Abstract
Tissue growth and function depend on vascularization, and vascular insufficiency or excess exacerbates many human diseases. Identification of the biological processes involved in angiogenesis will dictate strategies to modulate reduced or excessive vessel formation. We examine the essential role of pericytes. Their heterogeneous morphology, distribution, origins, and physiology have been described. Using double-transgenic Nestin-GFP/NG2-DsRed mice, we identified two pericyte subsets. We found that Nestin-GFP−/NG2-DsRed+ (type-1) and Nestin-GFP+/NG2-DsRed+ (type-2) pericytes attach to the walls of small and large blood vessels in vivo; in vitro, type-2, but not type-1, pericytes spark endothelial cells to form new vessels. Matrigel assay showed that only type-2 pericytes participate in normal angiogenesis. Moreover, when cancer cells were transplanted into Nestin-GFP/NG2-DsRed mice, type-1 pericytes did not penetrate the tumor, while type-2 pericytes were recruited during its angiogenesis. As inhibition of angiogenesis is a promising strategy in cancer therapy, type-2 pericytes may provide a cellular target susceptible to signaling and pharmacological manipulation in treating malignancy. This work also reports the potential of type-2 pericytes to improve blood perfusion in ischemic hindlimbs, indicating their potential for treating ischemic illnesses.
Keywords: angiogenesis, pericytes, stem cells, tumor
all tissues require healthy vasculature to supply nutrients and O2 and remove degradation products. Insufficient vascularization leads to ischemic conditions that inhibit tissue growth and survival, while tumors promote excessive and aberrant vascularization (36). Angiogenesis is the process by which new blood vessels form from existing vessels (65). It plays a central role in human physiology during fetal development, menstruation, wound healing, tissue repair after surgery or trauma, cancer, and various ischemic and inflammatory diseases (3, 46). Understanding angiogenesis well enough to manipulate it would create numerous therapeutic opportunities.
Successful angiogenesis requires the participation of various cell types and associated molecular signaling. Cells attached to the walls of microvessels, called pericytes, play a critical role in stabilizing blood vessels. On the basis of their markers, morphology, and origin, they are heterogeneous (15, 17–19, 34, 72, 75, 77, 90). Some have robust angiogenic potential (30), respond to angiogenic factors, guide newly formed vessels, and provide survival signals for endothelial cells. Whether all pericyte subpopulations have angiogenic potential remains unknown.
As pericytes participate in stem cell support, self-renewal, and proliferation (50), as well as tissue regeneration and repair (15, 18), targeting only the pericyte subpopulation involved in angiogenesis may provide more efficient control of blood vessel development and a target for treatments designed to decrease (cancer) or increase (ischemic diseases) vascularization.
Recently, using Nestin-GFP/NG2-DsRed transgenic mice, we identified two pericyte subtypes, type 1 (Nestin-GFP−/NG2-DsRed+) and type 2 (Nestin-GFP+/NG2-DsRed+). We found that they play diverse roles in different microenvironments (15, 17, 18): while type-2 pericytes generate new muscle tissue after injury, type-1 pericytes are fibrogenic and adipogenic in old and diseased skeletal muscle, respectively. Whether their angiogenic potential differs is unknown.
Here, using a Matrigel plug assay, we show that angiogenesis occurs when type-2, but not type-1, pericytes are cultured with endothelial cells or injected in vivo. Moreover, only endogenous type-2 pericytes are recruited during tumor angiogenesis. We also found that type-2 pericytes recover blood flow in a mouse model of hindlimb ischemia. We propose targeting type-2 pericytes, rather than all pericytes, for antiangiogenic cancer therapy and tissue revascularization. This approach will not interfere with normal type-1 pericyte functions, such as extracellular matrix deposition, needed for normal tissue remodeling.
MATERIALS AND METHODS
Animals.
Our colony of Nestin-GFP transgenic mice was maintained homozygous for the transgene on the C57BL/6 genetic background (54). Our colony of C57BL/6 wild-type mice was used as the control. Male athymic nude (nu/nu) mice (Taconic Farms, Germantown, NY) were used in transplantation studies. NG2-DsRed transgenic mice expressing DsRed-T1 under the control of the NG2 promoter (89) and β-actin-DsRed transgenic mice expressing the red fluorescent protein variant DsRed.MST under the control of the chicken β-actin promoter coupled with the cytomegalovirus immediate-early enhancer (84) were purchased from the Jackson Laboratory (Bar Harbor, ME). All tissues of β-actin-DsRed transgenic mice fluoresce red (84). Nestin-GFP mice were cross-bred with β-actin-DsRed mice to generate Nestin-GFP/β-actin-DsRed double-transgenic mice. Nestin-GFP/NG2-DsRed double-transgenic mice are described elsewhere (15, 17, 18). All colonies were housed in a pathogen-free facility of the Animal Research Program at Wake Forest School of Medicine under a 12:12-h light-dark cycle and fed ad libitum. Male and female homozygous mice were used, and their ages ranged from 3 to 5 mo. Animal handling and procedures were approved by the Wake Forest School of Medicine Animal Care and Use Committee.
Primary antibodies.
Table 1 shows the antibodies and source.
Table 1.
Antibodies and source
| Antibody | Source | Location |
|---|---|---|
| Rat anti-CD31 (PECAM-1) | BD Biosciences | San Jose, CA |
| Rat anti-mouse CD146 | BioLegend | San Diego, CA |
| Rabbit anti-PDGFRβ | Dr. W. Stallcup | Sanford-Burnham Medical Research Institute, La Jolla, CA |
| Rabbit anti-NG2 chondroitin sulfate proteoglycan | Chemicon-Millipore | Temecula, CA |
PECAM, platelet endothelial cell adhesion molecule; PDGFR, platelet-derived growth factor receptor.
Mesenteric vessel isolation.
Mesenteric vessels were isolated as described elsewhere (53). Briefly, the mice were anesthetized and their arteries were removed, with all precautions to ensure sterility. The abdominal wall was opened in layers, and the celiac gland was exposed. The superior mesenteric artery was then easily identified in close proximity to the gland, stripped of surrounding mesentery, and clamped proximally. Blood was cleared from the artery and its branches by injection of 1 ml of cold PBS solution into the artery, distal to the clamp. Tissues were kept moist with PBS solution during dissection. After surrounding mesentery was teased away, the artery and its branches were quickly removed and stripped of periarterial fat and fibrous tissue. The superior mesenteric artery and its main branches were then dissected out and observed under phase-contrast and fluorescence microscopy.
Skeletal muscle immunohistochemistry.
To detect DsRed and green fluorescent protein (GFP) fluorescence in 3-mo-old Nestin-GFP/NG2-DsRed mice, nondissociated extensor digitorum longus muscles were dissected; fixed in 4% paraformaldehyde (PFA) overnight; immersed in 10%, 20%, and 30% sucrose solutions for 60, 45, and 30 min, respectively; embedded in optimal cutting temperature (OCT) compound; and rapidly frozen in liquid nitrogen to prepare 10-μm-thick cryosections. Muscle sections were fixed with 4% PFA for 30 min, permeabilized in 0.5% Triton X-100 (Sigma, St. Louis, MO), and blocked to saturate nonspecific antigen sites using 5% (vol/vol) goat serum-PBS (Jackson ImmunoResearch Laboratories, West Grove, PA) overnight at 4°C. On the next day, the sections were incubated with primary antibodies at 1:100 dilution for 4 h at room temperature and visualized using appropriate species-specific secondary antibodies conjugated with Alexa Fluor 680 at 1:1,000 dilution (Invitrogen, Carlsbad, CA) (87, 88). Muscle sections were counterstained with Hoechst 33342, mounted on slides using fluorescence mounting medium (DakoCytomation, Carpinteria, CA), and examined under fluorescence microscopy.
Fluorescence-activated cell sorting.
Fluorescence-activated cell sorting (FACS) was carried out on a flow cytometer (Aria Sorter, BD Biosciences, San Jose, CA). Data acquisition and analyses were performed using FACSDiva 5.0.3 software (BD Biosciences), gated for a high level of GFP or allophycocyanin (APC) expression. The clear separation of GFP+ from GFP− cells (14) and APC+ from APC− cells, as well as the low flow rate, explains the ease and accuracy of sorting (17). Sorted cells were reanalyzed to confirm their fluorescence profile (14, 17).
Isolation of type-1 and type-2 DsRed+ pericytes.
Hindlimb muscle cells were freshly isolated from young adult (3- to 5-mo-old) Nestin-GFP/β-actin-DsRed mice as described elsewhere (16). Briefly, muscles were carefully dissected away from the surrounding connective tissue and minced, digested by gentle agitation in 0.2% (wt/vol) type-2 collagenase in Krebs solution at 37°C for 2 h, and dissociated by trituration and resuspension in 0.25% trypsin-0.05% EDTA in PBS for 15 min at 37°C. After centrifugation at 1,500 rpm for 5 min, the supernatant was removed, and the pellet was resuspended in growth medium. Aggregates were removed by passage through a 40-μm cell strainer prior to sorting. After they were counted, the cells were centrifuged at 1,500 rpm for 5 min and resuspended in 100 μl of 1% FBS in PBS per 106 cells. First, an aliquot was collected for use as unlabeled control (labeled with only the secondary APC anti-rabbit antibody, without the primary rabbit anti-NG2 antibody) to set the gate for APC. The remaining cells were incubated with the primary rabbit anti-mouse NG2 antibody for 45 min and washed in 1% FBS in PBS. They were then incubated for 30 min with APC anti-rabbit secondary antibody and washed in PBS with 1% FBS. The gate for GFP was set using cells isolated from skeletal muscle of wild-type mice. Sorting was based on GFP and APC fluorescence. Isolated Nestin-GFP+/NG2-APC+/β-actin-DsRed+ and Nestin-GFP−/NG2-APC+/β-actin-DsRed+ cells were used in angiogenic in vitro assays, in Matrigel plug in vivo assays, and in cell fate-tracking experiments to evaluate vessel formation in vivo after hindlimb ischemia. In contrast to Nestin-GFP/NG2-DsRed, all Nestin-GFP/β-actin-DsRed cells express β-actin-DsRed (21), and their red fluorescence persists even after their transdifferentiation, allowing us to track cell fate.
Cell culture.
Human umbilical vein endothelial cells (HUVECs) were obtained from the American Type Culture Collection. All experiments used endothelial cells between passages 2 and 4. HUVECs were maintained in Medium 200 with endothelial low-serum growth supplement (Invitrogen) at 37°C with 5% CO2. The medium was changed every other day. All cells were maintained as subconfluent cultures and split 1:3 at 24 h before use.
Angiogenic assays.
Angiogenic assays were performed as described elsewhere (4). Briefly, after overnight incubation at 4°C, 150 μl of precooled Matrigel-reduced growth factor (BD Matrigel 356230) were transferred to a 48-well plate on ice. After gel formation at 37°C for 30 min, 4 × 105 HUVECs were diluted in cell culture medium, and 500 μl of the suspension were added to the 48-well plate. HUVECs were cultured alone as a control or together with 1.5 × 105 type-1 DsRed+ purified pericytes or 1.5 × 105 type-2 DsRed+ purified pericytes in the same culture medium. Plating dishes were incubated at 37°C, and after 3 h the culture medium was gently removed and Matrigel (150 μl) was added to form a sandwich that was finally covered with 500 μl of culture medium. After 10 days, the formation of endothelial tubular networks was examined under the microscope.
Matrigel plug assays.
Matrigel plug assays were performed as described elsewhere (23). Briefly, 4 × 106 HUVECs and 5 × 105 type-1 DsRed+ purified pericytes or 5 × 105 type-2 DsRed+ purified pericytes were suspended in 500 μl of culture medium. The cell suspensions were mixed with 500 μl of liquid Matrigel-reduced growth factor (BD Matrigel 356230) at a ratio of 1:1 at 4°C. Nude mice (2–3 mo old) received a total of 1 ml of this mixture subcutaneously in the dorsal region, generating Matrigel plugs when warmed to body temperature (49). Plugs were recovered 2 wk later.
Intracerebral transplantation of glioblastoma cells.
To evaluate cell recruitment during brain tumor growth, orthotopic glioblastoma tumors were established by stereotaxic implantation of 2 × 105 actively growing G26-H2 murine glioma cells in Nestin-GFP/NG2-DsRed mice. Briefly, mice were anesthetized with a mixture of 114 mg/kg ketamine and 17 mg/kg xylazine, and a 0.45-mm burr hole was made 2 mm lateral and 0.5 posterior to the bregma in the right cerebral hemisphere through a scalp incision. Stereotaxic injection was performed on a Just For Mice stereotaxic apparatus (Harvard Apparatus, Holliston, MA), with insertion of a 10-μl syringe (Hamilton, Reno, NV) with a 30-gauge, 1-in. flat needle through the burr hole to a depth of 3.2 mm. A Nanomite programmable syringe pump (Harvard Apparatus) delivered constant infusion at a rate of 0.5 μl/min to a total volume of 5 μl (11, 12).
To prevent infection and alleviate pain and/or discomfort, all animals received an antibiotic (gentamicin, 5.8 mg/kg) and an analgesic (buprenorphine, 0.1–0.5 mg/kg). Mice were monitored for body weight and ambulatory, feeding, and grooming activities. Animals losing ≥20% of their body weight or having trouble ambulating or feeding were euthanized. Mice with tumors growing outside their intracranial space were eliminated from the study.
At 5 wk after implantation, mice were anesthetized again and transcardially perfused with cold PBS and then with 4% PFA solution in PBS. After decapitation, brains were rapidly dissected out, removed from the skull, postfixed for 24 h in the same fixative solution, and cryoprotected with 30% sucrose in PBS for 2 days. The brains were then placed in embedding cryomolds, covered with tissue embedding medium (OCT compound, Tissue-Tek, Sakura Finetek, Tokyo, Japan), snap-frozen in liquid nitrogen, and stored at −80°C.
Brain histological processing.
Serial, 20-μm-thick, coronal sections of frozen brains, obtained using a cryostat (Microm HM 500, Zeiss, Oberkochen, Germany) at −20°C, were mounted on SuperFrost Plus microscope slides in series of six (Fisher Scientific) and stored at −20°C. For experiments, the sections were dried at room temperature for 1 h, rehydrated in PBS, permeabilized with 0.5% Triton X-100 in PBS solution, and blocked to saturate nonspecific antigen sites using 5% (vol/vol) goat serum-PBS at 4°C overnight. On the next day, the sections were incubated, with Hoechst 33342 used as a nuclear marker. The sections were mounted on slides using fluorescence mounting medium and examined with fluorescence microscopy.
Subcutaneous transplantation of melanoma cells and tumor tissue processing.
To evaluate cell recruitment during peripheral tumor growth, actively growing B16 melanoma cells were implanted subcutaneously into Nestin-GFP/NG2-DsRed mice at 3 × 105 cells per mouse in 200 μl of PBS-Matrigel (BD Biosciences). When tumors reached a volume >1,000 mm3, the mice were killed. Tumor tissues and adjacent muscle were dissected, harvested, and fixed in 4% PFA overnight. Tissues were then immersed in 30% sucrose solution overnight, embedded in OCT, and rapidly frozen in liquid nitrogen to prepare 10-μm-thick cryosections, which were fixed with 4% PFA for 30 min, permeabilized in 0.5% Triton X-100, and blocked to saturate nonspecific antigen sites using 5% (vol/vol) goat serum-PBS overnight at 4°C. On the next day, the sections were incubated with the primary antibody anti-CD31 (platelet endothelial cell adhesion molecule) at room temperature for 4 h and visualized using appropriate species-specific secondary antibody conjugated with Alexa Fluor 680 at 1:1,000 dilution (Invitrogen). The sections were counterstained with Hoechst 33342, mounted on slides using fluorescence mounting medium, and examined with fluorescence microscopy.
Critical hindlimb ischemia model.
Two- to 3-mo-old athymic nude (nu/nu) male mice were anesthetized with xylazine (20 mg/kg) and ketamine (100 mg/kg) by intraperitoneal injection as described elsewhere (30) and subjected to hindlimb ischemia by femoral artery ligation and transection. Immediately after surgery, mice received 2 × 106 type-1 or type-2 DsRed+ pericytes or unconditioned DMEM (200 μl) by intramuscular injection into the ischemic leg. Limb perfusion was assessed with in vivo MRI angiography 10 days after treatment, followed by fluorescence microscopy analysis of muscle harvested from the ischemic limb.
In vivo MRI angiography.
All in vivo imaging was performed on a Bruker 70/30 horizontal-bore, small-animal MRI scanner equipped with a high-power gradient insert (60-mm inside diameter) capable of generating a maximal magnetic field gradient of 1,000 mT/m (Bruker Biospin, Ettlingen, Germany). Each animal was placed prone in a sliding mouse bed in an induction chamber initially filled with room air and then with a mixture of isoflurane (2%) and O2 (2 l/min) until the animal was anesthetized. A pillow over the abdomen monitored respiration rate, and a nose cone supplied isoflurane and O2 (typical levels: 1.5% and 1 l/min) during scanning. Thermostatically controlled warm air was blown into the bore of the magnet to keep the animal's skin temperature >35°C.
The animal's pelvis was placed at the center of the 7-T MRI magnet in a quadrature-volume radio-frequency coil with an inside diameter of 35 mm. A three-plane localizer scout scan was acquired using a rapid-acquisition-with-relaxation-enhancement (RARE) pulse sequence with the following parameters: repetition time (TR) = 1,500 ms, echo time (TE) = 35 ms, field of view (FOV) = 3 cm, matrix size = 128 × 128, slice thickness = 2.0 mm, and number of excitations (NEX) = 1.
The high-resolution, T1-weighted scan and angiography scan were planned using the three-plane localizer images. The T1-weighted scan was acquired using a RARE pulse sequence with the following parameters: TR = 3,000 ms, TE = 8 ms, matrix = 256 × 256, FOV = 2.2 cm, and NEX = 3, for a total acquisition time of 7 min and an in-plane resolution of 86 μm. High-resolution angiography scans were acquired using a fast low-angle shot two-dimensional gradient echo angiography sequence with the following parameters: TR = 15 ms, TE = 4 ms, FOV = 2.2 cm, matrix = 512 × 360, slice thickness = 0.40 mm, slice gap = 0.25 mm, and NEX = 16, for a total acquisition time of 1 h 14 min and an in-plane resolution of 43 × 61 μm. When scanning was complete, the mouse was removed from the scanner, placed on a warming pad, and allowed to regain consciousness.
Microscopy, cell imaging, and counting.
An inverted motorized fluorescence microscope (model IX81, Olympus, Tokyo, Japan) with an Orca-R2 Hamamatsu charge-coupled device camera was used for image acquisition. Camera drive and acquisition were controlled by a MetaMorph imaging system (Olympus, Center Valley, PA). Ten arbitrary microscopic fields were counted in each immunostained plate or tissue section, and values were pooled from parallel duplicates per time point and individual experiment.
Statistical analysis.
Values are means ± SE. Statistical significance was assessed by Student's t-test using GraphPad Prism (GraphPad Software, San Diego, CA). P < 0.05 was considered significant.
RESULTS
Two pericyte subtypes enwrap blood vessels of various calibers.
Pericytes have been reported around microvessels (6, 76) and possibly larger vessels (1, 7, 27, 31, 56, 83), and their heterogeneity has been described in various tissues (15, 17–19, 38). However, whether distinct classes of pericytes surround blood vessels of a certain caliber is unknown.
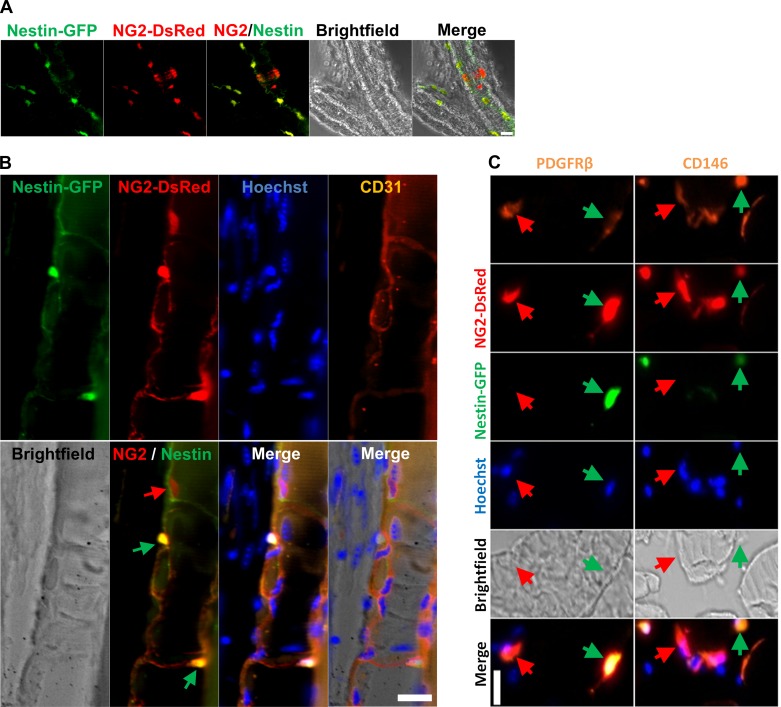
The marker most commonly used to identify pericytes in recent years is NG2, the neuron-glial 2 chondroitin sulfate proteoglycan (62, 63). We analyzed the small skeletal muscle capillaries and larger mesenteric blood vessels from NG2-DsRed/Nestin-GFP mice in which NG2 and Nestin regulatory elements control DsRed and GFP expression, respectively. We found the two types of pericytes, type 1 and type 2, around large blood vessel walls (Fig. 1A) and small capillaries (Fig. 1B). Type-1 and type-2 pericytes stained positive for platelet-derived growth factor receptor-β (PDGFRβ) (15, 18, 43) and CD146 (15, 18, 28, 68) (Fig. 1C). Our results indicate that both pericyte subpopulations line the outer surface of the vasculature, irrespective of its caliber.
Fig. 1.
Two pericyte subtypes are found in capillaries and larger blood vessels. A and B: pericyte subtypes in mesenteric vessels and skeletal muscle from Nestin-GFP/NG2-DsRed mice. A: pericytes surround mesenteric blood vessels. All panels show the same area for different channels (Nestin-GFP, NG2-DsRed, merged fluorescence, bright-field, and merged fluorescence and bright-field images). B: pericytes surround vessels in skeletal muscle. Muscle longitudinal sections show small blood vessels with CD31+ endothelial cells surrounded by NG2-DsRed+ pericytes. Red and green arrows indicate Nestin-GFP−/NG2-DsRed+ (type-1) and Nestin-GFP+/NG2-DsRed+ (type-2) pericytes, respectively. Blood vessels are labeled by the endothelial cell marker CD31. All panels show the same area for different channels (Nestin-GFP, NG2-DsRed, Hoechst, CD31 staining, bright-field, merged fluorescence, and merged fluorescence and bright-field images). C: pericyte markers platelet-derived growth factor receptor-β (PDGFRβ) and CD146 colocalize with skeletal muscle interstitial Nestin-GFP-/NG2-DsRed+ and Nestin-GFP+/NG2-DsRed+ cells. Panels show identical muscle areas from top to bottom: PDGFRβ or CD146 (orange), NG2-DsRed (red), Nestin-GFP+ (green), Hoechst (blue), bright-field, and merged images. Red arrow indicates type-1 pericytes (Nestin-GFP−/NG2-DsRed+); green arrow indicates type-2 pericytes (Nestin-GFP+/NG2-DsRed+). Scale bar, 20 μm.
Recently, we showed that pericyte subtypes differ in their differentiation capacity. Type-1 pericytes are responsible for ectopic adipocyte deposition (15) and fibrous tissue accumulation with aging (18), while type-2 pericytes differentiate into the neural lineage (16, 17) and participate in skeletal muscle regeneration after injury (15). Pericyte participation in engineered new blood vessels (30, 42) indicates their therapeutic potential, but whether the subtypes contribute differently to angiogenesis is unknown.
Only one pericyte subtype has angiogenic potential in vitro.
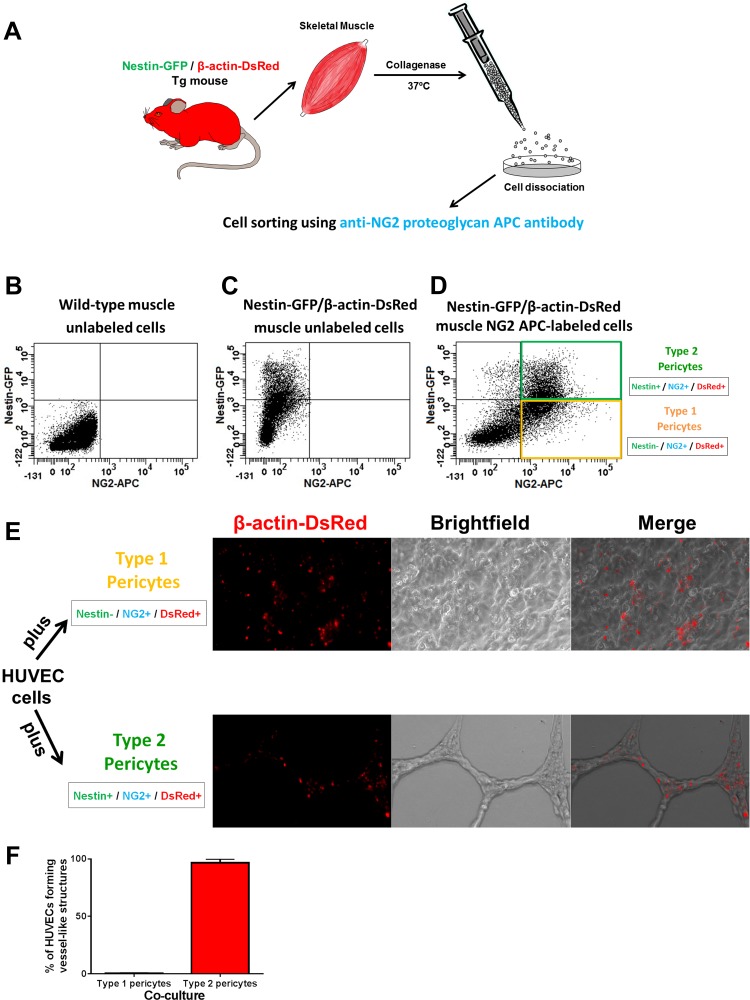
The angiogenic potential of pericyte subpopulations was initially evaluated in vitro by testing their ability to form vessel-like structures when incubated with HUVECs. All type-1 and type-2 pericytes, sorted from Nestin-GFP/β-actin-DsRed mice, expressed constitutive DsRed. In contrast to Nestin-GFP/NG2-DsRed mice, Nestin-GFP/β-actin-DsRed mice express β-actin-DsRed in all cells (21), and the red fluorescence persists even after their transdifferentiation, allowing us to track them after transplantation (Fig. 2, A–D). We confirmed their purity as described previously (18). HUVECs cultured alone in Matrigel usually form an unstable network that disappears after 5 days (57). All DsRed− cells are HUVECs, as they do not derive from β-actin-DsRed mice. After 10 days, we did not detect any vessel-like structures in the culture with type-1 pericytes (Fig. 2E). In contrast, type-2 pericytes from Nestin-GFP/β-actin-DsRed mice formed vessel-like networks in the in vitro Matrigel assay (Fig. 2, E and F). All DsRed+ cells were closely associated with HUVECs, assuming a clear periendothelial position (P < 0.05; Fig. 2, E and F). These results suggest that only type-2 pericytes are angiogenic in vitro.
Fig. 2.
Only type-2 pericytes are angiogenic in vitro. A: procedures for isolation of mononucleated cells from skeletal muscle of Nestin-GFP/β-actin-DsRed double-transgenic (Tg) mice. B: representative flow cytometry dot plot showing green fluorescent protein (GFP) vs. allophycocyanin (APC) fluorescence, with the gate set using cells isolated from wild-type mice. C and D: representative dot plots showing GFP vs. APC fluorescence using mononucleated cells from skeletal muscle of Nestin-GFP/β-actin-DsRed mice, unlabeled and after labeling with NG2 proteoglycan APC antibody. Two cell populations were isolated by sorting: Nestin-GFP−/NG2-APC+/β-actin-DsRed+ (type-1 pericytes, yellow) and Nestin-GFP+/NG2-APC+/β-actin-DsRed+ (type-2 pericytes, green). All cells are DsRed+ and can be tracked in vitro after they are mixed with human umbilical vein endothelial cells (HUVECs). E: in vitro Matrigel assay containing a mixture of HUVECs with type-1 or type-2 DsRed+ pericytes sorted from skeletal muscle of Nestin-GFP/β-actin-DsRed mice. DsRed fluorescence, bright-field, and merged images are shown. After 10 days, only HUVECs cultured with type-2 pericytes formed stable vessel-like networks. F: percentage of HUVECs forming vessel-like structures in coculture with type-1 or type-2 pericytes. Values are means ± SE.
Only type-2 pericytes form blood vessels in vivo.
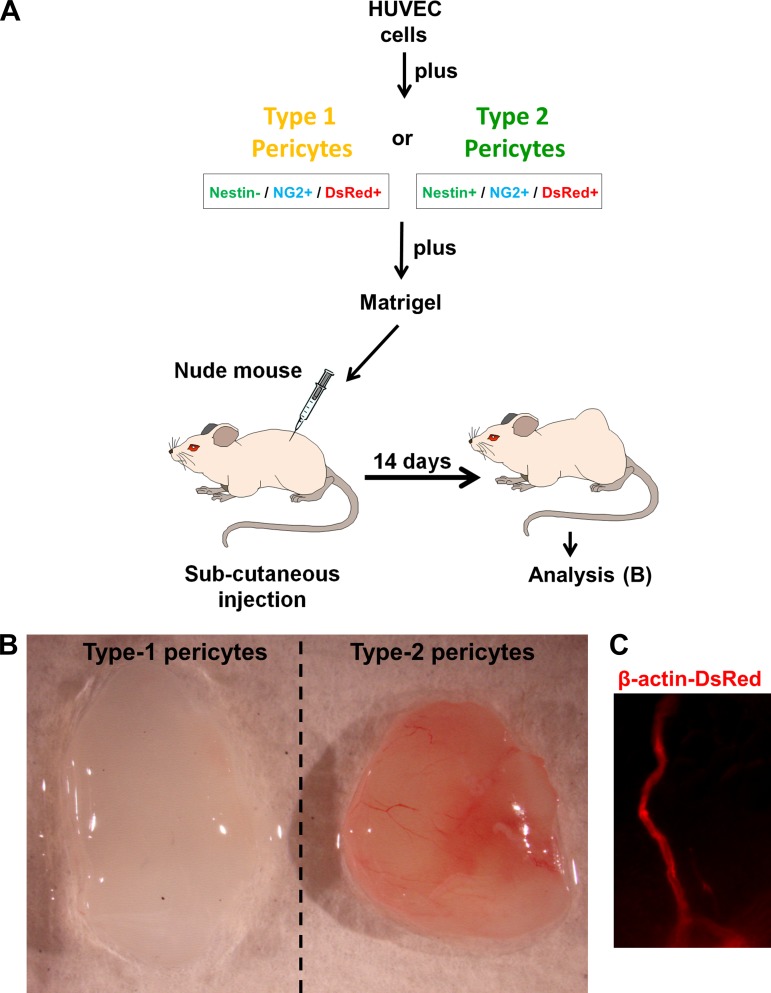
To examine the angiogenic potential of type-2 pericytes in vivo, we used the murine Matrigel plug assay. Matrigel, an extract of the Engelbreth-Holm-Swarm tumor composed of basement membrane components, is liquid at 4°C and forms a gel when warmed to 37°C (49). Pericyte subpopulations were isolated from Nestin-GFP/β-actin-DsRed mice. In this assay, Matrigel plus HUVECs, Matrigel plus HUVECs and type-1 pericytes, and Matrigel plus HUVECs and type-2 pericytes were injected separately and subcutaneously into the dorsal region of nude mice (Fig. 3A). The Matrigel plug solidified after the injection. At 2 wk after implantation, the Matrigel plug containing type-2 pericytes formed a large blood vessel network connected with the host vasculature. These vessels contained blood (n = 3). In contrast, the Matrigel plug containing only HUVECs (data not shown) or HUVECs plus type-1 pericytes displayed no functional vessels (n = 3; Fig. 3B). As the transplanted pericytes were marked with β-actin-DsRed fluorescence, we detected those cells in the Matrigel plugs in vivo participating in the vessel formation (Fig. 3C). These experiments indicate that type-2 pericytes are angiogenic in vivo.
Fig. 3.
Matrigel plug assay shows that only type-2 pericytes are angiogenic in vivo. A: transplantation scheme of an in vivo Matrigel plug assay. A mixture of HUVECs and either type-1 or type-2 pericytes sorted from skeletal muscle are embedded in Matrigel and implanted subcutaneously into nude mice. Matrigel plug is recovered after 2 wk for analysis. B: gross anatomy of freshly removed Matrigel plug with type-1 or type-2 pericytes 2 wk after implantation. Type-2 pericytes, together with endothelial cells, form blood vessels in vivo. C: β-actin-DsRed fluorescence around blood vessels in a Matrigel plug tracks type-2 pericytes in vivo.
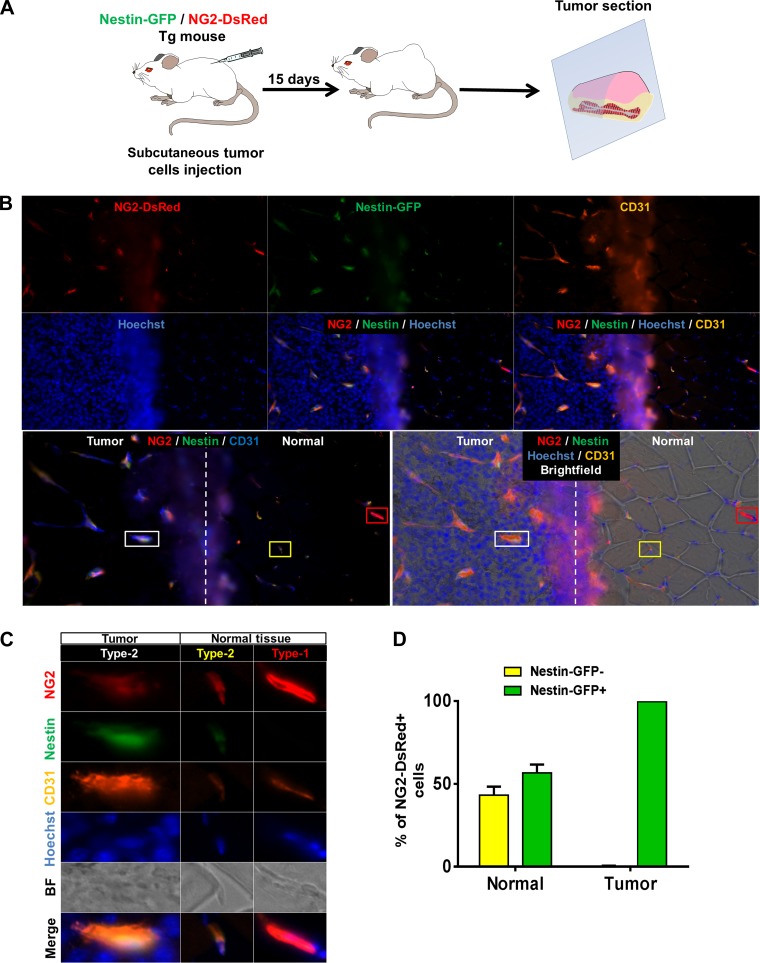
Brain tumor angiogenesis recruits NG2-DsRed+ cells that express Nestin-GFP.
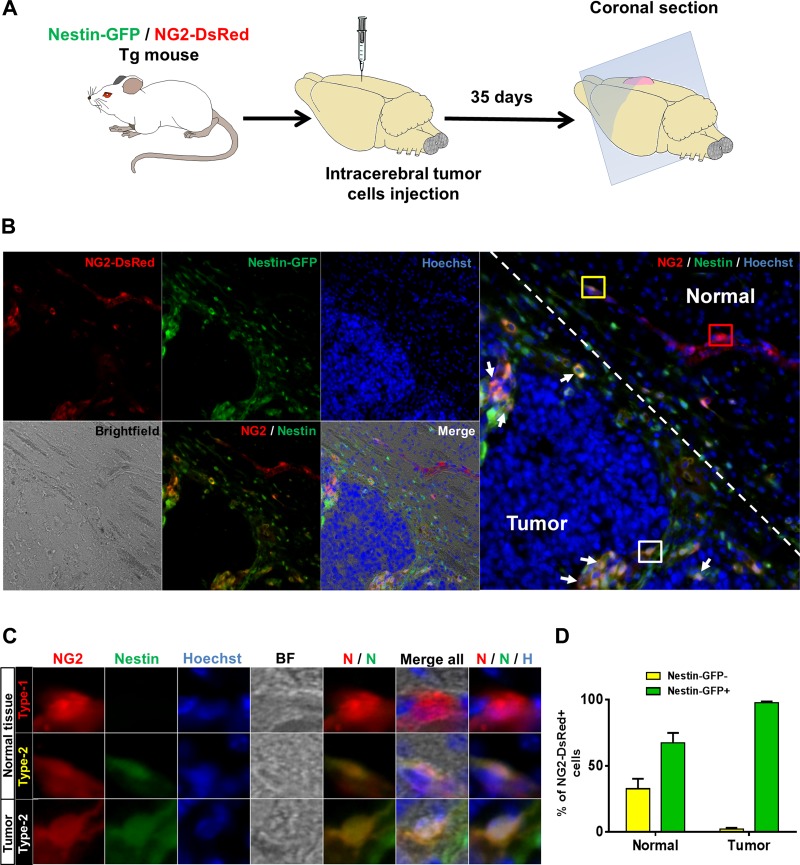
Angiogenic activity is enhanced in some pathological conditions, such as tumors, in which pericytes contribute to vessel formation (10, 71, 85). Pericytes stabilize the endothelium by surrounding the blood vessels and support angiogenesis by secreting VEGF (41). To examine whether one pericyte subtype or both are recruited during tumor angiogenesis, we injected actively growing glioblastoma tumor cells into the right brain hemisphere of 3-mo-old NG2-DsRed/Nestin-GFP mice (2 × 10 cells/5 μl, n = 3). After 5 wk, we removed the brains to examine the NG2-DsRed+ cells in the tumor margins and adjacent normal brain tissue in coronal sections (Fig. 4A). We detected NG2-DsRed+/Nestin-GFP− and NG2-DsRed+/Nestin-GFP+ cells in healthy normal brain tissue surrounding the tumor tissue (Fig. 4, B and C). Nestin-GFP+ cells represented 67.3 ± 7.5% of NG2-DsRed+ cells, while 32.7 ± 7.5% were Nestin-GFP−. In contrast, 97.7 ± 0.9% of NG2-DsRed+ cells penetrating the tumor expressed Nestin-GFP, while only 2.3 ± 0.9% were Nestin-GFP− (Fig. 4, B–D). Thus NG2-DsRed+/Nestin-GFP+ cells significantly increased, while NG2-DsRed+/Nestin-GFP− cells significantly decreased in the tumor (P = 0.02). These results support the conclusion that type-1 pericytes (NG2-DsRed+/Nestin-GFP−) are not recruited during tumor angiogenesis. Not all brain NG2-DsRed+/Nestin-GFP+ cells correspond to type-2 pericytes, as some are oligodendrocyte progenitors (33). Whether more type-2 than type-1 pericytes migrate toward the tumor or the cancer cells stimulate oligodendrocyte progenitor cell migration is unknown.
Fig. 4.
Nestin-GFP+/NG2-DsRed+, but not Nestin-GFP−/NG2-DsRed+, cells, invade brain tumor mass. A: intracranial injection of allograft tumor cells and brain preparation for histology. Growing G26-H2 murine glioblastoma cells were injected into the brain of a Nestin-GFP/NG2-DsRed double-transgenic mouse. B: representative image of a Nestin-GFP/NG2-DsRed mouse brain coronal section showing margin of a G26-H2 murine brain tumor. Dashed line indicates separation between tumor and normal surrounding tissue. Nestin-GFP+/NG2-DsRed+ cells are shown invading the tumor; Nestin-GFP-/NG2-DsRed+ cells are present only in adjacent healthy tissue. Left: identical areas in the brain section: NG2-DsRed (red), Nestin-GFP+ (green), Hoechst (blue), bright-field, NG2-DsRed (red) and Nestin-GFP+ (green) merged, and all images merged. Right: NG2-DsRed (red), Nestin-GFP+ (green), and Hoechst (blue) merged. Regions in red, yellow, and white boxes show Nestin-GFP−/NG2-DsRed+ and Nestin-GFP+/NG2-DsRed+ in normal tissue and Nestin-GFP+/NG2-DsRed+ cells invading the tumor tissue, respectively; these areas are magnified in C. White arrows indicate Nestin-GFP+/NG2-DsRed+ cells in the tumor. C: magnification of NG2-DsRed+ cells in normal and tumor cells in B. Identical brain tissue areas are shown for different channels: NG2-DsRed (red); Nestin-GFP+ (green); Hoechst (blue); bright-field (BF); merged NG2-DsRed (red) and Nestin-GFP+ (green) (N/N); all images merged; and merged NG2-DsRed (red), Nestin-GFP+ (green), and Hoechst (blue) (N/N/H). In normal tissue, NG2-DsRed+ cells express Nestin-GFP transgene or not, while only NG2-DsRed+/Nestin-GFP+ cells are present in the tumor. D: percentage of Nestin-GFP−/NG2-DsRed+ and Nestin-GFP+/NG2-DsRed+ cells in normal brain or tumor 35 days after cell implantation (n = 3 preparations). Predominantly type-2 pericytes invade the tumor.
Only type-2 pericytes are recruited during tumor vessel formation.
As oligodendrocyte progenitors are restricted to the central nervous system (60), we implanted growing B16 melanoma cells hypodermically into 3-mo-old Nestin-GFP/NG2-DsRed double-transgenic mice to evaluate cell recruitment during tumor growth outside the central nervous system (3 × 105 cells/200 μl, n = 3). After 2 wk, the tumors were surgically removed with a good margin of normal surrounding tissue (Fig. 5A). Consistent with a previous report (17), we found two pericyte subpopulations on the abluminal surface of vessels in the normal skeletal muscle surrounding the tumor (Fig. 5, B and C). Endothelial cells forming capillary tubes are located near pericytes (86). In the walls of small vessels, we found both pericyte subtypes associated with endothelial cells marked with CD31 (Fig. 5, B and C).
Fig. 5.
Type-2 pericytes are recruited during tumor angiogenesis. A: protocol for subcutaneous allograft tumor growth. B16 melanoma cells are injected into Nestin-GFP/NG2-DsRed double-transgenic mice, and tumor plus normal adjacent healthy tissue is surgically removed 2 wk later. B: representative immunofluorescence image of a Nestin-GFP/NG2-DsRed mouse tumor section stained with anti-CD31 antibody illustrating margin of the melanoma tumor and surrounding normal tissue. Vertical dashed line indicates separation between tumor and normal skeletal muscle. The same tissue area is shown for different channels: NG2-DsRed (red), Nestin-GFP+ (green), CD31 (orange or blue), Hoechst (blue), and merged images. Regions selected in red, yellow, and white boxes show type-1 pericytes in normal tissue, type-2 pericytes in normal tissue, and type-2 pericytes associated with tumor blood vessels, respectively. These areas are magnified in C. Only type-2 pericytes are associated with CD31+ blood vessel endothelial cells in the tumor, while type-1 and type-2 pericytes associate with these cells in the normal skeletal muscle. Type-1 pericytes are present only in adjacent healthy tissue. C: magnification of pericytes in normal and tumor tissues in B. Identical tissue areas are shown for different channels: NG2-DsRed (red), Nestin-GFP+ (green), CD31 (orange), Hoechst (blue), bright-field, and all fluorescent merged images. Type-1 and type-2 pericytes are associated with vessels (CD31+) in the normal tissue, while only type-2 pericytes associate with tumor vessels. D: percentage of Nestin-GFP−/NG2-DsRed+ (type-1) and Nestin-GFP+/NG2-DsRed+ (type-2) pericytes in normal and cancer tissues 15 days after tumor implantation (n = 3 preparations). Only type-2 pericytes are recruited during tumor blood vessel formation.
A fraction of NG2-DsRed+ cells (56.7 ± 5.0%) expressed Nestin-GFP, while 43.3 ± 5.0% were Nestin-GFP−. In contrast, we only detected NG2-DsRed+/Nestin-GFP+ cells (99.7 ± 0.3% of NG2-DsRed+ intratumoral cells), which showed a significant increase in the tumor (P = 0.0001), surrounding CD31+ microvessels (Fig. 5D). Our results indicate that only endogenous type-2 pericytes (NG2-DsRed+/Nestin-GFP+) are angiogenic.
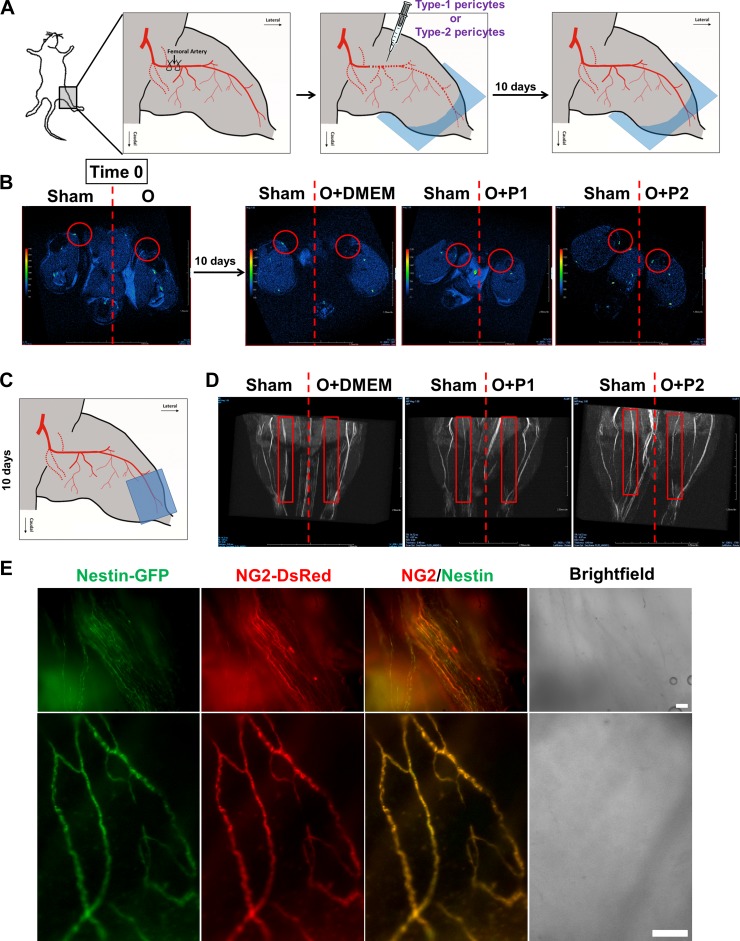
Type-2 pericytes improve blood flow in a mouse model of critical hindlimb ischemia.
Stimulation of angiogenesis can improve perfusion and function in ischemic tissues (81). Critical limb ischemia, a leading cause of nontraumatic amputation (61), is a condition of severe arterial obstruction in which blood flow to the legs and feet is not sufficient to maintain tissue viability. We used a well-established mouse model with unilateral hindlimb ischemia (30) to test whether pericyte subtypes can be used to promote angiogenesis. We transplanted type-1 β-actin-DsRed+ pericytes, type-2 β-actin-DsRed+ pericytes, or DMEM as a control into the ischemic leg of a nude mouse with unilateral hindlimb ischemia (Fig. 6A). After 10 days, we assessed limb perfusion using in vivo MRI angiography. We found that while type-1 pericytes did not recover femoral artery blood flow in the ischemic leg, type-2 pericytes induced partial recovery (Fig. 6, A–D) (the images are representative of 2 experiments). To test whether type-2 pericytes remain in the tissue, we analyzed muscles from these mice by fluorescence microscopy. We found that β-actin-DsRed+/Nestin-GFP+ type-2 pericytes formed the walls of newly formed blood vessels (Fig. 6E). These results indicate that type-2 pericytes can be used for vascular therapy.
Fig. 6.
Type-2 pericytes recover blood flow in a mouse model of hindlimb ischemia. A: hindlimb ischemia protocol in nude mice. Femoral artery is ligated and transected, type-1 or type-2 pericytes are intramuscularly injected into the ischemic leg, and limb perfusion is assessed with in vivo MRI angiography. B: high-resolution in vivo MRI. Angiography from mouse hindlimbs right after ligation of the femoral artery (time 0) and after 10 days of treatment. Red circles indicate femoral artery location. C: MRI angiography of hindlimbs below the knee 10 days after treatment. D: new, partially developed femoral artery in ischemic legs of mice injected with type-2 pericytes. Red rectangles indicate location of femoral arteries. E: DsRed and GFP fluorescence around vessels in whole muscles 10 days after type-2 pericyte transplantation. Scale bar, 100 μm. O, occlusion of femoral artery; P1, type-1 pericytes; P2, type-2 pericytes.
DISCUSSION
We propose that type-1 and type-2 pericytes in small capillaries and larger blood vessels vary in their angiogenic capacity. Only type-2 pericytes are angiogenic in vitro and in vivo and can be used in cell therapy to improve perfusion in ischemic tissues. In cancer, only type-2 pericytes from the surrounding normal tissue contribute to angiogenesis and can be a target for antiangiogenic therapy.
Pericytes are heterogeneous and differ in their regenerative capacity.
We propose that pericytes are multipotent, but their subtypes are oligopotent (18). They can behave like stem cells, but their potential in health and disease remains unknown (22). Despite their putative common identity, pericytes are a heterogeneous cell population and differ in their developmental origins. Their ontogeny indicates that those in the head derive from the neurectoderm, while those in other organs derive from the mesoderm (34). Pericytes from different anatomic locations differ morphologically, biochemically, and physiologically (6, 72), and different pericyte subsets occupy the periendothelial compartment (9).
Their multiplicity raises a question: Do pericyte subpopulations vary in function like stem cells do? Our previous work shows that pericyte subpopulations are oligopotent, differentially committed to specific lineages. Pericytes involved in repairing skeletal muscle differ from those that contribute to scar and fat formation. Our transplantation studies indicate that type-2 pericytes are myogenic (15), while type-1 pericytes do not form muscle but contribute to fat deposition in diseased skeletal muscle (15) and fibrous tissue deposition with aging (18).
Angiogenesis is the process of blood vessel assembly, beginning with cell clustering and ending with a vascular network. Angiogenic potential has been reported in pericytes (2, 30, 59, 79). They rapidly form neovasculature, which readily anastomoses with the host vasculature and significantly ameliorates hindlimb ischemia in a femoral artery ligation model (30). We show for the first time that only type-2 pericytes have angiogenic potential.
Blood vessels consist of endothelial and mural cells (32, 64), the latter of which refers to pericytes or smooth muscle cells (8). Pericytes' molecular markers can be down- or upregulated in various culture conditions, pathologies, and developmental states (9, 31, 32). Identification of pericytes in tissue sections relies on the combination of their anatomic location and specific markers such as the NG2 proteogylcan (39, 50, 55, 78). We distinguished two populations of pericytes in the skeletal muscle on the basis of Nestin-GFP expression (17); however, we do not know whether pericyte subtypes can interconvert. Two strategies, a pericyte subtype ablation in vivo and the discovery of additional specific markers, will address this question. We have shown that both pericyte subtypes can differentiate into smooth muscle cells in vitro (17). Whether pericytes differentiate into smooth muscle cells under specific conditions in vivo remains to be studied. Interestingly, we found that only Nestin-expressing (type-2) pericytes, which do not express neural markers, form neural progenitor cells (14, 16, 17).
The type-2 pericyte may be a cellular target for inhibiting tumor angiogenesis.
Cancer is characterized by excessive angiogenesis (24). Traditional antiangiogenic therapy aimed to inhibit as much as possible and to prune existing tumor vessels. The cellular targets of antiangiogenic drugs are normal host cells, such as pericytes or endothelial cells, which circumvents the acquired drug resistance when tumor cells are the target. Pericytes play an important role in stabilizing blood vessels in the microvasculature (58, 76) through cross talk with endothelial cells. Pericytes deposit matrix or releasing factors that can promote endothelial cell differentiation or quiescence (8). Tumor blood vessels associated intimately with pericytes are more functional and stable than those lacking the support of pericytes (25).
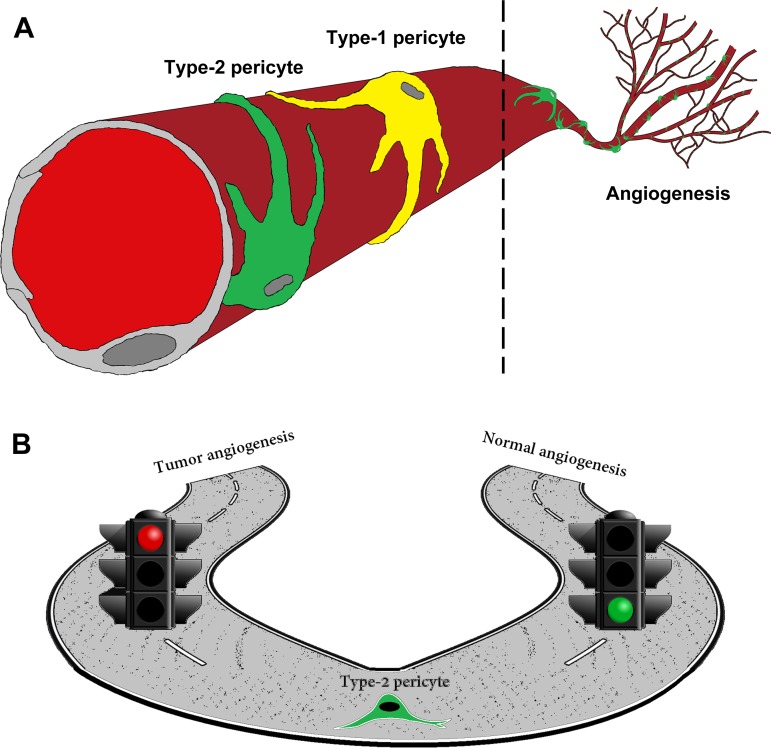
The combination of VEGF receptor and PDGFRβ inhibitors affects pericyte-mediated endothelial cell survival, resulting in regression of tumor blood vessels and inhibiting tumor growth (13). In parallel, the genetic depletion of pericytes using viral thymidine kinase slows primary tumor growth (26). However, this strategy can also increase invasiveness, possibly by increasing normal blood vessel leakage and reducing the barrier that tumor cells intravasate. Thus distinguishing tumor pericytes from normal pericytes could provide a specific cellular target for more efficient therapy. We found that only type-2 pericytes participate in new blood vessel formation during tumor angiogenesis (Fig. 7, A and B). Future work will analyze whether and how tumor type-2 pericytes differ from type-2 pericytes in normal vasculature. As pericytes are heterogeneous and subsets have different functions, targeting only the pericyte subpopulation involved in angiogenesis may be more efficient. Since antiangiogenic drugs are the leading therapy to arrest tumor growth, type-2 pericytes may provide a central cellular target susceptible to signaling and pharmacological manipulation.
Fig. 7.
Schematic representation of type-2 pericyte involvement in angiogenesis. A: type-1 (yellow) and type-2 (green) pericytes are associated with blood vessels. We propose that only type-2 pericytes are angiogenic. B: type-2 pericytes participate in angiogenesis associated with ischemic conditions and tumor progression.
Role of type-1 pericytes in tumor growth remains unclear.
Our results indicate that endogenous type-1 pericytes do not participate in tumor angiogenesis, but they do not exclude a role in tumor growth. In cancer, stromal cells may acquire a phenotype of activated fibroblasts (69). The signals that mediate the transition of normal cells into cancer-associated fibroblasts are not fully understood. Cancer-associated fibroblasts are commonly identified by their expression of α-smooth muscle actin (37, 67, 82), which pericytes express in culture (48). Phenotypic features of cancer-associated fibroblasts can be induced by transforming growth factor-β, which mediates fibroblast activation in organ fibrosis (51). Similar pathways may also be responsible for the emergence of cancer-associated fibroblasts in tumors (66). These cells produce an extracellular matrix rich in type I collagen, which is conducive to initiating tumor angiogenesis (20).
Recently, we showed that type-1 pericytes are fibrogenic and, when exposed to transforming growth factor-β, may differentiate into fibroblasts, which produce type I collagen (18). Additional studies are needed to determine whether type-1 pericytes can contribute to cancer-associated fibroblasts. However, using the Nestin-GFP/NG2-DsRed double-transgenic mice, we cannot track the fate of type-1 pericytes that change or lose their marker expression. We used NG2 and Nestin expression to distinguish type-1 pericytes (NG2+/Nestin−) in tissues, but we do not have a positive marker for them. As NG2 is expressed in type-1 and type-2 pericytes (15), we must explore the complete type-1 and type-2 pericyte transcriptome to find a specific marker for type-1 pericytes.
Type-2 pericytes and therapeutic angiogenesis.
Pathological changes in the vascular system, such as constriction and obstruction, may lead to ischemia and limb amputation. The goal of therapeutic angiogenesis is to treat ischemia by stimulating new blood vessel growth from existing vessels (35, 36, 44). Several cell types have been used to induce neovascularization. Of the available cell therapy approaches, endothelial progenitor cells are lineage-committed and grow slowly (47). By contrast, induced pluripotent stem cells exhibit high replicative capacity (80), but they have a tendency to lead to cancer.
Transplantion of mesenchymal stem cells (MSCs) induces neovascularization and improves blood flow to ischemic hindlimbs in animal models (40, 45, 74). The presence of MSCs in many adult tissues suggests a common origin. Some have proposed that MSCs are pericytes on the basis of shared markers in vivo and in vitro (22), and attention has turned to pericytes as stem cells with broad organ distribution (5, 28, 29, 52, 58, 70, 73). Consistently, pericytes have been proposed for angiogenic therapy on the basis of their role in forming and stabilizing engineered blood vessels (30, 42).
Because of their pivotal role in angiogenesis, pericytes represent a promising target for treatments designed to increase vascularization in ischemic diseases. Specific manipulation of type-2 pericytes, instead of the whole pericyte population, can preclude complications associated with excluding the benefits of type-1 functions. Future research should compare the angiogenic potency of various cell types: endothelial progenitor cells, embryonic stem cells, induced pluripotent stem cells, and MSCs. Our long-term studies will investigate the mechanisms underlying the angiogenic potential of type-2 pericytes and whether their ablation affects normal vascular function.
GRANTS
The present study was supported by a Wake Forest Pepper Center Pilot Project and PUSH grant from the Wake Forest Comprehensive Cancer Center (to O. Delbono and A. Mintz), National Institute on Aging Grants AG-13934 and AG-15820 (to O. Delbono) and P30-AG-21332 (to the Wake Forest Claude D. Pepper Older Americans Independence Center), and a Glenn/American Federation for Aging Research Scholarship for Research in the Biology of Aging (to A. Birbrair).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.B. and O.D. are responsible for conception and design of the research; A.B., T.Z., Z.-M.W., M.L.M., and J.D.O. performed the experiments; A.B. and O.D. analyzed the data; A.B., A.M., and O.D. interpreted the results of the experiments; A.B. and O.D. prepared the figures; A.B. and O.D. drafted the manuscript; A.B., A.M., and O.D. edited and revised the manuscript; O.D. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. G. N. Enikolopov (Cold Spring Harbor Laboratory) and Dr. W. Stallcup (Sanford-Burnham Medical Research Institute) for sharing with us the Nestin-GFP mouse and the rabbit anti-PDGFRβ antibody, respectively. Dr. James Wood (Wake Forest School of Medicine Comprehensive Cancer Center) contributed expertise on flow cytometry to our project.
REFERENCES
- 1.Abedin M, Tintut Y, Demer LL. Mesenchymal stem cells and the artery wall. Circ Res 95: 671–676, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Abramsson A, Berlin O, Papayan H, Paulin D, Shani M, Betsholtz C. Analysis of mural cell recruitment to tumor vessels. Circulation 105: 112–117, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8: 464–478, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Akhtar N, Dickerson EB, Auerbach R. The sponge/Matrigel angiogenesis assay. Angiogenesis 5: 75–80, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Alliot-Licht B, Hurtrel D, Gregoire M. Characterization of α-smooth muscle actin positive cells in mineralized human dental pulp cultures. Arch Oral Biol 46: 221–228, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs 169: 1–11, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Andreeva ER, Pugach IM, Gordon D, Orekhov AN. Continuous subendothelial network formed by pericyte-like cells in human vascular bed. Tissue Cell 30: 127–135, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res 97: 512–523, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21: 193–215, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Bagley RG, Weber W, Rouleau C, Teicher BA. Pericytes and endothelial precursor cells: cellular interactions and contributions to malignancy. Cancer Res 65: 9741–9750, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Baumann BC, Benci JL, Santoiemma PP, Chandrasekaran S, Hollander AB, Kao GD, Dorsey JF. An integrated method for reproducible and accurate image-guided stereotactic cranial irradiation of brain tumors using the small animal radiation research platform. Transl Oncol 5: 230–237, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumann BC, Dorsey JF, Benci JL, Joh DY, Kao GD. Stereotactic intracranial implantation and in vivo bioluminescent imaging of tumor xenografts in a mouse model system of glioblastoma multiforme. J Vis Exp 25: 4089, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest 111: 1287–1295, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O. Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle. PLos One 6: e16816, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev 22: 2298–2314, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Exp Cell Res 319: 45–63, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res 10: 67–84, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol 305: C1098–C1113, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bondjers C, He L, Takemoto M, Norlin J, Asker N, Hellstrom M, Lindahl P, Betsholtz C. Microarray analysis of blood microvessels from PDGF-B and PDGF-Rβ mutant mice identifies novel markers for brain pericytes. FASEB J 20: 1703–1705, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Brown LF, Guidi AJ, Schnitt SJ, Van De Water L, Iruela-Arispe ML, Yeo TK, Tognazzi K, Dvorak HF. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res 5: 1041–1056, 1999 [PubMed] [Google Scholar]
- 21.Bunnell TM, Burbach BJ, Shimizu Y, Ervasti JM. β-Actin specifically controls cell growth, migration, and the G-actin pool. Mol Biol Cell 22: 4047–4058, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caplan AI. All MSCs are pericytes? Cell Stem Cell 3: 229–230, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Cappellari O, Benedetti S, Innocenzi A, Tedesco FS, Moreno-Fortuny A, Ugarte G, Lampugnani MG, Messina G, Cossu G. Dll4 and PDGF-BB convert committed skeletal myoblasts to pericytes without erasing their myogenic memory. Dev Cell 24: 586–599, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 473: 298–307, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature 436: 193–200, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Cooke VG, LeBleu VS, Keskin D, Khan Z, O'Connell JT, Teng Y, Duncan MB, Xie L, Maeda G, Vong S, Sugimoto H, Rocha RM, Damascena A, Brentani RR, Kalluri R. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by Met signaling pathway. Cancer Cell 21: 66–81, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Covas DT, Piccinato CE, Orellana MD, Siufi JL, Silva WA, Jr, Proto-Siqueira R, Rizzatti EG, Neder L, Silva AR, Rocha V, Zago MA. Mesenchymal stem cells can be obtained from the human saphena vein. Exp Cell Res 309: 340–344, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008 [DOI] [PubMed] [Google Scholar]
- 29.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26: 2287–2299, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Dar A, Domev H, Ben-Yosef O, Tzukerman M, Zeevi-Levin N, Novak A, Germanguz I, Amit M, Itskovitz-Eldor J. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation 125: 87–99, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, Diaz-Flores L., Jr Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol 24: 909–969, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Dore-Duffy P, Cleary K. Morphology and properties of pericytes. Methods Mol Biol 686: 49–68, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8: 566–579, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 128: 1059–1068, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 438: 967–974, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Folkman J. Clinical applications of research on angiogenesis. N Engl J Med 333: 1757–1763, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 200: 500–503, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science 333: 238–242, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O'Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508: 55–60, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamano K, Li TS, Kobayashi T, Tanaka N, Kobayashi S, Matsuzaki M, Esato K. The induction of angiogenesis by the implantation of autologous bone marrow cells: a novel and simple therapeutic method. Surgery 130: 44–54, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 144: 646–674, 2011 [DOI] [PubMed] [Google Scholar]
- 42.He W, Nieponice A, Soletti L, Hong Y, Gharaibeh B, Crisan M, Usas A, Peault B, Huard J, Wagner WR, Vorp DA. Pericyte-based human tissue engineered vascular grafts. Biomaterials 31: 8235–8244, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126: 3047–3055, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Isner JM. Therapeutic angiogenesis: a new frontier for vascular therapy. Vasc Med 1: 79–87, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Iwase T, Nagaya N, Fujii T, Itoh T, Murakami S, Matsumoto T, Kangawa K, Kitamura S. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res 66: 543–551, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Johnstone CC, Farley A. The physiological basics of wound healing. Nursing Standard 19: 59–66, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA 97: 3422–3427, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katyshev V, Dore-Duffy P. Pericyte coculture models to study astrocyte, pericyte, and endothelial cell interactions. Methods Mol Biol 814: 467–481, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry 21: 6188–6193, 1982 [DOI] [PubMed] [Google Scholar]
- 50.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502: 637–643, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leask A, Abraham DJ. TGF-β signaling and the fibrotic response. FASEB J 18: 816–827, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev 17: 1053–1063, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindsey SH, Carver KA, Prossnitz ER, Chappell MC. Vasodilation in response to the GPR30 agonist G-1 is not different from estradiol in the mRen2.Lewis female rat. J Cardiovasc Pharmacol 57: 598–603, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in Nestin-GFP transgenic mice. J Comp Neurol 469: 311–324, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Mishra A, O'Farrell FM, Reynell C, Hamilton NB, Hall CN, Attwell D. Imaging pericytes and capillary diameter in brain slices and isolated retinae. Nat Protoc 9: 323–336, 2014 [DOI] [PubMed] [Google Scholar]
- 56.Montiel-Eulefi E, Nery AA, Rodrigues LC, Sanchez R, Romero F, Ulrich H. Neural differentiation of rat aorta pericyte cells. Cytometry A 81: 65–71, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Nakatsu MN, Sainson RC, Aoto JN, Taylor KL, Aitkenhead M, Perez-del-Pulgar S, Carpenter PM, Hughes CC. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and angiopoietin-1. Microvasc Res 66: 102–112, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Nehls V, Drenckhahn D. The versatility of microvascular pericytes: from mesenchyme to smooth muscle? Histochemistry 99: 1–12, 1993 [DOI] [PubMed] [Google Scholar]
- 59.Nielsen CM, Dymecki SM. Sonic hedgehog is required for vascular outgrowth in the hindbrain choroid plexus. Dev Biol 340: 430–437, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci 10: 9–22, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Novo S, Coppola G, Milio G. Critical limb ischemia: definition and natural history. Curr Drug Targets 4: 219–225, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn 222: 218–227, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Ozerdem U, Monosov E, Stallcup WB. NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvasc Res 63: 129–134, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem 54: 385–395, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Risau W. Mechanisms of angiogenesis. Nature 386: 671–674, 1997 [DOI] [PubMed] [Google Scholar]
- 66.Ronnov-Jessen L, Petersen OW. Induction of α-smooth muscle actin by transforming growth factor-β1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest 68: 696–707, 1993 [PubMed] [Google Scholar]
- 67.Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 76: 69–125, 1996 [DOI] [PubMed] [Google Scholar]
- 68.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131: 324–336, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Sappino AP, Skalli O, Jackson B, Schurch W, Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer 41: 707–712, 1988 [DOI] [PubMed] [Google Scholar]
- 70.Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, Peters H, Tang Z, Maxson R, Maas R. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet 24: 391–395, 2000 [DOI] [PubMed] [Google Scholar]
- 71.Schlingemann RO, Rietveld FJ, de Waal RM, Ferrone S, Ruiter DJ. Expression of the high molecular weight melanoma-associated antigen by pericytes during angiogenesis in tumors and in healing wounds. Am J Pathol 136: 1393–1405, 1990 [PMC free article] [PubMed] [Google Scholar]
- 72.Shepro D, Morel NM. Pericyte physiology. FASEB J 7: 1031–1038, 1993 [DOI] [PubMed] [Google Scholar]
- 73.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 18: 696–704, 2003 [DOI] [PubMed] [Google Scholar]
- 74.Shintani S, Murohara T, Ikeda H, Ueno T, Sasaki K, Duan J, Imaizumi T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation 103: 897–903, 2001 [DOI] [PubMed] [Google Scholar]
- 75.Sims DE. Diversity within pericytes. Clin Exp Pharmacol Physiol 27: 842–846, 2000 [DOI] [PubMed] [Google Scholar]
- 76.Sims DE. The pericyte—a review. Tissue Cell 18: 153–174, 1986 [DOI] [PubMed] [Google Scholar]
- 77.Sims DE. Recent advances in pericyte biology—implications for health and disease. Can J Cardiol 7: 431–443, 1991 [PubMed] [Google Scholar]
- 78.Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, Hepper I, Lauber K, Walzog B, Massberg S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and “instruct” them with pattern-recognition and motility programs. Nat Immunol 14: 41–51, 2013 [DOI] [PubMed] [Google Scholar]
- 79.Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood 116: 4720–4730, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676, 2006 [DOI] [PubMed] [Google Scholar]
- 81.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest 93: 662–670, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsukada T, McNutt MA, Ross R, Gown AM. HHF35, a muscle actin-specific monoclonal antibody. II. Reactivity in normal, reactive, and neoplastic human tissues. Am J Pathol 127: 389–402, 1987 [PMC free article] [PubMed] [Google Scholar]
- 83.Ugarte G, Cappellari O, Perani L, Pistocchi A, Cossu G. Noggin recruits mesoderm progenitors from the dorsal aorta to a skeletal myogenic fate. Dev Biol 365: 91–100, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vintersten K, Monetti C, Gertsenstein M, Zhang P, Laszlo L, Biechele S, Nagy A. Mouse in red: red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis 40: 241–246, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Wesseling P, Schlingemann RO, Rietveld FJ, Link M, Burger PC, Ruiter DJ. Early and extensive contribution of pericytes/vascular smooth muscle cells to microvascular proliferation in glioblastoma multiforme: an immuno-light and immuno-electron microscopic study. J Neuropathol Exp Neurol 54: 304–310, 1995 [DOI] [PubMed] [Google Scholar]
- 86.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci 14: 1398–1405, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang T, Birbrair A, Delbono O. Nonmyofilament-associated troponin T3 nuclear and nucleolar localization sequence and leucine zipper domain mediate muscle cell apoptosis. Cytoskeleton (Hoboken) 70: 134–147, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang T, Birbrair A, Wang ZM, Taylor J, Messi ML, Delbono O. Troponin T nuclear localization and its role in aging skeletal muscle. Age 35: 353–370, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 135: 145–157, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Zimmermann KW. Der feinere Bau der Blutkapillaren. Z Anat Entwicklungsgesch 68: 29–109, 1923 [Google Scholar]