Abstract
Previous experiments in cultures of neonatal rat myocytes demonstrated that the shape of Cai2+ transients measured using high-affinity Ca2+-sensitive dyes may be misrepresented. The purpose of this study was to examine the role of dye affinity in Cai2+ measurements in intact adult cardiac tissue by comparing optical recordings obtained with high- and low-affinity dyes. Experiments were carried out in porcine left ventricular (LV) wedge preparations stained locally by intramural injection via microcapillaries (diameter = 150 μm) with a low-affinity Ca2+-sensitive dye Fluo-4FF or Fluo-2LA (nominal Kd, ∼7–10 μmol/l), high-affinity dye Rhod-2 (Kd = 0.57 μmol/l), and Fluo-4 or Fluo-2MA (Kd, ∼0.4 μmol/l); in addition, tissue was stained with transmembrane potential (Vm)-sensitive dye RH-237. Optical recordings of Vm and Cai2+ were made using optical fibers (diameter = 325 μm) glued with the microcapillaries. The durations of Cai2+ transients measured at 50% level of recovery (CaD50) using high-affinity Fluo-4/Fluo-2MA dyes were up to ∼81% longer than those measured with low-affinity Fluo-4FF/Fluo-2LA at long pacing cycle lengths (CL). In Fluo-4/Fluo-2MA measurements at long CLs, Cai2+ transients often (∼50% of cases) exhibited slow upstroke rise and extended plateau. In Rhod-2 measurements, CaD50 was moderately longer (up to ∼35%) than in Fluo-4FF recordings, but Cai2+ transient shapes were similar. In all series of measurements, mean action potential duration values were not significantly different (P > 0.05). The delays between Vm and Cai2+ upstrokes were comparable for low- and high-affinity dyes (P > 0.05). In conclusion, measurements of Cai2+ transient in ventricular myocardium are strongly affected by the affinity of Ca2+ dyes. The high-affinity dyes may overestimate the duration and alter the shape of Cai2+ transients.
Keywords: intracellular calcium transient, optical mapping, optical fiber, dye affinity
changes in intracellular concentration of calcium ions (Cai2+) play important roles in myocardial function including regulation of contractility, electrical excitation, and arrhythmogenesis. Optical imaging with fluorescent dyes is presently the only available method for measurements of rapid Cai2+ changes during calcium transients, spontaneous oscillations, and waves. Optical Cai2+ imaging can be combined with simultaneous imaging of transmembrane potential (Vm) allowing studying interaction between Vm and Cai2+, which is crucial for understanding mechanisms of ECG alternans (2, 9, 11, 21) and cardiac arrhythmias (1, 12, 16, 18, 20, 22, 25, 28). An important requirement in such measurements is faithful reproduction of Cai2+ changes by fluorescent dyes. Previous studies in cultures of neonatal rat myocytes demonstrated that the shape of optical Cai2+ transients (CaT) can be strongly affected by the affinity of Ca2+-sensitive dyes (5, 6). Measurements that use high-affinity dyes such as Fluo-3, Fluo-4, and Rhod-2 (nominal Kd = 0.345, 0.35, and 0.57 μmol/l, respectively), which were often used in whole cardiac tissue (3, 4, 14, 15, 17, 18, 24), exhibited CaTs with a duration (CaD) approximately twice as long as action potential duration (APD) and significantly longer than CaDs measured using low-affinity dyes such as Fluo-4FF and Rhod-FF (Kd = 9.7 and 19 μmol/l, respectively) (5, 6). Mathematical modeling of ion-dye binding showed that this difference could be explained by the nonlinear response of high-affinity dyes to Cai2+ changes (13). Whether this effect applies to the intact adult myocardium is not known. The ionic properties and Cai2+ dynamics in adult and neonatal cells are different, which may result in a different relationship between Cai2+ changes and optical signals. Therefore, the goal of this study was to examine the role of dye affinity in measurements of CaT in the whole myocardium by comparing optical recordings obtained with high-affinity and low-affinity fluorescent dyes.
METHODS
Heart preparation.
Experiments were performed in coronary-perfused left ventricle (LV) wedge preparations isolated from porcine hearts. Animal use conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No.85-23, revised 1996). The protocol was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Pigs were anesthetized with telazol (4.4 mg/kg), xylazine (4.4 mg/kg), and antropine (0.04 mg/kg). Anesthesia was maintained with inhalation of isoflurane (1.3–2.5%) in oxygen during surgery. Heparin (500 unit/kg) was given 10 min before heart extraction. To improve heart preservation, cold cardioplegic solution containing (in mmol/l)110 NaCl, 16 KCl, 16 MgCl2, 1.2 CaCl2, and 10 NaHCO3 was infused into the clamped aorta before excising the heart, and then the coronary arteries were flushed with cardioplegic solution immediately after the heart was removed. A wedge preparation with dimensions of ∼2 × 5 cm2 was cut out from anterior LV wall. A branch of the left descending coronary artery was cannulated and perfused using a peristaltic pump with Tyrode's solution containing (in mmol/l) 128.5 NaCl NaCl, 4.7 KCl, 0.7 MgCl2, 0.5 NaH2PO4, 1.5 CaCl2, 28 NaHCO3, and 5 or 20 glucose bubbled with 95% O2 and 5% CO2 at a temperature of ≃37°C. The perfusion pressure was maintained at ∼70 mmHg.
To prevent motion artifact in optical recordings, muscle contraction was inhibited with 20 μmol/l of blebbistatin (Tocris Bioscience, Ellisville, MO). Since blebbistatin is light sensitive, preparation of blebbistatin solution and all experiments were performed in dark conditions. In addition, Tyrode's solution was supplemented with 1 μmol/l of anion transporter inhibitor probenecid (Sigma, St. Louis, MO) to prevent active extrusion of Fluo dyes from cells (6).
Dye staining.
Previous measurements of Cai2+ in whole cardiac tissue used high-affinity Ca2+-sensitive dyes delivered via coronary infusion. Preliminary experiments performed in this study demonstrated that this staining method was not successful for low-affinity dyes Fluo-4FF and Rhod-FF; coronary infusion of these dyes did not produce detectable optical signals. Therefore, we developed a different approach, in which tissue was stained with a Ca2+-sensitive dye locally via intramural injection. The dye was dissolved first in DMSO and then in Tyrode's solution. In the majority of injections (77%), dyes were used at a final concentration of 10 μmol/l. In some cases, dyes were also used at 5 and 20 μmol/l concentrations (each in 11% of injections). The data obtained at different dye concentrations were qualitatively similar; therefore, they were grouped together in statistical analysis. Thin plastic microcapillaries with external diameter of 200 μm and internal diameter of 150 μm were glued to optical fibers used for fluorescent light measurements and inserted into the ventricular wall at a depth of ∼4–6 mm, as shown schematically in Fig. 1. After ∼10 min, the dye solutions were delivered into the tissue via microcapillary at a flow rate of 0.05–0.2 ml/min for 5 min using syringe pumps.
Fig. 1.
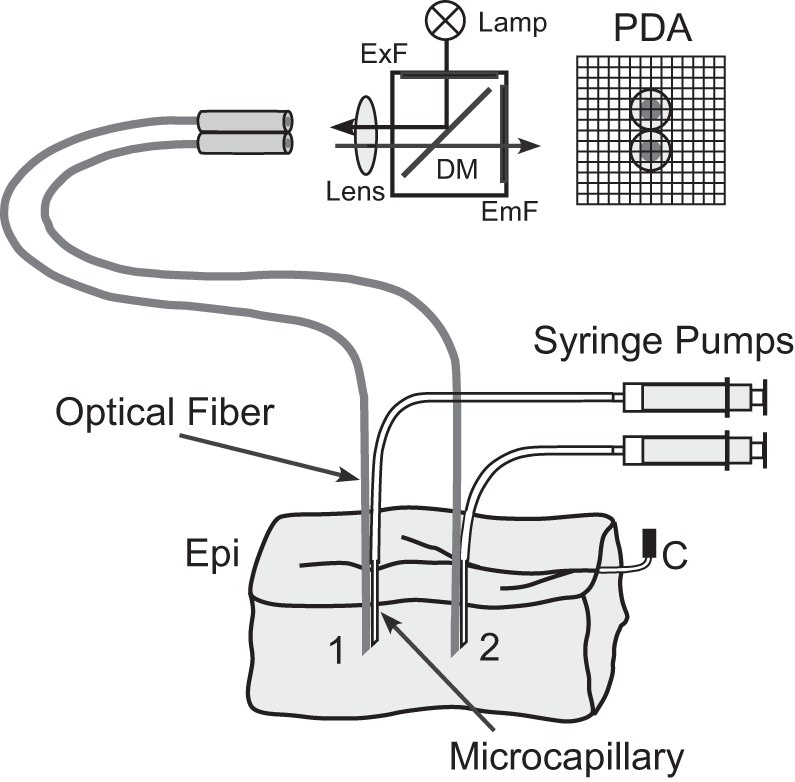
Schematic diagram of experimental setup with 2 microcapillary fiber bundles inserted into left ventricular (LV) wedge preparation. PDA, photodiode array; ExF, excitation filter; EmF, emission filter; DM, dichroic mirror; Epi, epicardium; C, perfusion cannula.
Several low-affinity Ca2+-sensitive dyes were tested in preliminary experiments for local staining including Fluo-4FF (Invitrogen, Carlsbad, CA), its analog Fluo-2 low-affinity (Fluo-2LA; Teflabs, Austin, TX), and Rhod-FF (Invitrogen). All dyes were used in their AM (membrane permeable) versions. Among these dyes, only Fluo-4FF and Fluo-2LA reliably produced optical signals of sufficient magnitude. Therefore, these dyes were used in subsequent experiments. In addition to staining with a low-affinity dye, tissue was simultaneously stained with a high-affinity dye via a second microcapillary, which was inserted at the same tissue depth as the first microcapillary. Several high-affinity dyes were tested including Fluo-4 (Invitrogen), its analog Fluo-2 medium affinity (Fluo-2MA; Teflabs, Austin, TX), and Rhod-2 (Invitrogen and Teflabs). All high-affinity dyes normally produced large optical signals. The nominal Kd values (measured in test solutions) of dyes are listed in Table 1.
Table 1.
Nominal dye Kd values and CaT parameters measured at selected cycle lengths
| CaT Duration at 80% Recovery, ms |
||||||
|---|---|---|---|---|---|---|
| Dye | Kd, μmol/l | CL = 300 ms | CL = 500 ms | CL = 2,000 ms | Transmembrane Potential-Ca Delay, ms | CaT Rise Time, ms |
| Fluo-4FF | 9.7 | 208 ± 34 | 256 ± 15 | 287 ± 53 | 12.4 ± 1.1 | 31.5 ± 2.8 |
| Fluo-2LA | 6.7 | 181 ± 55 | 232 ± 34 | 277 ± 57 | 10.8 ± 0.9 | 27.5 ± 3.4 |
| Rhod 2 | 0.57 | 213 ± 24 | 279 ± 23* | 353 ± 41* | 12.0 ± 1.0 | 28.8 ± 3.8 |
| Fluo-2MA | 0.39 | 233 ± 8* | 321 ± 35* | 531 ± 128* | 10.1 ± 1.1 | 24.7 ± 7.1 |
| Fluo-4 | 0.35 | 229 ± 15* | 342 ± 47* | 458 ± 84* | 11.4 ± 1.0 | 33.0 ± 9.3 |
Values are means ± SD. CaT, Cai2+ transient; CL, cycle length.
P < 0.05 vs. Fluo-4FF.
In addition to Ca2+-sensitive dye staining, the tissue was stained with a Vm-sensitive dye RH-237 (Biotium, Hayward, CA). This dye was either injected at a concentration of 5 μmol/l via microcapillaries simultaneously with Ca2+-sensitive dyes or it was delivered before Ca2+ dye staining as a 5-ml bolus of 300-μmol/l dye solution in the bubble trap. Optical measurements started ∼12 min after dye injection and continued for up to ∼1.5 h.
Optical recordings of Cai2+ and Vm.
Optical recordings were performed using silicon fibers with an outer diameter of 325 μm, core diameter of 300 μm, and numerical aperture of 0.39 (FT-300-UMT; Thorlabs, Newtown, NJ). At one end, the fibers were glued to dye injection microcapillaries. At the other end, fibers were flat-cleaved, polished, and mounted on inverted epi-fluorescent microscope (Zeiss Axiovert 135AV). Several fibers were bundled together and imaged simultaneously. Plastic jackets encompassing individual fibers ensured the lack of inter-fiber cross talk.
Figure 1 shows a schematic diagram of the optical setup and two microcapillary-fiber bundles inserted into LV wedge preparation. Excitation light was provided by a 200-W Hg/Xe lamp. The duration of excitation light exposure was controlled using an electromechanical shutter. To reduce dye photobleaching and possible degradation of blebbistatin by light, the duration of light exposure in each recording was typically limited to 1.2 s. The excitation light was focused on fiber ends using a 10× objective lens (Fluar; Carl Zeiss, Thornwood, NY). The same lens was used to collect fluorescent light and focus it on a 16 × 16 photodiode array (C4675–102; Hamamatsu, Japan). Detected optical signals were conditioned and sampled using a previously described mapping system (5) at a sample rate of 1 kHz. With the 10× lens, each fiber was projected onto at least four photodiodes. Signals from these photodiodes were averaged to improve the signal-to-noise ratio.
Vm and Ca2+-sensitive measurements were performed sequentially by changing optical filter sets. To measure fluorescence of Fluo dyes a filter set included a 480/40-nm excitation filter, a 535/50-nm emission filter, and a 505-nm dichroic mirror. Measurements of Rhod dyes used a 530/40-nm excitation filter, a 580/40-nm emission filter, and a 553-nm dichroic mirror. The RH-237 filter set included a 560/55-nm excitation filter, >720-nm emission filter, and a 600-nm dichroic mirror.
To check for cross talk between Vm and Cai2+ measurements, optical recordings were performed first by staining the tissue with only one dye (either Ca2+-sensitive dye or RH-237). Cross talk between Fluo dyes and RH-237 was undetectable. Cross talk between Rhod-2 and RH-237 was below the noise level.
Wedge preparations were stimulated via a bipolar electrode. Action potentials (APs) and CaTs were first recorded at a regular pacing cycle length (CL) of 500 ms and then repeated at CL of 1,000, 2,000, 300, and 250 ms. At the end of this series, measurements were repeated at the 500-ms CL to check for measurements reproducibility. The shapes of APs and CaTs measured at the 500-ms CL before and after the series were similar. To avoid possible motion artifact caused by pulsatile flow, the perfusion pump was stopped during the recording period. Optical Cai2+ recordings exhibited gradual decline of signal magnitude likely due to combination of dye photobleaching and washout. Because of this signal decline, optical recordings taken within 30 min after the start of measurements were used in data analysis.
Data analysis.
Signals were digitally filtered using a low-pass filter with a cutoff frequency of 0.1 kHz to reduce the high-frequency noise. Signals with sufficient signal-to-noise ratio were obtained in ∼70% of all recordings. Optical signals were corrected for photobleaching by subtracting a linear signal fit calculated using the baseline signal portion preceding AP upstrokes. The AP and CaT amplitudes were calculated as signal differences before and after the respective upstrokes. The arrival times of APs and CaTs were determined at 50% of signal amplitude. The signal rise times were measured as intervals between moments when signals reached levels of 10% and 90% of the total amplitude. The times to peak were measured as intervals between moments of 10% and 100% of signal values. The durations of APs and CaTs were measured as the time intervals between arrival and recovery times determined at 50% and 80% levels of signal recovery.
Data were expressed as means ± SD. Differences were compared using the two-tailed paired or unpaired t-test where appropriate. They were considered significant if P < 0.05.
RESULTS
Figure 2 shows representative optical recordings of Cai2+ and Vm obtained at different pacing CLs in porcine LV wedge preparation stained by microperfusion at two intramural sites with a high-affinity Ca2+ dye Rhod-2 (site 1) and a low-affinity dye Fluo-4FF (site 2). The Fluo-4FF traces had a lower signal-to-noise ratio than the Rhod-2 traces, likely due to lower affinity of Fluo-4FF. In addition, the preparation was stained by coronary infusion with Vm dye RH-237. The Vm recordings show that APs at the two sites had similar shapes and durations for all CLs. In contrast, CaTs measured with two dyes were different. The CaTs recorded with the low-affinity dye Fluo-4FF (red traces) had durations shorter than CaTs recorded with Rhod-2. At the CL of 300 ms (Fig. 2A), CaD80 measured with Fluo-4FF was ∼6% smaller than CaD80 measured with Rhod-2 (181 ms vs. 192 ms, respectively). This difference increased to ∼19% (262 ms vs. 322 ms, respectively) when CL was increased to 1,000 ms. Although the CaDs were different, the shapes of CaTs recorded with Rhod-2 and Fluo-4FF dyes were qualitatively similar. In comparison with the local APDs, CaDs measured with Fluo-4FF were somewhat shorter, whereas CaDs measured with Rhod-2 were similar.
Fig. 2.
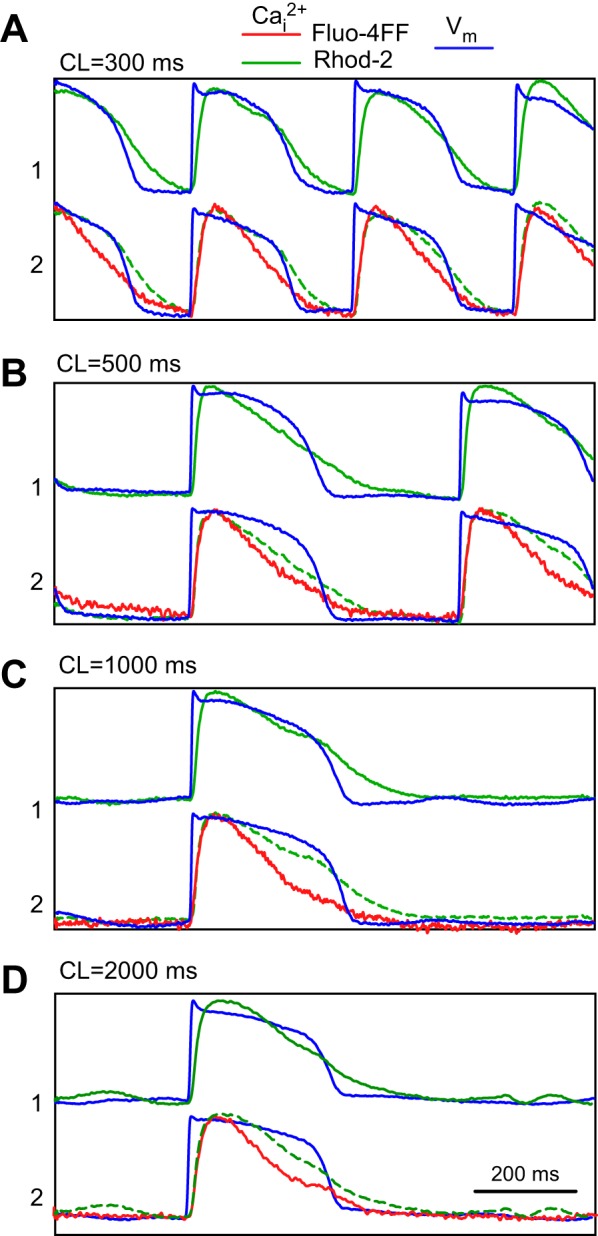
Comparison of Cai2+ transients measured using high-affinity dye Rhod-2 (site 1, green traces) and low-affinity dye Fluo-4FF (site 2, red traces), and corresponding transmembrane potential (Vm) recordings (blue traces) at different cycle lengths (CLs): 300 ms (A), 500 ms (B), 1,000 ms (C), and 2,000 ms (D). Green dashed lines duplicate Rhod-2 signals from site 1 to compare them with Fluo-4FF traces. Traces are individually normalized.
Figure 3 compares Cai2+ and Vm recordings obtained in another preparation stained at two sites with low-affinity Fluo-4FF and high-affinity Fluo-4. Similar to Fig. 2, the lower-affinity dye (Fluo-4FF) exhibited lower signal-to-noise ratio than the higher-affinity dye (Rhod-2), Also similar to Fig. 2, APs recorded at two sites had similar shapes and durations at all CLs. At the same time, CaT shapes recorded with Fluo-4FF and Fluo-4 were substantially different, especially at long CLs. At the CL of 300 ms (Fig. 3A), CaD80 measured with Fluo-4 was ∼26% longer than that one measured with Fluo-4FF (267 ms vs. 212 ms, respectively). This difference increased to ∼114% at a CL of 2,000 ms (557 ms vs. 260 ms, respectively) (Fig. 3D). The CaTs measured with Fluo-4 were significantly longer than the corresponding APs, especially at longer CLs. Thus, at the CL of 2,000 ms, the Fluo-4 CaD80 was ∼56% longer than the local APD at 80% level of recovery (557 ms vs. 356 ms, respectively).
Fig. 3.
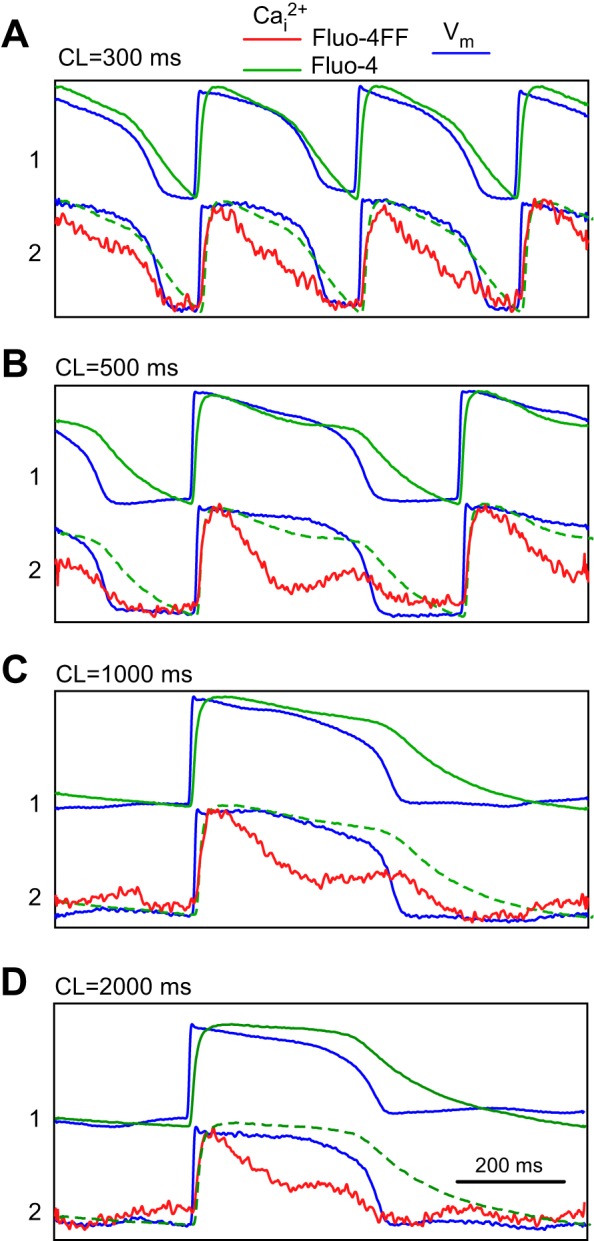
Comparison of Cai2+ transients measured using high-affinity dye Fluo-4 (site 1, green traces) and low-affinity dye Fluo-4FF (site 2, red traces), and corresponding Vm recordings (blue traces) at different CLs (A–D). Green dashed lines duplicate Fluo-4 signals from site 1 to compare them with Fluo-4FF traces. Traces are individually normalized.
In some cases, Cai2+ recordings demonstrated nonmonotonic recovery of CaTs. This is exemplified by the Fluo-4FF recording at CL of 500 ms (Fig. 3B, red trace). This recording shows that the initial Cai2+ recovery was followed by a second rise. In the Fluo-4 recording, this second rise was less apparent but still visible (green trace).
Another interesting feature of Cai2+ recordings is the presence of a long plateau in CaT measured with the Fluo-4 dye at long CLs (Fig. 3D, green trace). This feature could be even more pronounced in other preparations. Figure 4 shows Fluo-4 recordings obtained in another preparation at different CLs in comparison with Fluo-4FF and Rhod-2 recordings. Whereas Fluo-4 CaTs had a typical nearly triangular shape with a rapid rise at CL of 300 ms (lower black trace), the CaT measured at the CL of 500 ms (blue trace) exhibited a long plateau. At the CL of 1,000 and 2,000 ms (lower green and red traces), CaTs exhibited very slow rises with Cai2+ reaching peaks only ∼250 ms after the CaT onset. The following Cai2+ recovery was also much slower than at shorter CLs resulting in very long CaTs.
Fig. 4.
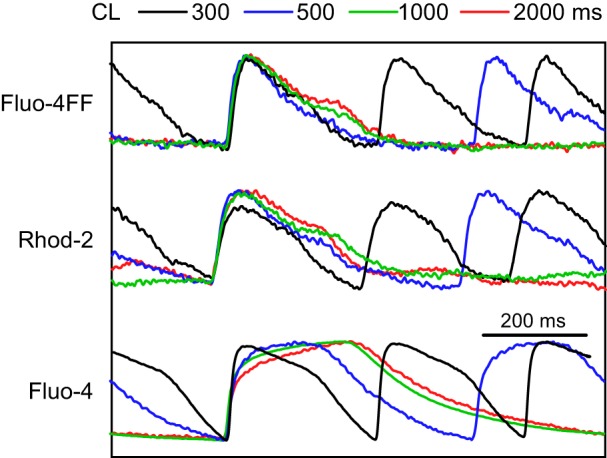
Superimposed Cai2+ traces measured with Fluo-4FF, Rhod-2, and Fluo-4 at different CLs. Traces are individually normalized.
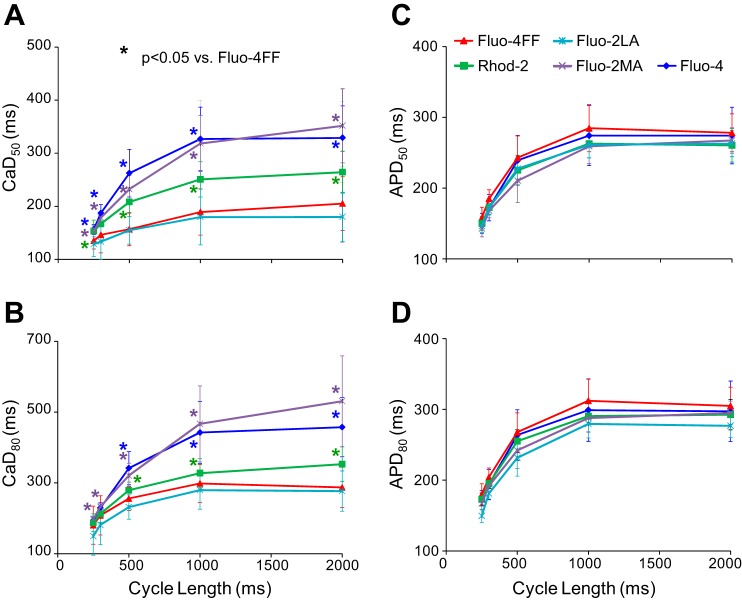
Similar results were obtained in a total of 13 preparations. Figure 5 presents data on the duration of CaTs measured using different dyes and corresponding APDs from all preparations. Parameters of Cai2+ transients and corresponding nominal dye Kd values at selected CLs are also presented in Table 1. In general, increase of dye affinity was associated with prolongation of CaTs. At each CL, the shortest CaTs were measured using the low-affinity dyes Fluo-4FF/Fluo-2LA and the longest CaTs were measured with high-affinity dyes Fluo-4/Fluo-2MA. Rhod-2 measurements exhibited CaTs with intermediate durations. There were no significant differences in average CaDs between the low-affinity dyes Fluo-4FF and Fluo-2LA (NS) or between the high-affinity dyes Fluo-4 and Fluo-2MA (NS). The largest difference in average CaD50 between Fluo-4/Fluo-2MA and Fluo-4FF/Fluo-2LA was ∼81% at CL 2,000 ms (Fig. 5A). The difference between Rhod-2 and Fluo-4FF/Fluo-2LA at this CL was ∼35% (Fig. 5A). The CaD80 measurements exhibited qualitatively similar differences between high- and low-affinity dyes. In contrast with CaD, the APD measurements (Fig. 5, C and D) were not significantly different in all series of measurements.
Fig. 5.
Comparison of Cai2+ transient (CaT) duration (CaD) and action potential duration (APD) at 50% and 80% levels of signal recovery (CaD50 and CaD80, APD50 and APD80, respectively) measured with different dyes as functions of CL (A–D). Blue, purple, green, light blue, and red represent measurements obtained with Fluo-4, Fluo-2MA, Rhod-2, Fluo-2LA, and Fluo-4FF, respectively. The colored star near each data point showed significance compared with Fluo-4FF.
The nonmonotonic recovery of CaT with the second Cai2+ rise exemplified in the Fluo-4FF trace in Fig. 3B was observed at long CL in 30–50% of recordings obtained with different dyes. The second Cai2+ rise appeared at the beginning of phase 3 of AP with an average delay of 256 ± 42 ms (n = 28; CL = 1,000 ms) after the AP upstroke.
The slow rise and signal plateauing shown in Fig. 4 were observed at long CLs in ∼50% of recordings with high-affinity Fluo-4/Fluo-2MA. They were not observed in recordings with low-affinity dyes Fluo-4FF/Fluo-2LA, as well as in Rhod-2 recordings. The slow Cai2+ upstroke rise in Fluo-4/Fluo-2MA recordings resulted in prolongation of the time-to-peak of CaTs upon increasing CL (Fig. 6A), which was measured as the time interval between the 10% level of CaT and its peak. For the low-affinity dyes, this parameter was not significantly changed. There was a trend showing larger CaT time-to-peak values for Fluo-4/Fluo-2MA at long CLs in comparison with the low-affinity dyes, but these differences were not statistically significant, probably due to large SD values. There were also no significant changes in the CaT rise times (Fig. 6B) and in the Vm-Ca delays (Fig. 6C) upon increasing CL for either low-affinity or high-affinity dyes. These parameters also exhibited no significant differences between low- and high-affinity dyes.
Fig. 6.
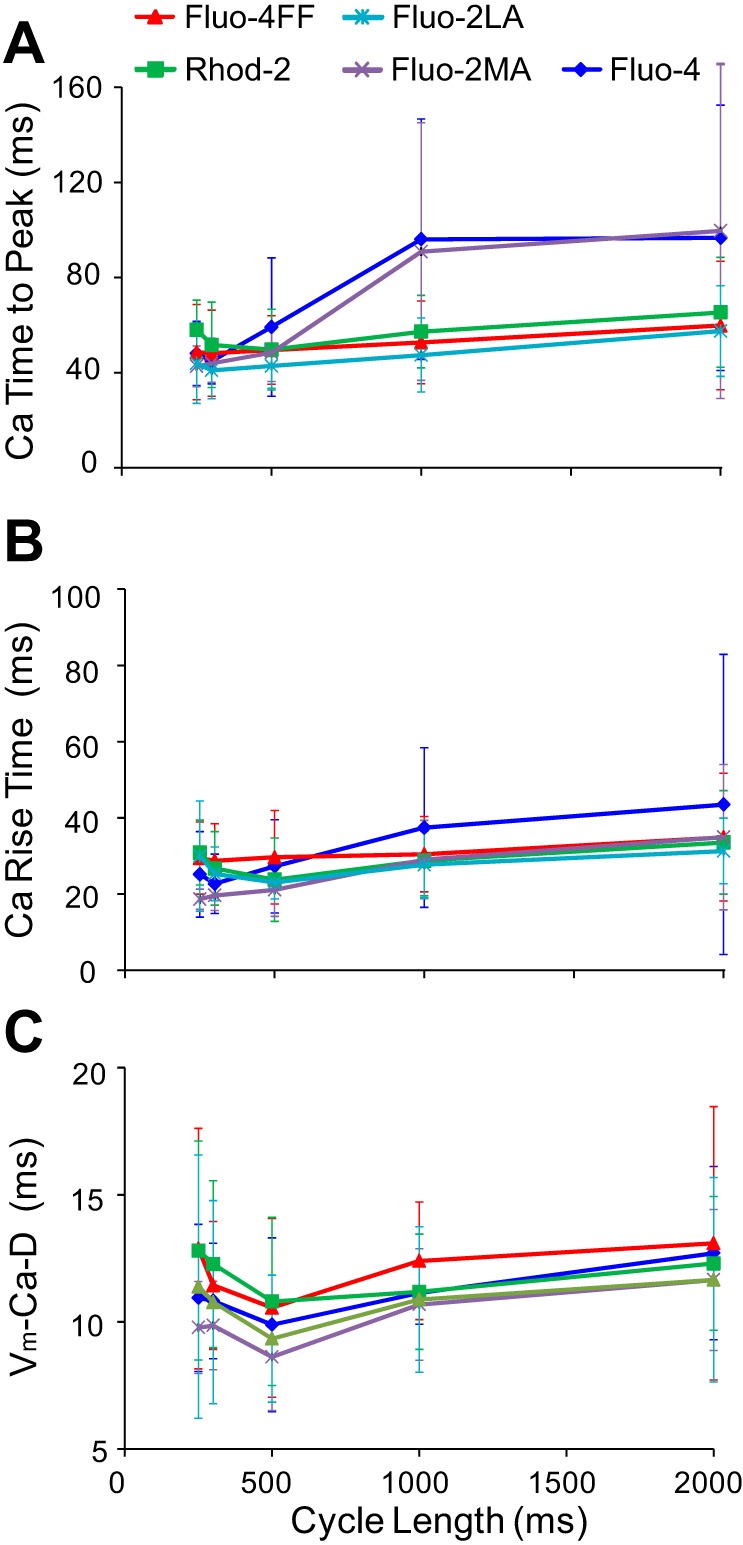
Parameters of CaT upstrokes measured with different dyes as functions of CL. A, CaT time to peak; B, CaT rise time; C, Vm-Ca upstroke delay.
DISCUSSION
This study investigated the effects of dye affinity on Cai2+ measurements in the whole porcine LV myocardium. The main findings are 1) measurements of Cai2+ transient duration were strongly dependent on the affinity of Ca2+-sensitive dyes. Dyes with the highest affinity to Ca2+ ions (Fluo-4 or Fluo-2MA) reported significantly longer CaT durations than dyes with the lowest affinity (Fluo-4FF or Fluo-2LA); the dye Rhod-2, which has affinity lower than Fluo-4/Fluo-2MA but higher than Fluo-4FF/Fluo-2LA, resulted in intermediate CaD values. 2) Cai2+ transients measured with high-affinity dyes often exhibited signal saturation, whereas saturation was absent in recordings obtained using low-affinity dyes and Rhod-2.
It was previously reported that dye affinity could affect Cai2+ measurements in cardiac cells. Optical mapping in cultures of neonatal rat myocytes demonstrated that CaD measured with high-affinity calcium dyes were twice as long as CaD measured with low-affinity dyes (5, 6). Whether this is true in whole cardiac tissue was not known. In this work, we compared Cai2+ recordings obtained using five Ca2+-sensitive dyes with different affinities to calcium ions. Because the standard method for staining myocardium with Ca2+-sensitive dyes via coronary infusion did not work for the low-affinity dyes, we developed a new technique, in which tissue was stained locally via dye injection through a microcapillary attached to a recording optical fiber. The more efficient microcapillary dye staining might be due to faster diffusion of dye molecules delivered via microcapillaries into cardiac cells, whereas in the case of coronary infusion the diffusion of dye molecules was hindered by the vessel walls. In addition to more efficient staining, the local dye injection had another advantage in requiring smaller dye amounts in comparison with the global staining. A disadvantage of the microcapillary staining was that it did not work every time (usable signals were obtained in ∼70% of injections), which could be due to local differences in the tissue structure. Concurrent tissue staining with a Vm-sensitive dye and optical recordings of Vm demonstrated that AP durations measured at different sites stained with different Ca2+-sensitive dyes were similar, indicating that electrophysiological tissue properties were similar in all series of measurements.
Optical recordings of intracellular calcium demonstrated that the shape of observed Cai2+ transients was strongly dependent on the dye affinity. Of the five Ca2+-sensitive dyes tested, the dyes Fluo-4 and Fluo-2MA with the highest affinity (Kd, ∼0.35 μmol/l) exhibited the longest Cai2+ transients in the intact cardiac tissue, whereas the two Ca2+-sensitive dyes with the lowest affinity (Fluo-4FF and Fluo-2LA, Kd = 7–10 μmol/l) demonstrated the shortest Cai2+ transients. The CaD differences between the high- and low-affinity dyes were most pronounced at longer CLs reaching ∼80% at CL of 2 s. The Rhod-2 dye, which has an intermediate Kd (∼0.6 μmol/l), reported moderately prolonged Cai2+ transients with the CaD difference from the low-affinity dyes of ∼35%. These findings are qualitatively similar to the results obtained in neonatal cell cultures (5, 6).
With the consideration of the Kd values of the dyes, it should be mentioned that these values were obtained in test solutions. Inside cells, the dye Kd may be substantially different. However, the nominal Kd is still useful because dyes that have relatively high affinity to Ca2+ ions in a test solution are likely to have a relatively high-affinity inside cells. Therefore, nominal Kd can be used to compare relative dye affinities, especially for dyes with similar molecular structures, such as dyes from the Fluo group. The Rhod-2 dye is different in that regard, which may explain the finding that, despite having a nominal Kd value similar to Fluo-4/Fluo-2MA, the CaD measurements obtained with Rhod-2 were different.
The effect of dye affinity on CaD measurements was most pronounced at long CLs but was less prominent at short CLs. For instance, CaD80 values measured with Rhod-2 and Fluo-4FF were not statistically different at CLs of 250 and 300 ms (Fig. 5A and Table 1). This suggests that the role of dye affinity in CaD measurements might be less important in studies of arrhythmias with high excitation rates than in Ca measurements during sinus rhythm. In addition, CaD differences between dyes with different affinities might be less pronounced in animals with rapid intrinsic heart rates such as rats and mice. We have performed similar CaD measurements in several experiments on isolated rabbit hearts paced at a short CL (CL = 300 ms, unpublished data). Although differences in CaD80 measured with different dyes were not statistically significant, these measurements demonstrated a trend toward longer CaD with increasing dye affinity.
A question can be asked which dye is more appropriate to measure the shape of Cai2+ transients and what is the role of dye affinity in these measurements. Unfortunately, there is no independent method that would allow measuring Cai2+ changes with sufficient speed to validate optical measurements. Although Cai2+ can be measured with calcium-sensitive microelectrodes (10, 23), their slow response time prevents its application for monitoring fast CaTs in the heart. An alternative method to resolve this problem is to calibrate optical Cai2+ measurements in situ following the physiological Cai2+ measurements (3). However, as discussed previously (5), such in situ dye calibration in the whole tissue is technically very difficult. Importantly, it requires the use of a ratiometric dye to compensate for signal decline caused by dye photobleaching and washout during the course of experiment. All dyes used in the present study, as well as dyes most often used in the whole tissue studies, are nonratiometric.
The role of dye affinity in CaT measurements can be evaluated by considering the linearity of the dye response to changes of Cai2+ concentration. This relationship can be presumed linear when Cai2+ changes do not exceed the dye Kd value, but it becomes nonlinear at Cai2+ concentrations much higher than the dye Kd (19). The Cai2+ concentration in cardiac myocytes at the peak of CaT was estimated to be around 0.7–1 μmol/l (3, 4), which significantly exceeds the Kd of high-affinity dyes. Modeling of dye response to Cai2+ changes demonstrated that high-affinity dyes caused changes in CaT shape, which were qualitatively similar to those measured experimentally, whereas low-affinity dyes reproduced CaTs without distortions (6). This qualitative correspondence between the modeling and experimental data supports the explanation that different CaT durations measured in the whole tissue with dyes of different affinities are caused by nonlinear response of the high-affinity dyes. It also indicates that, in the absence of in situ dye calibration, the low-affinity dyes are more appropriate for measurements of Cai2+ transients.
An unexpected finding in the present work was the observation of signal plateauing in measurements with high-affinity dyes Fluo-4 and Fluo-2MA. For these two dyes, CaTs frequently displayed long times to reach peak and long recovery times (Fig. 5). The mechanism of this effect is not clear. It cannot be explained by the nonlinear dye response considered above, which assumes that the underlying Cai2+ transients are not modified; in such cases, the nonlinear CaT transformation may actually slightly shorten the time to peak (6). A possible explanation of such signal plateauing might be related to strong buffering of calcium ions by the high-affinity dyes and the alteration of the dynamics of Cai2+ concentration changes. This question requires further investigation.
Another unexpected finding was the frequent observation of nonmonotonic Cai2+ transients with a secondary Cai2+ rise occurring during the repolarization phase of AP. A possible explanation of such Cai2+ rise could be the motion artifact but this is unlikely for two reasons. First, Vm traces showed little or no distortions at all, although typically optical Vm recordings are more sensitive to the motion artifact than Cai2+ measurements (7). Second, these secondary Cai2+ deflections were always upward, whereas motion-related deflections can be also downward or biphasic (7). Therefore, it is more likely that these signals reflect the real Cai2+ changes. The fact that these secondary Cai2+ rises were not paralleled by Vm rises or by large APD changes suggests that they were not caused by electrogenic Ca2+ fluxes such as the L-type calcium current (13, 26) or the reverse-mode NCX current (8). Therefore, it is more likely that these Cai2+ rises occurred due to spontaneous SR calcium release which per se is not electrogenic and does not directly affect the APD. Although it is known that spontaneous SR calcium release can produce Vm rises in the form of early afterdepolarizations (27), it was also reported that secondary Cai2+ rises could occur without accompanying early afterdepolarizations (20), similar to our present observations.
Summary.
In summary, measurements of calcium transients in porcine ventricular myocardium were strongly affected by the affinity of Ca2+ dyes. Overestimation of CaT duration and frequent signal plateauing indicate that high-affinity dyes Fluo-4 and Fluo-2MA misrepresent Cai2+ transients in the intact cardiac muscle. The dye Rhod-2 exhibited no signal plateauing and only moderately increased CaD. Because low-affinity Ca2+ dyes could not be used in whole tissue with staining by coronary infusion, Rhod-2 currently appears to be the most suitable dye among the tested dyes for qualitative measurements of Cai2+ transients, i.e., measurements of spontaneous calcium release or qualitative measurements of calcium alternans. However, it should be used with caution, especially for quantitatively precise CaD measurements. Ultimately, Cai2+ measurements in the whole tissue should use low-affinity dyes, which indicates the need for development of low-affinity dyes with a better loading efficiency.
Limitations.
Insertion of optical fibers may cause local tissue injury. However, the extent of such injury is likely to be much smaller than in previous measurements of intramural Vm and Cai2+ changes using wedge preparations. The other limitation is that recordings were made from only few intramural sites at the same time and not every local staining resulted in usable recordings. This limits the employment of the local staining approach for multisite mapping of Vm and Cai2+. Finally, a limitation of the intramural fiber-based optical measurements is that they were performed on the macroscopic scale with each fiber integrating light from multiple cells. However, the subcellular distribution of Cai2+ can be highly heterogeneous with different intracellular compartments generating different Cai2+ changes that may potentially contribute to the observed differences in Cai2+ transient measurements with dyes of different affinities. Further studies on the microscopic level may be needed to investigate this issue.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-067748 and HL-85370.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.K. performed experiments; W.K. and V.G.F. analyzed data; W.K. and V.G.F. interpreted results of experiments; W.K. prepared figures; W.K. drafted manuscript; W.K. and V.G.F. edited and revised manuscript; V.G.F. conception and design of research; V.G.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We would like to thank Frank Vance for technical support.
REFERENCES
- 1.Capogrossi MC, Houser SR, Bahinski A, Lakatta EG. Synchronous occurrence of spontaneous localized calcium release from the sarcoplasmic reticulum generates action potentials in rat cardiac ventricular myocytes at normal resting membrane potential. Circ Res 61: 498–503, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Clusin WT. Mechanisms of calcium transient and action potential alternans in cardiac cells and tissues. Am J Physiol Heart Circ Physiol 294: H1–H10, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Del Nido PJ, Glynn P, Buenaventura P, Salama G, Koretsky AP. Fluorescence measurement of calcium transients in perfused rabbit heart using rhod 2. Am J Physiol Heart Circ Physiol 274: H728–H741, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Du C, MacGowan GA, Farkas DL, Koretsky AP. Calibration of the calcium dissociation constant of Rhod(2) in the perfused mouse heart using manganese quenching. Cell Calcium 29: 217–227, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Fast VG. Simultaneous optical imaging of membrane potential and intracellular calcium. J Electrocardiol 38: 107–112, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Fast VG, Cheek ER, Pollard AE, Ideker RE. Effects of electrical shocks on Cai2+ and Vm in myocyte cultures. Circ Res 94: 1589–1597, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Fast VG, Ideker RE. Simultaneous optical mapping of transmembrane potential and intracellular calcium in myocyte cultures. J Cardiovasc Electrophysiol 11: 547–556, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Gaughan JP, Furukawa S, Jeevanandam V, Hefner CA, Kubo H, Margulies KB, McGowan BS, Mattiello JA, Dipla K, Piacentino V, Li S, 3rd, Houser SR. Sodium/calcium exchange contributes to contraction and relaxation in failed human ventricular myocytes. Am J Physiol Heart Circ Physiol 277: H714–H724, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Goldhaber JI, Xie LH, Duong T, Motter C, Khuu K, Weiss JN. Action potential duration restitution and alternans in rabbit ventricular myocytes: the key role of intracellular calcium cycling. Circ Res 96: 459–466, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Gorman AL, Levy S, Nasi E, Tillotson D. Intracellular calcium measured with calcium-sensitive micro-electrodes and Arsenazo III in voltage-clamped Aplysia neurones. J Physiol 353: 127–142, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi H, Shiferaw Y, Sato D, Nihei M, Lin SF, Chen PS, Garfinkel A, Weiss JN, Qu Z. Dynamic origin of spatially discordant alternans in cardiac tissue. Biophys J 92: 448–460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herron TJ, Milstein ML, Anumonwo J, Priori SG, Jalife J. Purkinje cell calcium dysregulation is the cellular mechanism that underlies catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 7: 1122–1128, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.January CT, Riddle JM. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ Res 64: 977–990, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Johnson PL, Smith W, Baynham TC, Knisley SB. Errors caused by combination of Di-4 ANEPPS and Fluo3/4 for simultaneous measurements of transmembrane potentials and intracellular calcium. Ann Biomed Eng 27: 563–571, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Kaneko T, Tanaka H, Oyamada M, Kawata S, Takamatsu T. Three distinct types of Ca2+ waves in Langendorff-perfused rat heart revealed by real-time confocal microscopy. Circ Res 86: 1093–1099, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Katra RP, Pruvot E, Laurita KR. Intracellular calcium handling heterogeneities in intact guinea pig hearts. Am J Physiol Heart Circ Physiol 286: H648–H656, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Lee P, Yan P, Ewart P, Kohl P, Loew LM, Bollensdorff C. Simultaneous measurement and modulation of multiple physiological parameters in the isolated heart using optical techniques. Pflügers Arch 464: 403–414, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lou Q, Li W, Efimov IR. Multiparametric optical mapping of the Langendorff-perfused rabbit heart. J Vis Exp 55: pii: 3160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer T, Wensel T, Stryer L. Kinetics of calcium channel opening by inositol 1,4,5-trisphosphate. Biochemistry 29: 32–37, 1990 [DOI] [PubMed] [Google Scholar]
- 20.Nemec J, Kim JJ, Gabris B, Salama G. Calcium oscillations and T-wave lability precede ventricular arrhythmias in acquired long QT type 2. Heart Rhythm 7: 1686–1694, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pruvot EJ, Katra RP, Rosenbaum DS, Laurita KR. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res 94: 1083–1090, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Salama G. Arrhythmia genesis: aberrations of voltage or Ca2+ cycling? Heart Rhythm 3: 67–70, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Takahashi A, Camacho P, Lechleiter JD, Herman B. Measurement of intracellular calcium. Physiol Rev 79: 1089–1125, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Tang L, Hwang GS, Hayashi H, Song J, Ogawa M, Kobayashi K, Joung B, Karagueuzian HS, Chen PS, Lin SF. Intracellular calcium dynamics at the core of endocardial stationary spiral waves in Langendorff-perfused rabbit hearts. Am J Physiol Heart Circ Physiol 295: H297–H304, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thandroyen FT, Morris AC, Hagler HK, Ziman B, Pai L, Willerson JT, Buja LM. Intracellular calcium transients and arrhythmia in isolated heart cells. Circ Res 69: 810–819, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Viswanathan PC, Rudy Y. Pause induced early afterdepolarizations in the long QT syndrome: a simulation study. Cardiovasc Res 42: 530–542, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Volders PG, Vos MA, Szabo B, Sipido KR, de Groot SH, Gorgels AP, Wellens HJ, Lazzara R. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: time to revise current concepts. Cardiovasc Res 46: 376–392, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Walker ML, Rosenbaum DS. Repolarization alternans: implications for the mechanism and prevention of sudden cardiac death. Cardiovasc Res 57: 599–614, 2003 [DOI] [PubMed] [Google Scholar]