Abstract
We recently showed that Helicobacter pylori (HP)-positive gastric ‘pure' diffuse large B-cell lymphoma (DLBCL) may respond to HP eradication therapy. However, whether these HP-related ‘pure' DLBCL of the stomach may differ fundamentally from those unrelated to HP remains unclear. In this study, we compared the clinicopathologic features of these two groups of patients who had been uniformly treated by conventional chemotherapy. Forty-six patients were designated HP-positive and 49 were HP-negative by conventional criteria. HP-positive patients had a lower International Prognostic Index score (0–1, 65% vs 43%, P=0.029), a lower clinical stage (I-IIE1, 70% vs 39%, P=0.003), a better tumor response to chemotherapy (complete pathologic response, 76% vs 47%, P=0.004) and significantly superior 5-year event-free survival (EFS) (71.7% vs 31.8%, P<0.001) and overall survival (OS) (76.1% vs 39.8%, P<0.001). To draw a closer biologic link with HP, HP-positive tumors were further examined for CagA expression in lymphoma cells. Compared with CagA-negative cases (n=16), CagA-positive cases (n=27) were associated with high phosphorylated SHP-2 expression (P=0.016), and even better 5-year EFS (85.2% vs 46.3%, P=0.002) and OS (88.9% vs 52.9%, P=0.003). HP-related gastric ‘pure' DLBCL may be a distinct tumor entity, which is less aggressive, and responds better to conventional chemotherapy.
Introduction
Previous studies have shown that gastric ‘pure' diffuse large B-cell lymphomas (DLBCLs), that is, tumors without any histologic evidence of mucosa-associated lymphoid tissue (MALT) origin, may be epidemiologically associated with Helicobacter pylori (HP) infection.1, 2 We recently reported that a substantial portion of patients with gastric ‘pure' DLBCLs can be cured by HP eradication therapy.3 These findings suggest that gastric ‘pure' DLBCLs can be further divided into HP-related and -unrelated subtypes. These two groups of gastric tumors may differ from each other in cell origins, carcinogenesis mechanisms and clinicopathologic features.
To explore the differences of these two groups of tumors, we reviewed all patients with primary gastric ‘pure' DLBCL who were treated with conventional chemotherapy as front-line therapy, and further divided them into HP-positive and -negative groups based on the presence or absence of HP infection. We analyzed the histomorphologic findings, molecular subclassification, clinical stage, International Prognostic Index (IPI) score, tumor response to chemotherapy, event-free survival and overall survival (OS) in these two patient groups. Because HP can be an innocent bystander infection in certain HP-positive cases, we further examined the HP-positive group for CagA tumor expression, a marker that we recently found useful in detecting a direct HP relevance of gastric low-grade MALT lymphoma.4 Our results indicated that HP-related gastric ‘pure' DLBCL, particularly that with CagA expression, is a distinct entity associated with less aggressive tumor behavior and a better patient prognosis.
Patients and methods
Patients, treatment and evaluation of tumors
The medical records and pathologic specimens of consecutive patients with histologically confirmed primary gastric DLBCL that had no histologic evidence of MALT origin were reviewed. These patients were all diagnosed at our institutions from 1 January 1999 to 30 December 2009. Patients with histologic features of MALT lymphoma, including dense infiltration of centrocyte-like cells in the lamina propria and typical lymphoepithelial lesions, in initial and in follow-up samples were excluded.5, 6, 7 Specimens were immunohistochemically stained with CD20, CD5, CD3 and CD43 for routine diagnostic purposes. In this study, the median number of gastric biopsies for each patient was 6 (range, 2–23). The presence of HP infection was confirmed in each case, using histologic examination, urease test or bacterial culture.3, 8
Staging workups included a physical examination with an inspection of Waldeyer's ring, a detailed history, a hemogram with leukocyte differential count, serum lactate dehydrogenase (LDH) evaluation, computed tomographic scan of the chest, abdomen and pelvis, bone marrow aspiration and biopsy, an upper gastrointestinal examination, using endoscopy and gallium scintigraphy or fluorine-18 fluorodeoxyglucose positron emission tomography. The staging and classification of lesions were based on the Musshoff modification of the Ann Arbor staging system. The diagnosis of primary gastric ‘pure' DLBCL must fulfill the modified criteria of Lewin et al.9 and Herrmann et al.:10 (1) the presence of a predominant gastric lesion, with or without expansion to regional lymph nodes, and no involvement of distal lymph nodes; (2) peripheral blood smears revealing no leukemic or lymphomatous abnormalities.11 This definition also excluded patients with gastric involvement who were detected following previously diagnosed extra-abdominal lymphoma.11 Patients were stratified according to the IPI for intermediate- and high-grade non-Hodgkin's lymphoma.12
Systemic chemotherapy consisting of either anthracycline- or anthracenedione-containing regimens or rituximab-containing regimens was administered as the initial therapy for all patients with primary gastric ‘pure' DLBCL. To exclude confounding factors (e.g., a synchronous therapy using HP eradication therapy and chemotherapy may potentially increase treatment efficacy in gastric DLBCL13), patients who received either surgical resection, HP eradication therapy or combined with HP eradication therapy and chemotherapy as their primary treatment were excluded in this study. Surgery and local radiotherapy were reserved for patients whose localized disease did not respond to chemotherapy or who developed treatment-related complications that warranted further treatment. Every patient underwent a follow-up endoscopic examination with a biopsy for suspicious lesions and an imaging examination to document their response to chemotherapy. Tumors were considered chemosensitive if complete pathologic remission (pCR) was documented after chemotherapy. The pathologic review and genetic studies of archived tumor tissues were approved by the Institutional review board of National Taiwan University Hospital.
Immunohistochemical staining and scoring
Immunohistochemical staining for CagA (A10; sc-28368; Santa Cruz Biotechnology, Santa Cruz, CA, USA), phosphorylated SHP-2 (p-SHP-2) (Thy542; AF3790; R&D Systems, Minneapolis, MN, USA), CD10 (clone 56C6; Novocastra, Newcastle upon Tyne, UK), BCL6 (clone PG-B6p; Dako, Glostrup, Denmark) and MUM-1 (clone MUM-1p; Dako) was performed on paraffin-embedded sections of pretreatment endoscopic biopsy specimens.4, 14, 15, 16, 17 Visualization was performed using an indirect immunoperoxidase method (retrieval buffer, sodium citrate buffer, pH 6.0) according to the manufacturer's instruction. Scoring for CagA, p-SHP-2, CD10, BCL6 or MUM-1 was independently performed by expert pathologists without the knowledge of the clinical data.
For the CagA maker and p-SHP-2, positive expression was defined as ⩾10% of cells with moderate or strong immunostaining (tumor cells with readily appreciable brown staining distinctly marking the tumor cell nucleus or cytoplasm) as described previously.4, 14 For CD10, BCL6 or MUM-1 markers, positive expression was defined as positive staining in >20% of cells as described previously.15, 16 For BCL6 and MUM-1, only diffuse or granular nuclear staining was considered positive; for CD10, only membrane staining was considered positive. The expression results of CD10, BCL6 and MUM-1 were used to classify all tumors into germinal center B-cell (GCB) or non-GCB subgroups as described previously.15, 16
Statistical analysis
The χ2 test and Fisher's exact test were used to compare the clinical characteristics, pathologic features and histologic subclassification of the two tumor subgroups. Analysis was conducted using the follow-up data available on 31 December 2012. Event-free survival (EFS) was calculated from the date of initial treatment until disease progression, relapse, discontinuation of treatment for any reason or death. OS was calculated from the date of initial treatment to the date of death from any cause. Survival was estimated using the Kaplan–Meier method and compared using the log-rank test. All prognostic variables found to be significant in univariate analysis were included in the multivariate analysis, using the Cox proportional hazards regression model. Differences between the results of the comparative tests were considered statistically significant if the two-sided P-value was <0.05.
Results
Clinicopathologic features
The clinicopathologic characteristics of the 95 patients who had gastric ‘pure' DLBCL are listed in Table 1. Of the 95 patients, 46 patients (48%) had HP-positive DLBCL and 49 patients (52%) had HP-negative DLBCL. The two subgroups did not differ histomorphologically for criteria such as pronounced nuclear pleomorphism, prominent nucleoli, a rim of basophilic cytoplasm, diffused or focal cluster distributions and morphologic variants of large cells.
Table 1. Clinicopathologic features of gastric ‘pure' DLBCL with and without HP infection.
| Variable | Total (%) | HP positive (%) | HP negative (%) | P-value |
|---|---|---|---|---|
| Total no. | 95 (100) | 46 (48) | 49 (52) | |
| Sex | ||||
| Female | 52 (55) | 23 (50) | 29 (59) | 0.369 |
| Male | 43 (45) | 23 (50) | 20 (41) | |
| Age (years old) | ||||
| <60 | 43 (45) | 20 (44) | 23 (47) | 0.735 |
| ⩾60 | 52 (55) | 26 (56) | 26 (53) | |
| Endoscopic features | ||||
| Ulceration or ulcerated mass | 54 (57) | 31 (67) | 23 (47) | 0.044 |
| Non-ulcerative lesionsa | 41 (43) | 15 (33) | 26 (53) | |
| Location of tumor (s) | ||||
| Proximal or ⩾2 components | 49 (52) | 19 (41) | 30 (61) | 0.052 |
| Distal | 46 (48) | 27 (59) | 19 (39) | |
| Presence of B symptoms | ||||
| Yes | 16 (17) | 3 (6) | 13 (27) | 0.009 |
| No | 79 (83) | 43 (94) | 36 (73) | |
| Stage | ||||
| I-IIE1 | 51 (62) | 32 (70) | 19 (39) | 0.003 |
| IIE2/III/IVb | 44 (38) | 14 (30) | 30 (61) | |
| ECOG | ||||
| 0–1 | 80 (84) | 42 (91) | 38 (78) | 0.066 |
| ⩾2 | 15 (16) | 4 (9) | 11 (22) | |
| LDH | ||||
| Normal | 53 (56) | 31 (67) | 22 (45) | 0.027 |
| High | 42 (44) | 15 (33) | 27 (55) | |
| IPI risk group | ||||
| 0–1 | 51 (54) | 30 (65) | 21 (43) | 0.029 |
| ⩾2 | 44 (46) | 16 (35) | 29 (57) | |
| Chemotherapy response | ||||
| pCR | 58 (61) | 35 (76) | 23 (47) | 0.004 |
| PR+SD+PDc | 37 (39) | 11 (24) | 26 (53) | |
| Histologic subclassification (n=78) | ||||
| GCB | 44 (56) | 25 (64) | 19 (49) | 0.171 |
| Non-GCB | 34 (44) | 14 (36) | 20 (51) | |
| Chemotherapy regimen | ||||
| Anthracycline-basedd | 61 (64) | 26 (57) | 35 (72) | 0.511 |
| Rituximab/anthracycline-basede | 22 (23) | 13 (28) | 9 (18) | |
| Rituximab/nonanthracycline-basedf | 5 (5) | 3 (6) | 2 (4) | |
| Othersg | 7 (8) | 4 (9) | 3 (6) | |
Abbreviation: ACE, doxorubicin, cyclophosphamide and etoposide; CEOP, cyclophosphamide, epirubicin (⩾70 mg/m2), vincristine and prednisolone; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisolone; COP, cyclophosphamide, vincristine and prednisolone; CNOP, cyclophosphamide, mitoxantrone, vincristine and prednisolone; DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; HP, H. pylori; IPI, International Prognostic Index; LDH, lactate dehydrogenase; PACE, prednisolone-ACE; pCR, complete pathologic remission; PR, partial remission; SD, stable disease; PD, progression; GCB, germinal center B-cell.
Proximal: Middle body, upper body, fundus or cardia; distal: antrum, angle or lower body.
Non-ulcerative lesions: gastritis-like or multiple erosion on infiltrative mucosa, erosions on giant nodular folds or mixed lesions.
Total, stage IIE2/III/IV, 14/23/7; HP-positive, stage IIE2/III/IV, 6/7/1; HP-negative, stage IIE2/III/IV, 8/16/6; stage IV (n=7), four bone marrow involvement, two bone involvement and one malignant pleural effusion.
PR, n=3; HP-positive, n=1; HP-negative, n=2.
Anthracycline-based: CHOP, CEOP, ACE and PACE.
Rituximab/anthracycline-based: rituximab-CHOP or rituximab-CEOP.
Rituximab/nonanthracycline-based: rituximab-COP.
Others: CNOP and COP.
HP-positive patients had more gastric ulceration/ulcerative mass lesions (67% vs 47%, P=0.044) and more tumors located in the distal (antrum, angle or lower body) portion of the stomach (59% vs 39%, P=0.052) (Table 1). The HP-positive patients had a lower clinical stage (I-IIE1, 70% vs 39%, P=0.003), a better performance status (Eastern Cooperative Oncology Group (ECOG) score ⩽1, 91% vs 78%, P=0.066), a higher probability of a normal LDH level (67% vs 45%, P=0.027), a significantly less B symptoms (6% vs 27%, P=0.009) and a significantly lower IPI score (0–1, 65% vs 43%, P=0.029) than that of HP-negative patients (Table 1). There were no significant differences in age, sex and chemotherapy regimens between the two groups.
Both HP-positive and -negative groups of patient received an identical median of six cycles chemotherapy. For patients responding satisfactorily to a particular regimen of chemotherapy, a minimum of six courses were given. Among those patients who did not achieve pCR with first-line chemotherapy, most received second-line chemotherapy, except for five patients who underwent gastrectomy (two HP-positive and three HP-negative) and one who received local radiation (HP-positive). The overall pCR rate was 76% for patients with HP infection and 47% for those without HP infection (P=0.004; Table 1). Among stage I-IIE1 patients (n=51), we showed that HP-positive group had a better trend for pCR than HP-negative group (88% vs 68%, P=0.097; Supplementary Table 1). For patients with stage I-IIE2 (n=65), HP infection was significantly associated with a pCR (84% vs 63%, P=0.05; Supplementary Table 1). Along the same line, for stage III–IV patients (n=30), HP-positive group had a trend for better pCR than HP-negative group (38% vs 27%, P=0.589; Supplementary Table 1).
We further evaluated 78 patients with available tumor specimens for CD10, BCL6 and MUM-1 expression. Based on the algorithm of Hans et al.15 (Supplementary Figure 1), we observed no differences in the distribution of GCB and non-GCB between HP-positive gastric pure DLBCL patients and their HP-negative counterparts (Table 1).
Prognosis differences between HP-positive and -negative gastric ‘pure' DLBCL
At a median follow-up of 5.6 years, the 5-year EFS and OS for all patients were 53.4% and 57.6%, respectively. Among patients who received rituximab/anthracycline-based regimens (n=22), the 5-year EFS and OS were 77.2% and 83.1%, respectively, whereas the 5-year EFS and OS were 52.3% and 57.4%, respectively, for patients with anthracycline-based chemotherapy (n=61; Table 1). Among patients with non-anthracycline-based regimen (n=12), the 5-year EFS and OS were 41.7% and 50.0%, respectively. Supplementary Table 1 lists the 5-year EFS and OS for different stages of patients (localized stages I-IIE1, I-IIE2 and III–IV).
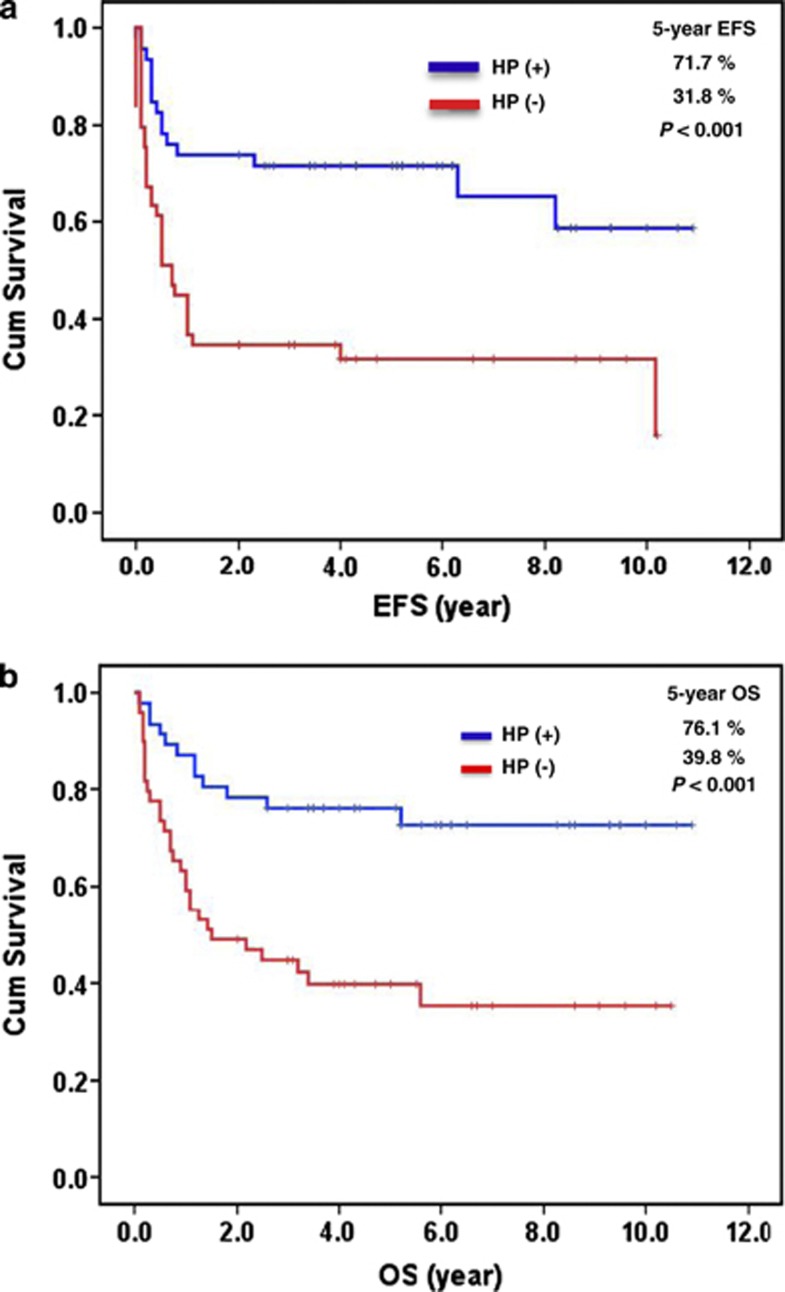
Patients with HP-positive gastric ‘pure' DLBCL had significantly better 5-year EFS and OS than HP-negative patients (5-year EFS, 71.7% vs 31.8%, P<0.001; OS, 76.1% vs 39.8%, P<0.001) (Figure 1). Among HP-positive patients, 11 patients died of progressive disease and other causes, two patients developed relapse, one patient developed gastric perforation and subsequently received gastrectomy, and one patient discontinued chemotherapy because of adverse effects. Of HP-negative patients, 28 patients died of progressive disease and other causes, one patient developed relapse, one patient experienced gastric perforation and subsequently received gastrectomy, and two patients discontinued treatment (one with chemotherapy toxicities and one with comorbidities). Among patients with pCR, we found that patients with HP-positive gastric ‘pure' DLBCL had a significantly better 5-year relapse-free survival than HP-negative patients (88.5% vs 63.8%, P=0.015). This finding indicates that the presence of HP infection is a prognostic maker for gastric ‘pure' DLBCL in pCR.
Figure 1.
Association of HP status with clinical outcome in gastric ‘pure' DLBCL. (a) Relationship of HP status to EFS. (b) Relationship of HP status to OS.
We tested the effects of clinical characteristics, and HP infection status on EFS and OS by using univariate analyses, which revealed the presence of six significant detrimental prognostic factors for EFS: (1) older age (P=0.048); (2) advanced clinical stage (P<0.001); (3) poor ECOG performance status (P<0.001); (4) elevated LDH level (P<0.001); (5) higher IPI score (P<0.001); and (6) absence of HP infection (P<0.001) (Table 2). These six factors also contributed to a higher risk in the context of OS (Table 2).
Table 2. Univariate analysis of stage, LDH, IPI score, HP status and histologic subclassification in EFS and OS of gastric ‘pure' DLBCL patients.
| Variable | 5-Year EFS (%) | S.e. (%) | P-value | 5-Year OS (%) | S.e. (%) | P-value |
|---|---|---|---|---|---|---|
| Age (years old) | ||||||
| <60 | 61.8 | 7.5 | 0.048 | 68.9 | 7.2 | 0.032 |
| ⩾60 | 42.3 | 7 | 48.5 | 6.9 | ||
| Sex | ||||||
| Male | 48.8 | 7.6 | 0.452 | 60.5 | 7.5 | 0.767 |
| Female | 53 | 7.1 | 55 | 7 | ||
| Stage | ||||||
| I-IIE1 | 73.8 | 6.3 | <0.001 | 82.2 | 5.4 | <0.001 |
| IIE2/III/IV | 24.8 | 6.5 | 28.7 | 7 | ||
| ECOG | ||||||
| 0–1 | 58 | 5.5 | <0.001 | 65.5 | 5.3 | <0.001 |
| ⩾2 | 7.7 | 7.4 | 7.7 | 7.4 | ||
| LDH | ||||||
| Normal | 71.5 | 6.3 | <0.001 | 81.3 | 5.3 | <0.001 |
| High | 24.4 | 6.7 | 26.2 | 7 | ||
| IPI risk group | ||||||
| 0–1 | 72.3 | 6.4 | <0.001 | 82.7 | 5.2 | <0.001 |
| ⩾2 | 25.6 | 6.7 | 27 | 6.9 | ||
| HP status | ||||||
| Positive | 71.7 | 6.7 | <0.001 | 76.1 | 6.3 | <0.001 |
| Negative | 31.8 | 6.8 | 39.8 | 7.1 | ||
Abbreviation: DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; EFS, event-free survival; EFS, event-free survival; LDH, lactate dehydrogenase; HP, H. pylori; IPI, International Prognostic Index; OS, overall survival; s.e., standard error.
Multivariate analysis identified the absence of HP infection (hazard ratio=2.509; P=0.007) and a poor ECOG performance status (HR=2.867; P=0.006) as independent predictors of worse EFS (Table 3). Similarly, the absence of HP infection (HR=2.666; P=0.009), an advanced clinical stage (HR=3.900; P=0.007) and a poor ECOG performance status (HR=4.308; P=0.001) was significantly associated with worse OS (Table 3).
Table 3. Multivariate analysis of prognostic factors and EFS and OS for gastric ‘pure' DLBCL patients.
| Characteristics |
EFS |
OS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| HP status | ||||||
| Negative vs positive | 2.509 | 1.279–4.924 | 0.007 | 2.666 | 1.279–5.553 | 0.009 |
| Age (years old) | ||||||
| ⩾60 vs <60 | 1.559 | 0.785–3.096 | 0.204 | 1.639 | 0.779–3.450 | 0.193 |
| Stage | ||||||
| IIE2/III/IV vs I-IIE1 | 2.174 | 0.854–5.533 | 0.103 | 3.9 | 1.451–10.479 | 0.007 |
| ECOG | ||||||
| ⩾2 vs 0–1 | 2.867 | 1.350–6.088 | 0.006 | 4.308 | 1.872–9.917 | 0.001 |
| LDH | ||||||
| High vs normal | 1.754 | 0.538–5.714 | 0.351 | 1.755 | 0.366–8.406 | 0.482 |
| IPI risk group | ||||||
| ⩾2 vs 0–1 | 1.139 | 0.296–4.379 | 0.849 | 1.306 | 0.244–6.998 | 0.755 |
Abbreviation: CI, confidence interval; DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; EFS, event-free survival; HP, H. pylori; HR, hazard ratio; IPI, International Prognostic Index; LDH, lactate dehydrogenase; OS, overall survival.
We further addressed whether HP infection was a significant prognostic factor for EFS and OS in stage I-IIE1 patients (n=51) and in patients receiving rituximab/anthracycline-based regimens (n=22). Among stage I-IIE1 patients, we showed that HP-positive group had better 5-year EFS and OS than HP-negative group (5-year EFS, 80.6% vs 46.8%, P=0.003; 5-year OS, 90.6% vs 68.0%, P=0.023). Among those who received rituximab/anthracycline-based regimens, we observed that HP-positive group had a better trend for 5-year EFS and OS than HP-negative group (5-year EFS, 84.8% vs 64.8%, P=0.176; 5-year OS, 93.3% vs 66.7%, P=0.091). Similarly, in patients treated with anthracycline-based chemotherapy (n=61; Table 1), HP-positive group had better 5-year EFS and OS than HP-negative group (5-year EFS, 71.8% vs 32.4%, P=0.006; 5-year OS, 76.0% vs 34.0%, P=0.001). Even in localized stage I-IIE1 patients, we found that HP infection was associated with a better 5-year EFS (87.7% vs 50%, P=0.078) and OS (88.9% vs 56.3%, P=0.017) for those treated with anthracycline-based chemotherapy. A similar trend of better 5-year EFS (87.5% vs 83.3%, P=0.274) and OS (100% vs 83.3%, P=0.221) was observed in those treated with rituximab/anthracycline-based regimens.
Prognostic significance of CagA expression in gastric ‘pure' DLBCL
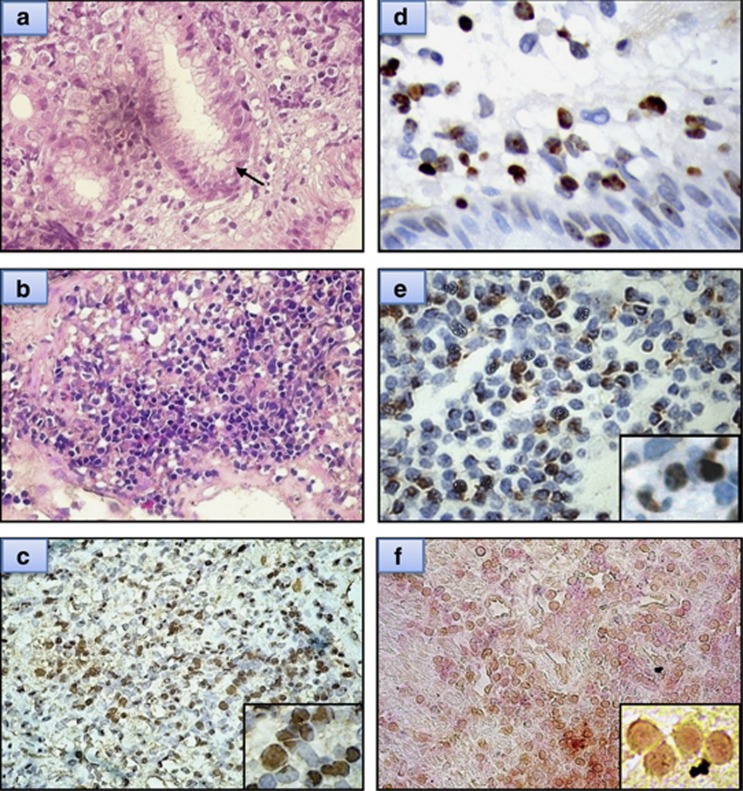
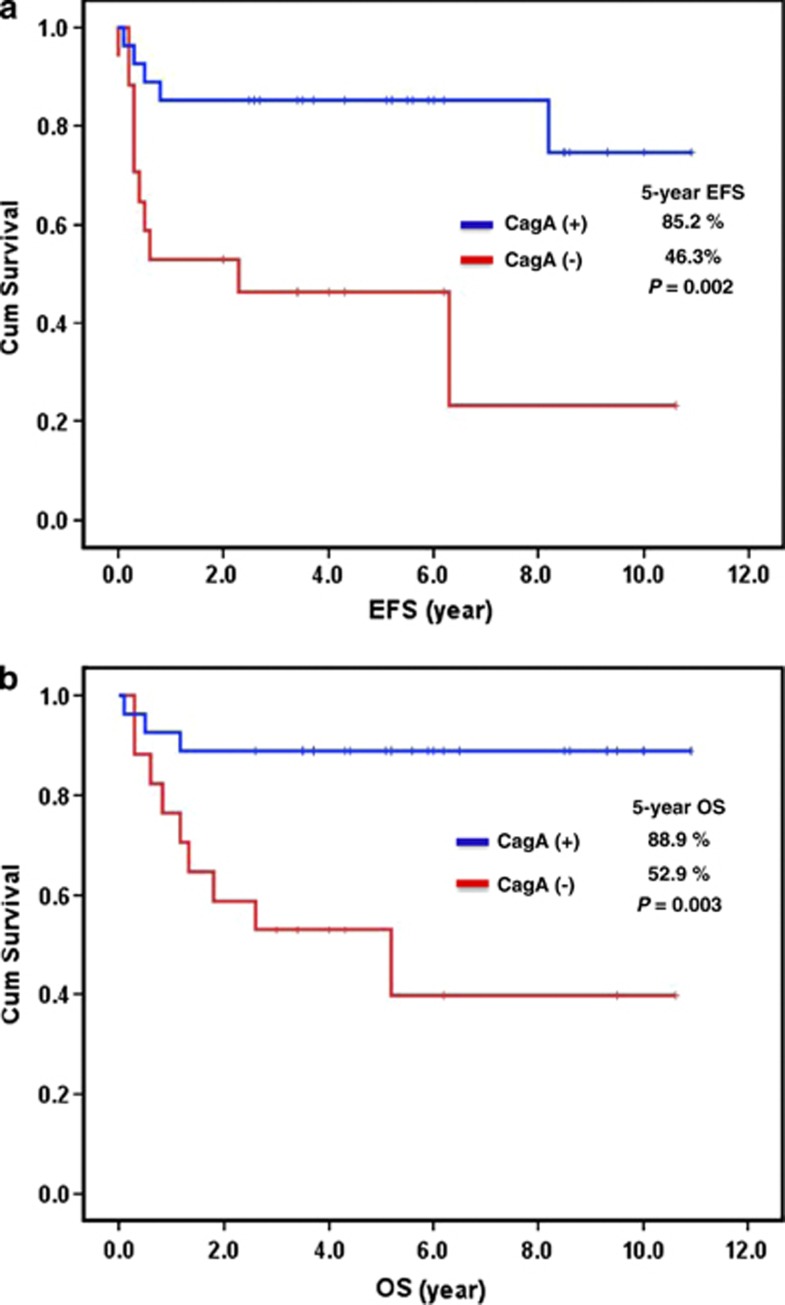
Using a technique that we described recently,4 43 HP-positive patients with available tumor specimens for CagA expression were analyzed. The CagA protein was expressed in gastric mucosa or submucosa tumor cells and showed scattered expression in the adjacent tumor-free gastric mucosa (Figure 2). Hematoxylin–eosin staining and immunohistochemical staining showed that most CagA-positive cells were morphologically abnormal and expressed CD20 (Figure 2). Positive CagA expression was observed in 27 (62.8%) of the 43 HP-positive cases and was closely associated with p-SHP-2 expression (17 (63.0%) of 27 CagA-positive cases vs 4 (25.0%) of 16 CagA-negative cases, P=0.016). Compared with the 16 HP-positive but CagA-negative cases, the 27 patients who were HP- and CagA-positive had a lower clinical stage (I-IIE1, 82% vs 47%, P=0.017), a better tumor response to chemotherapy (complete response, 89% vs 59% P=0.030) and significantly better 5-year EFS (85.2% vs 46.3%, P=0.002) and OS (88.9% vs 52.9%, P=0.003) (Figure 3).
Figure 2.
Examples of immunohistochemical analysis of CagA protein on tumor cells of gastric ‘pure' DLBCL. (a) Diffuse large cells infiltrating the mucosa are observable on histopathologic examination (hematoxylin–eosin (H&E), × 400) (arrow, HP). (b) Diffuse large cells infiltrating the submucosa are observable on histopathologic examination of an HP-positive case (H&E, × 400). (c) The same HP-positive case (b) shows CagA expression in the tumor cells (right bottom inset, × 1000). (d) HP-positive case shows CagA expression in the tumor cells of gastric mucosa. (e) HP-positive case shows CagA expression in the tumor cells of gastric submucosa (right bottom inset, × 1000). (f) Double stains: tumor cells with CagA nuclear staining (brown color) are also CD20-positive (red color) (right bottom inset, × 1000).
Figure 3.
Association of CagA expression with clinical outcome in HP-positive gastric ‘pure' DLBCL. (a) Relationship of CagA status to EFS. (b) Relationship of CagA status to OS.
Among HP-positive patients who received rituximab/anthracycline-based regimens (n=13), we observed that patients with CagA expression had a trend for better 5-year EFS and OS than those without CagA expression (5-year EFS, 83.3% vs 67.7%, P=0.052; 5-year OS, 100.0% vs 66.7%, P=0.058). Similarly, in patients treated with anthracycline-based chemotherapy (n=25), those with CagA expression had better 5-year EFS and OS than those without (5-year EFS, 91.7% vs 40.0%, P=0.002; 5-year OS, 91.7% vs 50%, P=0.005).
Discussion
We have shown that HP-positive gastric ‘pure' DLBCL has a lower clinical stage, a lower IPI score, a better tumor response to chemotherapy and superior 5-year EFS and OS. These findings indicate that HP-related gastric ‘pure' DLBCL shares clinicopathologic features of conventional gastric MALT lymphoma, suggesting an overlapping etiology between the two gastric lymphoma groups.
We fully understand that HP is a common infection in the general population, a coincidental infection of this microorganism in some cases of gastric ‘pure' DLBCL is highly possible. To improve the reliability of a true relationship, we provided evidence that CagA expression in the tumor cells may be a useful marker to distinguish a true from a spurious causative relationship between HP infection and lymphomagenesis of gastric ‘pure' DLBCL. For example, in HP-positive cases, we showed that CagA-positive cases had an even better response to chemotherapy and a favorable outcome and that CagA expression was closely associated with p-SHP-2 expression. These findings concur with our previous observation that translocation of CagA into human B lymphocytes biologically activates the relevant cellular pathways and promotes B lymphoid cell proliferation, and CagA expression in tumor cells is closely associated with HP dependence in gastric MALT lymphoma.4, 14 In a CagA-transgenic mouse model, Ohnishi et al.18 demonstrated that CagA has an important role in the development of HP-associated B lymphoma cells through SHP-2-tyrosine phosphorylation-dependent pathway. Other investigators have also demonstrated that translocated CagA can promote B-cell proliferation and inhibit apoptosis through ERK activation, BAD phosphorylation and p53 accumulation.14, 19, 20 Based on these findings, we hypothesized that HP-positive gastric ‘pure' DLBCLs, particularly those with CagA expression in tumor cells, are HP related, and are clinicopathologically distinct from HP-unrelated gastric ‘pure' DLBCLs. Further, the dependence on CagA-regulated signaling pathway2, 21 for the growth of malignant B-cell clones may explain the tendency of CagA-positive gastric ‘pure' DLBCLs to remain localized and thus enjoy a lower clinical stage of disease.
However, in light of the complex interaction between micromes and the genome/epigenome, conclusion of our study should be validated in another cohort of a diverse population. For example, epidemiologic studies have reported that the occurrence of gastric MALT lymphoma in East Asia is higher than in Western countries,22, 23, 24 and most of HP strains from East Asian are CagA-positive.25 East Asian CagA carries the lutamic acid-proline-isoleucinetyrosine-alanine (EPIYA)-D segment, which differs from the EPIYA-C motif of Western CagA.26 As EPIYA-D exhibits greater SHP-2-binding affinity and tyrosine phosphorylation activity than EPIYA-C,27 HP-positive lymphomas in this study may be more closely linked to HP-related signaling pathway.
The cell origin of HP-related ‘pure' DLBCL of stomach is obscure. Previous clonal, cytogenetic and transcriptional profiling studies suggest that a proportion of gastric ‘pure' DLBCLs may be transformed from the HP-related MALT lymphoma components.28, 29, 30, 31, 32 However, in contrast to the canonical concept that HP-related lymphomas are of marginal zone B-cell origin,8, 33, 34 a recent multicenter phase II study (HG-L1 trial) showed that a proportion of HP-dependent gastric ‘pure' DLBCLs are of GCB origin.35 In this study, we demonstrated that 25 (64%) of 39 HP-positive gastric ‘pure' DLBCL patients had the GCB immunophenotype. These crucial findings have raised a hypothesis that HP may transform GCB cells into lymphoma cells in certain HP-related gastric ‘pure' DLBCL, especially in HP-dependent ‘pure' DLBCL.35, 36, 37, 38
To exclude the possibilities that localized diseases may be associated with less aggressive behavior and nodal DLBCL may benefit more from rituximab/anthracycline-based regimens,39 we demonstrated that HP infection remains a superior prognostic factor for EFS and OS in patients with localized disease (stage I-IIE1), and in patients who received conventional anthracycline-based chemotherapy alone. Even with the same extent of rituximab/anthracycline-based regimens treatment, HP-positive patients still had a better outcome than HP-negative patients. A similar trend of better 5-year EFS and OS was both observed in localized stage I-IIE1 HP-positive patients treated with anthracycline-based and with rituximab/anthracycline-based regimens.
Our results indicate that HP-related gastric ‘pure' DLBCL, and particularly those with CagA expression in tumor cells, is associated with less aggressive tumor behavior, enhanced response to chemotherapy and a better prognosis. The cell origin of these lymphomas needs to be pursued.
Acknowledgments
We thank the Cancer Registry, Office of Medical Records and National Taiwan University Hospital for providing the necessary patient information. This study was supported by research grants NSC96-2321-B-002-013, NSC96-2321-B-002-014, NSC96-2314-B-002-164MY3, NSC 98-2314-B-002-087-MY3, NSC 101-2321-B-002-032, NSC 102-2321-B-002-032 and NSC 101-2314-B-002-157-MY3 from the National Science Council, Taiwan, NHRI-EX103-10239BI from the National Health Research Institutes, Taiwan, NTUH 103-S2387 from National Taiwan University Hospital, Taiwan, and DOH100-TD-B-111-001 from the Department of Health, Taiwan.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Suzuki T, Matsuo K, Ito H, Hirose K, Wakai K, Saito T, et al. A past history of gastric ulcers and Helicobacter pylori infection increase the risk of gastric malignant lymphoma. Carcinogenesis. 2006;27:1391–1397. doi: 10.1093/carcin/bgi334. [DOI] [PubMed] [Google Scholar]
- Kuo SH, Cheng AL. Helicobacter pylori and mucosa-associated lymphoid tissue: What's new. Hematol Am Soc Hematol Educ Program. 2013;2013:109–117. doi: 10.1182/asheducation-2013.1.109. [DOI] [PubMed] [Google Scholar]
- Kuo SH, Yeh KH, Wu MS, Lin CW, Hsu PN, Wang HP, et al. Helicobacter pylori eradication therapy is effective in the treatment of early-stage H pylori-positive gastric diffuse large B-cell lymphomas. Blood. 2012;119:4838–4844. doi: 10.1182/blood-2012-01-404194. [DOI] [PubMed] [Google Scholar]
- Kuo SH, Chen LT, Lin CW, Wu MS, Hsu PN, Tsai HJ, et al. Detection of the Helicobacter pylori CagA protein in gastric mucosa-associated lymphoid tissue lymphoma cells: clinical and biological significance. Blood Cancer J. 2013;3:e125. doi: 10.1038/bcj.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach W. Gastric mucosa-associated lymphoid tissue lymphoma; a challenge for endoscopy. Gastrointest Endosc. 2008;68:632–634. doi: 10.1016/j.gie.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Chan JK, Ng CS, Isaacson PG. Relationship between high-grade lymphoma and low-grade B-cell mucosa-associated lymphoid tissue lymphoma (MALToma) of the stomach. Am J Pathol. 1990;136:1153–1164. [PMC free article] [PubMed] [Google Scholar]
- Kuo SH, Yeh PY, Chen LT, Wu MS, Lin CW, Yeh KH, et al. Overexpression of B-cell activating factor of TNF family (BAFF) is associated with Helicobacter pylori-independent growth of gastric diffuse large B-cell lymphoma with histologic evidence of MALT lymphoma. Blood. 2008;112:2927–2934. doi: 10.1182/blood-2008-02-137513. [DOI] [PubMed] [Google Scholar]
- Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, Boot H, Du MQ, Megraud F, on behalf of the EGILS group et al. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011;60:747–758. doi: 10.1136/gut.2010.224949. [DOI] [PubMed] [Google Scholar]
- Lewin KJ, Ranchod M, Dorfman RF. Lymphomas of the gastrointestinal tract: a study of 117 cases presenting with gastrointestinal disease. Cancer. 1978;42:693–707. doi: 10.1002/1097-0142(197808)42:2<693::aid-cncr2820420241>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Herrmann R, Panahon AM, Barcos MP, Walsh D, Stutzman L. Gastrointestinal involvement in non-Hodgkin's lymphoma. Cancer. 1980;46:215–222. doi: 10.1002/1097-0142(19800701)46:1<215::aid-cncr2820460136>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Ferrucci PF, Zucca E. Primary gastric lymphoma pathogenesis and treatment: what has changed over the past 10 years. Br J Haematol. 2007;136:521–538. doi: 10.1111/j.1365-2141.2006.06444.x. [DOI] [PubMed] [Google Scholar]
- The Non-Hodgkin's Lymphoma Classification Project A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- Raderer M, Chott A, Drach J, Montalban C, Dragosics B, Jäger U, et al. Chemotherapy for management of localised high-grade gastric B-cell lymphoma: how much is necessary. Ann Oncol. 2002;13:1094–1098. doi: 10.1093/annonc/mdf178. [DOI] [PubMed] [Google Scholar]
- Lin WC, Tsai HF, Kuo SH, Wu MS, Lin CW, Hsu PI, et al. Translocation of Helicobacter pylori CagA into human B lymphocytes, the origin of mucosa-associated lymphoid tissue lymphoma. Cancer Res. 2010;70:5740–5748. doi: 10.1158/0008-5472.CAN-09-4690. [DOI] [PubMed] [Google Scholar]
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- Lin CH, Kuo KT, Chuang SS, Kuo SH, Chang JH, Chang KC, et al. Comparison of the expression and prognostic significance of differentiation markers between diffuse large B-cell lymphoma of central nervous system origin and peripheral nodal origin. Clin Cancer Res. 2006;12:1152–1156. doi: 10.1158/1078-0432.CCR-05-1699. [DOI] [PubMed] [Google Scholar]
- Kraan W, Horlings HM, van Keimpema M, Schilder-Tol EJ, Oud ME, Scheepstra C, et al. High prevalence of oncogenic MYD88 and CD79B mutations in diffuse large B-cell lymphomas presenting at immune-privileged sites. Blood Cancer J. 2013;3:e139. doi: 10.1038/bcj.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi N, Yuasa H, Tanaka S, Sawa H, Miura M, Matsui A, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci USA. 2008;105:1003–1008. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara S, Higashi H, Ohnishi N, Asaka M, Hatakeyama M. Effects of Helicobacter pylori CagA protein on the growth and survival of B lymphocytes, the origin of MALT lymphoma. Oncogene. 2003;22:8337–8342. doi: 10.1038/sj.onc.1207028. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Wang C, Huang J, Ge Z, Dong Q, Zhong X, et al. The Helicobacter pylori virulence factor CagA promotes Erk1/2-mediated Bad phosphorylation in lymphocytes: a mechanism of CagA-inhibited lymphocyte apoptosis. Cell Microbiol. 2007;9:952–961. doi: 10.1111/j.1462-5822.2006.00843.x. [DOI] [PubMed] [Google Scholar]
- Wang HP, Zhu YL, Shao W. Role of Helicobacter pylori virulence factor cytotoxin-associated gene A in gastric mucosa-associated lymphoid tissue lymphoma. World J Gastroenterol. 2013;19:8219–8226. doi: 10.3748/wjg.v19.i45.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelle LG, de Vries AC, Looman CW, Casparie MK, Boot H, Meijer GA, et al. Gastric MALT lymphoma: epidemiology and high adenocarcinoma risk in a nation-wide study. Eur J Cancer. 2008;44:2470–2476. doi: 10.1016/j.ejca.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Luminari S, Cesaretti M, Marcheselli L, Rashid I, Madrigali S, Maiorana A, et al. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol. 2010;21:855–859. doi: 10.1093/annonc/mdp402. [DOI] [PubMed] [Google Scholar]
- Huh J. Epidemiologic overview of malignant lymphoma. Korean J Hematol. 2012;47:92–104. doi: 10.5045/kjh.2012.47.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Wang FY, Wan HJ, Jin XX, Wei J, Wang ZK, et al. Amino acid polymorphisms flanking the EPIYA-A motif of Helicobacter pylori CagA C-terminal region is associated with gastric cancer in east China: experience from a single center. J Dig Dis. 2013;14:358–365. doi: 10.1111/1751-2980.12056. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M. Anthropological and clinical implications for the structural diversity of the Helicobacter pylori CagA oncoprotein. Cancer Sci. 2011;102:36–43. doi: 10.1111/j.1349-7006.2010.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Ohnishi N, Tanaka S, Yanagiya K, Hatakeyama M. Differential oncogenic potential of geographically distinct Helicobacter pylori CagA isoforms in mice. Int J Cancer. 2009;125:2497–2504. doi: 10.1002/ijc.24740. [DOI] [PubMed] [Google Scholar]
- Peng H, Du M, Diss TC, Isaacson PG, Pan L. Genetic evidence for a clonal link between low and high-grade components in gastric MALT B-cell lymphoma. Histopathology. 1997;30:425–429. doi: 10.1046/j.1365-2559.1997.5450786.x. [DOI] [PubMed] [Google Scholar]
- Kuo SH, Chen LT, Wu MS, Kuo KT, Yeh KH, Doong SL, et al. Differential response to H pylori eradication therapy of co-existing diffuse large B-cell lymphoma and MALT lymphoma of stomach—significant association with clonality and BCL10 expression of the tumor cells. J Pathol. 2007;211:296–304. doi: 10.1002/path.2117. [DOI] [PubMed] [Google Scholar]
- Barth TF, Bentz M, Leithauser F, Stilgenbauer S, Siebert R, Schlotter M, et al. Molecular-cytogenetic comparison of mucosa-associated marginal zone B-cell lymphoma and large B-cell lymphoma arising in the gastro-intestinal tract. Genes Chromosomes Cancer. 2001;31:316–325. doi: 10.1002/gcc.1150. [DOI] [PubMed] [Google Scholar]
- Barth TF, Barth CA, Kestler HA, Michl P, Weniger MA, Buchholz M, et al. Transcriptional profiling suggests that secondary and primary large B-cell lymphomas of the gastrointestinal (GI) tract are blastic variants of GI marginal zone lymphoma. J Pathol. 2007;211:305–313. doi: 10.1002/path.2096. [DOI] [PubMed] [Google Scholar]
- Flossbach L, Holzmann K, Mattfeldt T, Buck M, Lanz K, Held M, et al. High-resolution genomic profiling reveals clonal evolution and competition in gastrointestinal marginal zone B-cell lymphoma and its large cell variant. Int J Cancer. 2013;132:E116–E127. doi: 10.1002/ijc.27774. [DOI] [PubMed] [Google Scholar]
- Isaacson PG, Du MQ. Gastrointestinal lymphoma: where morphology meets molecular biology. J Pathol. 2005;205:255–274. doi: 10.1002/path.1703. [DOI] [PubMed] [Google Scholar]
- Du MQ. MALT lymphoma: many roads lead to nuclear factor-κb activation. Histopathology. 2011;58:26–38. doi: 10.1111/j.1365-2559.2010.03699.x. [DOI] [PubMed] [Google Scholar]
- Ferreri AJ, Govi S, Raderer M, Mulè A, Andriani A, Caracciolo D, et al. Helicobacter pylori eradication as exclusive treatment for limited-stage gastric diffuse large B-cell lymphoma: results of a multicenter phase 2 trial. Blood. 2012;120:3858–3860. doi: 10.1182/blood-2012-06-438424. [DOI] [PubMed] [Google Scholar]
- Möller P, Viardot A. Antibiotics as first-line therapy for Hp-associated gastric large B-cell lymphoma? Probably yes. Blood. 2012;119:4818–4819. doi: 10.1182/blood-2012-03-417345. [DOI] [PubMed] [Google Scholar]
- Caramuta S, Lee L, Ozata DM, Akçakaya P, Georgii-Hemming P, Xie H, et al. Role of microRNAs and microRNA machinery in the pathogenesis of diffuse large B-cell lymphoma. Blood Cancer J. 2013;3:e152. doi: 10.1038/bcj.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Ye H, Bacon CM, Goatly A, Liu H, Kerr L, et al. Translocations involving the immunoglobulin heavy chain gene locus predict better survival in gastric diffuse large B-cell lymphoma. Clin Cancer Res. 2008;14:3002–3010. doi: 10.1158/1078-0432.CCR-07-4946. [DOI] [PubMed] [Google Scholar]
- Griffin MM, Morley N. Rituximab in the treatment of non-Hodgkin's lymphoma—a critical evaluation of randomized controlled trials. Expert Opin Biol Ther. 2013;13:803–811. doi: 10.1517/14712598.2013.786698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.