Mutations in exon 12 of the nucleophosmin (NPM1) gene have been described as primary leukemogenic event in up to 35% of adult acute myelogenous leukemia (AML) cases, mainly those with normal karyotype.1 This type of AML is listed as provisional entity in the World Health Organization classification of tumors of the hematopoietic and lymphoid tissues 2008. NPM1 mutation status has improved risk stratification and therapy choice of the AML intermediate-risk group with normal karyotype, as NPM1-mutated cases without fms-related tyrosine kinase 3 (FLT3) gene internal tandem duplications have a favorable outcome and no need for bone marrow transplantation first line.2 Furthermore, mutations in other genes—for example, isocitrate dehydrogenase (IDH1 and IDH2 genes)—seem to influence prognosis as patients with both NPM1 and IDH mutations show improved overall 3-year survival, whereas NPM1-positive cases without IDH mutations have a much less favorable outcome.3
In addition to diagnosis and risk stratification, NPM1 mutation analysis can be used to monitor therapy response.4 However, although NPM1 mutations are regarded to be an early event in leukemogenesis, serial analysis of 245 initially NPM1-mutated AML cases revealed a loss of the mutation at relapse in 9% of the cases.5 This phenomenon is well known for genetic events occurring late in AML evolution, for example, FLT3 mutations.6, 7 For FLT3, also switches of the mutation type at progression of AML (initial diagnosis with D835 tyrosine kinase mutation, relapse with internal tandem duplication) but stable NPM1 mutation at both time points are documented.8
Here, we describe for the first time a switch of a NPM1 mutation type at relapse having profound impact on AML monitoring strategies.
Patient
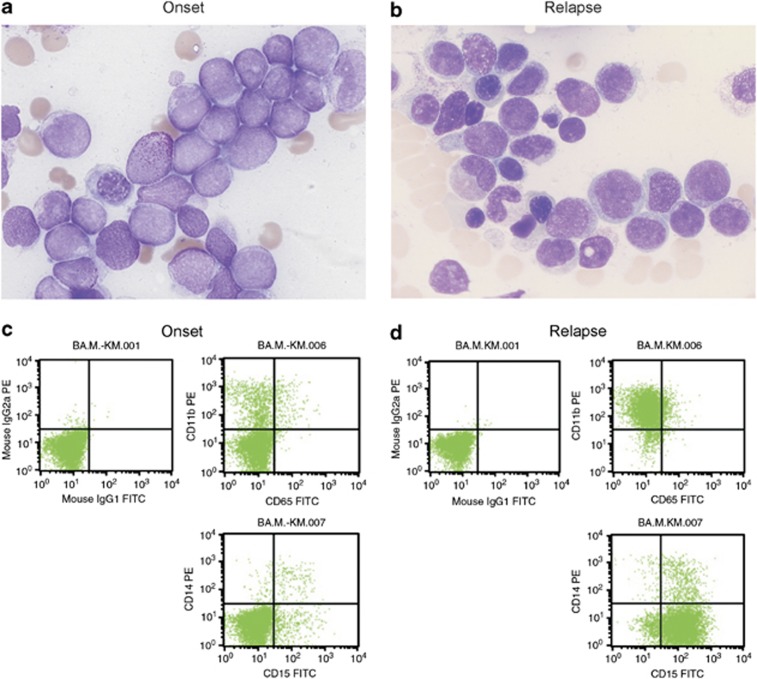
A 69-year-old female patient presented in 2007 with a peripheral blood leukocyte count of 140.76 × 106/l (91% blasts), a C-reactive protein of 6.6 mg/dl, hemoglobin 9.6 mg/dl and thrombocytopenia (84 × 109/l). Bone marrow cytology showed >90% blasts without Auer rods (Figure 1a); >95% of the blasts were peroxidase-positive and esterase staining was positive in 3%, confirming an AML with minimal maturation. The blast cell population was positive for MPO, CD117, CD13, CD33, CD38 and CD68, and negative for HLA-DR, CD11b, CD14, CD15, CD3, CD19, CD36 and TdT (Figure 1c).
Figure 1.
Comparison of blasts at onset of leukemia (left) and relapse (right), showing different morphologies (a, b). Flow profiles demonstrating the shift in CD11b and CD14 expression (c, d).
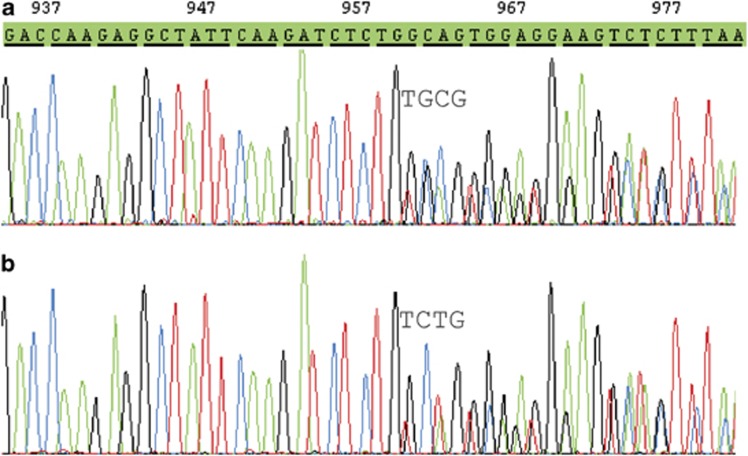
Conventional cytogenetics demonstrated a normal karyotype. PCR for RUNX1RUNX1T1, CBFB-MYH11, PML-RARA and MLL(KMT2A) rearrangements were negative; no FLT3–ITD or KIT exon 8 mutation could be detected. Fluorescence in situ hybridization excluded an atypical RARA rearrangement. Sequencing of the NPM1 gene revealed a c.959_960insTGCG mutation in exon 12 (Figure 2a).
Figure 2.
Mutations detected at onset of leukemia (a) and relapse (b).
The first cycle of chemotherapy (ICE protocol; ifosfamide, carboplatin, etoposide) resulted in a complete remission and the NPM1 mutation was no longer detectable via sequencing. A second cycle of ICE and two cycles of A-HAM were administered and a complete remission was documented.
After ∼6 years in remission the patient presented with anemia, normal leukocyte and thrombocyte count but some blasts in the peripheral smear. Bone marrow cytology with 40–50% blasts confirmed the first relapse of myeloid acute leukemia (Figure 1b).
Fluorescence-activated cell sorting (FACS) could demonstrate 46% myeloid blasts consisting of two cell populations: 13% of the blasts showed the original marker profile, whereas 87% in contrast to primary diagnosis were now CD117−/CD11b+/CD15+/HLA-DR+/CD36+/CD4+ (Figure 1d). Conventional cytogenetics showed a normal karyotype again; a high-resolution single-nucleotide polymorphisms array (CytoScan 750K, Affymetrix, Santa Clara, CA, USA) did not detect any gains or losses >50 kbp in the samples from relapse and primary diagnosis. By sequencing, an NPM1 mutation could be demonstrated; however, in contrast to primary diagnosis, at relapse a c.956_959dupTCTG mutation (type A) was detected, whereas the original c.959_960insTGCG mutation could not be found (Figure 2b). With a more sensitive quantitative PCR (Q-PCR) approach,4 samples from the primary diagnosis were reanalyzed for an NPM1 type A mutation, but the mutation was not detectable at diagnosis in contrast to the sample from relapse. Short tandem repeat analysis (PowerPlex 16 HS System, Promega, Mannheim, Germany) of the two samples at diagnosis and relapse excluded a sample mixup. The therapy consisted of two cycles ICE for elderly patients, followed by one cycle high-dose cytarabine, idarubicin, etoposide for elderly.
After the first induction cycle, blasts were reduced to 5% in bone marrow without any aberrant profile in flow cytometry. From that time point, no NPM1 mutation was detectable and the patient is waiting for bone marrow transplantation.
To our knowledge, we describe for the first time a switch of a NPM1 mutation at relapse, compatible with newer models of AML genesis. Relapses with loss of NPM1 mutation have been initially interpreted as secondary AML in the literature because the tumor cells gained independent genetic events, like MLL partial tandem duplications.5 According to this, a patient suffering from an acute monocytic leukemia became dramatically negative for the initial NPM1 mutation after induction therapy although no remission was achieved.9 Single cell analysis of FLT3 mutations has demonstrated tumor heterogeneity as minor clones with different FLT3–ITD mutations in the same patient were detected; however, FLT3 mutations are regarded as late events in leukemogenesis, whereas NPM1 mutations should be early ones.10 Recently, a next-generation deep-sequencing study has revealed discrepancies regarding RUNX1 mutations at primary diagnosis and relapse, also showing novel mutations at different regions of the RUNX1 gene.11
The appearance of a novel pathologic marker profile in the majority of blasts at relapse points to the occurrence of a secondary AML rather than the recurrence of the original leukemic clone. This is supported by the fact that the type A mutation of NPM1 was undetectable at diagnosis, even with a highly sensitive Q-PCR assay. As pointed out before, monitoring of minimal residual disease (MRD) along with therapy by Q-PCR using NPM1 mutations as marker is increasingly applied. This case demonstrates that it is reasonable to use more than one marker for MRD analysis, for example, Wilms tumor 1 (WT1) Q-PCR or multicolor FACS.
In conclusion, detecting two different NPM1 mutations at initial diagnosis and relapse in AML supports the model of a primary genetically instable hematopoietic stem cell with potential for gaining mutations in unique cell clones, eventually expanding in the clinical course. Our case also demonstrates the necessity of a broad genetic rescreening at relapse, not only at the onset of leukemia, as monitoring by NPM1 mutation type-specific methods would have missed the newly developed mutation at relapse.
The authors declare no conflict of interest.
References
- Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- Patel JP, Gönen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–1089. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittger S, Kern W, Tschulik C, Weiss T, Dicker F, Falini B, et al. Minimal residual disease levels assessed by NPM1 mutation-specific RQ-PCR provide important prognostic information in AML. Blood. 2009;114:2220–2231. doi: 10.1182/blood-2009-03-213389. [DOI] [PubMed] [Google Scholar]
- Krönke J, Schlenk RF, Jensen KO, Tschürtz F, Corbacioglu A, Gaidzik VI, et al. Monitoring of minimal residual disease in NMP1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011;29:2709–2716. doi: 10.1200/JCO.2011.35.0371. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Kiyoi K, Miyawaki S, Asou N, Ohno R, Saito H, et al. Molecular evolution of acute myeloid leukemia in relapse: unstable N-ras and FLT3 genes compared with p53 gene. Br J Haematol. 1999;104:659–664. doi: 10.1046/j.1365-2141.1999.01256.x. [DOI] [PubMed] [Google Scholar]
- Warren M, Luthra R, Yin CC, Ravandi F, Cortes JE, Kantarjian HM, et al. Clinical impact of change of FLT3 mutation status in acute myeloid leukemia patients. Modern Pathol. 2012;25:1405–1412. doi: 10.1038/modpathol.2012.88. [DOI] [PubMed] [Google Scholar]
- Radojkovic M, Tosic N, Colovic N, Ristic S, Pavlovic S, Colovic M. Reversal of FLT3 mutational status and sustained expression of NPM1 mutation in paired presentation, and relapse samples in a patient with acute myeloid leukemia. Ann Clin Lab Sci. 2012;42:186–190. [PubMed] [Google Scholar]
- Qiao C, Zhang R, Hong M, Wang L, Wu YJ, Qiu HR, et al. Heterogeneous leukemic clones identified by NPM1 mutation analysis in patient with acute monocytic leukemia. Leuk Lymphoma. 2012;53:886–890. doi: 10.3109/10428194.2011.635860. [DOI] [PubMed] [Google Scholar]
- Shouval R, Shlush LI, Yehudai-Resheff S, Ali S, Pery N, Shapiro E, et al. Single cell analysis exposes intra-tumor heterogeneity and suggests that FLT3-ITD is a late event in leukemogenesis Exp Hematol 2014. e-pub ahead of print 2 February 2014; doi: 10.1016/j.exphem.2014.01.010 [DOI] [PubMed]
- Kohlmann A, Nadarajah N, Alpermann T, Groddmann V, Schindela S, Dicker F, et al. Monitoring of residual disease by next-generation deep-sequencing of RUNX1 mutations can identify acute myeloid leukemia patients with resistant disease. Leukemia. 2014;28:129–137. doi: 10.1038/leu.2013.239. [DOI] [PubMed] [Google Scholar]