Abstract
The human visual system is extremely sensitive to the direction information retrieved from biological motion. In the current study, we investigate the functional impact of this sensitivity on attentional orienting in young children. We found that children as early as 4 years old, like adults, showed a robust reflexive attentional orienting effect to the walking direction of an upright point-light walker, indicating that biological motion signals can automatically direct spatial attention at an early age. More importantly, the inversion effect associated with attentional orienting emerges by 4 years old and gradually develops into a similar pattern found in adults. These results provide strong evidence that biological motion cues can guide the distribution of spatial attention in young children, and highlight a critical development from a broadly- to finely-tuned process of utilizing biological motion cues in the human social brain.
The ability to quickly recognize the motion of biological entities in the environment is extremely important for a species' survival, as it enables one to hunt prey, avoid predation, assess the intention of an approaching rival, and even identify a potential mate. Numerous studies have demonstrated that human observers are remarkably adept at detecting and recognizing biological motion signals in complex visual scenes, even when they are portrayed by just a handful of point lights attached to the head and major joints1. Notably, the ability to attend preferentially to biological motion arises at a very early age. For example, infants as young as four months can differentiate between upright and inverted human point-light walkers2 and show preference for the former3. Even newly hatched domestic chicks reared in the dark manifest a spontaneous preference for biological over non-biological motion patterns4. A similar phenomenon has also been observed with human neonates. 2- or 3-day-old infants have been able to distinguish the point-light biological motion from other random rigid motion displays and prefer to look at the former even if it depicts the other species' shape (i.e., a walking hen)5,6. The advantageous processing of biological motion continues to develop in childhood7,8, and the sensitivity to biological motion still improves into adolescence if the point-light displays are embedded in a noise mask9.
It is also worth noting that quite rich socially relevant information, such as gender, action, intention, and even emotion, can also be readily retrieved from these point-light biological motions10,11,12,13,14,15,16. Amongst them, walking direction is a particularly important attribute of biological motion, as it plays a major role in assessing another living creature's intention (e.g., moving towards or moving away from us). Previous studies in adults have found that the walking direction can be extracted even when the point-light displays are embedded in dynamic visual noise17,18,19 or are presented in the peripheral vision20. Intriguingly, peripheral walking direction information can automatically influence the processing of a centrally presented point-light walker21. Newly hatched chicks, lacking any visual experience, tend to align their bodies in the apparent direction of the upright but not inverted point-light animations22, suggesting that there might be an intrinsic sensitivity to walking direction in the visual system.
Some recent studies have also shown that the walking direction of biological motion can further affect human behavioral responses in adults23,24,25. For example, following a brief presentation of a central point-light walker walking toward either the left or right direction, observers' performance on a subsequent probe (i.e., Gabor patch) location discrimination task was significantly better when the probe was presented in the walking direction (congruent condition) than in the opposite direction (incongruent condition) even when observers were told explicitly that walking direction was not predictive of target location24. Moreover, such an effect disappeared when the biological motion displays were shown inverted, indicating a robust inversion effect that has been found widely in biological motion perception26,27,28,29,30. These findings suggest that the walking direction of an upright, but not inverted, point-light walker induces a reflexive attentional orienting effect in adult observers. However, it remains to be delineated whether the attentional effect of biological motion and the associated inversion effect emerge and develop at an early age. The answers to these questions not only add to our comprehensive understandings of biological motion processing in the human visual attention system, but also point to the functional development of a broad social cognitive system (i.e., the social brain) which involves the preferential processing of social information (e.g., face and biological motion)31.
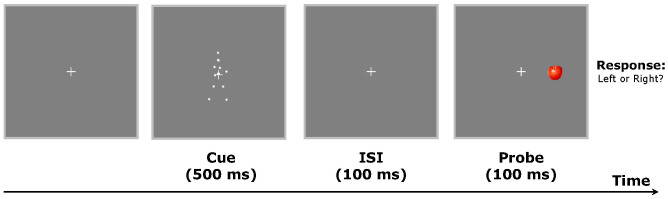
The present study thus aims to investigate the development of the reflexive attentional orienting effect induced by biological motion walking direction. Preschool children aged 4 to 6 years were examined with a similar central cueing paradigm as in the earlier study24, because children within this age range have enough sensitivity to readily discriminate both coarse and subtle differences in the direction of a point-light walker32,33. To make the task easier and more interesting to children, the Gabor-patch target was replaced with a red apple image (Figure 1). In order to directly compare the effects obtained from children with those from adults, adult participants were also tested with the same parameters. It should be noted that the modified central cueing paradigm involved aspects from both standard central cueing and peripheral cueing techniques34,35. A point-light walker, with the walking direction either towards the left or right of fixation, was located in the center of the screen. However, unlike traditional central arrow cues, the direction of the walker was not predictive of the probable location of the subsequent target. In this respect, it followed previous peripheral cueing studies that used spatially uninformative cues to investigate the reflexive response of attention36.
Figure 1. Schematic representation of the experimental paradigm.
Results
Trials with error responses (6.2% in children and 1.7% in adults) or reaction times longer than 3000 ms (0.1% in children and none in adults) were discarded before the statistical analysis. Accuracy and reaction time were analyzed separately through 2 × 2 × 2 mixed-design ANOVAs with Age Group (children vs. adults) being the between-subject factor and Cue Orientation (upright vs. inverted) and Target Congruence (congruent vs. incongruent) being the within-subject factors. Table 1 shows the mean accuracy for each condition and for each participant group. Since the observers were asked to respond as accurately as possible, their overall accuracies were high and didn't show much difference across conditions.
Table 1. Mean accuracies (with standard errors) in each condition.
| Upright | Inverted | |||
|---|---|---|---|---|
| Congruent | Incongruent | Congruent | Incongruent | |
| Children | 0.96 (0.01) | 0.91 (0.02) | 0.96 (0.01) | 0.93 (0.02) |
| Adults | 0.99 (0.01) | 0.98 (0.01) | 0.98 (0.02) | 0.99 (0.02) |
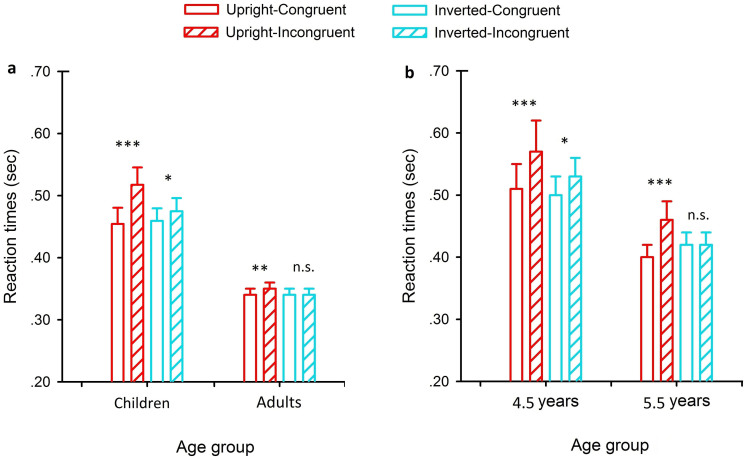
Figure 2a shows the mean reaction times for all test conditions and for both participant groups. Results revealed a significant main effect of Age Group, F(1, 38) = 18.57, p < .001, η2 = .33, and a significant main effect of Target Congruence, F(1, 38) = 53.48, p < .001, η2 = .59, whereas the main effect of Cue Orientation was not significant, F(1, 38) = 1.67, p> .10, η2 = .04. Results also showed a significant Target Congruence × Age Group interaction, F(1, 38) = 25.80, p < .001, η2 = .40, and a significant Cue Orientation × Target Congruence interaction, F(1, 38) = 17.12, p < .001, η2 = .31, whereas the Cue Orientation × Age Group interaction was not significant, F(1, 38) = .79, p > .10, η2 = .02. Importantly, results of this analysis revealed a significant three-way interaction of Cue Orientation × Target Congruence × Age Group, F(1, 38) = 6.98, p < .01, η2 = .16. Since the attentional orienting and its associated inversion effects are respectively indexed by the main effect of Target Congruency and the interaction between Cue Orientation and Target Congruence, we hence conducted the 2 × 2 repeated measures ANOVA (with Cue Orientation and Target Congruence being the within-subject factors) separately for the two participant groups (children and adults).
Figure 2. The mean reaction times in the upright and inverted conditions.
(a) Results for children aged 4 to 6 years and adults. (b) Results for 4.5- and 5.5-year-old children subgroups. Error bars show standard errors. *p < .05; **p < .01; ***p < .001; n.s., not significant.
For children, the analysis revealed that the main effect of Target Congruence was significant, F(1, 25) = 80.10, p< .001, η2 = .76, but the main effect of Cue Orientation was not significant, F(1, 25) = 2.32, p> .10, η2 = .09. The interaction of Cue Orientation × Target Congruence was significant, F(1, 25) = 22.99, p< .05, η2 = .48 (Figure 2a, left column). Further analyses revealed that the responses were significantly faster to a target probe when it was presented in the walking direction of the upright point-light walkers (congruent condition) compared with when the probe was presented in the opposite direction (incongruent condition) (p< .001). Interestingly, observers' attention was still influenced by the congruence of biological motion walking direction even in the inverted condition (p< .05).
For adults, the analysis revealed that the main effect of Target Congruence was significant, F(1, 13) = 6.63, p< .05, η2 = .34, but the main effect of Cue Orientation was not significant, F(1, 13) = .56, p> .10, η2 = .04. The interaction between Cue Orientation and Target Congruence was significant, F(1, 13) = 4.89, p< .05, η2 = .27 (Figure 2a, right column). Further analyses revealed a significant reflexive attentional orienting effect to biological motion walking direction in the upright (p < .01) but not the inverted condition (p > .5), replicating the earlier finding24.
Apparently, children, similar to adults, have exhibited a robust reflexive attentional effect to upright biological motion stimuli. However, the pattern of results from children in the inverted condition is not identical to that observed from adults. To further characterize the developmental profile of reflexive attentional effect to biological motion cues, particularly in the inverted condition, children's data were split into two subgroups according to their ages (13 children were assigned to 4.5-year-old group and 13 children were assigned to 5.5-year-old group). Figure 2b shows the mean reaction times for all test conditions and for the two children subgroups. Again, the data was analyzed with the 2 × 2 repeated measures ANOVA (with Cue Orientation and Target Congruence being the within-subject factors).
For the 4.5-year-old children, the analysis revealed that the main effect of Target Congruence was significant, F(1, 12) = 38.84, p < .001, η2 = .76, but the main effect of Cue Orientation was not significant, F(1, 12) = 1.43, p > .10, η2 = .11. Moreover, the interaction of Cue Orientation × Target Congruence was significant, F(1, 12) = 5.74, p < .05, η2 = .32 (Figure 2b, left column). Further analyses revealed that the responses were significantly faster to a target probe when it was presented in the walking direction of the upright point-light walkers (congruent condition) compared with when the probe was presented in the opposite direction (incongruent condition) (p < .001). Again, a weak but significant attentional orienting effect was also found in the inverted condition (p < .05).
For the 5.5-year-old children, the analysis revealed that there was a significant main effect of Target Congruence, F(1, 12) = 50.89, p < .001, η2 = .81, but not Cue Orientation, F(1, 12) = .83, p > .10, η2 = .06. Moreover, the Cue Orientation × Target Congruence interaction was significant, F(1, 12) = 20.85, p < .01, η2 = .64 (Figure 2b, right column). Further analyses revealed a significant reflexive attentional orienting effect to biological motion walking direction in the upright condition (p < .001). In contrast to the 4.5-year-old children, the 5.5-year-old children's attention was no longer influenced by the congruence of biological motion walking direction in the inverted condition (p > .5), consistent with the results of adults. Moreover, a direct comparison between 4.5- and 5.5-year-old children revealed a significant interaction between Target Congruence (congruent vs. incongruent) and Age Group (4.5 years vs. 5.5 years) in the inverted condition, F(1, 24) = 4.27, p = .05, η2 = .15, but not in the upright condition, F(1, 24) = .05, p > .10, η2 = .01.
Discussion
The present study employed a point-light walker as a central cue to investigate whether the reflexive attentional orienting effect to biological motion walking direction and the associated inversion effect emerge and develop at an early age. Using a modified central cueing paradigm, we found that children aged 4 to 6 years, similar to adults, showed a robust reflexive attentional effect to upright biological motion stimuli. In other words, the reflexive orienting of spatial attention to the biological motion walking direction develops as early as 4 years old. Our findings are in line with previous studies in non-human species showing that newly-hatched chicks tend to align their bodies to the walking direction conveyed by the upright rather than inverted walking hen stimuli22. The current study hence demonstrates that biological motion signals can guide the distribution of spatial attention in typically developing young children. Moreover, consistent with previous findings on developmental changes in attention37,38, the overall reaction times in children were slower than adults in the present study. This could occur because children were slower to detect the target, program a response, and/or execute that response.
Nevertheless, the emergence of the attentional orienting effect to biological motion walking direction in young children complements the attentional orienting effects induced by many social cues. For example, eye gaze and head direction, when used as central cues, can also elicit a similar reflexive attentional orientation in young children39,40. Since walking direction provides important cues for other people's mental states (e.g., current focus of attention, interests and goals), particularly when viewing them from a distance, it would be of obvious adaptive advantage for humans to pay more attention to their walking direction and enhance related information processing, allowing more resources for the extraction of others' intentions as well as the execution of appropriate reactions. In other words, the walking direction of the biological entity might also serve as another type of important attentional cue in young children. This is perhaps a more impressive capability for young children given that the point-light biological motion cues, different from human body parts such as face or eyes, are simply represented by an array of moving dots with which children are not so familiar.
Overall, observers' attentional orienting to biological motion cues was significantly reduced when the point-light walker was shown upside-down, reflecting a significant inversion effect associated with biological motion processing26,27,28,29,30. The inversion effect observed here resonates well with the previous findings on newborn chicks22. Interestingly, although adults' attention are no longer influenced by the inverted biological motion cues, younger children (4 years old) still show a weak but significant attentional effect to the walking direction of the inverted point-light walker, and this effect becomes diminished in older children (5 years old). In other words, there seems to exist a broadly-tuned attentional mechanism of utilizing biological motion cues in younger children, which becomes narrowed with age and is eventually specialized to the meaningful biological signals (e.g., upright biological motion cues). This notion is broadly compatible with the findings in the context of the functional development of the social brain. During the first few months of life, human infant's neural system manifests generalized responses to biological signals in the environment, and it may take several years for such a system to eventually develop into a functionally sophisticated social brain network in which the processing of face, biological motion, and other important social cues becomes highly specialized (see Grossmann & Johnson, 2007 for a review)31. This implication is also consistent with the evidence from comparative literature that both newly hatched chicks and human neonates show a generic and broad preference for biological visual patterns (e.g., face-like stimuli) irrespective of their specific forms, and this coarsely tuned preference present at birth will gradually be finely tuned throughout development41,42,43,44,45,46. Indeed, an earlier study has demonstrated a similar developmental trajectory of biological motion detection performance in infants47. Three-month-old infants have equivalent detection abilities for upright and inverted point-light walkers, whereas this ability is tuned to the upright biological motion signals in 5-month-old infants. We extend this previous research and demonstrate that the attentional orienting effect induced by biological motion walking direction is a fine-tuning (narrowing) process as well. It is important to note that the emergence of the narrowing process observed in the present study is right at the age when children are able to learn and fully acquire the meanings of biological motion signals7. It is possible that visual exposure plays a major role in shaping and narrowing the reflexive responses of the human visual attention system to biological signals. Rarely viewing the inverted biological signals over the course of development might substantially reduce the utilization of the inverted biological cues with age. Yet the developmental mechanism that initiates this specialization process remains an important and open question for future investigations.
In summary, the current study demonstrates that young children as early as 4 years old show a robust reflexive attentional orienting effect to upright biological motion cues. Moreover, the inversion effect associated with attentional orienting emerges by 4 years old and gradually develops into a similar pattern found in adults whose attention is no longer influenced by the congruency of the inverted biological motion walking direction. Taken together, our findings provide strong evidence that biological motion cues can guide spatial attention in young children, and highlight a critical development from a broadly- to finely-tuned process of utilizing biological motion cues in the human social brain.
Methods
Participants
Twenty-six kindergarten children (mean age = 5.2 years, SD = .5, range = 4.5–6.1 years, 16 boys) from Beijing, China were tested. Half of them were about 4.5 years old (mean age = 4.7 years, SD = .2, range = 4.5–4.9 years, 8 boys), and the other half were about 5.5 years old (mean age = 5.6 years, SD = .2, range = 5.4–6.1 years, 8 boys). Fourteen adults (mean age = 22.4 years, SD = 2.0, range = 20.6–24.2 years, 8 males) were also tested. Participants all had normal or corrected-to-normal vision and were naïve to the purpose of the experiment. All of the adult participants and the children' parents gave informed consent in accordance with procedures and protocols approved by the institutional review board of the Institute of Psychology, Chinese Academy of Sciences.
Stimuli and procedure
Stimuli were generated and displayed using MATLAB (Mathworks, Inc.) together with the Psychophysics Toolbox extensions48,49. Point-light biological motion sequences were adopted from Vanrie & Verfaillie50. In specific, the positions of 13 point lights attached to the head and the major joints (shoulders, elbows, wrists, hips, knees, and ankles) were encoded as motion vectors with initial starting positions in each frame. Each motion cycle was 1 s with 30 frames.
Visual stimuli were displayed on a 14-inch LCD monitor (1280 × 800 at 60 Hz). Each trial began with fixation on a central cross (0.8° × 0.8°, all visual angles were measured with a viewing distance of 50 cm) within a frame (24.5° × 24.5°) that extended beyond the outer border of the stimuli. After 1000 ms, a point-light walker stimulus (4.0° × 6.8°) in full side view without translational motion was presented as a cue on the central cross for 500 ms. The contrast between the point-light walker and the background was 58% (stimulus luminance: 110.2 cd/m2; background luminance: 29.42 cd/m2). After an inter-stimulus interval (ISI) of 100 ms, in which only the fixation cross was displayed, a small red apple image (2.5° × 2.5°) was presented as a probe on the left or right side of the fixation cross for 100 ms. The horizontal distance between the centers of the probe and the cross was 5.0°. Participants were required to press one of two buttons to indicate the position of the probe (left vs. right) as quickly as possible while minimizing errors (see Figure 1). There were a total of 80 trials with 40 trials for the upright and inverted conditions, respectively. Each condition (upright or inverted) consisted of 20 congruent trials and 20 incongruent trials. All participants took part in both the upright and inverted conditions that were run in separate blocks. The order of these two conditions was counter-balanced across participants.
At the beginning of the experiment, participants were told explicitly that walking direction would not predict target location, and that they were required to fixate at the central cross throughout the experiment. After practice for several trials, all children and adults were able to follow the instruction and perform well (with a mean accuracy >.90). In the actual experiment, participants had a break every 10 trials. Children were provided general encouragement and a little reward during the break if they successfully followed the instruction. According to the experimenter's observation, the task was child-friendly and all children were able to concentrate throughout the whole procedure.
Author Contributions
J.Z., L.W., S.L. and Y.J. designed the experiment. J.Z. and L.W. collected and analyzed the data. J.Z., L.W. and Y.J. prepared the figures. J.Z., L.W., S.L., Y.J., Y.W. and X.W. wrote the manuscript.
Acknowledgments
This research was supported by grants from the National Key Technology R & D Program of China (No. 2012BAI36B00), and the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB02010003), and the National Natural Science Foundation of China (No. 31070903, No. 31200767 and No. 81301175), and the Knowledge Innovation Program of Chinese Academy of Sciences (No. KSCX2-EW-BR-5 and No. KSCX2-EW-J-8).
References
- Johansson G. Visual perception of biological motion and a model for its analysis. Percept Psychophys 14, 195–204 (1973). [Google Scholar]
- Bertenthal B., Proffitt D. & Cutting J. Infant sensitivity to figural coherence in biomechanical motions. J Exp Child Psychol 37, 213–230 (1984). [DOI] [PubMed] [Google Scholar]
- Fox R. & McDaniel C. The perception of biological motion by human infants. Science 218, 486–487 (1982). [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Regolin L. & Marconato F. Visually inexperienced chicks exhibit spontaneous preference for biological motion patterns. PLoS Biol 3, e208 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidet-Ildei C., Kitromilides E., Orliaguet J.-P., Pavlova M. & Gentaz E. Preference for point-light human biological motion in newborns: Contribution of translational displacement. Dev Psychol 50, 113 (2014). [DOI] [PubMed] [Google Scholar]
- Simion F., Regolin L. & Bulf H. A predisposition for biological motion in the newborn baby. Proc Natl Acad Sci U S A 105, 809–813 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova M., Krageloh-Mann I., Sokolov A. & Birbaumer N. Recognition of point-light biological motion displays by young children. Perception 30, 925–933 (2001). [DOI] [PubMed] [Google Scholar]
- Freire A., Lewis T. L., Maurer D. & Blake R. The development of sensitivity to biological motion in noise. Perception 35, 647–657 (2006). [DOI] [PubMed] [Google Scholar]
- Hadad B. S., Maurer D. & Lewis T. L. Long trajectory for the development of sensitivity to global and biological motion. Dev Sci 14, 1330–1339 (2011). [DOI] [PubMed] [Google Scholar]
- Alaerts K., Nackaerts E., Meyns P., Swinnen S. P. & Wenderoth N. Action and emotion recognition from point light displays: an investigation of gender differences. PloS one 6, e20989 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov A. A., Krüger S., Enck P., Krägeloh-Mann I. & Pavlova M. A. Gender affects body language reading. Front Psychol 2, 1–6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runeson S. & Frykholm G. Kinematic specification of dynamics as an informational basis for person-and-action perception: expectation, gender recognition, and deceptive intention. J Exp Psychol General 112, 585–615 (1983). [Google Scholar]
- Krüger S., Sokolov A. N., Enck P., Krägeloh-Mann I. & Pavlova M. A. Emotion through Locomotion: Gender Impact. PloS one 8, e81716 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi S. Perception of point-light walker produced by eight lights attached to the back of the walker. Swiss J Psychol 59, 126–132 (2000). [Google Scholar]
- Troje N. F. Decomposing biological motion: a framework for analysis and synthesis of human gait patterns. J Vis 2, 371–387 (2002). [DOI] [PubMed] [Google Scholar]
- Pavlova M. A. Biological motion processing as a hallmark of social cognition. Cerebral Cortex 22, 981–995 (2012). [DOI] [PubMed] [Google Scholar]
- Bertenthal B. I. & Pinto J. Global processing of biological motions. Psychol Sci 5, 221–225 (1994). [Google Scholar]
- Neri P., Morrone M. C. & Burr D. C. Seeing biological motion. Nature 395, 894–896 (1998). [DOI] [PubMed] [Google Scholar]
- Thurman S. M. & Grossman E. D. Temporal “Bubbles” reveal key features for point-light biological motion perception. J Vision 8, 1–11 (2008). [DOI] [PubMed] [Google Scholar]
- Thompson B., Hansen B. C., Hess R. F. & Troje N. F. Peripheral vision: Good for biological motion, bad for signal noise segregation? J Vision 7, 1–7 (2007). [DOI] [PubMed] [Google Scholar]
- Thornton I. M. & Vuong Q. C. Incidental processing of biological motion. Curr Biol 14, 1084–1089 (2004). [DOI] [PubMed] [Google Scholar]
- Vallortigara G. & Regolin L. Gravity bias in the interpretation of biological motion by inexperienced chicks. Curr Biol 16, R279–280 (2006). [DOI] [PubMed] [Google Scholar]
- Hirai M., Saunders D. R. & Troje N. F. Allocation of attention to biological motion: Local motion dominates global shape. J Vision 11, 1–11 (2011). [DOI] [PubMed] [Google Scholar]
- Shi J., Weng X., He S. & Jiang Y. Biological motion cues trigger reflexive attentional orienting. Cognition 117, 348–354 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Yang X., Shi J. & Jiang Y. The feet have it: Local biological motion cues trigger reflexive attentional orienting in the brain. NeuroImage 84, 217–224 (2014). [DOI] [PubMed] [Google Scholar]
- Pavlova M. & Sokolov A. Orientation specificity in biological motion perception. Percept Psychophys 62, 889–899 (2000). [DOI] [PubMed] [Google Scholar]
- Proffit D. R. & Bertenthal B. I. Converging operations revisited: Assessing what infants perceive using discrimination measures. Percept Psychophys 47, 1–11 (1990). [DOI] [PubMed] [Google Scholar]
- Sumi S. Upside-down presentation of the Johansson moving light-spot pattern. Perception 13, 283–286 (1984). [DOI] [PubMed] [Google Scholar]
- Wang L. & Jiang Y. Life motion signals lengthen perceived temporal duration. P Natl Acad Sci USA 109, E673–E677 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhang K., He S. & Jiang Y. Searching for life motion signals visual search asymmetry in local but not global biological-motion processing. Psychol Sci 21, 1083–1089 (2010). [DOI] [PubMed] [Google Scholar]
- Grossmann T. & Johnson M. H. The development of the social brain in human infancy. Eur J Neurosci 25, 909–919 (2007). [DOI] [PubMed] [Google Scholar]
- Jordan H., Reiss J. E., Hoffman J. E. & Landau B. Intact perception of biological motion in the face of profound spatial deficits: Williams syndrome. Psychol Sci 13, 162–167 (2002). [DOI] [PubMed] [Google Scholar]
- Sweeny T. D., Wurnitsch N., Gopnik A. & Whitney D. Sensitive perception of a person's direction of walking by 4-year-old children. Dev Psychol 49, 2120–2124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J. Voluntary versus automatic control over the mind's eye's movement. Atten Perform IX 9, 187–203 (1981). [Google Scholar]
- Posner M. I. Orienting of attention. Q J of Ex Psychol 32, 3–25 (1980). [DOI] [PubMed] [Google Scholar]
- Frischen A., Bayliss A. P. & Tipper S. P. Gaze cueing of attention: visual attention, social cognition, and individual differences. Psychol Bull 133, 694–724 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. C., Maurer D. & Lewis T. L. Developmental changes in attention: The effects of endogenous cueing and of distractors. Dev Sci 4, 209–219 (2001). [Google Scholar]
- Ristic J. & Kingstone A. Rethinking attentional development: Reflexive and volitional orienting in children and adults. Dev Sci 12, 289–296 (2009). [DOI] [PubMed] [Google Scholar]
- Senju A., Csibra G. & Johnson M. H. Understanding the referential nature of looking: Infants' preference for object-directed gaze. Cognition 108, 303–319 (2008). [DOI] [PubMed] [Google Scholar]
- Yoon J. M. D. & Johnson S. C. Biological Motion Displays Elicit Social Behavior in 12-Month-Olds. Child Dev 80, 1069–1075 (2009). [DOI] [PubMed] [Google Scholar]
- Vallortigara G. Core knowledge of object, number, and geometry: A comparative and neural approach. Cognitive neuropsychology 29, 213–236 (2012). [DOI] [PubMed] [Google Scholar]
- Vallortigara G. Aristotle and the Chicken: Animacy and the Origins of Beliefs. The Theory of Evolution and Its Impact Fasolo, A. (ed.) 189–199 (Springer, New York, 2012). [Google Scholar]
- Rosa Salva O., Farroni T., Regolin L., Vallortigara G. & Johnson M. H. The evolution of social orienting: evidence from chicks (Gallus gallus) and human newborns. PLoS One 6, e18802 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa Salva O., Regolin L. & Vallortigara G. Inversion of contrast polarity abolishes spontaneous preferences for face-like stimuli in newborn chicks. Behav Brain Res 228, 133–143 (2012). [DOI] [PubMed] [Google Scholar]
- Rosa-Salva O., Regolin L. & Vallortigara G. Faces are special for newly hatched chicks: evidence for inborn domain-specific mechanisms underlying spontaneous preferences for face-like stimuli. Dev Sci 13, 565–577 (2010). [DOI] [PubMed] [Google Scholar]
- Mascalzoni E., Regolin L. & Vallortigara G. Innate sensitivity for self-propelled causal agency in newly hatched chicks. Proc Natl Acad Sci U S A 107, 4483–4485 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto J. Developing body representations: A review of infants' responses to biological motion displays. Human body perception from the inside out Thornton, I., Knoblich, G. & Shiffrar, M. (eds.) 305–322 (Oxford University Press, New York, 2006). [Google Scholar]
- Brainard D. H. The Psychophysics Toolbox. Spat Vis 10, 433–436 (1997). [PubMed] [Google Scholar]
- Pelli D. G. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10, 437–442 (1997). [PubMed] [Google Scholar]
- Vanrie J. & Verfaillie K. Perception of biological motion: a stimulus set of human point-light actions. Behav Res Methods Instrum Comput 36, 625–629 (2004). [DOI] [PubMed] [Google Scholar]