Abstract
Cough is important for airway clearance, particularly if penetration/aspiration of foreign material occurs during swallow. Measures of voluntary cough production from ten male participants with stage II–III Parkinson’s disease (PD) who showed no videofluorographic evidence of penetration/aspiration (Group 1) were examined and compared with those of ten male participants with stage II–III PD who showed videofluorographic evidence of penetration/aspiration (Group 2). The degree of penetration/ aspiration was expertly judged from the videofluorographic examinations of the participants’ sequential swallow of a thin, 30-cc bolus. Measured cough parameters included inspiratory phase duration, inspiratory peak flow, compression phase duration, expiratory peak flow, expiratory rise time, and cough volume acceleration. Results indicated significant group differences for the majority of cough measures, except for inspiratory phase duration and inspiratory peak flow. A modest relationship existed between voluntary cough parameters and penetration/aspiration scores. Decreased ability to adequately clear material from the airway with voluntary cough may exacerbate symptoms resulting from penetration/aspiration, particularly for those with neurodegenerative disease. Measurement of voluntary cough may be useful for the evaluation of airway clearance ability.
Keywords: Voluntary cough, Airflow, Penetration, Parkinson’s disease, Deglutition, Deglutition disorders
Symptoms of Parkinson’s disease (PD) may include bradykinesia (slowness of movement), resting tremor, rigidity, postural abnormalities [1, 2], respiratory difficulty (including dysrhythmias), [1, 3], respiratory muscle weakness [1, 4], and/or laryngeal muscle abnormalities [1]. Dysphagia (swallowing dysfunction) can occur in the early stages of PD and aspiration pneumonia is reported as the leading cause of death in PD patients [5, 6]. Dominant symptoms of dysphagia include decreased oral transit time, poor bolus formation, a delay in the triggering of the pharyngeal phase of the swallow, prolonged opening of the upper esophageal sphincter, and residue in the valleculae and piriform sinuses [7, 8]. Robbins et al. [9] proposed that many individuals with PD present as “silent aspirators” with little awareness of their dysphagia symptoms and little or no cough response to aspiration.
Voluntary cough is a mechanism of airway clearance and its relationship to aspiration has been highlighted by Smith et al. [10]. They demonstrated that patients who experienced stroke and severely aspirate had a more impaired voluntary cough compared with nonaspirators who experienced a stroke. Cough protects the airway by generating expiratory airflows that create a “scrubbing” action, removing material from the airway [10-12]. To generate high linear airflow velocities during voluntary cough, three distinct phases are needed: an inspiratory phase (involving chest wall and laryngeal inspiratory muscles), a compression phase (involving laryngeal closure and abdominal expiratory muscles), and an expiratory phase (involving chest wall and abdominal expiratory muscles). The inspiratory phase, initiated by diaphragmatic muscle activity [12, 13] and posterior cricoarytenoid and cricothyroid muscle activation [12], allows for thoracic cavity expansion and inspiratory airflow [14]. Because of chest wall rigidity, those with PD may have a decreased ability to inflate the lungs, thus decreasing the potential to generate expiratory airflow for cough [12, 15]. Decreases in cough reflex sensitivity, in response to a citric acid challenge with advanced stages of PD, suggest that the progression of the disease may not only compromise the intensity of the cough but impair the reflex sensitivity that contributes to an even higher potential level of aspiration risk [5]. While it is recognized that a voluntary cough maneuver is distinct from a reflexive cough, recent research suggests that a cough elicited by sensory stimulation is not entirely reflexive in an awake human. Davenport et al. [16, 17] demonstrated that cough, in response to a sensory stimulus, has two components, the first being the sensation of the stimulus (or Urge-to-Cough) and the second being the motor cough action. Of importance was the observation that with every cough response, individuals first have an Urge-to-Cough before the motor event. This consistent pattern, observed across all the participants in the above-mentioned studies, supports the hypothesis [16, 17] that in conscious humans there is a cognitive component to reflexive cough behavior.
To date, no study has examined the voluntary cough produced by persons with PD as it relates to the degree of penetration/aspiration defined from videofluorographic examination. It was hypothesized that the voluntary cough airflow patterns in those with PD with known penetration/ aspiration would be significantly more impaired than those with no evidence of penetration/aspiration. It was further predicted that there would be increased duration of the inspiratory and expiratory phases of cough and the laryngeal compression time and decreased peak expiratory flows and decreased cough volume acceleration in the group with penetration/aspiration into the larynx. In addition, it was predicted that the level of penetration/aspiration would positively relate to the voluntary cough flow parameters.
Methods
Group 1 included ten male participants with PD (average age = 67.6 years) who showed no visual evidence of penetration/aspiration into the laryngeal vestibule during a thin, 30-cc bolus, sequential swallow task. Group 2 included ten male participants with PD (average age = 72.9 years) who had, at a minimum, penetration into the laryngeal vestibule during a sequential swallow task of 30 cc of thin liquid. A qualified speech language pathologist judged the degree of penetration/aspiration from the videofluorographic examination of the sequential swallow task. The judge had over 20 years of experience in dysphagia. Scores indicating the level of penetration/aspiration were given using the Penetration/Aspiration Scale [22]. All of the videofluorographic exams were obtained using the Digital Swallow Station (Model 7200; Kay Elemetrics, Lincoln Park, NJ).
A certified movement disorders neurologist, affiliated with the University of Florida Movement Disorders Center, made the diagnosis of PD. All participants were staged at Hoehn and Yahr II and III (with one participant in the Group 1 at stage IV) (see Table 1). All participants were able to follow 3–4-step directions and none had a history of heart and pulmonary disease, stroke, tobacco use within the last five years, and cognitive deficits, including a diagnosis of dementia as determined from a neuropsychological battery of tests. Review of the participants’ medical charts revealed that all were on standard medications for treatment of the PD, and none were taking any prescription drugs that may have had potential influence on cough production, such as codeine. All videofluorographic examinations and cough productions were sampled in a medication “on” phase. Medication “on” was defined as 60 min after the ingestion of the medications. The Institutional Review Board at the University of Florida approved the study.
Table 1.
Participant demographics
| Participant | Group | Sex | Age | H & Y | P/A |
|---|---|---|---|---|---|
| 1 | 1 | M | 58 | 3 | 1 |
| 2 | 1 | M | 68 | 3 | 1 |
| 3 | 1 | M | 61 | 2 | 1 |
| 4 | 1 | M | 49 | 2.5 | 1 |
| 5 | 1 | M | 76 | 4 | 1 |
| 6 | 1 | M | 73 | 2.5 | 1 |
| 7 | 1 | M | 64 | 2.5 | 1 |
| 8 | 1 | M | 72 | 2.5 | 1 |
| 9 | 1 | M | 77 | 2 | 1 |
| 10 | 1 | M | 78 | 2.5 | 1 |
| 11 | 2 | M | 72 | 3 | 5 |
| 12 | 2 | M | 77 | 2.5 | 5 |
| 13 | 2 | M | 72 | 3 | 3 |
| 14 | 2 | M | 74 | 3 | 5 |
| 15 | 2 | M | 78 | 2.5 | 8 |
| 16 | 2 | M | 70 | 3 | 3 |
| 17 | 2 | M | 77 | 3 | 2 |
| 18 | 2 | M | 82 | 3 | 7 |
| 19 | 2 | M | 67 | 3 | 3 |
| 20 | 2 | M | 60 | 3 | 5 |
N = 10 in each group. Group 1 consisted of individuals judged to have no videofluorographic evidence of penetration/aspiration and Group 2 consisted of individuals judged to have videofluorographic evidence of penetration/aspiration
H & Y = Hoehn and Yahr score; P/A = Penetration/Aspiration score
Voluntary Cough Measures
Airflow produced during voluntary cough production was sampled using an oral pneumotachograph (MLT 1000, ADInstruments, Inc.) connected to a spirometer (ML141, ADInstruments, Inc.). A nose clip occluded nasal airflow. The airflow signal was measured and digitized at 1 kHz and displayed using Chart version 5 for Windows (Fig. 1).
Fig. 1.
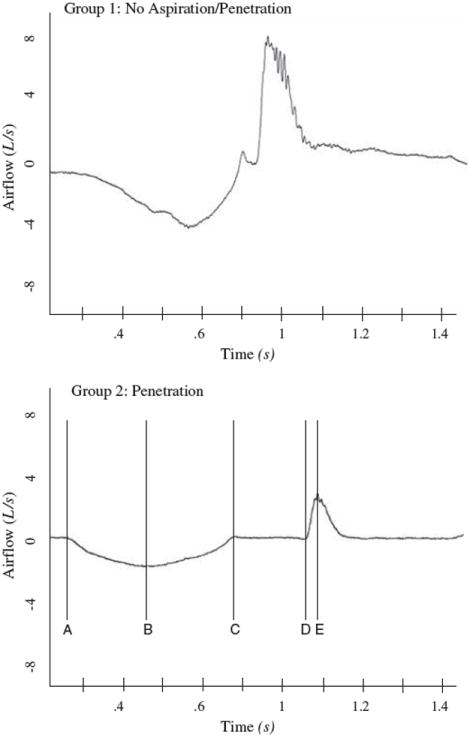
Examples of airflow waveforms during a voluntary cough task from Group 1 and Group 2. The vertical lines within the Group 2 waveform denote the phases of the cough waveform: A to C, inspiratory phase; C to D, compression phase; D to E, expiratory rise time
For each participant the production of voluntary cough followed a minimum of three cycles of tidal volume breathing. Participants were instructed to take a deep breath and cough hard into the pneumotachograph tube. The investigator visually ensured the lip seal around the pneumotachograph tube during the cough trials, and a nose clip was used to ensure no air leak. Three voluntary cough trials were produced. All three trials were analyzed for the purpose of this study.
The following measures were made from the cough flow waveform (Fig. 1):
Inspiratory phase duration (IPD) – defined as the onset of inspiration following tidal volume breathing to the end of inspiration before the compression phase (C to D) of cough (A to C)
Inspiratory peak flow (IPPF) – defined as the peak inspiratory flow during the inspiratory phase of the cough (B)
Compression phase duration (CPD) – defined as the time from the end of the inspiratory phase to the beginning of the expiratory phase (C to D)
Expiratory rise time (EPRT) – defined as time from the beginning of the expiratory phase to the peak expiratory flow (D to E)
Expiratory peak airflow (EP) – defined as the peak airflow during the expiratory phase of the cough (E)
Cough volume acceleration (CVA) – defined as the expiratory peak flow/expiratory phase rise time.
Means and standard deviations were calculated from the three trials of the cough productions. Intrameasurer reliability was calculated on 50% of the data set for the assessment of penetration/aspiration and 20% of the data set for the measures of the voluntary cough parameters. Group differences for each of the cough parameters were tested using a one-way analysis of variance with a between-subject factor of group (1 vs. 2). Spearman rho correlations were used to determine if the penetration/aspiration scale scores were related to the dependent variables extracted from the voluntary cough productions. Significance was set at p = 0.05 for all statistical testing.
Results
Intrameasurer reliability for the penetration/aspiration scores and the measurements made from the cough flow signals were measured using intraclass correlation coefficients (ICC). The ratings were significant for reliability for the penetration/aspiration scores (F = 4.400; p= 0.001) and the measurements from the cough flow signals (F = 1297.772; p < 0.001).
Group differences were evident for particular parameters of the cough waveform. Means and standard deviations are given in Table 2. Group 2 produced longer CPD (F = 35.872; p < 0.001), slower EPRT (F = 35.223; p < 0.001), decreased EP (F = 43.559; p < 0.001), and decreased CVA (F = 63.651; p < 0.001). No significant group differences were found for IPD (F = 2.329; p = 0.132) or IPPF (F = 3.073; p = 0.085). Figure 1 demonstrates the cough airflow patterns for the two groups. Results of the Spearman correlations showed modest but significant relationships between IPPF (r = −0.470), CPD (r = 0.593), EPRT (r = 0.744), EP (r = −0.669), and CVA (r = −0.808) relative to the penetration/aspiration scores.
Table 2.
Means and standard deviations calculated from the three trials for each participant
| Part | IPD | IPPF | EP | EPRT | CPD | CVA |
|---|---|---|---|---|---|---|
| 1 | 0.936 (0.035) | 3.071 (0.641) | 9.371 (0.893) | 0.249 (0.045) | 0.162 (0.068) | 38.110 (3.537) |
| 2 | 0.851 (0.221) | 6.761 (0.389) | 10.551 (0.475) | 0.165 (0.019) | 0.120 (0.013) | 35.685 (4.957) |
| 3 | 0.852 (0.028) | 5.483 (0.347) | 11.331 (0.726) | 0.325 (0.062) | 0.278 (0.062 | 35.685 (7.405) |
| 4 | 1.233 (0.155) | 4.977 (0.512) | 7.920 (0.306) | 0.323 (0.033) | 0.269 (0.068) | 24.759 (3.252) |
| 5 | 1.176 (0.078) | 2.240 (0.181) | 5.420 (0.338) | 0.257 (0.098) | 0.217 (0.094) | 23.357 (9.337) |
| 6 | 0.841 (0.072) | 4.616 (0.199) | 7.436 (0.309) | 0.150 (0.020) | 0.111 (0.030) | 50.259 (9.277) |
| 7 | 1.257 (0.026) | 3.195 (0.087) | 9.146 (1.813) | 0.273 (0.065) | 0.212 (0.055) | 33.978 (5.354) |
| 8 | 0.913 (0.311) | 4.343 (0.435) | 8.015 (0.286) | 0.141 (0.019) | 0.088 (0.019) | 57.732 (9.888) |
| 9 | 0.459 (0.046) | 7.754 (0.615) | 9.227 (0.367) | 0.121 (0.020) | 0.084 (0.020) | 77.315 (11.545) |
| 10 | 0.520 (0.014) | 1.360 (6.344) | 10.961 (0.679) | 0.145 (0.013) | 0.096 (0.021) | 75.531 (1.892) |
| 11 | 0.906 (0.145) | 4.297 (0.633) | 6.663 (1.892) | 0.557 (0.366) | 0.516 (0.357) | 16.570 (10.563) |
| 12 | 1.377 (0.613) | 3.129 (1.284) | 5.975 (0.127) | 0.518 (0.140) | 0.372 (0.122) | 12.068 (2.933) |
| 13 | 0.929 (0.111) | 4.989 (0.088) | 6.609 (0.807) | 0.319 (0.135) | 0.262 (0.126) | 22.730 (7.725) |
| 14 | 1.338 (0.354) | 3.029 (0.692) | 4.967 (1.463) | 0.370 (0.140) | 0.316 (0.137) | 13.873 (2.027) |
| 15 | 0.574 (0.076) | 3.741 (0.848) | 6.381 (1.912) | 0.275 (0.068) | 0.245 (0.068) | 24.851 (9.756) |
| 16 | 1.532 (0.069) | 1.806 (0.176) | 6.358 (1.610) | 0.414 (0.028) | 0.362 (0.017) | 15.250 (3.097) |
| 17 | 0.697 (0.062) | 4.968 (0.226) | 4.720 (0.081) | 0.576 (0.081) | 0.528 (0.079) | 8.162 (1.011) |
| 18 | 0.993 (0.134) | 3.077 (0.078) | 6.000 (0.236) | 0.319 (0.065) | 0.272 (0.072) | 19.397 (4.166) |
| 19 | 0.899 (0.134) | 2.898 (0.726) | 6.594 (1.868) | 0.379 (0.061) | 0.358 (0.057) | 16.954 (6.163) |
| 20 | 1.067 (0.141) | 2.900 (1.024) | 7.470 (0.559) | 0.399 (0.167) | 0.335 (0.165) | 20.337 (5.803) |
Values are mean (standard deviation)
Group 1 = participants 1–10; Group 2 = participants 11–20
IPD = inspiratory phase duration; IPPF = inspiratory peak flow; CPD = compression phase duration; EPRT = expiratory rise time; EP = expiratory peak airflow; CVA = cough volume acceleration
Discussion
In patient groups, reduced peak flows during voluntary cough are considered to be indicative of increased risk of respiratory complications [5, 10, 18]. The results from this study indicated that those with PD, with known swallow dysfunction as defined by degree of penetration/aspiration during a sequential swallow, have impaired voluntary cough. This was shown by significant differences in measures of cough parameters made from an airflow signal elicited during voluntary cough. Significant group differences existed for CPD, EPRT, EP, and CVA. Moreover, there were modest correlations between penetration and aspiration scores and voluntary cough airflow parameters, suggesting that the ability to cough voluntarily is related to the degree of airway protection.
The penetration/aspiration group produced significantly longer CPD and EPRT; the IPD was also increased, although not significantly. A longer CPD and ERPT indicate a general slowing down of the cough-related events, diminishing the final ballistic action of the cough. Reduced EP in the penetrator/aspirator group represented this. Group 2’s significantly weaker EP reduces the production of “shearing forces,” thus decreasing the ability to adequately clear material from the airway and creating greater risk for penetration and/or aspiration. Bach [19] found in healthy male subjects that expiratory peak airflow during a maximal voluntary cough ranged from 8.3 to 11.6 L/s. When compared with the results from our study, six of ten participants in Group 1 fell within this range, but within Group 2 none of the participants fell within the range. The longer CPD found for Group 2 could have been compensation for reduced motor drive to the expiratory muscles necessary to aid the buildup of subglottal pressure for the cough maneuver. This is speculative but could be verified by the additional measure of abdominal EMG. It is known that subcortical lesions, like that in PD, could be interrupting the cortical pathways necessary to facilitate a coordinated cough response [23]. Braak et al. [20] found lesions, beginning in PD stage I, in the dorsal motor group (within the medulla oblongata) that controls the glossopharyngeal and vagus nerves which allow for the activation of swallowing and the laryngeal activation for cough. The locations of lesions on sites that control both swallow and a portion of cough may be a factor that dictates changes to both the swallow and cough function in PD.
Continued studies of voluntary cough and reflexive cough in PD patients may be helpful in elucidating further the neural control mechanisms of cough and swallow as well as their relationship. For now, this current study suggests that alterations in voluntary cough may be an indicator of risk for penetration/aspiration, particularly in patient populations with known swallow dysfunction. The utility of voluntary cough as a screening tool for penetration and/or aspiration has interesting potential, but further supposition is limited by the small sample size of this study.
Summary
Dysphagia is a symptom of many neurologic conditions, (i.e., stroke, traumatic brain injury, motor neuron disease, pseudobulbar palsy, multiple sclerosis) [21]. This study suggests that for some with PD, the ability to produce an effective voluntary cough is impaired. This study revealed differences in cough airflow parameters between those with PD who had videofluorographic evidence of penetration/ aspiration and those with PD who did not demonstrate penetration/aspiration.
Acknowledgments
The authors extend their deep appreciation to the patients with PD who participated in this project and to Drs. Okun, Fernandez, and Rodriguez of the University of Florida Movement Disorders Center for their help with participant recruitment.
Contributor Information
Teresa Pitts, Email: tepitts@gmail.com, Department of Communication Sciences and Disorders, University of Florida, P.O. Box 117420, Gainesville, Florida 32610, USA.
Donald Bolser, Department of Physiological Sciences, University of Florida, P.O. Box 100144, Gainesville 32610, USA.
John Rosenbek, Department of Communicative Sciences, University of Florida, P.O. Box 100147, Gainesville, Florida 32610, USA.
Michelle Troche, Department of Communication Sciences and Disorders, University of Florida, P.O. Box 117420, Gainesville, Florida 32610, USA.
Christine Sapienza, Department of Communication Sciences and Disorders, University of Florida, P.O. Box 117420, Gainesville, Florida 32610, USA.
References
- 1.Fontana GA, Pantaleo T, Lavorini F, Benvenuti F, Gangemi S. Defective motor control of coughing in Parkinson’s disease. Am J Respir Crit Care Med. 1998;158:458–464. doi: 10.1164/ajrccm.158.2.9705094. [DOI] [PubMed] [Google Scholar]
- 2.Marsden CD. Problems with long-term levodopa therapy for Parkinson’s disease. Clin Neuropharmacol. 1994;17(Suppl 2):S32–S44. [PubMed] [Google Scholar]
- 3.De Keyser J, Vincken W. L-dopa-induced respiratory disturbance in Parkinson’s disease suppressed by tiapride. Neurology. 1985;35:235–237. doi: 10.1212/wnl.35.2.235. [DOI] [PubMed] [Google Scholar]
- 4.de Bruin PF, de Bruin VM, Lees AJ, Pride NB. Effects of treatment on airway dynamics and respiratory muscle strength in Parkinson’s disease. Am Rev Respir Dis. 1993;148:1576–1580. doi: 10.1164/ajrccm/148.6_Pt_1.1576. [DOI] [PubMed] [Google Scholar]
- 5.Ebihara S, Saito H, Kanda A, Nakajoh M, Takahashi H, Arai H, Sasaki H. Impaired efficacy of cough in patients with Parkinson disease. Chest. 2003;124:1009–1015. doi: 10.1378/chest.124.3.1009. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima K, Maeda M, Tabata M, Adachi Y, Kusumi M, Ohshiro H. Prognosis of Parkinson’s disease in Japan. Tottori University Parkinson’s Disease Epidemiology (TUPDE) Study Group. Eur Neurol. 1997;38(Suppl 2):60–63. doi: 10.1159/000113485. [DOI] [PubMed] [Google Scholar]
- 7.Miller N, Noble E, Jones D, Burn D. Hard to swallow: dysphagia in Parkinson’s disease. Age Ageing. 2006;35:614–618. doi: 10.1093/ageing/afl105. [DOI] [PubMed] [Google Scholar]
- 8.Potulska A, Friedman A, Krolicki L, Spychala A. Swallowing disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2003;9:349–353. doi: 10.1016/s1353-8020(03)00045-2. [DOI] [PubMed] [Google Scholar]
- 9.Robbins JA, Logemann JA, Kirshner HS. Swallowing and speech production in Parkinson’s disease. Ann Neurol. 1986;19:283–287. doi: 10.1002/ana.410190310. [DOI] [PubMed] [Google Scholar]
- 10.Smith Hammond CA, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56:502–506. doi: 10.1212/wnl.56.4.502. [DOI] [PubMed] [Google Scholar]
- 11.Bolser DC, Davenport PW. Functional organization of the central cough generation mechanism. Pulm Pharmacol Ther. 2002;15:221–225. doi: 10.1006/pupt.2002.0361. [DOI] [PubMed] [Google Scholar]
- 12.Fontana GA, Lavorini F. Cough motor mechanisms. Respir Physiol Neurobiol. 2006;152:266–281. doi: 10.1016/j.resp.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Tomori Z, Widdicombe JG. Muscular, bronchomotor and cardiovascular reflexes elicited by mechanical stimulation of the respiratory tract. J Physiol. 1969;200:25–49. doi: 10.1113/jphysiol.1969.sp008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macklem PT. Relationship between lung mechanics and ventilation distribution. Physiologist. 1973;16:580–588. [PubMed] [Google Scholar]
- 15.Bach JR. Mechanical insufflation-exsufflation. Comparison of peak expiratory flows with manually assisted and unassisted coughing techniques. Chest. 1993;104:1553–1562. doi: 10.1378/chest.104.5.1553. [DOI] [PubMed] [Google Scholar]
- 16.Davenport PW, Sapienza CM, Bolser DC. Psychophysical assessment of the urge-to-cough. Eur Respir Rev. 2002;12:1–5. [Google Scholar]
- 17.Davenport PW, Bolser DC, Vickroy T, Berry RB, Martin AD, Hey JA, Danzig M. The effect of codeine on the Urge-to-Cough response to inhaled capsaicin. Pulm Pharmacol Ther. 2007;20:338–346. doi: 10.1016/j.pupt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Addington WR, Stephens RE, Gilliland KA. Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke: an interhospital comparison. Stroke. 2999;30:1203–1207. doi: 10.1161/01.str.30.6.1203. [DOI] [PubMed] [Google Scholar]
- 19.Bach JR, Goncalves MR, Paez S, Winck JC, Leitao S, Abreu P. Expiratory flow maneuvers in patients with neuromuscular diseases. Am J Phys Med Rehabil. 2006;85:105–111. doi: 10.1097/01.phm.0000197307.32537.40. [DOI] [PubMed] [Google Scholar]
- 20.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 21.Buchholz DW. Neurogenic dysphagia: what is the cause when the cause is not obvious? Dysphagia. 1994;9:245–255. doi: 10.1007/BF00301918. [DOI] [PubMed] [Google Scholar]


