Abstract
Background
The aetiology of isolated clefts of the lip and/or palate remains obscure. Unaffected family members are treated as if their genetic risks are equivalent and low. Given the number of genes associated with both clefting and dental anomalies, the hypothesis that such anomalies contribute to the cleft phenotype should be explored.
Aim
To describe the dental characteristics of parents of children with non-syndromic cleft lip ± palate.
Design
Unaffected parents of Australian children with a cleft of the lip ± palate underwent dental examination including radiographs, photographs, and impressions. Dental anomalies were identified.
Results
Data were available on 101 parents (49 males, 52 females). Fifty-one participants had at least one dental anomaly. Twelve (11.8%) individuals had congenital absence of teeth, with seven missing multiple teeth. The tooth most commonly missing was the upper right lateral incisor. Five subjects (4.9%) had microdontia (upper lateral incisor most commonly affected). Four subjects (4.0%) had supernumerary teeth. Enamel defects were present in 27 (26.7%) cases with the incisors (46.8%) followed by premolars (24.2%) most affected.
Conclusions
This study supports previous work suggesting that ‘unaffected’ parents of children with clefts of the lip ± palate may present with dental anomalies.
Introduction
Clefts of the lip and/or palate are common structural birth defects occurring in 1 of 500–1000 live births worldwide1. They represent a significant burden to the affected individual, their family and health service providers. Despite this, the precise aetiology of CL/P remains unclear and both genetic and environmental factors are thought to play major roles2. Although some clefts occur as part of well-recognised syndromes with known gene mutations, the aetiology of the majority, described as isolated or non-syndromic (NS) clefts, is unclear. This lack of clarity combined with all unaffected family members essentially being treated as though their genetic risk is equivalent is particularly frustrating for individuals living with a cleft when considering the implications on future pregnancies and family planning. Although there are undoubtedly complex multifactorial gene–environment interactions associated with clefting, there is also great heterogeneity in the cleft phenotype itself, making it difficult to predict risk. To improve our understanding of the key aetiological factors underlying orofacial clefting, it is important to define reliable phenotypic characteristics in apparently unaffected family members of cleft patients who might potentially be harbouring genetic variants associated with an increased clefting risk3,4, thus improving both genetic studies to find new genes and genetic counselling.
The cleft phenotype is typically defined as a binary trait (i.e., either ‘affected’ or ‘unaffected’) encompassing various degrees of severity, ranging from a mild incomplete cleft of the lip to a complete bilateral cleft of the primary and secondary palate. The cleft phenotype, however, appears to be much more complex and characterised by a number of subclinical features4. These can be associated with skeletal, dental, and soft tissue abnormalities, all of which may represent an incomplete manifestation (i.e., forme fruste) of the clefting process. Examples of these markers include micro (subclinical)-defects of the orbicularis oris muscle5, the presence of dental anomalies6 and variations in face shape7. If these are included as part of the overall cleft phenotype, a previously apparently sporadic case of NS cleft might appear to segregate like a Mendelian trait2. Identifying unaffected individuals within cleft families who might harbour these subclinical phenotypes will lead to improved risk estimates and better understanding of the pathogenesis and treatment of NS clefting.
Dental anomalies are one such phenotypic marker. Teeth and lip/palate development have a close embryological relationship both in terms of timing and anatomical position. Furthermore, there is evidence of a shared genetic aetiology between dental anomalies and clefts. For clefting with hypodontia, at least four candidate genes show positive associations: IRF6, MSX1, PAX9, and TGFB38. These results suggest that the presence of dental anomalies may represent an additional subclinical marker for orofacial clefts. Individuals with clefts of the lip and/or palate have been reported to have an increased number of dental anomalies6,9–12. The anomalies most commonly described include tooth agenesis or hypodontia11, extra or supernumerary teeth10, enamel defects13,14, and microdontia15,16. The rationale is that if individuals with a cleft have an increased prevalence of dental anomalies, it is possible that their unaffected relatives may be similarly affected. Studies to date looking at unaffected family members have shown inconsistent results17–21.
The aim of this study is to further delineate the expanded dental cleft phenotype by describing the dental characteristics of a cohort of unaffected parents of Australian children born with an isolated (non-syndromic) cleft lip and palate. This study forms part of a larger genotype–phenotype correlation study (OzCleft) aimed at exploring the aetiology of orofacial clefts.
Methods
Approval for the OzCleft study was obtained from the Human Research Ethics Committee at the Royal Children’s Hospital (No 28132). Children with isolated clefts of the lip and palate were identified from a number of sources, including the registry of cleft patients held by the Melbourne Cleft Service based at the Royal Children’s Hospital Melbourne, CleftPALs (the cleft family support group) and the offices of individual cleft surgeons in the state of Victoria. Potentially eligible families were sent an invitation letter to participate and a ‘consent to contact’ form which they returned to the OzCleft coordinator. Phone contact was then made with each family, and a preliminary screening completed to ensure eligibility for participation. To be eligible to participate, the family had to have at least one child with a cleft of the lip and palate, no confounding medical conditions, at least one ‘unaffected’ sibling over the age of 5 years and both biological parents available. Families with children with cleft palate only (CPO) or any syndromic form of clefting were excluded as were those who had no siblings. Once assessed as eligible, the family were scheduled to attend a data collection clinic as a group on a Saturday morning. At the clinic, comprehensive data were collected across a range of clinical domains including oral health and dental development, lip ultrasounds, 3D facial imaging, plain photography, and speech evaluations. A comprehensive genetic assessment was completed by a clinical geneticist, and saliva collected for future genotyping and SNP analysis. This article contains preliminary details of the dental phenotype only; analysis of other clinical domains is ongoing.
Dental evaluation
Each participant underwent a comprehensive dental examination by a paediatric dentist or a dental therapist. This examination was carried out in the dental clinic using a dental light, mirror, probe, and air if needed. The examination included a full dental charting of the teeth present which were recorded on standardised data collection forms. Teeth were classified as sound, carious, or restored. Dental anomalies were recorded and classified as described in Table 1. An Orthopantomogram (OPG) radiograph was taken as well as alginate impressions of the upper and lower dental arches. Study models were constructed for later analysis. Intraoral photographs were taken using a Nikon D90 camera. Standardised views included teeth in occlusion, upper arch, lower arch, and buccal views. The images were saved for later analysis in conjunction with the radiographs and study models. Data on each participant were collated by one dentist (AA) who applied the criteria defined in Table 1 to complete the final dental phenotype data collection form.
Table 1.
Summary of the defining criteria for each dental anomaly recorded.
| Anomaly | Description |
|---|---|
| Missing tooth | The tooth is not visible on clinical examination, photographs, or study models. Confirmed absent on the OPG |
| Congenital | There is no record of previous extractions for orthodontic or other dental purposes (e.g., caries) |
| Extraction | If one premolar is missing in a quadrant and it is not stated as being extracted for orthodontic purposes, it is recorded as a congenitally absent second premolar |
| Other | If the subjects report previous dental extractions for trauma or caries, then a tooth is marked as missing for dental purposes. If premolars were extracted for orthodontic purposes, the missing teeth are recorded as the first premolars |
| If photographs, study models, and OPG reveal a missing tooth with obvious spacing, with tilting or rotation of other teeth into the space but no record of a previous extraction or trauma, then the missing tooth is marked as other as it was presumed (but not confirmed) to have been extracted | |
| Supernumerary tooth | An extra tooth is visible on clinical or radiographic examination. Confirmed by study models and photographs |
| Microdontia | The tooth appears smaller than normal for tooth type. Visible clinically and confirmed by examination of study models, photographs, and the OPG |
| Impacted | Tooth is not expected to erupt completely into its normal functional position. Sometimes visible clinically, confirmed by the OPG as being present and not fully erupted |
| Transposed | Positional interchange of two adjacent teeth, or the development or eruption of a tooth in a position normally occupied by a non-adjacent tooth. Visible clinically confirmed by photographs, study models, and OPG |
| Enamel Defects | Defects of the enamel visible clinically confirmed by photographs and in the case of hypoplasia also confirmed by study models and OPG. Enamel defects are not defined by the aetiology |
| Hypomineralisation | Appearance of brown, white, mottled enamel affecting any surface of the tooth, with no loss of tooth structure. Visible clinically and confirmed by examination of photographs |
| Hypoplasia | Loss of tooth structure, with the appearance of grooved/pitted uneven enamel surface. Visible clinically also confirmed by photographs, study models, and radiographs |
| Discolouration | Intrinsic staining of the tooth either generalised or chronological. Visible clinically and confirmed by dental photographs |
Results
One hundred and five parents were recruited, of whom four were excluded because they themselves had an overt cleft, leaving data on 101 parents available for analysis. Forty-nine were male (mean age 44.4 ± 5.9 years) and fifty two were female (mean age 42.3 ± 6.0 years). The majority of the subjects identified themselves as being of Australian descent (69.3%), followed by North-West European (9.9%) and South-East Asian (8.9%). Table 2 summarises the sample population by gender, age, and ethnicity. Among the families, 14 probands had just a cleft of the lip and the remaining 44 all had clefts of the lip and palate.
Table 2.
Demographics of the Australian sample population.
| Female (n = 52) | Male (n = 49) | |
|---|---|---|
| Mean age (years) | 42.3 ± 6.0 | 44.4 ± 5.9 |
| Ethnicity | ||
| Australian | 39 | 31 |
| North-West European | 3 | 7 |
| South-East Asian | 5 | 4 |
| Middle Eastern | 2 | 2 |
| North American/Canadian | 1 | 1 |
| Jewish | 0 | 1 |
Alterations in tooth number
Sixty-nine subjects (68.3%) were missing at least one permanent tooth (excluding third molars). Congenitally absent teeth were confirmed in 12 subjects (11.8%), of whom two-thirds (eight individuals) were female. The remaining missing teeth were identified either as ‘known extractions’ (in 46 individuals) or, if it was not clear whether a tooth had been extracted or was congenitally absent, they were recorded as ‘other’ (in 26 individuals). Of the 69 subjects with missing teeth, some had both congenital missing and extracted teeth and some also had teeth missing for ‘other’ reasons, explaining why the total numbers exceed 69. Of the 12 subjects with confirmed congenitally missing teeth, 7 (58.3%) had multiple congenitally missing teeth. The tooth most commonly missing was the upper right lateral incisor (tooth 12) followed by the lower left second premolar (tooth 35).
Four subjects (4.0%) had supernumerary teeth, all of whom were male. The most common area for supernumerary teeth was the maxillary arch in the upper lateral incisor region.
Alterations in tooth morphology
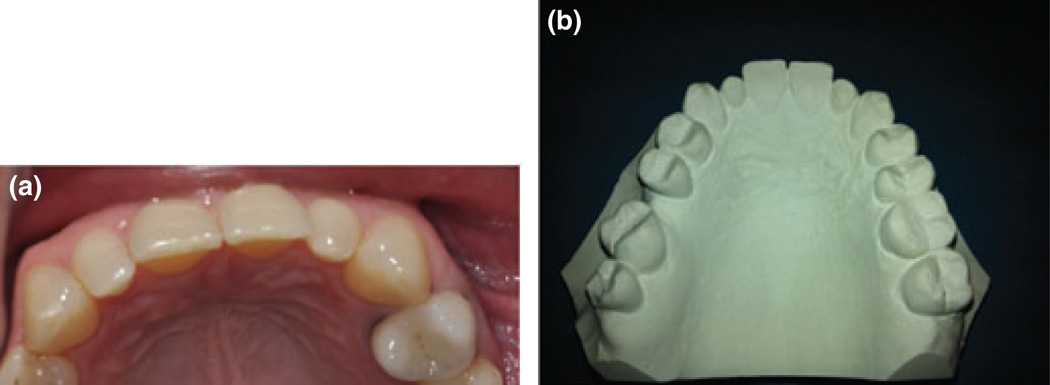
Five subjects (4.9%) had microdont teeth excluding wisdom teeth (three males and two females). The tooth most commonly affected was the upper lateral incisor, which occurred bilaterally in all cases (Fig. 1).
Fig. 1.
(a, b) Bilateral microdont lateral incisors.
Alterations in tooth structure
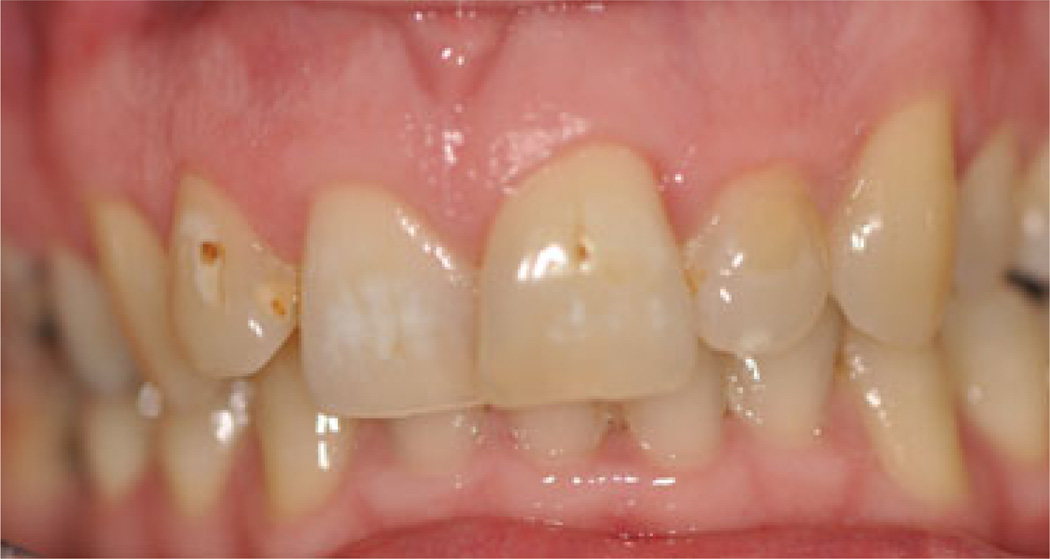
Enamel defects were common, affecting 27 (26.7%) subjects (12 male and 15 female). The teeth most frequently affected were the incisors (46.8%), the premolars (24.2%), and first permanent molars (21.0%). Figure 2 shows an example of a typical enamel defect presenting on the upper incisors.
Fig. 2.
Example of an enamel defect affecting the upper incisors.
The frequency of all dental anomalies, sex distribution, and teeth most commonly affected are summarised in Table 3. Chi-square and Fishers exact were performed to examine gender variation in the different anomalies. Statistical comparisons revealed a trend towards a higher number of males with supernumerary teeth than females (P = 0.052), but the numbers are too small to be significant. No other statistical significant gender differences were seen on all other variables.
Table 3.
Summary of the prevalence of each dental anomaly by gender.
| Total n = 101 | Female n = 52 | Male n = 49 | Gender difference? | Most common tooth/area affected | |
|---|---|---|---|---|---|
| Supernumeraries | 4 (3.9%) | 0 | 4 | P = 0.052 | Maxillary arch |
| Hypodontia | 12 (11.9%) | 8 | 4 | P = 0.360 | Upper right lateral incisor |
| Microdont | 5 (4.9%) | 2 | 3 | P = 0.672 | Upper lateral incisors |
| Transposition | 0 | 0 | 0 | – | – |
| Enamel defects | 27 (26.7%) | 15 | 12 | P = 0.621 | Upper incisors |
Discussion
The high prevalence of dental anomalies in this cohort of unaffected parents of children born with a cleft of lip and palate strongly supports the hypothesis that dental anomalies should be included in the routine diagnostic phenotyping of clefting conditions6.
Table 4 summarises the prevalence of the various dental anomalies in this Australian cohort in comparison with the (limited) international data. In contrast to two previous studies in which no predictors of orofacial clefting were found in the dental phenotypes of unaffected parents19,21, dental anomalies were very common in this study population, with 51% of the cohort presenting with at least one anomaly. Almost twelve per cent (11.9%) of this cohort had congenitally absent teeth, which is considerably greater than figures recently reported for similarly unaffected parents in a Brazilian study (4.9%)18. Non-cleft siblings, however, have been reported to have a similarly high prevalence of hypodontia; figures range from 11.1% in a Belgian study17 to 16% in an American cohort20. In both the Brazilian and Belgian studies, the prevalence of hypodontia in the unaffected cleft family members was statistically significantly greater than in matched non-cleft control groups. A matched Australian control group was beyond the remit of this study and, as there are well-recognised racial as well as gender differences in the prevalence of missing teeth22, direct comparison with these data is not entirely valid. A recent meta-analysis of international data on the prevalence of all dental anomalies, however, has suggested that hypodontia may be more common in Australians than other nationalities23, with a reported prevalence of between 6.3%24,25 and 8.1%26. Furthermore, the criteria against which participants in this study were attributed with hypodontia are much tighter thanks to the detailed clinical examination protocol than previous studies (Table 1). Given this, the figure of almost twelve per cent of the unaffected parents presented with hypodontia, a figure that is in excess of any other international normative data, the congenital absence of teeth may yet be a useful predictor of cleft risk.
Table 4.
Summary of the evidence with respect to the expanded dental phenotype.
| Dental anomaly | OzCleft parents n = 101 (%) |
Unaffected family members | International normative data |
|---|---|---|---|
| Hypodontia | 12 (11.9%) | 4.9% parents18 | 2.5–6.9% (Female > Male)23 |
| 0.9% parents19 | |||
| 3.6% parents21 | |||
| 11.1% siblings17 | |||
| 16.0% siblings20 | |||
| Supernumerary Teeth | 4 (3.9%) | 1.6% parents18 | 0.8–3.0% (M > F)27 |
| 0.8% parents19 | |||
| 3.6% parents21 | |||
| Microdontia | 5 (4.95%) | 8.2% parents (lateral incisor only)18 | 2% (F > M) |
| Enamel defects | 26.7% | Not reported | 2.8–25% MIH30 |
Similarly, the prevalence of both supernumerary teeth (3.9%) and microdontia (4.95%) was high in this Australian population. In a recent meta-analysis of the prevalence of supernumerary teeth, 1.6% of non-cleft White European populations presented with extra teeth27. This same meta-analysis clearly showed that the prevalence of supernumerary teeth was higher in males than females regardless of ethnicity, which is supported in this study with supernumerary teeth found only in fathers. Although microdontia has been reported in individuals with clefts of the lip and palate15, there are less data available on non-cleft relatives. Five parents (4.95%) in this study had markedly smaller upper lateral incisors; in all instances, it was bilateral (see Fig. 2). Haria and co-workers, reporting on a small cohort of similar parents (n = 42), found three (5.4%) participants had ‘abnormalities of crown formation’ of which two were microdont upper lateral incisors (unilateral only) and the third had ‘abnormal’ lateral incisors and canines21.
Developmental defects of enamel were common in this study population, affecting just over a quarter (26.7%) of the participants. Prevalence data on enamel defects vary widely due to differences in diagnostic criteria and study methodology. In this study, all types of enamel defect were included based on clinical appearance, from isolated enamel opacities to generalised discolouration and hypoplasia, which may have led to an over-reporting of this anomaly. Individuals with clefts of the lip and/or palate commonly have enamel defects affecting the incisors in the line of the cleft28,29. Although it is possible that genetic factors may be associated with these enamel defects, mechanical disruption to the tooth bud at the time of primary surgery may also play a role in the affected proband, but is obviously not the case in their unaffected parent. Multiple genetic and environmental factors are thought to influence the presence of enamel defects. The lack of consistency throughout the literature in the diagnosis of such defects, however, makes it difficult to explore the relative contributions of genetic as opposed to environmental factors to their presence specifically in cleft families. The role of this particular phenotype characteristic in the clefting spectrum therefore remains unclear at this time, although further well-controlled studies with clearly defined diagnostic criteria would be beneficial.
This is the first study to report on the dental phenotype in Australian families with cleft lip ± palate. We note that the lack of an appropriately matched Australian non-cleft control group and the small number of individual anomalies are limitations in this study; however, this is the largest cohort of unaffected parents of individuals with CL ± palate studied systematically for dental phenotypes and provides useful pilot data for further validation.
Conclusions
There is strong evidence that children with clefts of the lip and/or palate have a greater number of dental anomalies than the otherwise healthy population. The accurate delineation of this dental phenotype in the otherwise apparently unaffected family members is less well known. Studies such as this underscore the need to estimate heritability based on an ‘extended cleft phenotype’ that encompasses a range of subphenotypes that are part of the overall cleft spectrum, such as VPI, distinct facial asymmetries, dental anomalies, interruptions in the oris orbicularis muscle, differences in brain morphology, etc. This approach aims to improve recurrence risk estimation and provide more informative and genetically homogeneous pedigrees for gene mapping. The fact that a high proportion of dental anomalies were observed in our data reinforces our hypothesis that these anomalies are part of the extended cleft phenotype, explaining their ‘enrichment’ in our data. A formal comparison against normative data, which we are in the process of gathering currently, will certainly help in shedding more light into this. Although such analysis is undoubtedly complex, it has the potential to improve the diagnosis and management of children with clefts and better inform individualised genetic counselling replacing empiric risk formulation.
Why is this important to paediatric dentists.
Paediatric dentists should be part of the multidisciplinary team caring for individuals with clefts of the lip and/or palate.
Paediatric dentists are in a unique position to identify dental anomalies in both individuals with clefts of the lip and/or palate and their family members.
By understanding the implications of the dental phenotype, paediatric dentists can play a key role in informing the diagnosis and management of individuals with clefts of the lip and/or palate.
Acknowledgements
This work was supported by the Victorian Government’s Operational Infrastructure Support Program. OzCleft is funded by the National Health and Medical Research Council of Australia (grant no 607396) and by the United States National Institute of Health grant No R01-DE16148 (held by MM). Contents of the published material are solely the responsibility of the Administering Institution, individual authors, and do not reflect the views of NHMRC.
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Murray JC. Gene/environment causes of cleft lip and/or palate. Clin Genet. 2002;61:248–256. doi: 10.1034/j.1399-0004.2002.610402.x. [DOI] [PubMed] [Google Scholar]
- 2.Dixon MJ, Marazita ML, Beaty TH, Murray JC. Cleft lip and palate: understanding genetic and environmental influences. Nat Rev Genet. 2011;12:167–178. doi: 10.1038/nrg2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jugessur A, Farlie PG, Kilpatrick NM. The genetics of isolated orofacial clefts: from genotype to subphenotype. Oral Dis. 2009;15:437–453. doi: 10.1111/j.1601-0825.2009.01577.x. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg SM, Neiswanger K, Martin RA, et al. The Pittsburgh Oral-Facial Cleft study: expanding the cleft phenotype. Background and justification. Cleft Palate Craniofac J. 2006;43:7–20. doi: 10.1597/04-122r1.1. [DOI] [PubMed] [Google Scholar]
- 5.Marazita ML. Subclinical features in non-syndromic cleft lip with or without cleft palate (CL/P): review of the evidence that subepithelial orbicularis oris muscle defects are part of an expanded phenotype for CL/P. Orthod Craniofac Res. 2007;10:82–87. doi: 10.1111/j.1601-6343.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- 6.Menezes R, Vieira AR. Dental anomalies as part of the cleft spectrum. Cleft Palate Craniofac J. 2008;45:414–419. doi: 10.1597/07-064.1. [DOI] [PubMed] [Google Scholar]
- 7.Mossey PA, Batra P, McIntyre GT. The parental dentocraniofacial phenotype: an orofacial clefting microform. Cleft Palate Craniofac J. 2010;47:22–34. doi: 10.1597/08-158.1. [DOI] [PubMed] [Google Scholar]
- 8.Vieira AR. Unraveling human cleft lip and palate research. J Dent Res. 2008;87:119–125. doi: 10.1177/154405910808700202. [DOI] [PubMed] [Google Scholar]
- 9.Akcam MO, Evirgen S, Uslu O, Memikoglu UT. Dental anomalies in individuals with cleft lip and/or palate. Eur J Orthod. 2010;32:207–213. doi: 10.1093/ejo/cjp156. [DOI] [PubMed] [Google Scholar]
- 10.Letra A, Menezes R, Granjeiro JM, Viera AR. Defining subphenotypes for oral clefts based on dental development. J Dent Res. 2007;86:986–991. doi: 10.1177/154405910708601013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro LL, Teixeira Das Neves L, Costa B, Ribeiro Gomide M. Dental anomalies of the permanent lateral incisors and prevalence of hypodontia outside the cleft area in complete unilateral cleft lip and palate. Cleft Palate Craniofac J. 2003;40:172–175. doi: 10.1597/1545-1569_2003_040_0172_daotpl_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 12.Tannure PN, Oliveira CA, Maia LC, Vieira AR, Granjeiro JM, de Castro Costa M. Prevalence of dental anomalies in nonsyndromic individuals with cleft lip and palate: a systematic review and meta-analysis. Cleft Palate Craniofac J. 2012;49:194–200. doi: 10.1597/10-043. [DOI] [PubMed] [Google Scholar]
- 13.Vichi M, Franchi L. Abnormalities of the maxillary incisors in children with cleft lip and palate. J Dent Child. 1995;62:412–417. [PubMed] [Google Scholar]
- 14.Lucas VS, Gupta R, Ololade O, Gelbier M, Roberts GJ. Dental health indices and caries associated microflora in children with unilateral cleft lip and palate. Cleft Palate Craniofac J. 2000;37:447–452. doi: 10.1597/1545-1569_2000_037_0447_dhiaca_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 15.Werner SP, Harris EF. Odontometrics of the permanent teeth in cleft lip and palate: systemic size reduction and amplified asymmetry. Cleft Palate Craniofac J. 1989;26:36–41. [PubMed] [Google Scholar]
- 16.Jaksic N, Sepan I, Glisic B, Stamenic E, Stamenkovik Z. Mesiodistal size of deciduous teeth in subjects wiht unilateral cleft lip and palate. Orthod Craniofac Res. 2002;5:17–21. doi: 10.1034/j.1600-0544.2002.01180.x. [DOI] [PubMed] [Google Scholar]
- 17.Eerens K, Vlietinck R, Heidbuchel K, et al. Hypodontia and tooth formation in groups of children with cleft, siblings without cleft, and nonrelated controls. Cleft Palate Craniofac J. 2001;38:374–378. doi: 10.1597/1545-1569_2001_038_0374_hatfig_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 18.Küchler EC, da Motta LG, Vieira AR, Granjeiro JM. Side of dental anomalies and taurodontism as potential clinical markers for cleft subphenotypes. Cleft Palate Craniofac J. 2011;48:103–108. doi: 10.1597/09-159. [DOI] [PubMed] [Google Scholar]
- 19.Anderson PJ, Moss AL. Dental findings in parents of children with cleft lip and palate. Cleft Palate Craniofac J. 1996;33:436–439. doi: 10.1597/1545-1569_1996_033_0436_dfipoc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder DC, Green LJ. Frequency of dental trait anomalies in cleft, sibling and non-cleft groups. J Dent Res. 1975;54:802–807. doi: 10.1177/00220345750540041801. [DOI] [PubMed] [Google Scholar]
- 21.Haria S, Noar JH, Sanders R. An investigation of the dentition of parents of children with cleft lip and palate. Cleft Palate Craniofac J. 2000;37:395–405. doi: 10.1597/1545-1569_2000_037_0395_aiotdo_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 22.Brook AH. Variables and criteria in prevalence studies of dental anomalies of number, form and size. Community Dent Oral Epidemiol. 1975;3:288–293. doi: 10.1111/j.1600-0528.1975.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 23.Polder BJ, Van’t Hof MA, Van der Linden FPGM, Kuijpers-Jagtman AM. A meta-analysis of the prevalence of dental agenesis of permanent teeth. Community Dent Oral Epidemiol. 2004;32:217–226. doi: 10.1111/j.1600-0528.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 24.Lynham A. Panoramic radiographic survey of hypodontia in Australian Defence Force recruits. Aust Dent J. 1989;35:19–22. doi: 10.1111/j.1834-7819.1990.tb03021.x. [DOI] [PubMed] [Google Scholar]
- 25.Davies PL. Agenesis of teeth in the permanent dentition: a frequency study in Sydney school children. Aust Dent J. 1968;13:146–150. doi: 10.1111/j.1834-7819.1968.tb02254.x. [DOI] [PubMed] [Google Scholar]
- 26.Thongudomporn U, Freer T. Prevalence of dental anomalies in orthodontic patients. Aust Dent J. 1998;43:395–398. [PubMed] [Google Scholar]
- 27.Anthonappa R, King NM, Rabie ABM. Diagnostic tools used to predict the prevalence of supernumerary teeth: a meta-analysis. Dentomaxillofac Radiol. 2012;41:444–449. doi: 10.1259/dmfr/19442214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camporesi M, Baccetti T, Marinelli A, Defraia E, Franchi L. Maxillary dental anomalies in children with cleft lip and palate: a controlled study. Int J Paediatr Dent. 2010;20:442–450. doi: 10.1111/j.1365-263X.2010.01063.x. [DOI] [PubMed] [Google Scholar]
- 29.Maciel SP, Costa B, Marcia Ribeiro G. Difference in the prevalence of enamel alterations affecting Central Incisors of Children With Complete Unilateral Cleft Lip and Palate. Cleft Palate Craniofac J. 2005;42:392–395. doi: 10.1597/02-152.1. [DOI] [PubMed] [Google Scholar]
- 30.Willmott NS, Bryan RAE, Duggal M. Molar-Incisor-Hypomineralisation. Eur Arch Paediatr Dent. 2008;9:172–179. doi: 10.1007/BF03262633. [DOI] [PubMed] [Google Scholar]