Myeloproliferative neoplasms (MPNs) are a heterogeneous group of leukemias with defective regulation of myeloid stem cell proliferation. They include four distinct diseases: chronic myeloid leukemia, polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF).1, 2 In 2005, four independent studies have concurred to the identification in MPN patients of a specific mutation in the Janus kinase 2 (JAK2) protein (1849 G→T in exon 14; 617Val→Phe) in the majority of MPNs.1, 2 Indeed, this JAK2V617F variant was identified in 95% of PV, 55% of ET and 65% of PMF patients. The amino-acid change triggers a constitutive activation of the JAK2 protein, independently of cytokines. Whether JAK2V617F is the initiating event in these MPNs remains under debate; however, JAK2V617F seems to drive the phenotype of the disease.
JAK2 gene is located on human chromosome 9, at p24.1 locus. It is composed of 25 exons and encodes a 1132 amino-acid protein of 130.7 kDa apparent molecular weight. The gene is expressed only in blood cells, bone marrow and lymph nodes. Occurrence of JAK2V617F in association with phenotypically different classes of MPNs raised a controversy as to the real function of the mutated JAK2 and the possible involvement of additional genetic determinants in these phenotypic discrepancies. Recent whole genomic studies have identified few single-nucleotide polymorphisms (SNPs) in the vicinity of exon 143, 4, 5 (Figure 1a). Notably, it has been anticipated that the 46/1 haplotype predisposes to the JAK2V617F mutation.5 Other studies have suggested that several SNPs could be implicated in MPN phenotype disparity.6 The question remains as to the possible role of these SNPs in MPN phenotypic discrepancies.
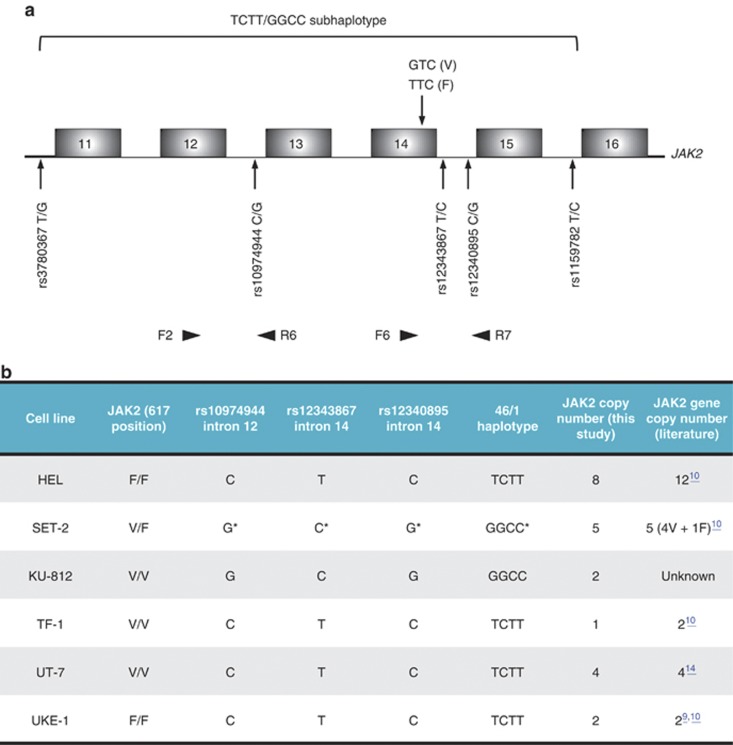
Figure 1.
DNA analysis. (a) Schematic representation of JAK2 gene region surrounding exon 14. The codon 617 mutation in JAK2V617F variant, together with the five SNPs defining the TCTT/GGCC subhaplotype of 46/1 haplotype are depicted, and their positions indicated by arrows. The polymorphic sites were found in linkage disequilibrium (see Text); hence, we focused on sequencing genomic PCR fragments encompassing the three sites surrounding the V617F mutation, and the mutation itself, using the two pairs of primers F2/R6 and F6/R7 (Supplementary Table 2), indicated at the bottom of the scheme. (b) Genetic characterization of the cell lines used. Genomic sequencing analysis led to confirm the JAK2 status regarding the amino acid at position 617, and to determine the three SNPs rs10974944, rs12343867 and rs1230895, as well as the deduced haplotypes. The predominant haplotype associated with the JAK2617V allele in SET-2 cells is indicated by an asterisk. Real-time PCR of a genomic region encompassing exon 8–intron 8 of JAK2, normalized to SDHA (succinate dehydrogenase complex subunit A) genomic fragment, served to determine JAK2 copy number in the tested cell lines, using the forward and reverse primers presented in Supplementary Table 2. DNA from peripheral blood lymphocytes of a healthy blood donor without any hematological anomaly was used as control.
This 46/1 haplotype is a 280-kb-long region on chromosome 9 that includes JAK2, INSL4 and INSL6 genes. Four SNPs define the ‘GGCC' part of this haplotype and are located in the JAK2 gene: rs3780367 T/G in intron 10, rs10974944 C/G in intron 12, rs12343867 T/C in intron 14 and rs1159782 T/C in intron 15 (Figure 1a). All four SNPs are in complete linkage disequilibrium.7 The 46/1 haplotype includes the sequences frequently mutated in MPNs like mutations in exons 12 and 14. On another hand, the rs10974944G minor allele was significantly more common in PV than in ET conditions.3 In addition, it was shown that other JAK2 SNPs, namely rs7046746, rs10815148 and rs12342421, were also significantly associated with PV and ET but not with PMF.6
In this work, we hypothesized that the JAK2V617F and/or the 46/1 haplotype could have an impact on JAK2 mRNA level or/and pre-mRNA maturation. To provide new insights into the JAK2 mRNA expression and splicing, six cell lines, derived from patients with MPNs, were chosen on the basis of their JAK2 status (Figure 1b), and cultured in appropriate media (Supplementary Table 1). It has been suggested that both in human and mouse, the phenotypic expression of the disease depends on the intensity of the signaling, which is thought to be modulated only by the number of JAK2V617F copies.8 Moreover, it was recently emphasized that the number of JAK2 genes in UKE-1 cell line increases with time in culture.9 We therefore determined the actual number of JAK2 genes in the six cell lines used in our laboratory before further investigation (Figure 1b). The number of JAK2 genes in SET-2, UT-7 and UKE-1 cell lines was as previously reported, whereas only eight JAK2 gene copies were found in HEL cell line. KU-812 cell line was found to have two copies of the gene. As for TF-1, the cell line was described with two copies of the JAK2 gene,10 however, the subclone in our laboratory displayed only one copy. It is possible that a loss of heterozygosity occurred, leading to a haploid cell line. Comparative data are gathered in Figure 1b.
We next established the genotypes and haplotypes around the JAK2 gene for the six JAK2V617-negative or -positive cell lines (Figure 1b). To define the JAK2-associated haplotypes, genomic DNA obtained from the six cell lines was amplified with specific primers: one set of primers allows the amplification of a 3047-bp fragment containing the two intron 14 SNPs rs12340895 and rs12343867, in 3′ of the JAK2V617F mutation, along with the JAK2V617F mutation itself (Figure 1a). Another set of primers was used to characterize the intron 12 SNP rs10974944, in 5′ of the JAK2V617F mutation. PCR fragments were then fully sequenced and sequence polymorphisms characterized.
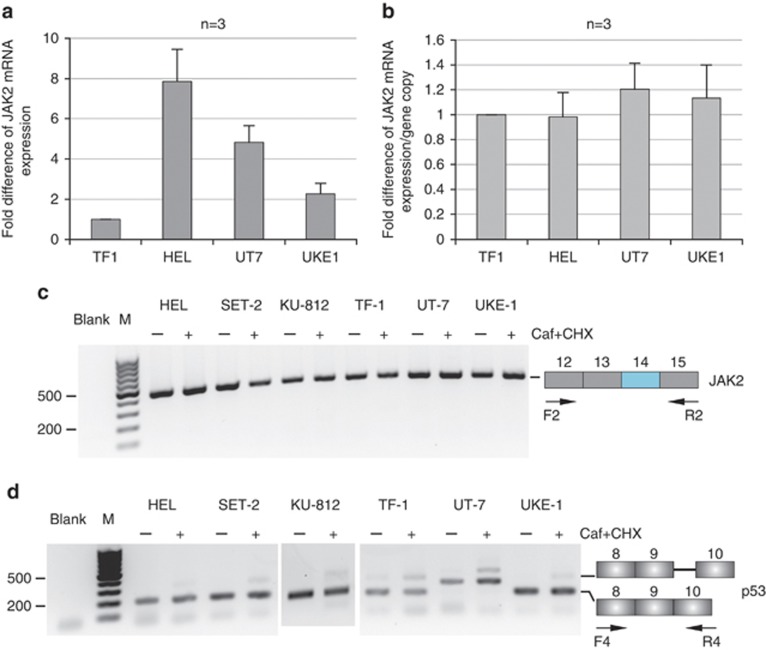
The HEL, TF-1, UT-7 and UKE-1 cell lines were then chosen to test whether the JAK2V617F mutation affects the steady-state level of JAK2 mRNA. These cells have the same ‘TCTT' haplotype; two of them, HEL and UKE-1, are homozygous for the JAK2V617F mutation, whereas the two others, TF-1 and UT-7, contain only the wild-type allele (Figure 1b). Quantitative analyses revealed that accumulated JAK2 mRNA level differs widely from one cell line to another (Figure 2a). However, once correlated to JAK2 gene copy number, these data showed no difference between cell lines, suggesting that each copy of the gene yields about the same amount of JAK2 mRNA molecules accumulating in each cell line, independently of the JAK2V617F mutation (Figure 2b). Collectively, JAK2 mRNA steady-state level correlates with JAK2 gene copy number, regardless of the presence or absence of exon 14 single-nucleotide mutation.
Figure 2.
mRNA analysis. (a, b) JAK2 steady-state mRNA expression. Real-time reverse transcription (RT)-PCR experiments were performed on cells expressing either the wild-type (WT) JAK2 or the JAK2V617F variant. Primers for the PCR step were designed upstream of the exon 14 mutation, within exon 8 (forward primer) and exon 9 (reverse primer) (Supplementary Table 2). Data were expressed as fold difference of JAK2 mRNA level relative to TF-1 single gene copy, and standardized to SDHA mRNA expression13 (a). JAK2 mRNA steady-state level per gene copy was determined for each cell line (b). (c, d) JAK2 mRNA splicing analysis. JAK2 mRNA deriving from the WT or JAK2V617F allele was analyzed by RT-PCR, using pairs of primers surrounding exon 14 (Supplementary Table 2). Cells were treated with caffeine (Caf) and cycloheximide (CHX) to stabilize nonsense mRNA molecules.11 RT-PCR experiments were performed on untreated (−) or treated (+) cells. No abnormal mRNA species was detected at exon 14 and flanking exons (c). An alternative splicing event, generating the inclusion in mature p53 mRNA of an additional sequence from intron 9, leads to the accumulation of a nonsense p53 mRNA species. This alternative splicing event was analyzed concomitantly in the same samples (d) to ascertain the efficiency of cell treatment. In this splicing event, 133 bp of p53 intron 9 are included in the mRNA. After treatment with caffeine and cycloheximide, two bands at 174 and 307 bp were visible on agarose gel, whereas only the 174 bp band was visible in the absence of cell treatment (d). In the UT-7 cell line, the same pattern is observed, although the two bands were higher than expected. Most likely, an additional splicing event must occur specifically in this cell line.
To identify potential exonic and intronic motifs and predict the effects of mutations on pre-mRNA splicing, a preliminary bioinformatics study was performed using ‘Human Splicing Finder' algorithm http://www.umd.be/HSF/. This analysis showed a differential binding of several splicing factors between the wild-type and the mutated sequence (Supplementary Figure 1). In particular, one potential site for the serine–arginine-rich (SR) protein SRSF6 is lost in the mutated sequence compared with the wild type, whereas a putative site for SRSF2, another member of the SR protein family, appears (Supplementary Figure 1). In silico analysis also revealed that the G to C mutation favors the occurrence of two exonic splicing silencers (ESSs): a putative ESS (PESS) octamer, and Fas-ESSs (Supplementary Figure 1).
On the basis of this preliminary in silico analysis, we hypothesized that the JAK2V617F mutation itself or the associated haplotype could affect the JAK2 mRNA splicing or/and stability, and therefore modulate the protein expression and activity, which would affect the phenotypic discrepancies of JAK2V617F-associated MPNs.
Alteration of pre-mRNA splicing due to the JAK2V617F mutation would lead either to an inframe translatable mRNA species or to an out-of-frame mRNA, which most likely contains a premature termination codon. Such nonsense mRNAs are harmful for cellular functions, and therefore, they are intercepted in most cases by a potent mechanism, called nonsense-mediated mRNA decay (NMD), and targeted to rapid degradation.
We analyzed the JAK2 mRNA splicing pattern, focusing on exon 14 region, in cells with or without the JAK2V617F mutation. The six cell lines were treated with cycloheximide, which inhibits translation, and caffeine, which inhibits UPF1 phosphorylation and therefore blocks the NMD mechanism.11 RNA was isolated and reverse transcription-PCRs were performed with specific primers around exon 14. Using a set of primers in exons 12 and 15, a single band of 480 bp was revealed in all cell lines, in untreated as well as in treated cells (Figure 2c). In the same manner, a single band of 445 bp was amplified with primers designed in exons 13 and 16 (Supplementary Figure 2). Additional PCRs around the mutation were performed between exons 12 and 16 (data not shown); yet no abnormal band appeared. To ascertain the efficiency of the NMD blockage with the two drugs, we tested a nonsense p53 mRNA generated from a physiological alternative splicing around exon 9 and known to be stabilized through NMD blockage12 (Figure 2d).
Altogether, mRNA investigations showed no splicing defects around exon 14, and a constant level of mRNA accumulation per JAK2 gene copy, regardless of the presence or absence of exon 14 JAK2V617F mutation. Furthermore, no splicing alteration of the JAK2 mRNA was found around exon 14, in association with the two different haplotypes investigated, hence rebutting the hypothesis suggested by the preliminary bioinformatics study. However, it cannot be excluded that a distant splicing defect, yet linked to the mutation, would occur on one or/and the other haplotype.
Although no abnormal splicing was detectable in JAK2V617F, we believe that most of the mutations recently discovered in MPNs might benefit from our approach to unravel the pathophysiology of MPNs, and better understand the different phenotypic manifestations resulting from the same mutations. We will extend our investigation to specific missense mutations found in a variety of genes in association with MPNs.
Acknowledgments
We thank Dr S Mazoyer for technical advice. This work was supported by grants from the Ligue Nationale contre le Cancer, Comité de la Loire, and from the CNRS. The authors were supported by the INSERM, the University Lyon 1 and the Hospices Civils de Paris.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Author contributions
PN performed most of the molecular studies and drafted the first version of the manuscript. FD contributed to the characterization of MPN cell lines. FB supervised and conceived the molecular studies, and drafted the final manuscript. All authors read and approved the manuscript.
Supplementary Material
References
- Milosevic JD, Kralovics R. Genetic and epigenetic alterations of myeloproliferative disorders. Int J Hematol. 2013;97:183–197. doi: 10.1007/s12185-012-1235-2. [DOI] [PubMed] [Google Scholar]
- Vainchenker W, Delhommeau F, Constantinescu SN, Bernard OA. New mutations and pathogenesis of myeloproliferative neoplasms. Blood. 2011;118:1723–1735. doi: 10.1182/blood-2011-02-292102. [DOI] [PubMed] [Google Scholar]
- Kilpivaara O, Mukherjee S, Schram AM, Wadleigh M, Mullally A, Ebert BL, et al. A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet. 2009;41:455–459. doi: 10.1038/ng.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olcaydu D, Harutyunyan A, Jager R, Berg T, Gisslinger B, Pabinger I, et al. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet. 2009;41:450–454. doi: 10.1038/ng.341. [DOI] [PubMed] [Google Scholar]
- Jones AV, Chase A, Silver RT, Oscier D, Zoi K, Wang YL, et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009;41:446–449. doi: 10.1038/ng.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardanani A, Fridley BL, Lasho TL, Gilliland DG, Tefferi A. Host genetic variation contributes to phenotypic diversity in myeloproliferative disorders. Blood. 2008;111:2785–2789. doi: 10.1182/blood-2007-06-095703. [DOI] [PubMed] [Google Scholar]
- Hermouet S, Vilaine M. The JAK2 46/1 haplotype: a marker of inappropriate myelomonocytic response to cytokine stimulation, leading to increased risk of inflammation, myeloid neoplasm, and impaired defense against infection. Haematologica. 2011;96:1575–1579. doi: 10.3324/haematol.2011.055392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J, et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111:3931–3940. doi: 10.1182/blood-2007-08-107748. [DOI] [PubMed] [Google Scholar]
- Buors C, Douet-Guilbert N, Morel F, Lecucq L, Cassinat B, Ugo V. Clonal evolution in UKE-1 cell line leading to an increase in JAK2 copy number. Blood Cancer J. 2012;2:e66. doi: 10.1038/bcj.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnucci G, Amicarelli G, Salmoiraghi S, Spinelli O, Guinea Montalvo ML, Giussani U, et al. A novel, highly sensitive and rapid allele-specific loop-mediated amplification assay for the detection of the JAK2V617F mutation in chronic myeloproliferative neoplasms. Haematologica. 2012;97:1394–1400. doi: 10.3324/haematol.2011.056184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinière M, Delhommeau F, Calender A, Ribeiro L, Delaunay J, Baklouti F. Nonsense-mediated mRNA decay (NMD) blockage promotes nonsense mRNA stabilization in protein 4.1R deficient cells carrying the 4.1R Coimbra variant of hereditary elliptocytosis. Blood Cells Mol Dis. 2010;45:284–288. doi: 10.1016/j.bcmd.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Anczukow O, Ware MD, Buisson M, Zetoune AB, Stoppa-Lyonnet D, Sinilnikova OM, et al. Does the nonsense-mediated mRNA decay mechanism prevent the synthesis of truncated BRCA1, CHK2, and p53 proteins. Hum Mutat. 2008;29:65–73. doi: 10.1002/humu.20590. [DOI] [PubMed] [Google Scholar]
- Baklouti F, Morinière M, Haj-Khelil A, Fénéant-Thibault M, Gruffat H, Couté Y, et al. Homozygous deletion of EPB41 genuine AUG-containing exons results in mRNA splicing defects, NMD activation and protein 4.1R complete deficiency in hereditary elliptocytosis. Blood Cells Mol Dis. 2011;47:158–165. doi: 10.1016/j.bcmd.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Komatsu N, Nakauchi H, Miwa A, Ishihara T, Eguchi M, Moroi M, et al. Establishment and characterization of a human leukemic cell line with megakaryocytic features: dependency on granulocyte-macrophage colony-stimulating factor, interleukin 3, or erythropoietin for growth and survival. Cancer Res. 1991;51:341–348. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.