Abstract
Impaired exercise capacity is common after the Fontan procedure and is attributed to cardiovascular limits. The Fontan circulation, however, is also distinctively vulnerable to unfavorable lung mechanics. This study aimed to define the prevalence and physiological relevance of pulmonary dysfunction in patients with Fontan physiology. We analyzed data from the Pediatric Heart Network Fontan Cross-Sectional Study to assess the prevalence and pattern of abnormal spirometry in Fontan patients (6–18 yr old) and investigated the relationship between low forced vital capacity (FVC) and maximum exercise variables, including peak O2 consumption (V̇o2peak), among those who demonstrated adequate effort (n = 260). Average ages at the time of exercise testing and Fontan completion were 13.2 ± 3.0 and 3.5 ± 2.2 yr old, respectively. Aerobic capacity was reduced (V̇o2peak: 67.3 ± 15.6% predicted). FVC averaged 79.0 ± 14.8% predicted, with 45.8% having a FVC less then the lower limit of normal. Only 7.8% demonstrated obstructive spirometry. Patients with low FVC had lower V̇o2peak (64.4 ± 15.9% vs. 69.7 ± 14.9% predicted, P < 0.01); low FVC independently predicted lower V̇o2peak after adjusting for relevant covariates. Among those with V̇o2peak < 80% predicted (n = 204/260), 22.5% demonstrated a pulmonary mechanical contribution to exercise limitation (breathing reserve < 20%). Those with both low FVC and ventilatory inefficiency (minute ventilation/CO2 production > 40) had markedly reduced V̇o2peak (61.5 ± 15.3% vs. 72.0 ± 14.9% predicted, P < 0.01) and a higher prevalence of pulmonary mechanical limit compared with patients with normal FVC and efficient ventilation (36.1% vs. 4.8%). In conclusion, abnormal FVC is common in young patients after the Fontan procedure and is independently associated with reduced exercise capacity. A large subset has a pathologically low breathing reserve, consistent with a pulmonary mechanical contribution to exercise limitation.
Keywords: Fontan procedure, congenital heart disease, exercise physiology, pulmonary, pulmonary heart disease
the fontan procedure is the most common surgical palliation for patients with single ventricle physiology, a severe form of congenital heart disease (CHD) associated with early mortality. While the Fontan procedure addresses both the hypoxemia and systemic ventricular volume loading inherent to other forms of palliation, it is associated with impaired exercise capacity, an array of evolving complications, and premature death (19). As patients enter adulthood and acquire additional comorbidities related and unrelated to CHD (27), it is increasingly critical to understand the mechanisms of exercise limitation to facilitate the development of novel strategies to support the tenuous underlying cardiopulmonary physiology.
It is commonly understood that physical work with Fontan physiology is limited by inadequate cardiac output, due to an inability to augment ventricular preload (13). Pulmonary mechanics may also play a role in exercise intolerance in various types of CHD. Forced vital capacity (FVC) correlates with exercise capacity (14) and predicts survival among adults with CHD (5). The relationship could be due to effects of the underlying cardiovascular disease (e.g., pulmonary congestion or cardiomegaly) on lung mechanics. Studies in adults without known heart disease, however, have reported an association between FVC and risks for myocardial infarction, heart failure, and death (41). Proposed mechanisms include inflammation and long-term effects of less favorable in utero and early developmental environments (30, 39).
Reports have described the abnormal pulmonary physiology and pathology associated with various forms of CHD, including small studies of Fontan patients (12, 17, 23, 25, 35). Abnormal lung function may be common and important in Fontan patients. While there are likely differences in the hemodynamic response by Fontan subtypes (26, 36, 37), none has pulsatile flow or a subpulmonary energy source. As a consequence, augmentation of pulmonary flow and pressure are limited, reducing the normal exercise-related recruitment and distension of the pulmonary vasculature and the attendant drop in pulmonary vascular resistance. Modest intrathoracic pressure shifts can, therefore, have a major influence on systemic venous and pulmonary arterial blood flows (32–34).
This study aimed to explore the prevalence and pattern of abnormal spirometry and relationships between spirometry and aerobic capacity after the Fontan procedure.
METHODS
Subjects.
We analyzed data from the Pediatric Heart Network Fontan Cross-Sectional Study from patients 6–18 yr old who had undergone a Fontan procedure. Ethics board approval and informed consent/assent were obtained according to local guidelines. A cycle ergometry cardiopulmonary exercise test was performed on 411 of 546 subjects (75%) enrolled in the overall study; exclusions were height < 115 cm, refusal, inability to cooperate, or a medical condition precluding exercise (31).
Spirometry.
Subjects underwent spirometry and a 10-s maximal voluntary ventilation (MVV) maneuver. Both were repeated more than three times to ensure reproducibility as recommended by American Thoracic Society guidelines (8). Forced expiratory volume in 1 s (FEV1), FVC, and the ratio of FEV1 to FVC were compared with predicted values as recommended by the American Thoracic Society [National Health and Nutrition Examination Survey (NHANES) III], with the lower limit of normal (LLN) as the 5th percentile of the general population. NHANES III equations were derived on data from subjects 8 yr of age or older. Only 7 of 260 subjects in this analysis were <8 yr old; excluding these subjects did not substantively alter the results. An obstructive pattern on spirometry was defined as a FEV1-to-FVC ratio < LLN, whereas FVC < LLN defined restrictive spirometry. Analyses were repeated using an alternative predictive equation to confirm the robustness of the results (21) and using FEV1 × 40 to estimate MVV. We reported the most conservative estimates of the pulmonary mechanical limit to exercise using the higher of the two values (MVVcons) to calculate the breathing reserve (BR).
Cardiopulmonary exercise testing.
Subjects pedaled unloaded for 3 min, and the workload was subsequently continuously increased at a rate aimed to achieve a symptom-limited test after 10–12 min. Minute ventilation (V̇e) was measured continuously, as were breath-by-breath O2 uptake (V̇o2) and CO2 output (V̇co2). Peak V̇o2 (V̇o2peak) was defined as the highest V̇o2 averaged over 20 s indexed to body weight (in ml/kg) and expressed as a percentage of the predicted values (% predicted) (10). The ventilatory anaerobic threshold (VAT) was estimated using the V-slope method. The ventilatory equivalent for CO2 (V̇e/V̇co2) was measured at the VAT. Other variables, including the respiratory exchange ratio (RER), BR, arterial O2 saturation, heart rate (HR), and chronotropic index, were calculated as previously described (31). A pulmonary mechanical exercise limitation was defined as BR < 20% and V̇o2peak < 80% predicted.
Statistical analysis.
Interpretable exercise testing with baseline spirometry data was available for n = 401/411. Analysis was limited to n = 260 with either peak RER > 1.05 or peak HR > 80% predicted. Average V̇o2peak of the subjects meeting one of these criteria was 67.4 ± 14.7% predicted, similar to n = 147 who met the single criterion of peak RER > 1.09 (66.7 ± 14.8% predicted). FVC (79.0 ± 14.8% vs. 78.5 ± 9.4% predicted) and peak HR (78.5 ± 10.0% vs. 78.5 ± 9.4% predicted) were also similar. Sensitivity analysis was performed to confirm equivalent results using the stricter classification. Continuous variables are presented as means ± SD, and categorical variables are presented as counts and percentages. Unpaired t-tests were used to compare values for normally distributed variables between FVC groups, whereas Wilcoxon rank-sum tests were used for non-normally distributed variables. Tests between subgroups for categorical variables were performed with Fisher's exact test. Multiple linear regression was used to test the association of FVC with the percent predicted V̇o2peak, adjusting for variables shown in Table 1 using forward selection (P < 0.10 for entry). Two-way interactions were assessed. Logistic regression was used to identify predictors of percent predicted V̇o2peak above the sample median value (V̇o2peak > 67.9% predicted). The interaction between FVC and V̇e/V̇co2 was explored given the borderline statistical significance of an interaction term (P = 0.10) and physiological rationale that low FVC may have a greater impact on exercise capacity in the setting of ventilatory inefficiency. Given the strong correlation between FEV1 and FVC (absolute r = 0.97 and r = 0.99 overall and percent predicted r = 0.87 and r = 0.93 for the subset without obstructive spirometry), the results for FEV1 and FVC were similar. Only the FVC analysis is presented. Two-sided P values of <0.05 were considered significant. Analysis was performed with SAS 9.3 for Windows (SAS Institute, Cary, NC). This study was approved by the Institutional Review Board of Boston Children's Hospital.
Table 1.
Demographic and clinical characteristics
| Characteristic | Total | FVC < LLN | FVC ≥ LLN | P Value |
|---|---|---|---|---|
| n | 260 | 119 | 141 | |
| Age, yr | ||||
| At earliest Fontan | 3.5 ± 2.2 | 3.7 ± 2.4 | 3.3 ± 2.1 | 0.09 |
| At exercise testing | 13.1 ± 3 | 13.6 ± 2.7 | 12.8 ± 3.3 | 0.03 |
| Height, cm | 150.8 ± 14.6 | 152.2 ± 13.2 | 149.7 ± 15.6 | 0.16 |
| Weight, kg | 45.3 ± 15.7 | 44.6 ± 14.4 | 46 ± 16.7 | 0.49 |
| Body surface area, m2 | 1.4 ± 0.3 | 1.36 ± 0.27 | 1.37 ± 0.32 | 0.78 |
| BMI | ||||
| kg/m2 | 19.3 ± 3.7 | 18.8 ± 3.6 | 19.8 ± 3.8 | 0.03 |
| Z-score | 0.0 ± 1.1 | −0.4 ± 1.2 | 0.3 ± 0.9 | <0.0001 |
| Sex, n (%) | ||||
| Male | 155 (59.6) | 71 (59.7) | 84 (59.6) | 1 |
| Female | 105 (40.4) | 48 (40.3) | 57 (40.4) | 1 |
| Race, n (%)* | 0.1 | |||
| Caucasian | 225 (86.5) | 101 (84.9) | 124 (87.9) | |
| African-American | 19 (7.3) | 6 (5.0) | 13 (9.2) | |
| Asian | 8 (3.1) | 6 (5.0) | 2 (1.4) | |
| Ventricular morphology, n (%) | 0.68 | |||
| Left | 142 (54.6) | 68 (57.1) | 74 (52.5) | |
| Right | 78 (30) | 35 (29.4) | 43 (30.5) | |
| Both | 40 (15.4) | 16 (13.5) | 24 (17.0) | |
| Type of Fontan, n (%) | 0.20 | |||
| APC | 43 (16.5) | 23 (19.3) | 20 (14.2) | |
| Intracardiac lateral tunnel | 152 (58.5) | 68 (57.1) | 84 (59.6) | |
| Extracardiac lateral tunnel | 3 (1.2) | 0 (0) | 3 (2.1) | |
| Extracardiac conduit | 57 (21.9) | 24 (20.2) | 33 (23.4) | |
| Other | 5 (1.9) | 4 (3.4) | 1 (0.7) | |
| Pacemaker present, n (%) | 30 (11.6) | 15 (12.6) | 15 (10.7) | 0.54 |
| Orthopedic problem, n (%) | 27 (10.6) | 17 (14.5) | 10 (7.2) | 0.07 |
| Respiratory problem, n (%) | 28 (10.9) | 18 (15.5) | 10 (7.1) | 0.04 |
| Asthma, n (%) | 32 (12.7) | 17 (14.8) | 15 (10.9) | 0.45 |
| Neurological deficit, n (%) | 9 (3.6) | 8 (7.0) | 1 (0.8) | 0.01 |
| Developmental delay, n (%) | 23 (10.4) | 14 (13.7) | 9 (7.6) | 0.18 |
| Postoperative chylothorax, n (%) | 12 (4.6) | 8 (6.7) | 4 (2.8) | 0.15 |
| Postoperative pleural effusions, n (%) | 68 (26.2) | 28 (23.5) | 40 (28.4) | 0.40 |
Continuous variables are presented as means ± SD; categorical variables are presented as number of subjects (n) and percentages. Demographic and clinical characteristics by forced vital capacity (FVC) category [less than vs. greater than or equal to the lower limit of normal (LLN)] are shown.
Other and unknown races are not shown. BMI, body mass index; APC, atiropulmonary connection.
RESULTS
Patient characteristics.
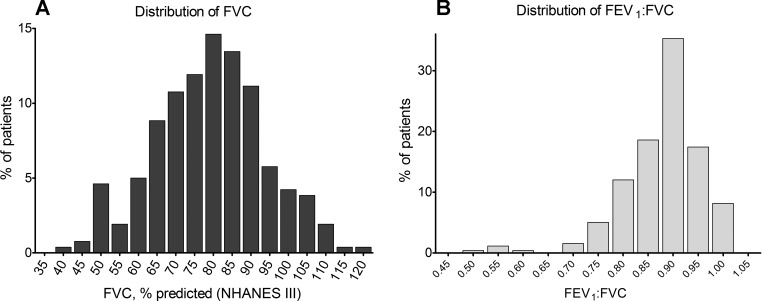
The NHANES III percent predicted FVC was normally distributed [79.0 ± 14.8% (median: 79.9%, interquartile range: 68.6–88.1%), P = 0.72 by Shapiro-Wilk test; Fig. 1A]. Almost half of the subjects (45.8%) had FVC < LLN (low FVC; Table 1). Those with low FVC were slightly older and more likely to report clinical respiratory, orthopedic, or neurological problems. While there was no significant difference in either height or weight, the mean body mass index (BMI) was lower in the low FVC group. There were no demographic (sex and race) or clinical (underlying anatomic cardiac diagnosis, ventricular morphology, or Fontan type) differences between those with low and normal FVC. There was no significant relationship between the number of surgical procedures before Fontan (P = 0.79), total number of cardiac surgeries (P = 0.17), or age at first palliative surgery (P = 0.83) and low FVC.
Fig. 1.
Distribution of National Health and Nutrition Examination Survey (NHANES) III percent predicted (%pred) values for forced vital capacity (FVC; A) and forced expiratory volume in 1 s (FEV1)-to-FVC ratio (B).
Obstructive spirometry (i.e., FEV1-to-FVC ratio < LLN) was present in only 7.8% (Fig. 1B). There were no differences in the prevalence of obstructive spirometry (6.8% vs. 8.5%, P = 0.65) or mean FEV1-to-FVC ratio (0.89 ± 0.08 vs. 0.87 ± 0.07, P = 0.07) between those with low and normal FVC. Knudson and NHANES III percent predicted values for FVC correlated well(r = 0.95), with a mean percent predicted FVC for the entire sample of 83.1 ± 17.3% and 79.0 ± 14.8% using Knudson and NHANES III equations, respectively; FVC < 80% predicted was present among a similar proportion (43.9% and 45.8%) for each predictive equation.
Relationship between baseline spirometry and cardiopulmonary exercise variables.
Both weight-normalized and percent predicted V̇o2peak were lower in the low FVC group (Table 2). Peak HR was lower in the low FVC group, and there was a trend toward a lower O2 pulse. Those with low FVC also tended to have lower peak O2 saturation and higher V̇e/V̇co2. Low FVC was associated with both lower peak tidal volume (VT) and higher peak respiratory rate (RR). The increase in RR did not fully compensate for the limited VT, and those with low FVC had lower peak V̇e. The BR at peak exercise averaged 31.5 ± 16.1% (using MVVcons), and BRs of <30%, <20%, and <10% were present in 45.0%, 26.5%, and 9.6% of the subjects, respectively. A pulmonary ventilatory exercise limitation, defined as BR < 20% and V̇o2peak < 80% predicted, was present in 19.4% of the subjects overall and in 26.9% and 13.2% of those with low and normal FVC, respectively. Among those with an aerobic limitation (V̇o2peak < 80%predicted), a pulmonary limit was present in 22.5% of the subjects (29.3% and 16.2% of those with low and normal FVC, respectively).
Table 2.
Exercise testing characteristics of those with and without FVC below LLN
| Total | FVC < LLN | FVC ≥ LLN | P Value | r Value for Continuous Percent Predicted FVC | P Value for Continuous Percent Predicted FVC | |
|---|---|---|---|---|---|---|
| V̇o2peak | ||||||
| ml·kg−1·min−1 | 27.4 ± 6.5 | 26.1 ± 6.7 | 28.5 ± 6.2 | 0.004 | 0.18 | 0.005 |
| % predicted | 67.3 ± 15.6 | 64.4 ± 15.9 | 69.7 ± 14.9 | 0.006 | 0.16 | 0.01 |
| V̇o2 at VAT, % predicted | 79.5 ± 24.4 | 75.2 ± 23.8 | 83 ± 24.5 | 0.01 | 0.13 | 0.04 |
| Maximum work | ||||||
| W | 90.2 ± 36.3 | 84.7 ± 28 | 94.8 ± 41.6 | 0.03 | 0.22 | 0.0003 |
| % predicted | 65.2 ± 16.2 | 63.4 ± 16.8 | 66.6 ± 15.5 | 0.11 | 0.15 | 0.02 |
| V̇e/V̇co2 | 42.1 ± 8.0 | 43.1 ± 8.4 | 41.3 ± 7.5 | 0.07 | −0.19 | 0.004 |
| Breathing reserve, % | 31.5 ± 16.3 | 27.7 ± 15.9 | 34.7 ± 15.7 | 0.0004 | 0.36 | <0.0001 |
| Peak HR | ||||||
| beats/min | 162.4 ± 21 | 159.2 ± 23.7 | 165.1 ± 18.1 | 0.02 | 0.13 | 0.04 |
| % predicted | 78.5 ± 10 | 77.1 ± 11.3 | 79.7 ± 8.6 | 0.04 | 0.11 | 0.07 |
| Chronotropic index | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.7 ± 0.1 | 0.03 | 0.11 | 0.08 |
| Peak O2 pulse | ||||||
| ml·m−2·beat−1 | 5.6 ± 1.3 | 5.4 ± 1.3 | 5.7 ± 1.3 | 0.06 | 0.16 | 0.01 |
| % predicted | 88.5 ± 21.8 | 86.9 ± 23.1 | 89.8 ± 20.7 | 0.28 | 0.07 | 0.29 |
| Peak O2 saturation, % | 90.8 ± 6.1 | 90.2 ± 6.7 | 91.3 ± 5.6 | 0.14 | 0.21 | 0.001 |
| Peak V̇e, l/min | 57.1 ± 20.2 | 53.9 ± 16 | 59.7 ± 22.9 | 0.02 | 0.18 | 0.004 |
| Peak VT, liters | 1.13 ± 0.46 | 1.03 ± 0.37 | 1.21 ± 0.5 | 0.002 | 0.28 | <0.0001 |
| Peak RR, breaths/min | 52.7 ± 12.3 | 54.2 ± 12.2 | 51.4 ± 12.3 | 0.07 | −0.20 | 0.002 |
Data are presented as means ± SD. Exercise testing characteristics by FVC category (less than vs. greater than or equal to LLN) are shown. There were no significant between-group differences in peak respiratory exchange ratio, resting heart rate (HR), or resting O2 saturation (data not shown). V̇o2peak, peak O2 consumption (V̇o2); VAT, ventilatory anaerobic threshold; V̇e, minute ventilation; V̇co2, CO2 production; VT, tidal volume; RR, respiratory rate.
Characteristics associated with percent predicted V̇o2peak.
Higher percent predicted FVC was associated with higher percent predicted V̇o2peak (β = +1.68/10% increase in percent predicted FVC, r2 = 0.03, P = 0.01). Conversely, obstructive spirometry was not associated with percent predicted V̇o2peak (+3.0%, P = 0.61). The association between percent predicted FVC and percent predicted V̇o2peak was strengthened by adjustment for important covariates (Table 3). Other variables predictive of percent predicted V̇o2peak paralleled those previously reported (31). Limiting the analysis to patients with peak RER > 1.09 yielded equivalent results (n = 145, multivariate β = +3.0/10% increase in percent predicted FVC, P < 0.0001), with other significant predictors being BMI and sex (P < 0.0001 and 0.03, respectively).
Table 3.
Multivariable regression, dependent variable percent predicted V̇o2peak (n = 259, r2 = 0.35)
| Independent Variable | β | 95% Confidence Interval | Partial r2 Value | P Value |
|---|---|---|---|---|
| Intercept | 72.9 | 50.6, 95.2 | <0.0001 | |
| Percent predicted FVC, 10% | 2.58 | 1.46, 3.71 | 0.08 | <0.0001 |
| BMI, kg/m2 | −2.17 | −2.72, −1.62 | 0.19 | <0.0001 |
| Age, yr | −1.14 | −2.01, −0.28 | 0.03 | 0.006 |
| Female sex | 3.86 | 0.62, 7.10 | 0.02 | 0.02 |
| Height, cm | 0.17 | −0.01, 0.36 | 0.01 | 0.06 |
| Morphological right ventricle | −3.43 | −6.91, 0.04 | 0.01 | 0.06 |
Shown is the model derived using forward selection (P < 0.10 for entry) for variables included in Table 1. Use of the BMI Z-score instead of absolute BMI did not change the results.
Normal FVC, relative to those with low FVC, was associated with a higher odds of having V̇o2peak above the median [67.9% predicted (univariate odds ratio (OR) = 2.1, 95% confidence interval: 1.3–3.5), P = 0.003] even after adjusting for age and BMI (multivariable OR = 3.7, 95% confidence interval: 2.0–6.9, P < 0.0001, with the c statistic increasing from 0.74 for a model including only age and BMI to 0.84 with addition of FVC < LLN); the inclusion of V̇e/V̇co2 did not affect the result (multivariable OR = 4.5, 95% confidence interval: 2.2–9.4, P < 0.0001, c statistic = 0.85).
Additive effects of ventilatory inefficiency and low FVC.
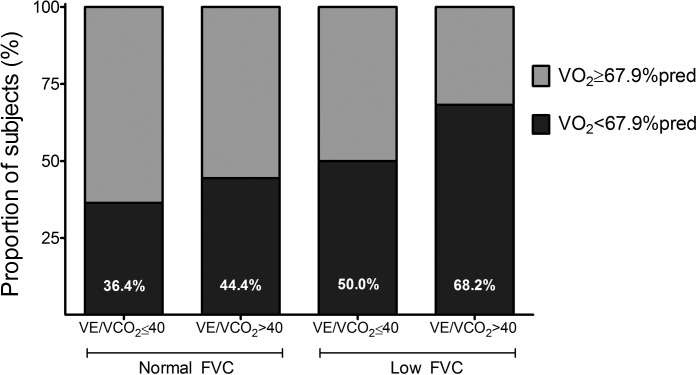
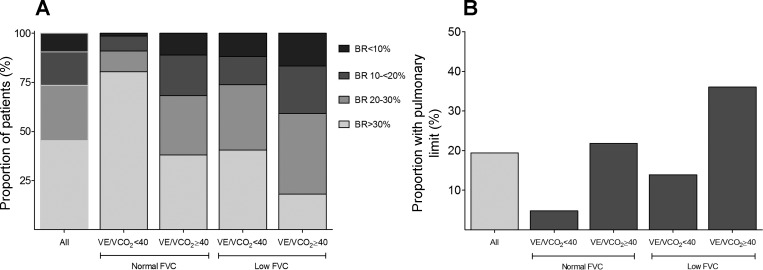
FVC and V̇e/V̇co2 each independently predicted V̇o2peak, and there was an additive effect: the combination of high V̇e/V̇co2 (>40) and low FVC was associated with a much lower odds of having V̇o2peak > median (Table 4 and Fig. 2), even after adjustment for age and BMI. Those with V̇e/V̇co2 > 40 but normal FVC were able to achieve a peak V̇e equivalent to patients with more efficient ventilation as the result of increased RR in the setting of low peak VT; those with both elevated V̇e/V̇co2 and low FVC, however, were unable to further increase RR despite an even more depressed peak VT. Those with either inefficient ventilation or low FVC were more likely to have a low BR; among those with both characteristics, the BR averaged 24.6 ± 15.0%, and 65.1% had BR < 30% (Fig. 3). A pulmonary mechanical exercise limitation was present in 4.8% of those with normal V̇e/V̇co2 and FVC compared with 36.1% among patients with a combination of high V̇e/V̇co2 and low FVC.
Table 4.
Exercise testing characteristics by V̇e/V̇co2 and FVC category
| V̇e/V̇co2 | FVC | n | V̇o2peak, % Predicted | V̇e/V̇co2 | Breathing Reserve, % | Peak HR, % predicted | Peak O2 Pulse, % predicted | Peak V̇e, l/min | Peak VT, liters | Peak VT/FVC, % | Peak RR, breaths/min | Less Than Median V̇o2peak, % | OR | 95% Confidence Interval | Multivariable OR* | 95% Confidence Interval |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤40 | >LLN | 66 | 72.0 ± 14.9 | 35.4 ± 3.5 | 40.8 ± 13.5 | 78.3 ± 9.8 | 93.6 ± 20.2 | 61.1 ± 22.8 | 1.37 ± 0.5 | 45.4 ± 7.3 | 46.3 ± 10.5 | 63.6 | 3.7 | 1.8, 7.7 | 16.3 | 5.8, 45.4 |
| <40 | <LLN | 63 | 68.5 ± 14.6 | 47.4 ± 5.5† | 28.3 ± 15.1† | 80.5 ± 7.5 | 88.3 ± 21.0 | 61.0 ± 22.9 | 1.12 ± 0.4† | 45.0 ± 7.7 | 55.6 ± 12.1† | 55.6 | 2.7 | 1.3, 5.5 | 4.2 | 1.7, 10.3 |
| ≤40 | >LLN | 42 | 69.4 ± 15.8 | 35.7 ± 3.2 | 30.9 ± 17.6 | 76.0 ± 11.8 | 93.2 ± 21.5 | 57.9 ± 18.5 | 1.19 ± 0.4 | 50.4 ± 10.3† | 50.4 ± 10.3‡ | 50.0 | 2.1 | 1.0, 4.8 | 4.0 | 1.6, 10.5 |
| >40 | <LLN | 66 | 61.5 ± 15.3† | 47.9 ± 7.1† | 24.6 ± 15.0† | 77.6 ± 11.2 | 83.6 ± 24.3‡ | 53.5 ± 13.4‡ | 0.98 ± 0.3† | 49.8 ± 9.9† | 56.8 ± 12.8† | 31.8 | Ref | Ref |
Adjusted for age and BMI.
P < 0.01 compared with V̇e/V̇co2 ≤ 40 (FVC > LLN);
P < 0.05 compared with V̇e/V̇co2 ≤ 40 (FVC > LLN).
Fig. 2.
Aerobic exercise capacity as a function of FVC and minute ventilation (V̇e)/CO2 production (V̇co2). Shown is the proportion of subjects with low peak O2 consumption (V̇o2; less than a median value of 67.9% predicted) by category of FVC and V̇e/V̇co2.
Fig. 3.
Prevalence of low breathing reserve (PR) and a pulmonary mechanical limit to exercise by FVC and V̇e/V̇co2 category. A and B: proportion of subjects by BR (A) and with limited aerobic capacity (peak V̇o2 < 80% predicted) with a pulmonary mechanical limit to exercise (BR < 20%; B) by both category of FVC and V̇e/V̇co2.
Characteristics of patients with a pulmonary mechanical exercise limit.
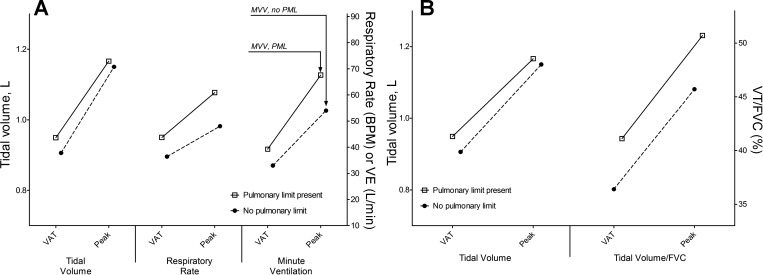
Patients with a pulmonary exercise limit were compared with those with a presumed cardiovascular limit (i.e., V̇o2peak < 80% predicted but BR > 20%); anthropometric, demographic, and clinical characteristics were similar, although men tended to have a higher probability of a pulmonary limit (26.6% vs. 16.3%, P = 0.09). Those with and without a pulmonary limit underwent a similar number of surgical procedures overall or before the initial Fontan procedure (4.0 ± 1.8 vs. 3.5 ± 1.5, P = 0.13, and 2.3 ± 1.1 vs. 2.5 ± 1.4, P = 0.33), and there was no difference in age at the first palliative surgery (0.5 ± 1.3 vs. 0.5 ± 1.0 yr old, P = 0.99). There were no differences in peak RER, V̇o2, workload, HR, or O2 pulse between the two groups. A pulmonary limit was associated with both lower FVC and higher V̇e/V̇co2 (Table 5). The presence of both lower MVVcons and higher peak V̇e contributed to the lower BR. There was no difference in VT at either VAT or peak exercise between those with and without a pulmonary limit; those with a pulmonary limit, however, had a markedly higher RR at both time points (Table 5 and Fig. 4A). In addition, whereas absolute VT was equivalent, the ratio of VT to FVC was greater among patients with a pulmonary limit at both VAT and peak exercise (Fig. 4B). Interestingly, the average FEV1-to-FVC ratio tended to be slightly lower for those with a pulmonary limit, and almost half of the patients with obstructive spirometry had a pulmonary exercise limit (7 of 15 patients, 46.7% vs. 19.8%, P = 0.02). We performed multivariable logistic regression using forward selection inclusive of univariate predictors of a pulmonary limit as well as age and BMI. Higher V̇e/V̇co2 (χ2 = 11.9, P = 0.0006), higher BMI (χ2 = 10.8, P = 0.001), and lower percent predicted FVC (χ2 = 10.4, P = 0.0016) independently predicted the presence of a pulmonary limit (model c-statistic: 0.81); neither the FEV1-to-FVC ratio as a continuous variable nor obstructive spirometry was an independent predictor.
Table 5.
Characteristics of those with and without a pulmonary exercise limitation
| Pulmonary Limit |
|||
|---|---|---|---|
| Characteristic | No | Yes | P Value |
| n | 158 | 46 | |
| Age, yr | |||
| At earliest Fontan | 3.7 ± 2.5 | 3.3 ± 2.1 | 0.23 |
| At exercise testing | 13.7 ± 3.2 | 13 ± 2.6 | 0.19 |
| Height, cm | 153.1 ± 14.8 | 150.7 ± 13.5 | 0.34 |
| Weight, kg | 47.8 ± 16 | 47.4 ± 16.5 | 0.89 |
| BMI | |||
| kg/m2 | 19.8 ± 3.8 | 20.2 ± 4.2 | 0.52 |
| Z-score | 0.0 ± 1.2 | 0.2 ± 1.3 | 0.21 |
| V̇o2peak | |||
| ml·kg−1·min−1 | 25 ± 5.6 | 26.6 ± 4.5 | 0.07 |
| % predicted | 60.9 ± 12.7 | 63.7 ± 8.8 | 0.17 |
| Peak work | |||
| W | 90.6 ± 38 | 91 ± 33.1 | 0.96 |
| % predicted | 61.1 ± 15.9 | 63.1 ± 12.2 | 0.42 |
| Peak HR | |||
| beats/min | 160.4 ± 22.4 | 161.9 ± 19.6 | 0.69 |
| % predicted | 77.7 ± 10.6 | 78.2 ± 9.6 | 0.77 |
| Peak O2 pulse, % predicted | 81.5 ± 19.4 | 83.6 ± 15.7 | 0.52 |
| Peak O2 saturation, % | 90.5 ± 6.5 | 89.6 ± 6.3 | 0.47 |
| V̇e/V̇co2 | 41.3 ± 7.4 | 47.2 ± 9.1 | <0.0001 |
| Maximal voluntary ventilation, liters | 90.9 ± 29.9 | 76.5 ± 28.1 | 0.004 |
| Breathing reserve,% | 39.6 ± 11.6 | 11.0 ± 7.7 | <0.0001 |
| Peak V̇e, l/min | 54.0 ± 18.6 | 67.6 ± 24.1 | <0.0001 |
| Peak VT, liters | 1.17 ± 0.45 | 1.15 ± 0.49 | 0.84 |
| Peak RR, breaths/min | 48.2 ± 11.2 | 60.9 ± 8.7 | <0.0001 |
| VT/FVC at VAT, % | 36.4 ± 8.8 | 41.1 ± 6.5 | <0.0001 |
| Peak VT/FVC, % | 45.7 ± 8.1 | 50.7 ± 7.1 | 0.0002 |
| FEV1, liters | 2.24 ± 0.74 | 1.89 ± 0.71 | 0.006 |
| FVC, liters | 2.56 ± 0.88 | 2.2 ± 0.87 | 0.01 |
| FEV1, % predicted | 79.4 ± 14.8 | 68.7 ± 14 | <0.0001 |
| FVC, % predicted | 80 ± 14.6 | 71.9 ± 13.7 | 0.001 |
| FEV1-to-FVC ratio | 0.88 ± 0.07 | 0.86 ± 0.09 | 0.09 |
There was no significant between-group difference in resting HR, chronotropic index, resting O2 saturation, peak O2 pulse (in ml·m−2·beat−1) or peak respiratory exchange ratio (data not shown). FEV1, forced expiratory volume in 1 s.
A pulmonary limit was defined as V̇o2peak < 80% predicted and breathing reserve < 20%.
Fig. 4.
Characteristics of the pulmonary ventilatory response to exercise among those with normal and low BR. A: tidal volume (VT), respiratory rate, and V̇e at the ventilatory anaerobic threshold (VAT) and peak exercise for those with a pulmonary exercise limit compared with patients with peak V̇o2 < 80% predicted but normal BR. Vertical arrows in A represent BR. B: whereas absolute VT was equivalent, VT relative to FVC was significantly higher at both time points. BPM, breaths per minute; MVV, maximal voluntary ventilation; PML, pulmonary mechanical limit.
DISCUSSION
The physiological data from this large multicenter cohort highlight the central role of the lungs in the exercise response among young patients who have had a Fontan procedure. FVC is below the normal range in almost half of patients with Fontan physiology, and low FVC independently predicts impaired exercise capacity. This association between FVC and V̇o2peak was stronger than that seen for ventricular morphology or markers of ventricular dysfunction and represents one of our key findings. Low FVC also appears to limit the ability to compensate for ventilatory inefficiency, a common finding in this population. As a result, aerobic capacity may be limited by pulmonary in addition to cardiovascular factors in a sizable subset of Fontan patients.
The underlying cause of low FVC is unclear, but there are likely developmental, mechanical, and functional contributors. Lung development is abnormal in many types of CHD without intervention, and surgical interventions themselves also impact pulmonary development. Both the pulmonary vasculature (4, 11, 16, 17, 35) and parenchyma may be affected (17, 35). Alveolarization begins in the last trimester of pregnancy, and rapid alveolar growth continues after birth, with continued development for at least the first several years of life; 95% of the adult alveolar surface area forms after birth, and disruption of vascular development inhibits alveolar growth (2, 39). Thus, both prenatal and early postnatal environmental, particularly vascular, deprivation may affect lung development (6). There are few data on pulmonary parenchymal effects of the Fontan circulation, but animal studies have demonstrated acute development of bronchiolitis after Fontan completion (18). Chest wall abnormalities, including scoliosis and pectus deformities, are common in patients with cyanotic CHD, and this can cause low FVC through mechanical constraints and impaired lung growth. Marked cardiomegaly, pleural adhesions, or ascites may also mechanically limit lung volume. These findings are not common in Fontan patients in this age group, however. Diaphragmatic paralysis is a known surgical complication, and more subtle respiratory and skeletal muscle weakness is prevalent (15). While postoperative pleural effusions could presumably have long-term consequences, the present data do not support a relationship between this phenomenon and low FVC. Similarly, while there was a trend toward lower FVC with postoperative chylothorax, this was not statistically significant, and only a small number of patients suffer postoperative chylothorax (9).
The present analysis was limited to spirometry data. While we observed a reduction in FVC, it cannot be concluded that Fontan patients have restrictive lung disease as defined by low total lung capacity. While spirometry suggests that obstructive lung disease is uncommon in patients with Fontan physiology, smaller studies have reported elevated residual volume/total lung capacity, suggesting the presence of air trapping (23, 28).
The mechanisms for the robust relationship between low FVC and low V̇o2peak remain undefined. A simple explanation may be that smaller lungs have fewer pulmonary blood vessels, thereby precluding a physiological decrease in pulmonary vascular resistance with increased flow. Low FVC may reflect smaller than normal lungs with fewer pulmonary vessels relative to body size, limiting the ability to recruit vessels in response to increased flow. This impediment would be in addition to demonstrated pulmonary vascular histological changes and endothelial dysfunction in patients with Fontan circulation (3, 20, 45). The prepulmonary Fontan circulation, lacking a ventricle, would be unable to generate the power needed to compensate for an abnormal pulmonary vascular response to exercise, resulting in impaired cardiac output augmentation.
The present data do not allow confirmation of the above explanation, and there are alternative potential mechanisms. The Fontan circulation is vulnerable to small changes in intrathoracic pressure and loading conditions due to lack of a subpulmonary power source as well as elevated systemic venous pressures preventing the normal respiratory collapse of veins entering the thoracic cavity (with negative right atrial pressure), resulting in a continuous transmission of intrathoracic venous pressure to the periphery. Studies have demonstrated striking effects on venous and pulmonary blood flow with changes in ventilation strategy (32, 33, 40, 44). Evidence for the importance of a “pulmonary pump” in generating forward flow is mixed (38); adverse pulmonary mechanics may limit this positive effect or impede forward momentum. The absence of normal pulsatile flow and pressure augmentation may limit normal exercise-related dilation and upper lung pulmonary vascular recruitment in the upright position and, consequently, increase alveolar dead space. In combination with the lower VT relative to FVC, this would increase the ratio of physiological dead space ventilation to VT and V̇e/V̇co2. One study (29) has reported increased hypercapnic chemosensitivty in a subset of Fontan patients (n = 10/42), and this was associated with abnormal end-tidal CO2 and V̇e/V̇co2. We are not aware of studies of directly measured arterial Pco2 suggesting an abnormal resting or exercise response, although if present this would contribute to the elevated V̇e/V̇co2. The higher RR seen in patients with low FVC may itself be disadvantageous; physiological modeling suggests that respiration with exercise causes enhanced energy dissipation in the total cavopulmonary anastomosis (22). While not as widely appreciated, inspiratory muscle fatigue itself, in normal human subjects, results in reflex sympathetic activation with associated skeletal muscle vasoconstriction and increases in arterial pressure and HR, responses that would adversely impact Fontan hemodynamics (42). In addition, progressive air trapping could presumably compress the pulmonary vascular bed and limit systemic ventricular preload. While the present data allow little more than speculation on the underlying mechanisms, this discussion highlights the complex interaction between the pulmonary mechanical pump, pulmonary vasculature, neuromuscular physiology, and cardiac structures. The rich interplay between markers of pulmonary vascular dysfunction (V̇e/V̇co2) and pulmonary mechanical phenomena (FVC) in limiting maximal O2 delivery argues that aerobic capacity is limited by multiple factors. It follows that a deeper understanding of the interactions between the vasculature, heart, and lung in the Fontan circulation may identify novel noncardiac therapeutic targets.
The data presented must be interpreted with an appreciation of the study design. Only spirometry data were available, and low FVC does not necessarily correspond to low total lung capacity (1). There are no data on inspiratory muscle function or chest wall deformities, and very limited data on diagnosed lung disease. We used V̇e/V̇co2 at VAT to represent ventilatory efficiency; opinion varies on the merits of alternatives such as the V̇e/V̇co2 slope, although some support using V̇e/V̇co2 at VAT (43).
The BR is a gross marker of pulmonary limitation and does not provide mechanistic insights. We used a strict definition for the pulmonary mechanical limit, choosing FEV1 ×40 (as opposed to ×35) to provide a conservative estimate of the prevalence of a pulmonary limit (43). Likewise, while BR < 30% has defined pulmonary limit in a previous study (24), we used a stricter criterion (BR < 20%). The average BR in normal adults is ∼39% (7), similar to the group of Fontan patients with normal FVC and V̇e/V̇co2, suggesting that the technique did not preclude normal ventilatory findings. Thus, we believe that our estimate of the proportion of patients with a physiologically important pulmonary mechanical contribution to exercise limitation underestimates the true prevalence. Even the cautious values presented suggest that the role of pulmonary mechanics in Fontan physiology deserves greater attention.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants U01-HL-068269, U01-HL-068270, U01-HL-068279, U01-HL-068281, U01-HL-068285, U01-HL-068292, U01-HL-068290, and U01-HL-068288.
DISCLAIMER
The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). None of the authors' potential conflicts of interests apply to the study submitted.
AUTHOR CONTRIBUTIONS
Author contributions: A.R.O., M.J.L., M.G.E., J.K.T., A.C., and J.R. conception and design of research; A.R.O. analyzed data; A.R.O., M.J.L., M.G.E., A.C., D.A.E., D.S., S.M.P., and J.R. interpreted results of experiments; A.R.O. prepared figures; A.R.O. drafted manuscript; A.R.O., M.J.L., M.G.E., F.M.W., J.K.T., A.C., D.A.E., D.S., S.M.P., and J.R. edited and revised manuscript; A.R.O., M.J.L., M.G.E., F.M.W., J.K.T., A.C., D.A.E., D.S., S.M.P., and J.R. approved final version of manuscript; S.M.P. and J.R. performed experiments.
ACKNOWLEDGMENTS
The authors are grateful to Mario Santos for insightful review of the manuscript.
REFERENCES
- 2.Aaron SD, Dales RE, Cardinal P. How accurate is spirometry at predicting restrictive pulmonary impairment? Chest 115: 869–873, 1999 [DOI] [PubMed] [Google Scholar]
- 1.Ad hoc Statement Committee, American Thoracic Society. Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med 170: 319–343, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Adachi I, Ueno T, Hori Y, Sawa Y. Alterations in the medial layer of the main pulmonary artery in a patient with longstanding Fontan circulation. Interact Cardiovasc Thorac Surg 11: 682–683, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Alderson PO, Boonvisut S, McKnight RC, Hartman AF., Jr Pulmonary perfusion abnormalities and ventilation-perfusion imbalance in children after total repair of tetralogy of Fallot. Circulation 53: 332–337, 1976 [DOI] [PubMed] [Google Scholar]
- 5.Alonso-Gonzalez R, Borgia F, Diller GP, Inuzuka R, Kempny A, Martinez-Naharro A, Tutarel O, Marino P, Wustmann K, Charalambides M, Silva M, Swan L, Dimopoulos K, Gatzoulis MA. Abnormal lung function in adults with congenital heart disease: prevalence, relation to cardiac anatomy, and association with survival. Circulation 127: 882–890, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Balinotti JE, Tiller CJ, Llapur CJ, Jones MH, Kimmel RN, Coates CE, Katz BP, Nguyen JT, Tepper RS. Growth of the lung parenchyma early in life. Am J Respir Crit Care Med 179: 134–137, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackie SP, Fairbarn MS, McElvaney NG, Wilcox PG, Morrison NJ, Pardy RL. Normal values and ranges for ventilation and breathing pattern at maximal exercise. Chest 100: 136–142, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Brusasco V, Crapo R, Viegi G. Coming together: the ATS/ERS consensus on clinical pulmonary function testing. Eur Respir J 26: 1–2, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Chan EH, Russell JL, Williams WG, Van Arsdell GS, Coles JG, McCrindle BW. Postoperative chylothorax after cardiothoracic surgery in children. Ann Thorac Surg 80: 1864–1870, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Cooper DM, Weiler-Ravell D. Gas exchange response to exercise in children. Am Rev Respir Dis 129: S47–S48, 1984 [DOI] [PubMed] [Google Scholar]
- 11.De Troyer A, Yernault JC, Englert M. Lung hypoplasia in congenital pulmonary valve stenosis. Circulation 56: 647–651, 1977 [DOI] [PubMed] [Google Scholar]
- 12.Fredriksen PM, Therrien J, Veldtman G, Warsi MA, Liu P, Siu S, Williams W, Granton J, Webb G. Lung function and aerobic capacity in adult patients following modified Fontan procedure. Heart 85: 295–299, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gewillig M, Brown SC, Eyskens B, Heying R, Ganame J, Budts W, La Gerche A, Gorenflo M. The Fontan circulation: who controls cardiac output? Interact Cardiovasc Thorac Surg 10: 428–433, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Ginde S, Bartz PJ, Hill GD, Danduran MJ, Biller J, Sowinski J, Tweddell JS, Earing MG. Restrictive lung disease is an independent predictor of exercise intolerance in the adult with congenital heart disease. Congenit Heart Dis 8: 246–254, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greutmann M, Le TL, Tobler D, Biaggi P, Oechslin EN, Silversides CK, Granton JT. Generalised muscle weakness in young adults with congenital heart disease. Heart 97: 1164–1168, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Haworth SG, Reid L. Quantitative structural study of pulmonary circulation in the newborn with aortic atresia, stenosis, or coarctation. Thorax 32: 121–128, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hislop A, Reid L. Structural changes in the pulmonary arteries and veins in tetralogy of Fallot. Br Heart J 35: 1178–1183, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanakis MA, Katsimpoulas M, Kavantzas N, Kostomitsopoulos N, Dimitriou C, Lioulias A, Kostakis A, Mitropoulos F. Acute histological changes of the lung after experimental Fontan circulation in a swine model. Med Science Monit 18: BR362–BR365, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khairy P, Fernandes SM, Mayer JE, Jr, Triedman JK, Walsh EP, Lock JE, Landzberg MJ. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 117: 85–92, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Khambadkone S, Li J, de Leval MR, Cullen S, Deanfield JE, Redington AN. Basal pulmonary vascular resistance and nitric oxide responsiveness late after Fontan-type operation. Circulation 107: 3204–3208, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis 127: 725–734, 1983 [DOI] [PubMed] [Google Scholar]
- 22.Marsden AL, Vignon-Clementel IE, Chan FP, Feinstein JA, Taylor CA. Effects of exercise and respiration on hemodynamic efficiency in CFD simulations of the total cavopulmonary connection. Ann Biomed Eng 35: 250–263, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Matthews IL, Fredriksen PM, Bjornstad PG, Thaulow E, Gronn M. Reduced pulmonary function in children with the Fontan circulation affects their exercise capacity. Cardiol Young 16: 261–267, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Medoff BD, Oelberg DA, Kanarek DJ, Systrom DM. Breathing reserve at the lactate threshold to differentiate a pulmonary mechanical from cardiovascular limit to exercise. Chest 113: 913–918, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Moller P, Weitz M, Jensen KO, Dubowy KO, Furck AK, Scheewe J, Kramer HH, Uebing A. Exercise capacity of a contemporary cohort of children with hypoplastic left heart syndrome after staged palliation. Eur J Cardiothorac Surg 36: 980–985, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Nakazawa M, Nakanishi T, Okuda H, Satomi G, Nakae S, Imai Y, Takao A. Dynamics of right heart flow in patients after Fontan procedure. Circulation 69: 306–312, 1984 [DOI] [PubMed] [Google Scholar]
- 27.O'Leary JM, Siddiqi OK, de Ferranti S, Landzberg MJ, Opotowsky AR. The changing demographics of congenital heart disease hospitalizations in the United States, 1998 through 2010. JAMA 309: 984–986, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Ohuchi H, Ohashi H, Takasugi H, Yamada O, Yagihara T, Echigo S. Restrictive ventilatory impairment and arterial oxygenation characterize rest and exercise ventilation in patients after fontan operation. Pediatr Cardiol 25: 513–521, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Ohuchi H, Wakisaka Y, Watanabe K, Kishiki K, Yamada O, Echigo S. Impact of central hypercapnic chemosensitivity on enhanced ventilation in patients after the Fontan operation. Int J Cardiol 121: 36–43, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Opotowsky AR. Abnormal spirometry in congenital heart disease: where do we go from here? Circulation 127: 865–867, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS, Margossian R, Mital S, Russell J, Rhodes J. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol 52: 99–107, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Penny DJ, Hayek Z, Redington AN. The effects of positive and negative extrathoracic pressure ventilation on pulmonary blood flow after the total cavopulmonary shunt procedure. Int J Cardiol 30: 128–130, 1991 [DOI] [PubMed] [Google Scholar]
- 33.Penny DJ, Redington AN. Doppler echocardiographic evaluation of pulmonary blood flow after the Fontan operation: the role of the lungs. Br Heart J 66: 372–374, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redington AN, Penny D, Shinebourne EA. Pulmonary blood flow after total cavopulmonary shunt. Br Heart J 65: 213–217, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reid L. Edward B. D. Neuhauser lecture: the lung: growth and remodeling in health and disease. Am J Roentgenol 129: 777–788, 1977 [DOI] [PubMed] [Google Scholar]
- 36.Rosenthal M, Bush A, Deanfield J, Redington A. Comparison of cardiopulmonary adaptation during exercise in children after the atriopulmonary and total cavopulmonary connection Fontan procedures. Circulation 91: 372–378, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Shachar GB, Fuhrman BP, Wang Y, Lucas RV, Jr, Lock JE. Rest and exercise hemodynamics after the Fontan procedure. Circulation 65: 1043–1048, 1982 [DOI] [PubMed] [Google Scholar]
- 38.Shafer KM, Garcia JA, Babb TG, Fixler DE, Ayers CR, Levine BD. The importance of the muscle and ventilatory blood pumps during exercise in patients without a subpulmonary ventricle (Fontan operation). J Am Coll Cardiol 60: 2115–2121, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaheen S, Barker DJ. Early lung growth and chronic airflow obstruction. Thorax 49: 533–536, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shekerdemian LS, Bush A, Shore DF, Lincoln C, Redington AN. Cardiopulmonary interactions after Fontan operations: augmentation of cardiac output using negative pressure ventilation. Circulation 96: 3934–3942, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 127: 1952–1959, 2005 [DOI] [PubMed] [Google Scholar]
- 42.St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol 529: 493–504, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. Philadelphia, PA: Lippincott, Williams & Wilkins, 2005, p. xvi [Google Scholar]
- 44.Williams DB, Kiernan PD, Metke MP, Marsh HM, Danielson GK. Hemodynamic response to positive end-expiratory pressure following right atrium-pulmonary artery bypass (Fontan procedure). J Thorac Cardiovasc Surg 87: 856–861, 1984 [PubMed] [Google Scholar]
- 45.Zongtao Y, Huishan W, Zengwei W, Hongyu Z, Minhua F, Xinmin L, Nanbin Z, Hongguang H. Experimental study of nonpulsatile flow perfusion and structural remodeling of pulmonary microcirculation vessels. Thorac Cardiovasc Surg 58: 468–472, 2010 [DOI] [PubMed] [Google Scholar]