significant and detrimental gastric mucosal inflammation and adverse outcomes. In fact, recent genetic analysis suggests the organism (which has probably coevolved with humans for 40–50,000 years) is dying out as an endemic commensal in the Western world. Use of antibiotics and acid reducing regimens to eliminate the bacterium were established soon after Warren and Marshall's discovery. These approaches have been adopted as an evidence-based clinical approach for PUD. Despite this, research continues to evaluate whether colonization and commensal coexistence between human hosts and H. pylori might provide certain health benefits, specifically in terms of digestion and metabolic benefits. Reduced H. pylori colonization, for example, in the Western world may relate to increased rates of gastroesophageal reflux disease, esophageal cancer, and various allergic and asthmatic conditions.
Other recent research discoveries and preliminary approaches in clinical medicine have promoted a focus on dysbiosis and specific disease states. The rise of C. difficile as a common and serious pathogen has been clearly linked to the extensive modern use of broad spectrum antibiotics. These antibiotics, often with a potential for eliminating an array of both gram-positive and gram-negative bacteria, may leave an individual in a “dysbiotic state” with a high disposition for overgrowth and colonic infection with C. difficile. Severe diarrhea, dehydration, and weight loss often result from overgrowth of this pathogen, and the condition is much more common in older adults with comorbid diseases. It is also likely that the common microbial shifts tied to aging (as described above) may also predispose to risk for this form of “colitis.” C. difficile colitis may respond to the addition of multiple agents within the arsenal of antibiotic therapy (typically either motronidazole or oral vancomycin), but many individuals remain at risk for recurrence. New regimens developed for those with recurrent “C. dif” include “fecal transplantation,” a technique currently receiving a great deal of attention and study by gastroenterologists and infectious disease specialists. Both direct small intestinal and colonic delivery of “healthy microbial” samples are being tested clinically and by industry. The impact of C. difficile on population health and geriatric medicine is likely to grow in coming decades and this pathogen will spur additional research on probiotics, prebiotics, and dysbiosis.
IBS has also been linked in some studies to changes in the gastrointestinal microbial environment. IBS is characterized by frequent and recurrent abdominal pain, bloating, and alternations between diarrhea and constipation. In studies comparing IBS sufferers to normal controls, relative reductions in Lactobacilli and Bifidobacteria have been seen (28, 137). Although IBS may not be flora related in all cases, the therapeutic value of various antibiotics, prebiotics, and probiotics for the disorder lends some support to the connection. Additional studies should provide useful information.
IBD, such as Crohn's disease and ulcerative colitis, has also been studied as a microbial condition. Some dysfunction of the normal microbiome and host immune system symbiosis is likely responsible for the chronic inflammation and secondary complications of these diseases. Unfortunately, probiotic treatment trials have yielded conflicting results to date. Additional studies evaluating the initial development of IBD and the potential for certain microbiome profiles in disease prevention are underway.
Metabolic disorders such as obesity and Type 2 diabetes mellitus have also been linked to modern era dysbiosis. Alterations in the gut microbiome have led to changed carbohydrate and nutrient mobilization within the intestine, potentially aiding caloric exposure and nutrient processing.
In pediatric disorders, a number of conditions have been tied to dysbiosis. Conditions such as atopic dermatoses in infants, diarrhea, necrotizing enterocolitis in preterms, and caries infections have been improved with probiotic treatments. Probiotic therapies have been shown effective for reducing diarrhea, constipation, and food intolerance in infants transitioning from breast milk to a complex diet.
Finally, the influence of microbiome on cancer in general and colorectal cancer (CRC) in particular, is poorly understood. Some recent studies showed differences in microbial profiles between those with and without CRC (19, 65, 87, 125, 151). One study found greater Bacteroides and Prevotella species in CRC patients than in stool from cancer-free patients. Fusobacterium was found at higher levels in CRC tissue analysis (19, 65). Interestingly, an elegant review recently discussed many organs, such as the liver and pancreas, that do not have their own defined microbiome but may be influenced by bacterial molecular patterns and metabolites because of anatomic links to the gut. GI microbes and metabolites may have various health-promoting or deleterious influences even in adjacent organ systems (117). Thus gastrointestinal microbes and microbial metabolites can have varied health promoting and deleterious influences.
Fecal Transplant and the Human Microbiome
Human fecal transplantation [or fecal microbiota transplantation (FMT)] has been gaining increased attention as a potential mechanism to treat infectious colonic disease and to change the human microbiome. Several methods have been used clinically and several additional methods are under development. Transplant has most often been used to treat persistent C. difficile infection (57). As described above, C. difficile infection can cause chronic diarrhea, weight loss, and even death in the elderly if resistant to standard antimicrobials. Recent randomized trials show encouraging results (144).
Clinical setups for FMT have included rectal retention enema, where 150 g of stool from health volunteers is mixed with 300 ml of sterile saline, then administered rectally. Other methods have included feces cultured in culture media, then introduced as a 30-ml suspension by enema, colonoscopy, or nasogastric tube (56). Some clinical protocols have used purified intestinal bacterial cultures (99). Patients receiving transplants are often pretreated with antibiotics that are discontinued just prior to the receipt of the donor fecal material. Regimens may involve a single administration or daily administrations for a short course.
The limited literature on FMT suggests that the procedure is successful ∼90% of the time (58, 84, 99, 111). There is a great need for additional randomized controlled trials that can verify outcomes for various disorders and assess safety. In addition to colonic disease, FMT may also prove useful to treat various other conditions, including colitis, constipation, IBS, and some neuropsychiatric conditions (6).
Diet and Microbiome
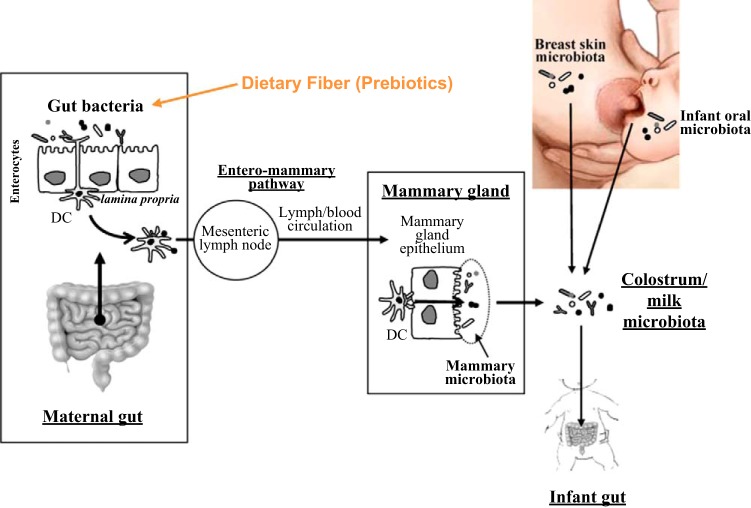
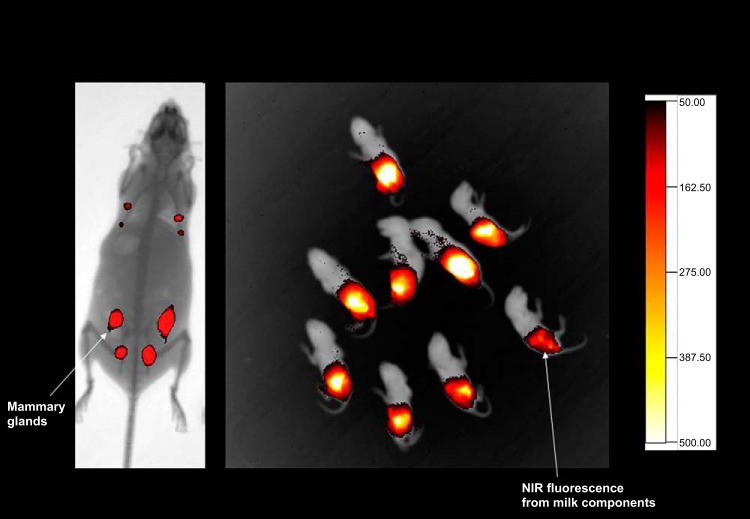
Interactions between the gut microbiome and the diet are complex and dynamic. Diet is the primary determinant in the development of the microbiota colonization pattern from the first stages of life. In infants exclusively on breast milk, the gut microbiota is enriched in bifidobacteria and lactic acid bacteria, whereas the community in babies on formula diet is dominated by bifidobacteria, Bacteroides spp., Clostridium spp., and facultative anaerobes (10). Breast milk, which is usually rich in oligosaccharides, apart from contributing to the growing nutritional requirements of infants, also influences the infant immune system while providing a degree of protection against pathogens. Milk is also a good source of microbes required to colonize the baby's gut (Fig. 2). The bacterial genera include Weissella, Leuconostoc, Staphylococcus, Streptococcus, and Lactococcus. The role of natural products in modulating the infant's immune system through the mother can be assessed by using genetically engineered microbiomes in animal models. Using near infrared fluorescent (NIRF) compound that is capable of binding to the phospholipids on the bacterial surface, the mother to infant transfer of the NIRF signals in the guts of mouse pups (Fig. 3) can be monitored and evaluated (R. V. L. Papineni and S. Umar, personal observation). The introduction of solid food leads to a dramatic shift into the intestinal microbiota composition (37). Intriguingly, comparison of the fecal microbiota by 16S rDNA sequencing of children from Burkina Faso consuming a rural African diet with children from Italy consuming a modern Western diet showed no significant differences in the microbiota composition between the two cohorts during the breastfeeding period (32). After weaning, however, enrichment in Bacteroidetes, Prevotella, and Xylanibacter was observed in Burkina Faso children in addition to higher levels of SCFAs, the main products of polysaccharide fermentation. In the Italian children, on the other hand, these features were completely absent. Gordon's group (163) also recently reported parallel ontogenetic changes in the microbiome of Malawian, Venezuelan, and American populations from infants to adults, associated with differences in diet, culture, and lifestyle. Interestingly, the authors observed that the gut microbiomes of the Malawian and Venezuelan populations, which consume a “rural” diet, were enriched in Prevotella spp., similar to the observation of De Filippo et al. (32) in the Burkina Faso cohort. In the American population, since the diet is higher in protein, the microbiome is enriched with genes necessary for breaking down amino acids. Moreover, the microbiome of the American tends to shift to a more Bacteroides-enriched gut community (11). These results support previous findings that carnivore microbiomes are enriched in protein degradation genes, whereas herbivore microbiomes are enriched in genes necessary to break down starch (90). Thus these studies solidify the notion that nutrition is a driving factor in shaping gut microbiota composition and its functional maturation, from birth to adulthood. More studies are needed, however, to determine what factors or events during gut microbiota maturation might contribute to the development of metabolic disorders.
Fig. 2.
Potential sources of the bacteria present in human colostrum and milk. Prebiotics (arrow) such as pectin, can promote the growth of “good bacteria” in the mother's gut. DC, dendritic cell. [Adapted from Fernandez L, Langa S, Martín V, Maldonado A, Jiménez E, Martín R, Rodríguez JM. Pharmacol Res 69: 1–10, 2013, by permission from Elsevier].
Fig. 3.
Near-infrared (NIR) fluorescence to detect mother-to-infant transfer of the NIR fluorescence signals. Real-time monitoring of mother-to-infant transfer of milk components (unpublished data).
Epidemiologically, fruits and vegetables protect against chronic diseases including cardiovascular disease, cancer, obesity, and diabetes. Dietary fibers are thought to contribute significantly toward the protective nature of plant structural and storage polysaccharides (12, 29). Interestingly, both potentially interact with the gut microbiota: for many polyphenols microbial transformation modifies bioavailability and activity, and fiber is the major energy source for fermentation in the colon, the dominant metabolic activity of the gut microbiota. Polyphenols are partially absorbed in the small intestine, being modified by phase I and II reactions in the liver, producing glucuronides and sulfates that can be shunted back into the intestine via bile. Microbial conversion then takes place in the colon, directing the degradation of complex metabolites into simpler forms, leading to absorption, systemic circulation, or urinary and fecal excretion. Enterococcus casseliflavus is described to be involved in the hydrolysis of sugar moieties, such as in quercetin-3-glucoside, releasing the aglycone quercetin, with acetate, lactate, formate, and ethanol production. Eubacterium ramulus, Eubacterium oxidoreducens, Flavonif ractor plautii, as well as several Clostridium strains have been associated with the fermentation of the aglycone quercetin, leading to the formation of taxifolin, 3,4-dihydroxyphenyl-acetic acid, acetate, and butyrate(67, 116, 161). The catabolism of polyphenols is associated with the production of benzoic acid, which normally undergoes glycine conjugation in the liver, leading to the production of hippuric acid, easily detected in urine. To better assess the modulation of polyphenols in the gut microbiome, bacterial groups have recently been associated with specific cocoa and coffee polyphenols. For example, the flavanol monomer (+)-catechin significantly increases the growth of the Clostridium coccoides-Eubacterium rectale group, Bifidobacterium spp., and E. coli and inhibits the growth of the Clostridium histolyticum group (142). A metabolic pathway for the catabolism of (epi)catechins where ring rupture takes place and simple phenolics are produced, such as 3,4-dihydroxybenzoic acid and 3-hydroxybenzoic acid. In vitro digestion of water-insoluble cocoa fractions with gastrointestinal enzymes was carried out to investigate the biotransformation of polyphenols. Interestingly, bacterial fermentation of the insoluble material was associated with an increase of bifidobacteria and lactobacilli as well as butyrate production. Flavanols were converted into phenolic acids by the microbiota, resulting in an increasing concentration of 3-hydroxyphenylpropionic acid. These microbial changes were associated with significant reductions in plasma triacylglycerol and C-reactive proteins, suggesting the potential benefits associated with the dietary inclusion of flavanol-rich foods.
A European Commission in 2008 defined dietary fiber as “carbohydrate polymers with three or more monomeric units, which are neither digested nor absorbed in the human small intestine and include: edible carbohydrate polymers naturally occurring in the food as consumed, edible carbohydrate polymers which have been obtained from food raw material by physical, enzymatic, or chemical means and which have a beneficial physiological effect demonstrated by generally accepted scientific evidence and edible synthetic carbohydrate polymers. Importantly, this definition recognizes the role of dietary fiber in maintaining human health. Fiber is consistently found to be inversely associated with chronic human diseases such as cancer and cardiovascular disease in large-scale human epidemiological studies. Similarly, studies in laboratory animals have provided mechanistic data linking high dietary fiber (typically 10% w/w diet) and protection from these same diseases. This is particularly true for the prebiotic dietary fibers that are selectively fermented to affect specific changes, in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon host health” (54). Dietary prebiotics have been repeatedly shown in both animals and humans to modulate the gut microbiota by state of the art culture-independent microbiological techniques, and they typically increase the relative abundance of bifidobacteria. In the CR model, we have discovered that dietary intervention with pectin leads to restoration of commensals such as B. vulgatus, S. gordonii, and L. lactis, which were lost during CR-induced colitis, suggesting potent prebiotic effects (personal observation). There is also strong animal data linking prebiotics with protection from metabolic syndrome, obesity, Type 2 diabetes, colon cancer, and IBD and fortifying the gut microbiota against invading gastrointestinal pathogens (40, 78, 104). However, few human studies have been conducted with levels of dietary fiber or prebiotic demonstrated in animals to protect against these chronic diseases.
The estimates of fiber intake for Western populations are in the range 20–25 g per day compared with populations consuming traditional diets rich in fruits, vegetables, and grains and our ancestral hunter-gatherers, who consume or consumed between 70 and 120 g fiber per day (102, 120). Plant nonstarch structural polysaccharides, by nature of their chemical makeup, resist the degradative activities of human digestive enzymes in the stomach and small intestine. Plant storage carbohydrates such as starch may also be rendered physically or chemically inaccessible to human digestive enzymes and reach the colon (120). For example, polyphenol-rich beverages when consumed at meal times can affect starch digestion by inhibiting starch-degrading enzymes in the upper gut and thus blunting postprandial glucose peaks. Similarly, the Maillard reaction in cooked foods can increase the recalcitrance of food macromolecules to digestion, leading to increased survival of both carbohydrates and proteins until they reach the colon (48). However, once these food compounds reach the colon, they become available to the fermentative activities of the human colonic microbiota. Recent metagenomic studies have highlighted that the gut microbiota is specifically evolved for the digestion of complex plant polysaccharides, possessing a range of polysaccharide- and glycan-degrading enzymes not present in the human genome (48). In this way, the human gut microbiota can be viewed as a closely coevolved microbial partner to the human genome, extending host-encoded functions and allowing the host to derive energy and other biologically active compounds from food components that would otherwise remain inaccessible and be excreted as waste.
Carbohydrate fermentation is the chief energy source for the gut microbiota, and the proximal colon can be viewed as a saccharolytic environment, where the dominant fermentative activity is carbohydrate fermentation leading to the production of the SCFAs such as acetate, propionate, and butyrate. These small organic acids have diverse functions in the host, not just supplying energy to the intestinal mucosa, heart, brain, and muscle but also playing important roles in human cell differentiation, proliferation, and programmed cell death; regulation of immune function; thermogenesis; and lipid metabolism (15). In individuals following a Western-style diet, as dietary fiber and colonic carbohydrate are used up in the proximal colon, saccharolytic fermentation decreases along the transverse and distal colon as the concentration of substrate decreases. Microorganisms then switch to other energy sources including dietary- or host-derived proteins and amino acids. The end products of amino acid fermentation include SCFA but also branched-chain fatty acids, amines, indoles, sulfides, and phenols, some of which are potentially harmful, being variably genotoxic, cytotoxic, and carcinogenic. Human populations with higher intakes of dietary fiber and whole plant foods tend to have higher concentrations of SCFA in their feces, suggesting that in these populations carbohydrate fermentation may be extended along the length of the colon. This may thus avoid buildup of toxic or harmful metabolites produced when bacteria switch their fermentation substrate from carbohydrate to amino acids. It has recently been shown that a threefold increase in dietary fiber results in a proportional increase in SCFA production by the gut microbiota and extends saccharolytic fermentation into the transverse and distal colon using an in vitro three-stage model of the human colonic microbiota (139). It will be very interesting to confirm in humans following a low-fiber Western-style diet whether intervention with high levels of dietary fiber can modulate the health-promoting saccharolytic activities of the gut microbiota to a similar degree in vivo, not only along the length of the gastroenterological tract but in anatomically linked organs such as liver and pancreas.
Understanding the resilience of the gut microbiota is crucial for determining the efficacy of therapeutic diets. Consuming a carbohydrate- or fat-restricted low-calorie diet for a year, or a high-fat and a low-fiber, or low-fat and high-fiber diet for 10 days can induce statistically significant changes in the gut microbiota. However, these changes in species and gene content are small compared with the baseline variations that occur between individuals. Long-term dietary surveys and cross-cultural comparisons suggest that changes to diet might lead to regime changes over longer periods of time, perhaps by eroding the landscape of alternative stable states.
In conclusion, diet is a major force that shapes the composition and activity of the gut microbiota (Table 1). Interactions between different dietary factors and gut microbes may lead to dysbiosis that exerts distinct immune responses in the host, resulting in higher susceptibility to various gastrointestinal and systemic diseases. Probiotics and fecal microbiota transplantation may all be effective, inexpensive, and safe remedies to prevent and/or treat diseases.
Table 1.
Effect of select group of compounds on microbiota
| Compounds | Dietary Source | Molecular Targets | Microbiota Involved |
|---|---|---|---|
| Sulforaphane Phenethylisothiocyanate Benzylisothiocyanate | Cruciferous vegetables, Cabbage, Cauliflower, Turnips, Broccoli | AKT, NF-κB, AP-1 BclII, Survivin, CyclinD1 p53, Bax, COX-2, iNOS VEGF, MMPs | In vivo bacterial glucosinolate metabolism but no direct link to specific bacterial species. |
| Quercetin | Onions, Broccoli, Apples, Berries | iNOS, COX-2, AKT Caspases | Eubacterium casseliflavus, Eubacterium ramulus, Eubacterium oxidoreducens, Flavonif ractor plautii |
| EGCG, (+)-Catechin | Tea | EGFR, AKT, NF-κB, Cyclin D1, VEGF, COX-2, AP-1, MMP-2/-9, Bcl-2, Bax, IL-12 | Clostridium coccoides-Eubacterium rectale group; Bifidobacterium spp., Escherichia coli: ↑ Clostridium histolyticum: ↓ |
| Genistein | Soybeans, Red clover | Caspases, ASK-1, AKT, NF-κB, Survivin, Bcl-2, Bax, STAT-3/-5, CDK, VEGF | Slackia isoflavoniconvertens |
| Lycopene | Tomato, Orange, Papaya | Bcl-2, Bcl-xL, Bax, p53, Caspases, Cyclin D1, AKT, NF-κB, MMP-9, BAD, Sp-1, Cytochrome c, IGF-BP3, PCNA | None specified |
| Curcumin/Curcuma-Pt | Turmeric | AKT, EGFR, Her2, NF-κB, IGF-1R, Bcl-2, COX-2, ERK, AP-1, VEGF, MMP-2/-9, p53, p21, Bax, STAT-3/-5, survivin, iNOS | No change in total bacterial content; No change in either Bifidobacterium spp./Lactobacillus spp., or Bacteroides/Prevotella spp. |
| Resveratrol | Grapes | NF-κB, iNOS, COX-2, STAT-3, p53, Survivin, p53, p21, Bax, SOD, Catalase, GSH, Cyclin D1, CDK, VEGF | Bifidobacterium spp./Lactobacillus spp.↑ |
| Pectin | Apples, Oranges, Guavas, Plums, Gooseberries | NF-κB, β-catenin, Cyclin D1, CXCL1, Caspase-1, BclII, HDACs | Lactococcus lactis, Streptococcus gordonii, Bacteroides vulgatus:↑ |
GRANTS
This work was partially supported by R01 grant from the National Cancer Institute (R01-CA-131413) and by start-up funds from The University of Kansas Medical Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.K.G., R.V.L.P., and S.U. edited and revised manuscript; A.K.G., R.V.L.P., and S.U. approved final version of manuscript; R.V.L.P. performed experiments; R.V.L.P. and S.U. analyzed data; R.V.L.P. and S.U. prepared figures; R.V.L.P. and S.U. drafted manuscript; S.U. conception and design of research.
REFERENCES
- 1.Adlercreutz H, Honjo H, Higashi A, Fotsis T, Hamalainen E, Hasegawa T, Okada H. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am J Clin Nutr 54: 1093–1100, 1991 [DOI] [PubMed] [Google Scholar]
- 2.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet 342: 1209–1210, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr 30: 173–199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed I, Chandrakesan P, Tawfik O, Xia L, Anant S, Umar S. Critical roles of Notch and Wnt/beta-catenin pathways in the regulation of hyperplasia and/or colitis in response to bacterial infection. Infect Immun 80: 3107–3121, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn J, Lee H, Kim S, Ha T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/β-catenin signaling. Am J Physiol Cell Physiol 298: C1510–C1516, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Curr Opin Gastroenterol 29: 79–84, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Attari F, Sepehri H, Delphi L, Goliaei B. Apoptotic and necrotic effects of pectic acid on rat pituitary GH3/B6 tumor cells. Iran Biomed J 13: 229–236, 2009 [PubMed] [Google Scholar]
- 8.Avivi-Green C, Polak-Charcon S, Madar Z, Schwartz B. Apoptosis cascade proteins are regulated in vivo by high intracolonic butyrate concentration: correlation with colon cancer inhibition. Oncol Res 12: 83–95, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Avivi-Green C, Polak-Charcon S, Madar Z, Schwartz B. Dietary regulation and localization of apoptosis cascade proteins in the colonic crypt. J Cell Biochem 77: 18–29, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Balmer SE, Wharton BA. Diet and faecal flora in the newborn: breast milk and infant formula. Arch Dis Child 64: 1672–1677, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bi X, Zhao Y, Fang W, Yang W. Anticancer activity of Panax notoginseng extract 20(S)-25-OCH3-PPD: targetting beta-catenin signalling. Clin Exp Pharmacol Physiol 36: 1074–1078, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Bird AR, Brown IL, Topping DL. Starches, resistant starches, the gut microflora and human health. Curr Issues Intest Microbiol 1: 25–37, 2000 [PubMed] [Google Scholar]
- 13.Blumberg R, Powrie F. Microbiota, disease, and back to health: a metastable journey. Sci Transl Med 4: 137rv137, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bose M, Hao X, Ju J, Husain A, Park S, Lambert JD, Yang CS. Inhibition of tumorigenesis in ApcMin/+ mice by a combination of (-)-epigallocatechin-3-gallate and fish oil. J Agric Food Chem 55: 7695–7700, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Brahe LK, Astrup A, Larsen LH. Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obes Rev 14: 950–959, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr 69: 30–42, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Cao Y, Gao X, Zhang W, Zhang G, Nguyen AK, Liu X, Jimenez F, Cox CS, Jr, Townsend CM, Jr, Ko TC. Dietary fiber enhances TGF-beta signaling and growth inhibition in the gut. Am J Physiol Gastrointest Liver Physiol 301: G156–G164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carvalho FA, Koren O, Goodrich JK, Johansson ME, Nalbantoglu I, Aitken JD, Su Y, Chassaing B, Walters WA, Gonzalez A, Clemente JC, Cullender TC, Barnich N, Darfeuille-Michaud A, Vijay-Kumar M, Knight R, Ley RE, Gewirtz AT. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe 12: 139–152, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P, Allen-Vercoe E, Moore RA, Holt RA. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 22: 299–306, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandrakesan P, Ahmed I, Anwar T, Wang Y, Sarkar S, Singh P, Peleg S, Umar S. Novel changes in NFκB activity during progression and regression phases of hyperplasia: role of MEK, ERK, and p38. J Biol Chem 285: 33485–33498, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandrakesan P, Ahmed I, Chinthalapally A, Singh P, Awasthi S, Anant S, Umar S. Distinct compartmentalization of NF-kappaB activity in crypt and crypt-denuded lamina propria precedes and accompanies hyperplasia and/or colitis following bacterial infection. Infect Immun 80: 753–767, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, Young VB. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis 197: 435–438, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Charpentier MS, Whipple RA, Vitolo MI, Boggs AE, Slovic J, Thompson KN, Bhandary L, Martin SS. Curcumin targets breast cancer stem-like cells with microtentacles that persist in mammospheres and promote attachment. Cancer Res 74: 1250–1260, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho Y, Kim H, Turner ND, Mann JC, Wei J, Taddeo SS, Davidson LA, Wang N, Vannucci M, Carroll RJ, Chapkin RS, Lupton JR. A chemoprotective fish oil- and pectin-containing diet temporally alters gene expression profiles in exfoliated rat colonocytes throughout oncogenesis. J Nutr 141: 1029–1035, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho Y, Turner ND, Davidson LA, Chapkin RS, Carroll RJ, Lupton JR. A chemoprotective fish oil/pectin diet enhances apoptosis via Bcl-2 promoter methylation in rat azoxymethane-induced carcinomas. Exp Biol Med (Maywood) 237: 1387–1393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu YF, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem 50: 6910–6916, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Clausen MR, Bonnen H, Tvede M, Mortensen PB. Colonic fermentation to short-chain fatty acids is decreased in antibiotic-associated diarrhea. Gastroenterology 101: 1497–1504, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Codling C, O'Mahony L, Shanahan F, Quigley EM, Marchesi JR. A molecular analysis of fecal and mucosal bacterial communities in irritable bowel syndrome. Dig Dis Sci 55: 392–397, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Cummings JH, Englyst HN. Measurement of starch fermentation in the human large intestine. Can J Physiol Pharmacol 69: 121–129, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Dashwood WM, Orner GA, Dashwood RH. Inhibition of beta-catenin/Tcf activity by white tea, green tea, and epigallocatechin-3-gallate (EGCG): minor contribution of H2O2 at physiologically relevant EGCG concentrations. Biochem Biophys Res Commun 296: 584–588, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Davidson LA, Wang N, Shah MS, Lupton JR, Ivanov I, Chapkin RS. n-3 Polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colon. Carcinogenesis 30: 2077–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 107: 14691–14696, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol 8: 523–531, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Elamin MH, Shinwari Z, Hendrayani SF, Al-Hindi H, Al-Shail E, Khafaga Y, Al-Kofide A, Aboussekhra A. Curcumin inhibits the Sonic Hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Mol Carcinog 49: 302–314, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Eliaz I, Hotchkiss AT, Fishman ML, Rode D. The effect of modified citrus pectin on urinary excretion of toxic elements. Phytother Res 20: 859–864, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Erdman JW, Jr, Ford NA, Lindshield BL. Are the health attributes of lycopene related to its antioxidant function? Arch Biochem Biophys 483: 229–235, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fallani M, Amarri S, Uusijarvi A, Adam R, Khanna S, Aguilera M, Gil A, Vieites JM, Norin E, Young D, Scott JA, Dore J, Edwards CA. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 157: 1385–1392, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Fares F, Azzam N, Appel B, Fares B, Stein A. The potential efficacy of 3,3′-diindolylmethane in prevention of prostate cancer development. Eur J Cancer Prev 19: 199–203, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Fimmel CJ, Etienne A, Cilluffo T, von Ritter C, Gasser T, Rey JP, Caradonna-Moscatelli P, Sabbatini F, Pace F, Buhler HW, et al Long-term ambulatory gastric pH monitoring: validation of a new method and effect of H2-antagonists. Gastroenterology 88: 1842–1851, 1985 [DOI] [PubMed] [Google Scholar]
- 40.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104: 13780–13785, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frank DN, Zhu W, Sartor RB, Li E. Investigating the biological and clinical significance of human dysbioses. Trends Microbiol 19: 427–434, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao Z, Xu Z, Hung MS, Lin YC, Wang T, Gong M, Zhi X, Jablon DM, You L. Promoter demethylation of WIF-1 by epigallocatechin-3-gallate in lung cancer cells. Anticancer Res 29: 2025–2030, 2009 [PubMed] [Google Scholar]
- 43.Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, Gordon JI, Onderdonk AB, Glimcher LH. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe 8: 292–300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gianduzzo TR, Holmes EG, Tinggi U, Shahin M, Mactaggart P, Nicol D. Prostatic and peripheral blood selenium levels after oral supplementation. J Urol 170: 870–873, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Hawrelak JA, Myers SP. The causes of intestinal dysbiosis: a review. Altern Med Rev 9: 180–197, 2004 [PubMed] [Google Scholar]
- 46.Hebert JR, Hurley TG, Olendzki BC, Teas J, Ma Y, Hampl JS. Nutritional and socioeconomic factors in relation to prostate cancer mortality: a cross-national study. J Natl Cancer Inst 90: 1637–1647, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Heitman DW, Hardman WE, Cameron IL. Dietary supplementation with pectin and guar gum on 1,2-dimethylhydrazine-induced colon carcinogenesis in rats. Carcinogenesis 13: 815–818, 1992 [DOI] [PubMed] [Google Scholar]
- 48.Helou C, Marier D, Jacolot P, Abdennebi-Najar L, Niquet-Leridon C, Tessier FJ, Gadonna-Widehem P. Microorganisms and Maillard reaction products: a review of the literature and recent findings. Amino Acids 46: 267–277, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482: 179–185, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res 55: 224–236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang CS, Liao JW, Hu ML. Lycopene inhibits experimental metastasis of human hepatoma SK-Hep-1 cells in athymic nude mice. J Nutr 138: 538–543, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Jacobsen BK, Knutsen SF, Fraser GE. Does high soy milk intake reduce prostate cancer incidence? The Adventist Health Study (United States). Cancer Causes Control 9: 553–557, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Jaiswal AS, Marlow BP, Gupta N, Narayan S. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene 21: 8414–8427, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Jakobsdottir G, Nyman M, Fak F. Designing future prebiotic fiber to target the metabolic syndrome. Nutrition 30: 497–502, 2014 [DOI] [PubMed] [Google Scholar]
- 55.Jian L, Lee AH, Binns CW. Tea and lycopene protect against prostate cancer. Asia Pac J Clin Nutr 16, Suppl 1: 453–457, 2007 [PubMed] [Google Scholar]
- 56.Jorup-Ronstrom C, Hakanson A, Sandell S, Edvinsson O, Midtvedt T, Persson AK, Norin E. Fecal transplant against relapsing Clostridium difficile-associated diarrhea in 32 patients. Scand J Gastroenterol 47: 548–552, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Kassam Z, Hundal R, Marshall JK, Lee CH. Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Arch Intern Med 172: 191–193, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol 108: 500–508, 2013 [DOI] [PubMed] [Google Scholar]
- 59.Kim J, Zhang X, Rieger-Christ KM, Summerhayes IC, Wazer DE, Paulson KE, Yee AS. Suppression of Wnt signaling by the green tea compound (-)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. J Biol Chem 281: 10865–10875, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Kimura Y, Okuda H. Resveratrol isolated from Polygonum cuspidatum root prevents tumor growth and metastasis to lung and tumor-induced neovascularization in Lewis lung carcinoma-bearing mice. J Nutr 131: 1844–1849, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Kipp A, Banning A, van Schothorst EM, Meplan C, Schomburg L, Evelo C, Coort S, Gaj S, Keijer J, Hesketh J, Brigelius-Flohe R. Four selenoproteins, protein biosynthesis, and Wnt signalling are particularly sensitive to limited selenium intake in mouse colon. Mol Nutr Food Res 53: 1561–1572, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Kiuchi F, Goto Y, Sugimoto N, Akao N, Kondo K, Tsuda Y. Nematocidal activity of turmeric: synergistic action of curcuminoids. Chem Pharm Bull (Tokyo) 41: 1640–1643, 1993 [DOI] [PubMed] [Google Scholar]
- 63.Kolar S, Barhoumi R, Jones CK, Wesley J, Lupton JR, Fan YY, Chapkin RS. Interactive effects of fatty acid and butyrate-induced mitochondrial Ca2+ loading and apoptosis in colonocytes. Cancer 117: 5294–5303, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kolar SS, Barhoumi R, Callaway ES, Fan YY, Wang N, Lupton JR, Chapkin RS. Synergy between docosahexaenoic acid and butyrate elicits p53-independent apoptosis via mitochondrial Ca2+ accumulation in colonocytes. Am J Physiol Gastrointest Liver Physiol 293: G935–G943, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, Baselga J, Liu C, Shivdasani RA, Ogino S, Birren BW, Huttenhower C, Garrett WS, Meyerson M. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 22: 292–298, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koushik A, Hunter DJ, Spiegelman D, Beeson WL, van den Brandt PA, Buring JE, Calle EE, Cho E, Fraser GE, Freudenheim JL, Fuchs CS, Giovannucci EL, Goldbohm RA, Harnack L, Jacobs DR, Jr, Kato I, Krogh V, Larsson SC, Leitzmann MF, Marshall JR, McCullough ML, Miller AB, Pietinen P, Rohan TE, Schatzkin A, Sieri S, Virtanen MJ, Wolk A, Zeleniuch-Jacquotte A, Zhang SM, Smith-Warner SA. Fruits, vegetables, and colon cancer risk in a pooled analysis of 14 cohort studies. J Natl Cancer Inst 99: 1471–1483, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Krumholz LR, Crawford RL, Hemling ME, Bryant MP. A rumen bacterium degrading quercetin and trihydroxybenzenoids with concurrent use of formate or H2. Progr Clin Biol Res 213: 211–214, 1986 [PubMed] [Google Scholar]
- 68.Kuang HB, Miao CL, Guo WX, Peng S, Cao YJ, Duan EK. Dickkopf-1 enhances migration of HEK293 cell by beta-catenin/E-cadherin degradation. Front Biosci 14: 2212–2220, 2009 [DOI] [PubMed] [Google Scholar]
- 69.Kucuk O, Sarkar FH, Djuric Z, Sakr W, Pollak MN, Khachik F, Banerjee M, Bertram JS, Wood DP., Jr Effects of lycopene supplementation in patients with localized prostate cancer. Exp Biol Med (Maywood) 227: 881–885, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Kucuk O, Sarkar FH, Sakr W, Djuric Z, Pollak MN, Khachik F, Li YW, Banerjee M, Grignon D, Bertram JS, Crissman JD, Pontes EJ, Wood DP., Jr Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol Biomarkers Prev 10: 861–868, 2001 [PubMed] [Google Scholar]
- 71.Kurahashi N, Sasazuki S, Iwasaki M, Inoue M, Tsugane S. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol 167: 71–77, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients 2: 1266–1289, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lawaetz O, Blackburn AM, Bloom SR, Aritas Y, Ralphs DN. Effect of pectin on gastric emptying and gut hormone release in the dumping syndrome. Scand J Gastroenterol 18: 327–336, 1983 [DOI] [PubMed] [Google Scholar]
- 74.Leclere L, Cutsem PV, Michiels C. Anti-cancer activities of pH- or heat-modified pectin. Front Pharmacol 4: 128, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee SM, Donaldson GP, Mikulski Z, Boyajian S, Ley K, Mazmanian SK. Bacterial colonization factors control specificity and stability of the gut microbiota. Nature 501: 426–429, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leow PC, Tian Q, Ong ZY, Yang Z, Ee PL. Antitumor activity of natural compounds, curcumin and PKF118–310, as Wnt/beta-catenin antagonists against human osteosarcoma cells. Invest New Drugs 28: 766–782, 2010 [DOI] [PubMed] [Google Scholar]
- 77.Lepage P, Hasler R, Spehlmann ME, Rehman A, Zvirbliene A, Begun A, Ott S, Kupcinskas L, Dore J, Raedler A, Schreiber S. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology 141: 227–236, 2011 [DOI] [PubMed] [Google Scholar]
- 78.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Li Y, Wang Z, Kong D, Li R, Sarkar SH, Sarkar FH. Regulation of Akt/FOXO3a/GSK-3beta/AR signaling network by isoflavone in prostate cancer cells. J Biol Chem 283: 27707–27716, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, Yu Y, Clouthier SG, Schwartz SJ, Wicha MS, Sun D. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res 16: 2580–2590, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Limpens J, van Weerden WM, Kramer K, Pallapies D, Obermuller-Jevic UC, Schroder FH. Re: Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst 96: 554; author reply 554–555, 2004 [DOI] [PubMed] [Google Scholar]
- 82.Liu JZ, Jellbauer S, Poe AJ, Ton V, Pesciaroli M, Kehl-Fie TE, Restrepo NA, Hosking MP, Edwards RA, Battistoni A, Pasquali P, Lane TE, Chazin WJ, Vogl T, Roth J, Skaar EP, Raffatellu M. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe 11: 227–239, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2: 119–129, 2007 [DOI] [PubMed] [Google Scholar]
- 84.MacConnachie AA, Fox R, Kennedy DR, Seaton RA. Faecal transplant for recurrent Clostridium difficile-associated diarrhoea: a UK case series. QJM 102: 781–784, 2009 [DOI] [PubMed] [Google Scholar]
- 85.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, Theriaque D, Li N, Sharma R, Hudak M, Neu J. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PloS One 6: e20647, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, Roca J, Dore J. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 55: 205–211, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marchesi JR, Dutilh BE, Hall N, Peters WH, Roelofs R, Boleij A, Tjalsma H. Towards the human colorectal cancer microbiome. PloS One 6: e20447, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McLaughlin SD, Walker AW, Churcher C, Clark SK, Tekkis PP, Johnson MW, Parkhill J, Ciclitira PJ, Dougan G, Nicholls RJ, Petrovska L. The bacteriology of pouchitis: a molecular phylogenetic analysis using 16S rRNA gene cloning and sequencing. Ann Surg 252: 90–98, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Minich DM, Bland JS. A review of the clinical efficacy and safety of cruciferous vegetable phytochemicals. Nutr Rev 65: 259–267, 2007 [DOI] [PubMed] [Google Scholar]
- 90.Muegge BD, Kuczynski J, Knights D, Clemente JC, Gonzalez A, Fontana L, Henrissat B, Knight R, Gordon JI. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 332: 970–974, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Murillo G, Peng X, Torres KE, Mehta RG. Deguelin inhibits growth of breast cancer cells by modulating the expression of key members of the Wnt signaling pathway. Cancer Prev Res (Phila) 2: 942–950, 2009 [DOI] [PubMed] [Google Scholar]
- 92.Nho CW, Jeffery E. Crambene, a bioactive nitrile derived from glucosinolate hydrolysis, acts via the antioxidant response element to upregulate quinone reductase alone or synergistically with indole-3-carbinol. Toxicol Appl Pharmacol 198: 40–48, 2004 [DOI] [PubMed] [Google Scholar]
- 93.Obrenovich ME, Nair NG, Beyaz A, Aliev G, Reddy VP. The role of polyphenolic antioxidants in health, disease, and aging. Rejuvenation Res 13: 631–643, 2010 [DOI] [PubMed] [Google Scholar]
- 94.Ohkami H, Tazawa K, Yamashita I, Shimizu T, Murai K, Kobashi K, Fujimaki M. Effects of apple pectin on fecal bacterial enzymes in azoxymethane-induced rat colon carcinogenesis. Jpn J Cancer Res 86: 523–529, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Olano-Martin E, Gibson GR, Rastell RA. Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. J Appl Microbiol 93: 505–511, 2002 [DOI] [PubMed] [Google Scholar]
- 96.Olano-Martin E, Rimbach GH, Gibson GR, Rastall RA. Pectin and pectic-oligosaccharides induce apoptosis in in vitro human colonic adenocarcinoma cells. Anticancer Res 23: 341–346, 2003 [PubMed] [Google Scholar]
- 97.Pahlke G, Ngiewih Y, Kern M, Jakobs S, Marko D, Eisenbrand G. Impact of quercetin and EGCG on key elements of the Wnt pathway in human colon carcinoma cells. J Agric Food Chem 54: 7075–7082, 2006 [DOI] [PubMed] [Google Scholar]
- 98.Paul S, DeCastro AJ, Lee HJ, Smolarek AK, So JY, Simi B, Wang CX, Zhou R, Rimando AM, Suh N. Dietary intake of pterostilbene, a constituent of blueberries, inhibits the beta-catenin/p65 downstream signaling pathway and colon carcinogenesis in rats. Carcinogenesis 31: 1272–1278, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Petrof EO, Gloor GB, Vanner SJ, Weese SJ, Carter D, Daigneault MC, Brown EM, Schroeter K, Allen-Vercoe E. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome 1: 3, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Petrovski G, Gurusamy N, Das DK. Resveratrol in cardiovascular health and disease. Ann NY Acad Sci 1215: 22–33, 2011 [DOI] [PubMed] [Google Scholar]
- 101.Polakis P. Wnt signaling and cancer. Genes Dev 14: 1837–1851, 2000 [PubMed] [Google Scholar]
- 102.Power SE, O'Toole PW, Stanton C, Ross RP, Fitzgerald GF. Intestinal microbiota, diet and health. Br J Nutr 111: 387–402, 2014 [DOI] [PubMed] [Google Scholar]
- 103.Prasad CP, Rath G, Mathur S, Bhatnagar D, Ralhan R. Potent growth suppressive activity of curcumin in human breast cancer cells: modulation of Wnt/beta-catenin signaling. Chem Biol Interact 181: 263–271, 2009 [DOI] [PubMed] [Google Scholar]
- 104.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490: 55–60, 2012 [DOI] [PubMed] [Google Scholar]
- 105.Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, Mak TW, Tsolis RM, Bevins CL, Solnick JV, Dandekar S, Baumler AJ. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 5: 476–486, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ramakrishna BS, Mathan VI. Colonic dysfunction in acute diarrhoea: the role of luminal short chain fatty acids. Gut 34: 1215–1218, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ransohoff DF. Fecal DNA tests for colorectal cancer. N Engl J Med 346: 1912–1913; discussion 1912–1913, 2002 [DOI] [PubMed] [Google Scholar]
- 109.Roccaro AM, Leleu X, Sacco A, Moreau AS, Hatjiharissi E, Jia X, Xu L, Ciccarelli B, Patterson CJ, Ngo HT, Russo D, Vacca A, Dammacco F, Anderson KC, Ghobrial IM, Treon SP. Resveratrol exerts antiproliferative activity and induces apoptosis in Waldenstrom's macroglobulinemia. Clin Cancer Res 14: 1849–1858, 2008 [DOI] [PubMed] [Google Scholar]
- 110.Roeselers G, Ponomarenko M, Lukovac S, Wortelboer HM. Ex vivo systems to study host-microbiota interactions in the gastrointestinal tract. Best Pract Res Clin Gastroenterol 27: 101–113, 2013 [DOI] [PubMed] [Google Scholar]
- 111.Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol 44: 567–570, 2010 [DOI] [PubMed] [Google Scholar]
- 112.Roth GN, Chandra A, Nair MG. Novel bioactivities of Curcuma longa constituents. J Nat Prod 61: 542–545, 1998 [DOI] [PubMed] [Google Scholar]
- 113.Ryu MJ, Cho M, Song JY, Yun YS, Choi IW, Kim DE, Park BS, Oh S. Natural derivatives of curcumin attenuate the Wnt/beta-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochem Biophys Res Commun 377: 1304–1308, 2008 [DOI] [PubMed] [Google Scholar]
- 114.Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer Invest 21: 744–757, 2003 [DOI] [PubMed] [Google Scholar]
- 115.Sarkar FH, Li Y, Wang Z, Padhye S. Lesson learned from nature for the development of novel anti-cancer agents: implication of isoflavone, curcumin, and their synthetic analogs. Curr Pharm Des 16: 1801–1812, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schneider H, Schwiertz A, Collins MD, Blaut M. Anaerobic transformation of quercetin-3-glucoside by bacteria from the human intestinal tract. Arch Microbiol 171: 81–91, 1999 [DOI] [PubMed] [Google Scholar]
- 117.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer 13: 800–812, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schwartz SE, Levine GD, Starr CM. Effects of dietary fiber on intestinal ion fluxes in rats. Am J Clin Nutr 36: 1102–1105, 1982 [DOI] [PubMed] [Google Scholar]
- 119.Scott EN, Gescher AJ, Steward WP, Brown K. Development of dietary phytochemical chemopreventive agents: biomarkers and choice of dose for early clinical trials. Cancer Prev Res (Phila) 2: 525–530, 2009 [DOI] [PubMed] [Google Scholar]
- 120.Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res 69: 52–60, 2013 [DOI] [PubMed] [Google Scholar]
- 121.Shah MS, Schwartz SL, Zhao C, Davidson LA, Zhou B, Lupton JR, Ivanov I, Chapkin RS. Integrated microRNA and mRNA expression profiling in a rat colon carcinogenesis model: effect of a chemo-protective diet. Physiol Genomics 43: 640–654, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shin HW, Park SY, Lee KB, Jang JJ. Down-regulation of Wnt signaling during apoptosis of human hepatic stellate cells. Hepatogastroenterology 56: 208–212, 2009 [PubMed] [Google Scholar]
- 123.Slusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, Narula AS, MacDonald RS, Besch-Williford CL, Lubahn DB. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Res 70: 3382–3390, 2010 [DOI] [PubMed] [Google Scholar]
- 124.Smith EA, Macfarlane GT. Enumeration of human colonic bacteria producing phenolic and indolic compounds: effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J Appl Bacteriol 81: 288–302, 1996 [DOI] [PubMed] [Google Scholar]
- 125.Sobhani I, Tap J, Roudot-Thoraval F, Roperch JP, Letulle S, Langella P, Corthier G, Tran Van Nhieu J, Furet JP. Microbial dysbiosis in colorectal cancer (CRC) patients. PloS One 6: e16393, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Dore J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis 15: 1183–1189, 2009 [DOI] [PubMed] [Google Scholar]
- 127.Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, Dougan G, von Mering C, Hardt WD. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol 5: 2177–2189, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stelter C, Kappeli R, Konig C, Krah A, Hardt WD, Stecher B, Bumann D. Salmonella-induced mucosal lectin RegIIIbeta kills competing gut microbiota. PloS One 6: e20749, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Strimpakos AS, Sharma RA. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 10: 511–545, 2008 [DOI] [PubMed] [Google Scholar]
- 130.Su Y, Simmen FA, Xiao R, Simmen RC. Expression profiling of rat mammary epithelial cells reveals candidate signaling pathways in dietary protection from mammary tumors. Physiol Genomics 30: 8–16, 2007 [DOI] [PubMed] [Google Scholar]
- 131.Su Y, Simmen RC. Soy isoflavone genistein upregulates epithelial adhesion molecule E-cadherin expression and attenuates beta-catenin signaling in mammary epithelial cells. Carcinogenesis 30: 331–339, 2009 [DOI] [PubMed] [Google Scholar]
- 132.Subramaniam D, Ponnurangam S, Ramamoorthy P, Standing D, Battafarano RJ, Anant S, Sharma P. Curcumin induces cell death in esophageal cancer cells through modulating Notch signaling. PloS One 7: e30590, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis 30: 300–307, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun J, Chu YF, Wu X, Liu RH. Antioxidant and antiproliferative activities of common fruits. J Agric Food Chem 50: 7449–7454, 2002 [DOI] [PubMed] [Google Scholar]
- 135.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 3: 768–780, 2003 [DOI] [PubMed] [Google Scholar]
- 136.Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut 53: 1–4, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil 22: 512–519, e114–e115, 2010 [DOI] [PubMed] [Google Scholar]
- 138.Tang GQ, Yan TQ, Guo W, Ren TT, Peng CL, Zhao H, Lu XC, Zhao FL, Han X. (-)-Epigallocatechin-3-gallate induces apoptosis and suppresses proliferation by inhibiting the human Indian Hedgehog pathway in human chondrosarcoma cells. J Cancer Res Clin Oncol 136: 1179–1185, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tarini J, Wolever TM. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab 35: 9–16, 2010 [DOI] [PubMed] [Google Scholar]
- 140.Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, Sterzenbach T, Tsolis RM, Roth JR, Baumler AJ. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc Natl Acad Sci USA 108: 17480–17485, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tvede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet 1: 1156–1160, 1989 [DOI] [PubMed] [Google Scholar]
- 142.Tzounis X, Vulevic J, Kuhnle GG, George T, Leonczak J, Gibson GR, Kwik-Uribe C, Spencer JP. Flavanol monomer-induced changes to the human faecal microflora. Br J Nutr 99: 782–792, 2008 [DOI] [PubMed] [Google Scholar]
- 143.Umar S, Morris AP, Kourouma F, Sellin JH. Dietary pectin and calcium inhibit colonic proliferation in vivo by differing mechanisms. Cell Prolif 36: 361–375, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van Nood E, Dijkgraaf MG, Keller JJ. Duodenal infusion of feces for recurrent Clostridium difficile. N Engl J Med 368: 2145, 2013 [DOI] [PubMed] [Google Scholar]
- 145.Vanamala J, Glagolenko A, Yang P, Carroll RJ, Murphy ME, Newman RA, Ford JR, Braby LA, Chapkin RS, Turner ND, Lupton JR. Dietary fish oil and pectin enhance colonocyte apoptosis in part through suppression of PPARdelta/PGE2 and elevation of PGE3. Carcinogenesis 29: 790–796, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vanamala J, Reddivari L, Radhakrishnan S, Tarver C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer 10: 238, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Velmurugan B, Singh RP, Kaul N, Agarwal R, Agarwal C. Dietary feeding of grape seed extract prevents intestinal tumorigenesis in APCmin/+ mice. Neoplasia 12: 95–102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328: 228–231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Walker AW, Sanderson JD, Churcher C, Parkes GC, Hudspith BN, Rayment N, Brostoff J, Parkhill J, Dougan G, Petrovska L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol 11: 7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang M, Karlsson C, Olsson C, Adlerberth I, Wold AE, Strachan DP, Martricardi PM, Aberg N, Perkin MR, Tripodi S, Coates AR, Hesselmar B, Saalman R, Molin G, Ahrne S. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol 121: 129–134, 2008 [DOI] [PubMed] [Google Scholar]
- 151.Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, Jia W, Cai S, Zhao L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J 6: 320–329, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57–63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152a.Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 321: 1273–1275, 1983 [PubMed] [Google Scholar]
- 153.Watanabe K, Reddy BS, Weisburger JH, Kritchevsky D. Effect of dietary alfalfa, pectin, and wheat bran on azoxymethane- or methylnitrosourea-induced colon carcinogenesis in F344 rats. J Natl Cancer Inst 63: 141–145, 1979 [PubMed] [Google Scholar]
- 154.Wattenberg LW. Chemoprevention of cancer. Prev Med 25: 44–45, 1996 [DOI] [PubMed] [Google Scholar]
- 155.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455: 1109–1113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Weng YL, Liao HF, Li AF, Chang JC, Chiou RY. Oral administration of resveratrol in suppression of pulmonary metastasis of BALB/c mice challenged with CT26 colorectal adenocarcinoma cells. Mol Nutr Food Res 54: 259–267, 2010 [DOI] [PubMed] [Google Scholar]
- 157.Wertz K. Lycopene effects contributing to prostate health. Nutr Cancer 61: 775–783, 2009 [DOI] [PubMed] [Google Scholar]
- 158.Willing B, Halfvarson J, Dicksved J, Rosenquist M, Jarnerot G, Engstrand L, Tysk C, Jansson JK. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn's disease. Inflamm Bowel Dis 15: 653–660, 2009 [DOI] [PubMed] [Google Scholar]
- 159.Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z, Jarnerot G, Tysk C, Jansson JK, Engstrand L. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 139: 1844–1854.e1, 2010 [DOI] [PubMed] [Google Scholar]
- 160.Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol 9: 233–243, 2011 [DOI] [PubMed] [Google Scholar]
- 161.Winter J, Moore LH, Dowell VR, Jr, Bokkenheuser VD. C-ring cleavage of flavonoids by human intestinal bacteria. Appl Environ Microbiol 55: 1203–1208, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, Roth JR, Baumler AJ. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467: 426–429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature 486: 222–227, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhou H, Shang L, Li X, Zhang X, Gao G, Guo C, Chen B, Liu Q, Gong Y, Shao C. Resveratrol augments the canonical Wnt signaling pathway in promoting osteoblastic differentiation of multipotent mesenchymal cells. Exp Cell Res 315: 2953–2962, 2009 [DOI] [PubMed] [Google Scholar]