Abstract
Cognitive conflict detection and resolution develops with age across childhood and likely supports age-related increases in other aspects of cognitive and emotional development. Little is known about the neural correlates of conflict detection and resolution in early childhood. In the current study, we investigated age-related change in neural recruitment during a blocked spatial-incompatibility task (Simon task) in children ages 5–10 years using fMRI. Cortical thickness was measured using structural MRI. Across all children, there was greater activation in right prefrontal and bilateral parietal cortices for incompatible than compatible conditions. In older children, compared with younger children, there was decreased activation and decreased gray matter thickness in the medial pFC. Thickness and activation changes across age were associated within participants, such that thinner cortex was associated with less activation in the rostral ACC. These findings suggest that developmental change in medial pFC activation supports performance on cognitive control tasks in early childhood.
INTRODUCTION
As children grow up, they improve in their ability to self-regulate (Vazsonyi & Huang, 2010; Murray & Kochanska, 2002). This development is thought to result from the enhanced ability to identify appropriate actions from a number of alternatives and suppress less task-relevant representations (Best, Miller, & Jones, 2009; Garon, Bryson, & Smith, 2008). In adults, the combined ability to perform these functions is described as cognitive control. Cognitive control is hypothesized to be a fundamental cognitive capacity whose developmental progression across childhood supports the maturation of self-regulatory competence in both cognitive and emotional domains (Lahat & Fox, 2013; Munakata, Snyder, & Chatham, 2012; Diamond, 2002). Some researchers propose that particularly in early childhood, before the age of 7 years, changes in cognitive control drive cognitive development across a range of tasks, including theory of mind and task switching (Benson, Sabbagh, Carlson, & Zelazo, 2012; Carlson & Zelazo, 2011; Diamond, Carlson, & Beck, 2005). Thus, one important goal is understanding the neural correlates of development in this set of fundamental cognitive capacities during early childhood.
Cognitive control is not a unitary function, but instead is comprised of a number of cognitive functions including suppression or inhibitory control processes, working memory, task switching, response conflict, and error monitoring, which may rely on different neural substrates and have different developmental trajectories. The neural correlates of development in working memory (Thomason et al., 2009), task switching (Crone, Donohue, Honomichl, Wendelken, & Bunge, 2006; Crone,Wendelken, Donohue, & Bunge, 2006), and inhibitory control (Rubia, Smith, Taylor, & Brammer, 2007; Rubia et al., 2006; Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002) have been widely studied. However, fewer studies to date have specifically focused on the development of conflict monitoring/error detection, particularly those employing fMRI to identify changes in activation across age. In addition, all early studies of cognitive control have focused on children in middle childhood, at least 8 years. It is well documented that early childhood is characterized by large changes in cognitive ability (e.g., Davidson, Amso, Anderson, & Diamond, 2006); here we examine the neural underpinnings of cognitive control in children ages 5– 10 years.
Conflict monitoring theory asserts that one aspect of cognitive control is the identification of situations in which more than one incompatible behavioral response is actively represented (e.g., press right and press left), resulting in conflicting possible behaviors; among these, the task-relevant response must be identified and executed (Botvinick, Cohen, & Carter, 2004; MacDonald, Cohen, Stenger, & Carter, 2000; Carter et al., 1998). Numerous studies indicate that, in adults, a neural substrate of conflict monitoring is the ACC, which is more active in conditions where multiple responses are actively maintained (e.g., the color naming condition of the color–word Stroop task) compared with conditions where one response is primarily active (e.g., the reading condition of the Stroop task; Botvinick et al., 2004; MacDonald et al., 2000).
The development of response conflict from ages 10 through adulthood has implicated ACC as a region of functional maturation (Rubia et al., 2007). Participants ages 10–42 performed an adaptive stop signal task to assess ACC recruitment across age in conditions of increased response conflict. The stop task presents participants with two “go” stimuli, each of which is paired with a specific response (e.g., press the right hand to a rightward pointing arrow, press the left hand to a leftward pointing arrow). On a minority of trials (~20%), participants are instructed to withhold responses if a stop signal (e.g., an upward pointing arrow) is presented. To isolate conflict monitoring instead of general inhibition or suppression processes, activation for trials on which participants pressed to a usual “go” signal was compared with activation for trials on which participants were unable to withhold a response and erroneously pressed to a “stop” signal (thus, two instances of “going” one with increased conflict monitoring demands). Across all participants, a network of cognitive control-related areas were more active for failed stop trials compared with go trials including parietal regions, but only ACC increased activation with age for this comparison (Rubia et al., 2007).
In the current study, we investigated the functional brain development of conflict monitoring in younger children ages 5–10 years. To accommodate children in this young age range, we used a very simple spatial response incompatibility task, the Simon task (Simon & Small, 1969), presented in a blocked design. This Simon task has been widely used and in adults typically elicits neural activation in the ACC, dorsolateral pFC (DLPFC), superior parietal cortex, and precuneus (Liu, Banich, Jacobson, & Tanabe, 2004; Fan, Flombaum, McCandliss, Thomas, & Posner, 2003; Dassonville et al., 2001). In addition, this task has been used successfully with children as young as 4 years who perform this task at greater than 90% accuracy (Davidson et al., 2006). The use of a block design response incompatibility task permitted a relatively short experimental session, which is needed for children as young as 5 years. This is the first study, to our knowledge, examining the neural correlates of conflict monitoring in children as young as 5 years.
We report a whole-brain analysis followed by hypothesis-driven investigation of four a priori defined ROIs: the right inferior frontal gyrus (IFG), the right and left ACC, and bilateral DLPFC, all areas previously associated with task performance on this and other cognitive control tasks (Rubia et al., 2007; Botvinick et al., 2004; Liu et al., 2004; Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004; Fan et al., 2003; Bunge et al., 2002; Dassonville et al., 2001; MacDonald et al., 2000). In a previous study with this sample, we observed that estimates of gray matter thickness in ACC mediated age-related improvement in resolving response conflict on this task (Kharitonova, Martin, Gabrieli, & Sheridan, 2013), whereas gray matter thickness in the right IFG was not associated with resolution of response conflict. Thus, here we predict that we will observe age-related change in activation in ACC but not the right IFG for trials on which response conflict is high.
Right IFG
The right IFG has previously been hypothesized to specifically support inhibitory control (Aron, 2007; Aron, Robbins, & Poldrack, 2004; Hazeltine, Bunge, Scanlon, & Gabrieli, 2003), suggesting that this region might be particularly sensitive to incongruency in the Simon task and that activation of this region may change with age. If age-related improvement in this task results from development of inhibitory control and its neural substrates, we predict that increased activation in the right IFG for the incongruent > congruent contrast will be associated with age.
ACC
ACC is activated in support of the detection and resolution of conflicting response possibilities (Botvinick et al., 1999, 2004) and in support of error detection. Because the Simon task requires resolution of response conflict in the incongruent blocks (pressing on the side of the screen opposite of the stimulus), we predict increased ACC activation for incongruent > congruent contrast. If age-related changes in conflict monitoring are what underlie changing performance on this inhibitory control task, we hypothesize increased ACC recruitment associated with age (as in Rubia et al., 2007, for older children and adults). An alternate explanation would be that age is associated with change in errors for the incongruent > congruent contrast, and thus, age-related change in ACC activation to this contrast reflects changes in error detection or monitoring (Ridderinkhof et al., 2004). This explanation would be predicated on the observation of age-related change in task performance for incongruent relative to congruent trials. A final possibility is that neural activity becomes more efficient with age, which would predict decreased ACC activation with increasing age.
DLPFC
In adults, the DLPFC is often observed to activate during spatial incompatibility tasks (Ridderinkhof et al., 2004; Fan et al., 2003; MacDonald et al., 2000). It is hypothesized that where ACC performs an error or conflict detection role, the DLPFC may perform a role more consistent with on-line adjustment of behavior to meet task demands in the face of conflict or an implementation of cognitive control (MacDonald et al., 2000). If differences in task performance across 5–10 years are primarily because of increases in one’s ability to implement cognitive control strategies, we would expect changes in DLPFC function to be associated with age.
METHODS
Participants
Forty children were included as participants in this study. Of the original 40, 7 were excluded from further analysis: 2 because they could not tolerate the fMRI scanner, 2 because they moved excessively during the functional scans, and 3 because they did not understand task instructions. This resulted in a final sample of 33 children (Mage = 8.1 years, SD = 1.66 years, range = 5.7–10.7 years; 14 female).
We are examining age-related change in activation in children who are younger than previously reported in fMRI studies, and some children’s data could not be included because of movement or tolerating the scanner. Because of this, one concern would be that younger participants were differentially selected than older participants. To address this concern, we report associations between age, gender, IQ, and number of movement or signal change-related outliers using bivariate correlation or two-sample t tests.
Procedure
All participants came to the Athinoula A. Martinos Center for Brain Imaging at Massachusetts Institute for Technology (MIT). The child’s mother, father, or legal guardian provided written consent on the same day that the child participated in the mock scanning session. Researchers explained mock and real scanning and testing procedures to parents and children, answered questions, and provided written consent documents. All procedures were approved by the institutional review board at MIT and were conducted in accordance with the Helsinki declaration on human participants.
Mock Scan Session
Children first participated in a preliminary session where they lay in a mock brain scanner, listened to recordings of the sounds they would hear in the scanner, and participated in a training paradigm that taught them to lie still. During this training, they watched a movie while their head motion was monitored using a motion detector. If they moved their head more than 3–4 mm during the movie, it would pause to indicate they had moved too much. This training and experiencing the mock scanner took approximately 30 min. Children also practiced the task they would perform in the real scanner while lying in the mock scanner. Children ages 5–7 years participated in the “mock scan session” on a separate day from the real scan. Children ages 8–10 years participated in the mock scan session on the same day before the real scan.
Behavioral Testing
Following fMRI scanning, children completed a standardized estimate of IQ (KBIT). Two participants did not complete the KBIT because they had recently completed this test in another study and their responses would be invalid.
Spatial Response Incompatibility Task (Simon Task)
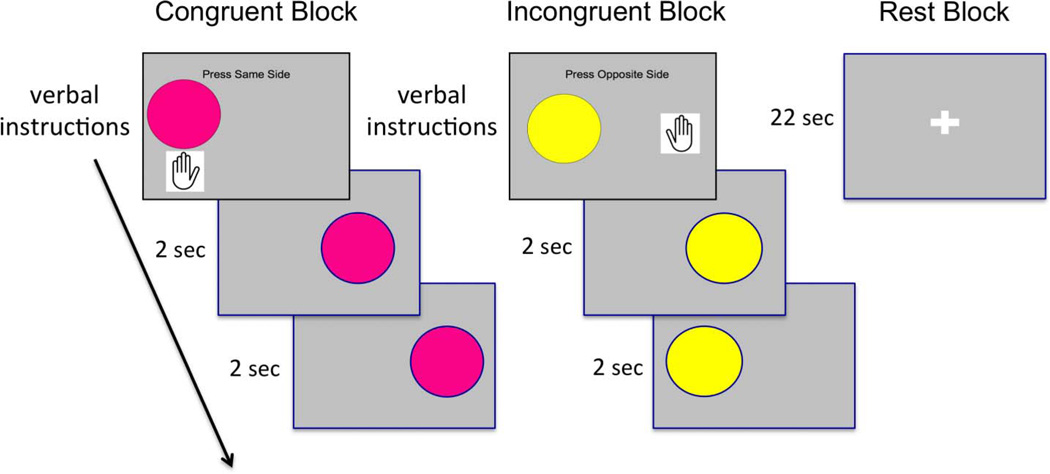
During fMRI scanning, children performed a “Simon task” (Figure 1). In this task (adapted from Davidson et al., 2006), children were presented with two kinds of stimuli (e.g., pink and yellow circles). These stimuli were presented one at a time on either the right or left side of the screen. Children were told to press the side-congruent button for one of these stimuli (e.g., for pink circles presented on the right, press with your right finger) and the side incongruent button for the other stimuli (e.g., for yellow circles presented on the right, press with your left finger). This task requires response conflict monitoring and inhibitory demands: Both the side-congruent and side-incongruent trials have general spatial attention and response selection demands in that the correct motor response must be selected and executed. Only the side-incongruent trials have an additional cognitive control demand. On side-incongruent trials, the prepotent side-congruent response is automatically represented, but task demands require that participants additionally represent side-incongruent responses and ultimately suppress the side-congruent response while selecting the side-incongruent response. Thus, the incongruent > congruent contrast isolates activation supporting resolving response conflict.
Figure 1.
Design of the Simon task.
The two task conditions were presented separately by block. There were three types of blocks: side congruent, side incongruent, and fixation, each lasting 22 sec. During fixation, participants were told to relax and look at the fixation cross-hair. At the beginning of each task block, participants were reminded of the rule they should use with an instruction screen that was presented for 2 sec. Next, they saw 10 trials, lasting 2 sec each, of the task condition indicated by the instruction screen. Each child participated in two runs, each of which included 12 blocks (4 congruent, 4 incongruent, 4 fixation). The order of block was random but fixed within run across participants, and the order of runs was counterbalanced across participants. Altogether, the participants saw 80 instances of each task condition (side congruent and side incongruent). For each trial, accuracy and RT were recorded.
MRI Data Acquisition
All MRIs were acquired at Athinoula A. Martinos Imaging Center at MIT on a 3T Siemens Magnetom Tim Trio scanner. Structural MRI was obtained using an MP-RAGE sequence producing T1-weighted images (echo time = 1.64 msec, repetition time [TR] = 2530 msec, flip angle = 7°, 176 slices with 1 × 1 × 1 mm isometric voxels) using a 32-channel head coil. To reduce the imaging acquisition time, parallel imaging was used with an acceleration factor of 3. BOLD signal was obtained using a gradient-echo T2*-weighted EPI sequence (echo time = 30 msec, TR = 2000 msec, flip angle = 90°, bandwidth = 2300, echo spacing = 0.5, field of view = 192 × 192, matrix size = 64 × 64, resulting in in-plane resolution of 3 mm × 3 mm). Thirty-two 3-mm-thick slices were acquired positioned parallel to the AC–PC line using noninterleaved acquisition. Before each scan, four images were acquired and discarded to allow longitudinal magnetization to reach equilibrium. PACE, an on-line prospective motion correction algorithm, was included to reduce the effect of motion artifacts.
Data Analysis
fMRI Data Analysis
Processing and statistical analysis of fMRI data were performed using a Nipype, a Python-based software platform that incorporates components of several fMRI data analysis packages (nipy.sourceforge.net/nipype/). Following reconstruction of images into 3-D space, realignment and slice timing correction was run using statistical parametric mapping (SPM8; www.fil.ion.ucl.ac.uk/spm/) to correct for subject movement within the scanner. Next, artifact detection (ART tool; web.mit.edu/swg/software.htm) was used to identify for which frames or TRs we should exclude them from the overall analysis; these frames are referred to as “outliers.” Outliers are fluctuations in signal that exceed a certain threshold of signal intensity or movement; they are excluded from analyses by creating unique covariates for each outlier image. Outliers were determined in two ways: (1) by identifying signal intensity flux which was 3 standard deviations above or below the mean and (2) movement in excess of 2 (combined rotation and translation movement parameters). Finally, a 6-mm FWHM isotropic smoothing kernel was used to smooth the data. The number of frames with extreme movement and signal fluctuations (outliers) were negatively correlated with age (r(33) = − 38, p = .03); children ages 5–7 years (M = 12.5, SD = 14.4) had more outliers than children ages 8–10 years (M = 4.5, SD = 4.1). To determine if increased movement accounted for the effects observed across all participants, the four participants with the most outliers were excluded and analyses were repeated. Exclusion of these four participants made the association between age and outliers nonsignificant (r(28) = .1, p = .61). After excluding these four participants, all significant associations increased in strength, and no reported association decreased in strength or became insignificant. We understood this, coupled with the fact that increased outliers necessarily means less data contributing to sample estimates, to indicate that these participants were likely adding noise to our sample; thus, we report the analyses without those four participants here.
Identification of activations was performed using the SPM8 analysis package (Friston, Frith, Frackowiak, & Turner, 1995). Covariates to model blocks of incongruent, congruent, and fixation were entered into the general linear model. Nuisance covariates for motion parameters, outliers (from artifact detection), and run effects were also included in this general linear model. Contrasts were constructed by directly contrasting BOLD-related activity in one type of block with the other (e.g., incongruent–congruent). These individual contrasts were then input into a group-level analysis. A one-sample t test was performed across all participants to identify significant activation for the incongruent > congruent contrast. A two-sample t test was performed to compare activation in younger children (ages 5–7 years) to older children (ages 8–10 years). Clusters are reported if they reached significance at p < .05, corrected using a false discovery rate cluster correction for multiple comparisons implemented in SPM8, with a voxel level significance of p < .01.
To examine the a priori hypotheses concerning neural recruitment across age and task performance, we used an ROI analysis. We examined incongruent > congruent activity in four a priori defined ROIs, the right IFG, the bilateral middle frontal gyrus, and the right and left ACC. ROIs were identified structurally using the Free-Surfer image analysis suite, which is documented and freely available for download on-line (surfer.nmr.mgh.harvard.edu). FreeSurfer morphometric procedures have demonstrated good test–retest reliability across scanner manufacturers and field strengths (Han et al., 2006). In addition, these procedures have been successfully used in studies of children as young as 4 years (Ghosh et al., 2010).
FreeSurfer processing includes motion correction of a volumetric T1-weighted image, removal of nonbrain tissue using a hybrid watershed/surface deformation procedure (Ségonne et al., 2004), automated Talairach transformation, previously validated in pediatric populations (Burgund et al., 2002), and segmentation of the sub-cortical white matter and deep gray matter volumetric structures, separately validated for use with pediatric populations (Ghosh et al., 2010).
FreeSurfer provided thickness estimates for 148 cortical regions (74 for each hemisphere) according to the 2009 Destrieux Atlas (Destrieux et al., 2010). Of these regions, we examined three a priori ROIs outlined above based on hypotheses about regions that should be most critical for performance on the Simon task. The middle frontal gyrus region was calculated by examining activation collapsed across the left and the right middle frontal gyrus (designated as G_frontal_middle), and the ACC region was calculated by examining activation in the left and the right ACC region (designated as G_and_S_cingul_Ant_thickness). Finally, the right IFG was calculated based on activation in the right pars triangularis region (designated as G_frontal_inf-Triangular_part; see freesurfer.net/fswiki/DestrieuxAtlasChanges for further information on segmentations in this atlas). ACC and right IFG ROIs used here are the same as those used to identify associations between task performance and gray matter thickness in a previously published report (Kharitonova et al., 2013).
For functional analysis, first, each participant’s structural and functional data were coregistered using bbregister an alignment technique used by FreeSurfer in the normalization process (freesurfer.net/fswiki/bbregister#Description) and then normalized using standard FSL algorithms. For each participant, functional data were extracted for their FreeSurfer region corresponding to each ROI (e.g., right ACC). This method allowed for the use of independently defined structural ROIs without relying on regions that had been normalized to group space. Additionally, because we previously reported cortical thickness in these same participants, we were able to examine the association between thickness in these ROIs and functional recruitment in the same region using linear regression.
The ROI analysis was conducted using REX on unstandardized beta values from the first-level general linear model analysis (web.mit.edu/swg/software.htm) for the incongruent > baseline, congruent > baseline, and incongruent > congruent contrast. One participant had beta values for BOLD activation in the right IFG for incongruent > fixation that were more than 3 standard deviations from the mean beta value of all participants. As a result, this participant was understood to be an outlier and excluded from analyses of the right IFG. To identify associations between age and activation in these ROIs, an ordinary least squares linear regression analysis was performed. In each regression analysis, we controlled for gender and IQ composite. In our linear regression model, we estimated the association between age and the incongruency effect by estimating the association between age and activation for the incongruent > baseline trials while controlling for activation on congruent > baseline trials. Although many researchers have used difference scores when examining tasks like the Simon task, which use the concept of cognitive subtraction, others have pointed out that using difference scores is less powerful, less reliable, and less accurate than examining the specific measure of interest (e.g., incongruent activation) when controlling for the baseline condition (e.g., congruent activation) to identify the effect of interest (DeGutis, Wilmer, Mercado, & Cohan, 2013; Cronbach & Fruby, 1970), the approach we use here.
Behavioral Data Analysis
The effect of our task manipulation on behavior was estimated using paired subject t tests to compare RT and accuracy on congruent and incongruent trials. To identify the association between task performance and age, we performed regression analyses identical to those described for the ROI analyses. We used these analyses to identify the association between age and behavioral performance for congruent trials, incongruent trials, and the congruency effect.
Brain Behavior Associations
For ROIs that were significantly associated with age, we examined the association between brain and behavior using bivariate correlation.
RESULTS
Demographics
Across all children, there was no significant difference in age or the number of motion outliers between boys and girls (age: t(26) = 0.31, p = .75; outliers: t(26) = 0.47, p = .59). In addition, there was not a significant correlation between age and composite IQ as measured by the KBIT (r(29) = .12, p = .56). Finally, there was no association between IQ and number of outliers (r(29) = −.23, p = .27). IQ and gender were included as covariates in all subsequent regression analyses.
Behavior
All participants were slower when performing incongruent (M = 664 msec, SD = 114 msec) compared with congruent (M = 620 msec, SD = 113 msec) trials (t(27) = 5.93, p < .001), but there was no significant difference in accuracy across conditions (incongruent M = 92%, SD = 11, congruent M = 91%, SD = 12, t(27) = 0.297, p = .77). Controlling for gender and IQ, there were significantly associations between age and accuracy for both congruent trials (age: β = .54, p = .005) and incongruent trials (age: β = .38, p = .04) where children improved their accuracy on all trials with age. There was a nonsignificant trend toward a negative association between age and RT for congruent trials (age: β = −.37, p = .10) and a nonsignificant association between age and RT for incongruent trials (age: β = −.27, p = .24). There was no significant association between age and the incongruency effect for RT (age: β = .08, p = .34) or accuracy (age: β = −.02, p = .86).
BOLD Response
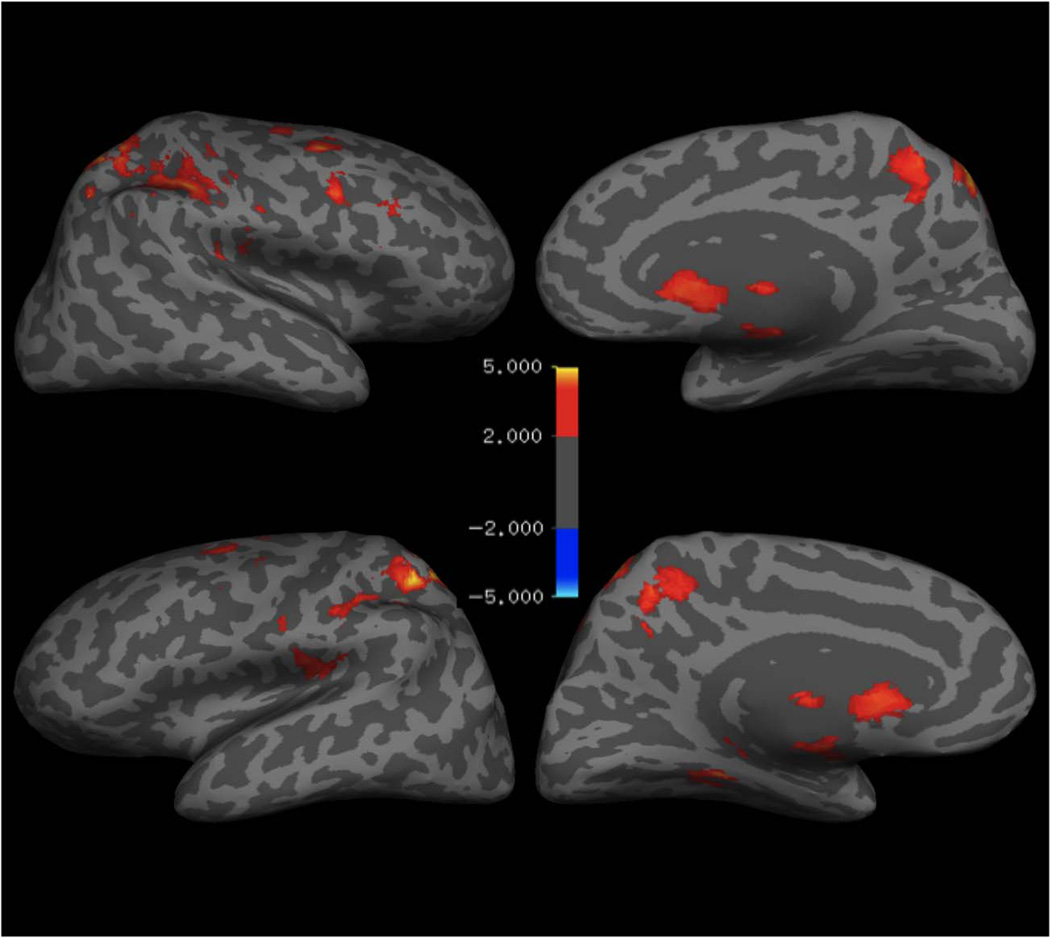
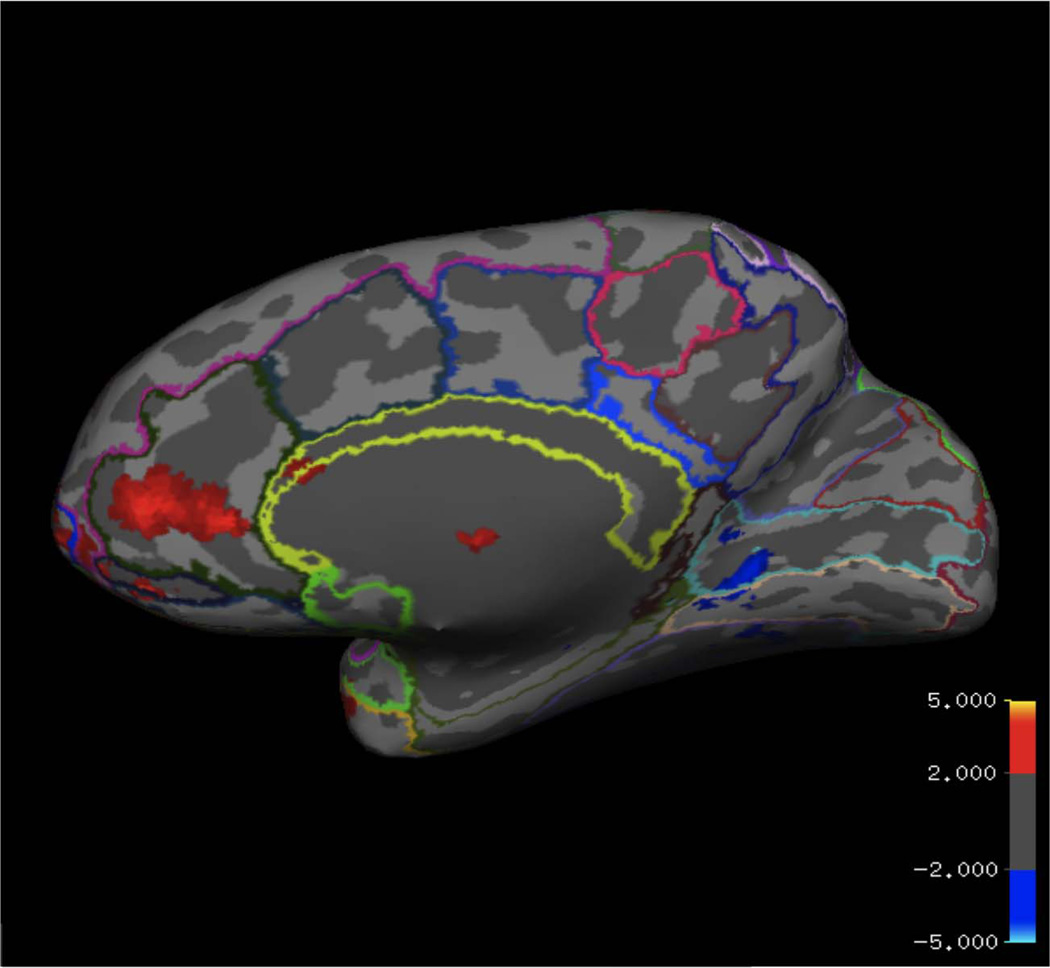
Consistent with previous investigations of the Simon task (Liu et al., 2004; Fan et al., 2003; Dassonville et al., 2001), across all participants there was significantly greater activation for incongruent relative to congruent trials in a network of regions including bilateral superior parietal cortex, inferior parietal cortex, pFC, caudate, thalamus, and brainstem (Table 1; Figure 2). In contrast, when younger children were directly compared with older children in a whole-brain analysis, we observed that children ages 5–7 years activated only the rostral/subgenual ACC more than children ages 8– 10 years for the incongruent > congruent contrast (Figure 3). To further explore the association between age and prefrontal activation in this task, we examined the four independently identified ROIs described above.
Table 1.
Areas of Activation for the Incongruent > Congruent Contrast across All Participants and for that Contrast Compared between Younger (5–7 Years) and Older (8–10 Years) Children
| Region | x | y | z | Voxels | t | |
|---|---|---|---|---|---|---|
| Incongruent > congruent | MFG [R] | 24 | −4 | 46 | 718 | 4.62 |
| MFG [L] | −24 | 12 | 54 | 272 | 3.40 | |
| Caudate [R&L] | −10 | 4 | 16 | 1052 | 4.12 | |
| Brain stem | −2 | −30 | −6 | 468 | 4.2 | |
| Hippocampus [L] | −26 | −42 | 2 | 277 | 4.01 | |
| Superior parietal [R] | 20 | −64 | 56 | 2080 | 5.14 | |
| Superior parietal [L] | −20 | −58 | 62 | 1271 | 5.19 | |
| Young > old for the incongruent > congruent contrast | ACC | 0 | 38 | 4 | 330 | 3.47 |
| Medial pFC | −2 | 64 | −12 | 959 | 3.68 |
Activation in the ACC for the young > old contrast was only marginally significant.
Figure 2.
BOLD activity for the incongruent > congruent contrast presented on a representative inflated FreeSurfer surface. Areas are shown if they survive false discovery rate cluster level correction at p < .05. Please refer to Table 1 for a list of significant activations for this contrast.
Figure 3.
BOLD activity for the incongruent > congruent contrast was significantly greater in the rostral ACC/medial pFC for younger compared with older children. Overlaid on top of this brain are outlines of the segmentation labels from 2009 Destrieux FreeSurfer Atlas. In dark green is the ACC ROI.
Right ACC
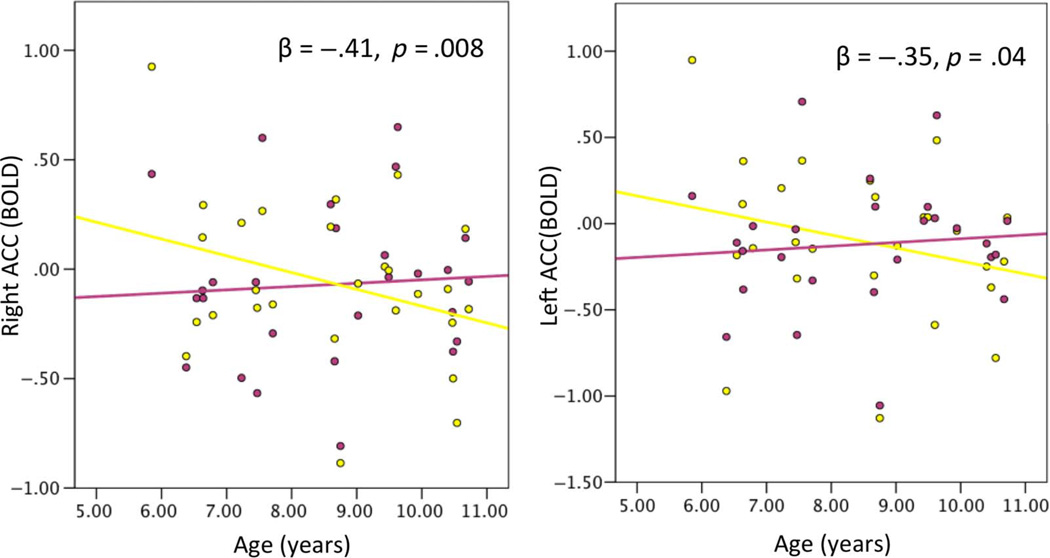
There was a nonsignificant effect of age on activation for incongruent (age: β = −.36, p = .11), but not congruent trials (age: β = .07, p = .76) and a significant association between age and the activation for the incongruency effect (age: β = −.41, p = .008; Figure 4) where children activated the right ACC less with age for incongruent > congruent trials.
Figure 4.
Association between age and activation in the right and left ACC as defined by the FreeSurfer segmentation for each participant. Beta values represent association between age and the incongruent > congruent contrast controlling for gender and estimated IQ. Yellow dots represent activation for incongruent stimuli, and pink dots represent activation for congruent stimuli.
Left ACC
There was no effect of age on activation for incongruent (age: β = −.26, p = .26) or congruent trials (age: β = .11, p = .66). However, there was a significant association between age and the activation for the incongruency effect (age: β = −.35, p = .04; Figure 4) where children activated the left ACC less with age for incongruent > congruent trials.
Right IFG
There was no significant effect of age on activation for congruent trials, incongruent trials, or the incongruency effect.
DLPFC
There was no significant effect of age on activation for incongruent (age: β = −.16, p = .48) and congruent trials (age: β = .14, p = .53) and a trend toward an association between age and the activation for the incongruency effect (age: β = −.27, p = .1) where children activated the DLPFC less with age for incongruent > congruent trials.
Structure–Function Relationships
Thinning in right ACC was associated with less activation for the incongruency effect: β = .32, p = .05. The same association was not significant for left ACC (incongruency effect: β = .11, p = .52).
DISCUSSION
We examined activation in children ages 5–10 years performing a spatial response incompatibility task. Consistent with previous studies of behavioral performance on spatial incompatibility tasks in this age range, we observed a significant effect of side incongruency on RT across all participants, but no association between the incongruency effect and age for either accuracy or RT (Davidson et al., 2006). Across all the children, there was greater activation for incongruent compared with congruent trials in a network of brain regions implicated in cognitive control, including inferior parietal cortex, superior parietal cortex, and right middle frontal gyrus. The only brain region in which activation varied with age was the right ACC, observed both in the ROI analysis and in the whole-brain two-sample t test. Activation for in-congruent relative to congruent trials decreased in ACC as age increased. These findings suggest a specific role of ACC in the neural development of response conflict monitoring and resolution.
In a previous study in this sample, this same ACC ROI exhibited cortical thinning with increasing age (Kharitonova et al., 2013). Decreasing thickness in ACC could reflect selective pruning of redundant connections, so that decreased activation in this same region reflects increased neural efficiency in ACC processing. However, there are several alternate explanations for decreasing activation with age in this region that should be considered. First, decreasing activation might be described as cognitive efficiency. It may be that, as response conflict in this task becomes easier to resolve, ACC is recruited less strongly in support of task demands (perhaps because of development of other cognitive abilities). To further test the neural efficiency hypothesis, we examined the relationship between neural activation and cortical thickness in this region. We observed that thickness and activation were associated specifically within the right ACC, enhancing our support for the neural efficiency hypothesis.
Another potential explanation for decreases in ACC recruitment between 5 and 10 years is that ACC is recruited in the service of error monitoring, not conflict detection or resolution. There is evidence from work with adults that ACC can be recruited in the service of error monitoring (Liu et al., 2004) and response uncertainty (Aarts, Roelofs, & van Turennout, 2008) even in the absence of explicit response conflict. Although we did observe increased accuracy across all kinds of trials in our study between younger and older children, we did not observe this selectively for the incongruency effect, and across all participants, there was no difference in accuracy for in-congruent compared with congruent trials, decreasing (but not eliminating) the likelihood that ACC activation for incongruent > congruent trials was because of error monitoring in this case.
The area of ACC that we define a priori in our ROI or observe to be different between groups in our whole-brain analysis is commonly activated as a part of the “default” network. That is, this area is commonly activated more during rest compared with task. It is possible that areas of the brain more active during rest compared with task will influence findings, a phenomenon sometimes referred to as the “Task B” problem (Church, Petersen, & Schlaggar, 2010). In the current study, we do not think this could account for our findings because (a) there were no significant differences between children ages 5–7 years and children ages 8–10 years in ACC activation during fixation (all ps > .51) and (b) whereas children ages 8–10 years deactivated ACC in response to both task conditions (see Figure 4, incong mean = −.13,congmean = −.04), children ages 5–7 years activated ACC in response to incongruent stimuli (incong mean = .04, cong mean = —.05).
We observed age-related change in ACC in both whole-brain and ROI analyses, consistent with a previous study of the development of conflict monitoring (Rubia et al., 2007). However, our results differed in two ways from this previous study. First, we observed decreases in ACC recruitment with age, whereas that study observed increases in ACC recruitment. There are two possible explanations for this divergence in findings: Rubia and colleagues investigated participants ages 10–42 years, whereas our participants were 5–10 years. It may be that there are nonlinear changes in ACC recruitment in the context of conflict monitoring with age. However, it is more likely that this discrepancy is accounted for by our divergent approaches to equating difficulty across participants: We equated task difficulty by ensuring that all children achieved a high level of accuracy (~90%) and that there was no association between age and the incongruency effect. In contrast, Rubia and colleagues ensured that children and adults achieved similarly low levels of accuracy by manipulating task demands around their contrast of interest so that all participants performed at about 50% accuracy. This difference may account for the difference in direction of the association between age and ACC activation in these two studies. Second, the location of the peak of activation in our study was more ventral than peak activation observed by Rubia and others during response conflict tasks (Rubia et al., 2007; Liu et al., 2004; MacDonald et al., 2000). The peak difference activation observed in the whole-brain analysis between younger and older children is located at the genu of the ACC (see Figure 3). Our a priori defined ACC ROI encompasses this area and more dorsal areas that are commonly activated in adults in the context of response conflict. The locus of the peak of this activation is in an area of the medial pFC sometimes associated with self-reflection and with representing the mental states of others (Jenkins & Mitchell, 2011). However, even within the context of representing the mental states of others, the medial pFC appears to be more active in the context of more ambiguous or conflicting information about others’ mental states (Jenkins & Mitchell, 2010). It may be that increased recruitment of this more ventral-medial pFC region by younger children in the context of a response conflict task is evidence for a less specialized medial pFC, highlighting the underdeveloped nature of pFC in this age range. This lack of specialization is not incompatible with the previously described “neural inefficiency” hypothesis. However, it is also possible that children are selectively representing the mental state of the experimenter or engaging in self-reflection more during incongruent relative to congruent blocks. Because we did not (intentionally) experimentally manipulate self-reflection or mentalizing, we cannot speak to this possibility in the current study.
Limitations
Here we report associations between age and neural recruitment, which we tentatively interpret in light of developmental change in neural processing. Although we observed no association between age and gender or IQ, without a longitudinal sample we are unable to rule out the possibility that our older participants differed from our younger participants on unmeasured variables. Finally, younger children move more than older children; however, we were able to partially account for this confound by eliminating participants with the greatest number of movement-related outliers in the fMRI data. After doing this, we observed no association between age and outliers, and all associations between age and neural activity were strengthened. We feel that these analyses indicate that our findings were robust to movement differences across age.
Conclusions
This is the first investigation of neural recruitment supporting cognitive control in children as young as 5 years using fMRI. Consistent with previous ERP studies (Torpey, Hajcak, Kim, Kujawa, & Klein, 2012; Maguire, White, & Brier, 2011; Cragg, Fox, Nation, Reid, & Anderson, 2009; Davidson et al., 2006) and studies of older children using fMRI (Durston et al., 2006; Booth et al., 2003; Tamm, Menon, & Reiss, 2002), we observe decreased recruitment of ACC and general improvement in task performance across age. We argue that the neural recruitment in older children during this cognitive control task is more efficient than that of younger children and that this efficiency may be related to cortical thinning.
Interestingly, this pattern of associations between age and neural recruitment is substantially different than that observed for other forms of executive function, specifically, working memory (e.g., Thomason et al., 2009) where increased recruitment of task-relevant regions in pFC and improved performance is observed across age. It may be that the cognitive function underlying task performance on cognitive control tasks develops very early, and therefore, decreased neural recruitment may represent honing of an existing skill accompanied by increased differentiation among neural pathways in the medial pFC.
Acknowledgments
We thank our child and family participants, Anisha Keshavan and Susan Whitfield-Gabrieli for statistical support, and NIMH (M.S. K01MH092555) and Robert Wood Johnson Society (M.S. Cohort 5, Health and Society Scholars at Harvard) for funding.
Footnotes
UNCITED REFERENCE
REFERENCES
- Aarts E, Roelofs A, van Turennout M. Anticipatory activity in anterior cingulate cortex can be independent of conflict and error likelihood. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28:4671–4678. doi: 10.1523/JNEUROSCI.4400-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Benson JE, Sabbagh MA, Carlson SM, Zelazo PD. Individual differences in executive functioning predict preschoolers’ improvement from theory-of-mind training. Developmental Psychology. 2012 doi: 10.1037/a0031056. [DOI] [PubMed] [Google Scholar]
- Best JR, Miller PH, Jones LL. Executive functions after age 5: Changes and correlates. Developmental Review: DR. 2009;29:180–200. doi: 10.1016/j.dr.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, et al. Neural development of selective attention and response inhibition. Neuroimage. 2003;20:737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cerebral Cortex (New York, N.Y.: 1991) 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM, Zelazo PD. The value of control and the influence of values. Proceedings of the National Academy of Sciences, U.S.A. 2011;108:16861–16862. doi: 10.1073/pnas.1113235108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science (New York, NY) 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Church JA, Petersen SE, Schlaggar BL. The “Task B problem” and other considerations in developmental functional neuroimaging. Human Brain Mapping. 2010;31:852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg L, Fox A, Nation K, Reid C, Anderson M. Neural correlates of successful and partial inhibitions in children: An ERP study. Developmental Psychobiology. 2009;51:533–543. doi: 10.1002/dev.20391. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ, Fruby L. How should we measure “change”: Or should we? Psychological Bulletin. 1970;74:68–80. [Google Scholar]
- Crone EA, Donohue SE, Honomichl R, Wendelken C, Bunge SA. Brain regions mediating flexible rule use during development. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26:11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cerebral Cortex (New York, NY.: 1991) 2006;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Lewis SM, Zhu X-H, Ugurbil K, Kim S-G, Ashe J. The effect of stimulus-response compatibility on cortical motor activation. Neuroimage. 2001;13:1–14. doi: 10.1006/nimg.2000.0671. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGutis J, Wilmer J, Mercado RJ, Cohan S. Using regression to measure holistic face processing reveals a strong link with face recognition ability. Cognition. 2013;126:87–100. doi: 10.1016/j.cognition.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex, from birth to young adulthood: Cognitive functions, anatomy and biochemistry. Principles of frontal lobe function. Oxford, UK: Oxford University Press; 2002. 2002. [Google Scholar]
- Diamond A, Carlson SM, Beck DM. Preschool children’s performance in task switching on the dimensional change card sort task: Separating the dimensions aids the ability to switch. Developmental Neuropsychology. 2005;28:689–729. doi: 10.1207/s15326942dn2802_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: A multivariate approach. Neuroimage. 1995;2:166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Garon N, Bryson SE, Smith IM. Executive function in preschoolers: A review using an integrative framework. Psychological Bulletin. 2008;134:31–60. doi: 10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Bunge SA, Scanlon MD, Gabrieli JDE. Material-dependent and material-independent selection processes in the frontal and parietal lobes: An event-related fMRI investigation of response competition. Neuropsychologia. 2003;41:1208–1217. doi: 10.1016/s0028-3932(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Jenkins AC, Mitchell JP. Mentalizing under uncertainty: Dissociated neural responses to ambiguous and unambiguous mental state inferences. Cerebral Cortex (New York, NY.: 1991) 2010;20:404–410. doi: 10.1093/cercor/bhp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins AC, Mitchell JP. Medial prefrontal cortex subserves diverse forms of self-reflection. Social Neuroscience. 2011;6:211–218. doi: 10.1080/17470919.2010.507948. [DOI] [PubMed] [Google Scholar]
- Kharitonova M, Martin RE, Gabrieli JDE, Sheridan MA. Cortical gray-matter thinning is associated with age-related improvements on executive function tasks. Developmental Cognitive Neuroscience. 2013;6C:61–71. doi: 10.1016/j.dcn.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat A, Fox NA. The neural correlates of cognitive control and the development of social behavior. Chap. 23. In: Rubenstein J, Rakic P, editors. Neural circuit development and function in the brain. Oxford, UK: Academic Press; 2013. pp. 413–427. Retrieved from www.sciencedirect.com/science/article/pii/B9780123972675000601. [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. Neuroimage. 2004;22:1097–1106. doi: 10.1016/j.neuroimage.2004.02.033. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science (New York, N.Y.) 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Maguire MJ, White J, Brier MR. How semantic categorization influences inhibitory processing in middle-childhood: An event related potentials study. Brain and Cognition. 2011;76:77–86. doi: 10.1016/j.bandc.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata Y, Snyder HR, Chatham CH. Developing cognitive control: Three key transitions. Current Directions in Psychological Science. 2012;21:71–77. doi: 10.1177/0963721412436807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KT, Kochanska G. Effortful control: Factor structure and relation to externalizing and internalizing behaviors. Journal of Abnormal Child Psychology. 2002;30:503–514. doi: 10.1023/a:1019821031523. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science (New York, N.Y.) 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JR, Small AM., Jr Processing auditory information: Interference from an irrelevant cue. The Journal of Applied Psychology. 1969;53:433–435. doi: 10.1037/h0028034. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Race E, Burrows B, Whitfield-Gabrieli S, Glover GH, Gabrieli JDE. Development of spatial and verbal working memory capacity in the human brain. Journal of Cognitive Neuroscience. 2009;21:316–332. doi: 10.1162/jocn.2008.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Kim J, Kujawa A, Klein DN. Electrocortical and behavioral measures of response monitoring in young children during a Go/No-Go task. Developmental Psychobiology. 2012;54:139–150. doi: 10.1002/dev.20590. [DOI] [PMC free article] [PubMed] [Google Scholar]