Abstract
In this review, I outline the indirect evidence for the formation of singlet oxygen (1O2) obtained from experiments with the isolated PSII reaction center complex. I also review the methods we used to measure singlet oxygen directly, including luminescence at 1,270 nm, both steady state and time resolved. Other methods we used were histidine-catalyzed molecular oxygen uptake (enabling 1O2 yield measurements), and dye bleaching and difference absorption spectroscopy to identify where quenchers of 1O2 can access this toxic species. We also demonstrated the protective behavior of carotenoids bound within Chl–protein complexes which bring about a substantial amount of 1O2 quenching within the reaction center complex. Finally, I describe how these techniques have been used and expanded in research on photoinhibition and on the role of 1O2 as a signaling molecule in instigating cellular responses to various stress factors. I also discuss the current views on the role of 1O2 as a signaling molecule and the distance it might be able to travel within cells.
Keywords: Chlorophyll, Photosynthesis, Reactive oxygen species, Triplet states
Introduction
Singlet oxygen (1O2) is an electronically excited state of molecular oxygen which is extremely reactive (Ogilby 2010). It attacks and oxidizes proteins, lipids and nucleic acids, and consequently it is an important reactive oxygen species (ROS) in biological systems. It is less stable than triplet oxygen (3O2), and may be formed in a variety of ways; however, a common way is by electronic energy transfer from the triplet state of a photosensitized pigment or dye molecule.
| (1) |
where S is a sensitizer molecule, dye or pigment. During oxygenic photosynthesis (Blankenship 2014), 1O2 is easily formed as Chl molecules are very good photosensitizers and the nature of the photosynthetic process means that there is always plenty of ground state, 3O2, around.
Photosensitization of the triplet state of Chl leads to formation of 1O2 unless Chl triplets are removed rapidly before 1O2 formation can take place (Ogilby 2010). Carotenoid (Car) molecules are very effective quenchers of triplet Chl (Frank and Cogdell 1996) and also directly of 1O2 (Hirayama et al. 1994) in photosynthetic systems. However, despite their effectiveness in the protection of photosynthetic organisms, high light intensities do bring about loss of photosynthetic activity in oxygenic organisms as reflected by the physiological phenomenon of photoinhibition (Prasil et al. 1992, Aro et al. 1993, Adir et al. 2003).
The phenomenon of photoinhibition has been localized mainly to the photosynthetic reaction center (RC) of PSII. High light initially causes a decrease in the rate of electron transport through PSII and preferential degradation of the Dl protein, an intrinsic subunit of the complex. Restoration of activity requires de novo protein synthesis. Molecular oxygen has been implicated in photoinhibition (Prasil et al. 1992), and damaging oxygen species, 1O2 and other ROS, are likely to be the agents that activate Dl protein degradation (Barber and Andersson 1992, Fischer et al. 2013). Keren et al. (1997) have also argued that PSII can be inactivated at low light levels and that formation of the Chl triplet state in PSII and 1O2 is involved. This is discussed in more detail later.
Here I will describe the history of the detection of 1O2 formed by isolated photosynthetic complexes and demonstrate the protective behavior of Cars bound within Chl–protein complexes, and then relate this information to current research in photoinhibition and its function as a signaling molecule in instigating cellular responses to various stress factors.
Photosynthetic Pigment–Protein complexes
Photosynthetic electron transport is carried out by a series of Chl–protein complexes. The antenna pigments are bound to light-harvesting pigment–protein complexes (LHCI and LHCII), which absorb light, producing the first excited singlet state of Chl, and then there are a series of energy transfer reactions, between the antenna Chl and the RCs. Here the first photochemical step occurs in which a specialized Chl molecule (P) on excitation to its excited singlet state passes on an electron to an acceptor molecule (A) to form the primary radical pair, P+A–. During oxygenic photosynthesis, two photochemical reactions occur in series catalyzed by two pigment–protein complexes known as PSII and PSI. In PSII, the oxidized electron donor is re-reduced by electrons extracted from water (a by-product being molecular oxygen after extraction of four electrons from two water molecules), while in PSI the reduced acceptor donates two electrons to NADP+ to form NADPH.
The four pigment–protein complexes of green plants (PSI, PSII, and the antenna complexes LHCI and LHCII) all bind approximately 1 Car molecule per 4 Chl molecules and it is the Cars that normally prevent the formation of 1O2. Car-deficient mutants can grow from seed but are bleached and die as soon as they see normal light (Walles 1965).
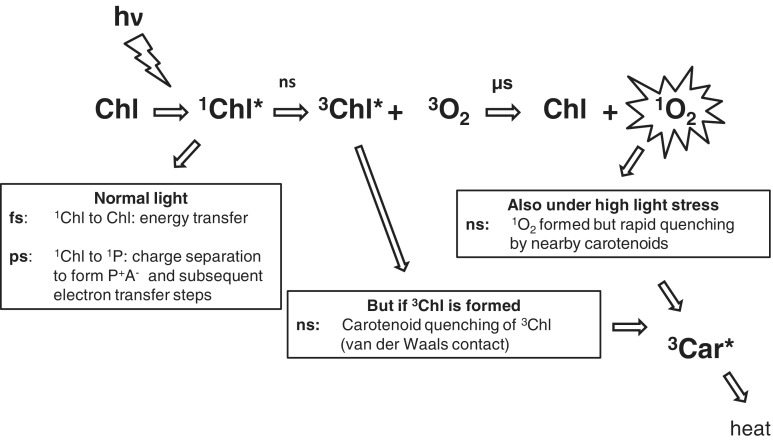
Car molecules, provided they are bound within van der Waals distance of the Chl, are extremely efficient quenchers of Chl triplets (Fig. 1). One of the earliest experiments demonstrating the transfer of energy from the triplet excited state of Chl to Car was the so-called ‘valve reaction’ of Witt (1971) in which an increase in the size of an absorbance change (due to 3Car formation) was seen only once photosynthetic electron transfer was light saturated. It then continued to rise more or less linearly with the intensity of the laser flash energy.
Fig. 1.
Avoidance of 3Chl and 1O2 formation: time scales involved.
There are two mechanisms by which Chl triplets are formed. In the antenna complexes it is by intersystem crossing:
| (2) |
while in the PSII reaction centre it is by the radical pair (RP) mechanism:
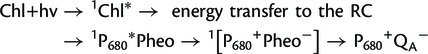 |
(3) |
P680+QA– recombination occurs either directly or indirectly:
| (4) |
| (5) |
where P680 is the primary Chl electron donor in PSII, Pheo is the primary electron acceptor in PSII, and QA is a plastoquinone molecule, which is the second electron acceptor in PSII. The indirect pathway leads to formation of 1O2 while the direct pathway does not. In experiments where the midpoint potential of the secondary electron acceptor QA was made more positive (and hence decreased the likelihood of the indirect pathway), the yield of 1O2 was lowered while when it was made more negative the yield was increased (Krieger-Liszkay and Rutherford 1998, Fufezan et al. 2002).
The two mechanisms of Chl triplet formation can be distinguished by their electron paramagneitc resonance (EPR) signal properties. The radical pair triplet is only formed after formation of P680+Pheo–, which gives a spin-polarized EPR triplet signal, after spin dephasing, which has a characteristic absorption (A) and emission (E) spectrum (AEEAAE) as opposed to the pattern seen in triplets formed by intersystem crossing (AEAEAE) (van Mieghem et al. 1991).
The D1D2 Reaction Center Complex of PSII and Indirect Evidence for 1O2 Formation
It was the instability, in the presence of molecular oxygen, of the PSII RC complex (also known as the D1D2 complex) isolated by Satoh and colleagues (Nanba and Satoh 1987) which first led to the suggestion that large amounts of 1O2 were being formed by this complex due to interaction of the radical pair triplet state with molecular oxygen (Barber et al. 1987, Durrant et al. 1990) (see Fig. 2).
Fig. 2.
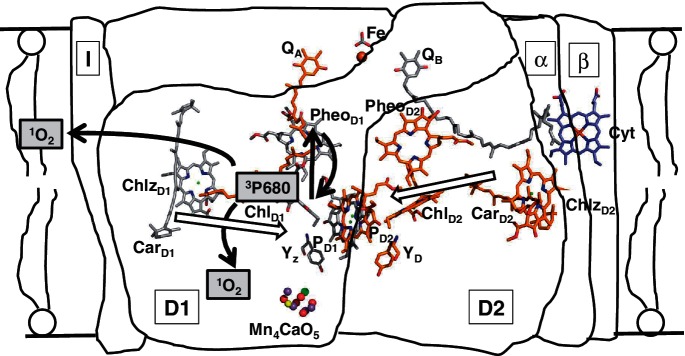
Schematic diagram of the electron transfer reactions occurring in the membrane-bound PSII RC and the formation of singlet oxygen at the site of 3P680. The purified complex (D1, D2, α and β subunits of Cyt b559 and the PsbI protein) has lost both of the secondary electron acceptors, QA and QB, the non-heme iron (Fe) and also the water-splitting Mn cluster, Mn4CaO5. The figure shows that if the triplet state of P680 is formed it will be quenched by ground state oxygen to form 1O2 which can damage either the pigment–protein complex or the lipid membrane. The cofactor arrangement in the RC complex shows the distance of the two β-carotenes from the four central Chl cofactors, based on Umena et al. (2011).
The presence of molecular oxygen was found to bleach the sample and to inactivate P680, the primary electron donor, it also shortened the P680 triplet lifetime from 1 ms (under anaerobic conditions) to 33 µs (Durrant et al. 1990). The first observation was that the complex showed a high yield of the radical pair recombination triplet state (Okamura et al. 1987), which shows the distinctive and specific absorption and emission EPR spectrum (AEEAAE), indicative of the formation of the P680 triplet state via the RP mechanism (see Equations 3–5). There was virtually no triplet Car formed by the D1D2 RC complex (Takahashi et al. 1987, Durrant et al. 1990).
The question arose as to why the two Cars bound to the RC were not protecting against 1O2 formation by quenching the RP triplet (Telfer 2002). This was shown by De Las Rivas et al. (1993) to be because the Car is oxidized by P680+ if this highly oxidizing species is allowed to persist for any length of time, i.e. in the presence of an added artificial electron acceptor which can stabilize P680+ (Barber et al. 1987). The oxidized Car is very unstable and its absorption (420–520 nm) is rapidly irreversibly bleached (De Las Rivas et al. 1993).
1O2 Production by Photosynthetic Pigment–Protein Complexes
Conditions arise where 1O2 can be formed in photosynthetic light-harvesting antenna complexes by triplet–triplet excitation transfer (intersystem crossing), as was seen by Wolff and Witt (1969) at high light intensities, when electron transport is light saturated and the Car triplet yield is increased substantially. Here Cars can prevent 1O2 formation as they are bound in the antenna complexes within van der Waals contact and so quench Chl triplets which are formed on a nanosecond time scale (Schödel et al. 1998). The Car triplets then decay harmlessly, releasing heat.
It is very unlikely that Chl triplets are formed in the PSI RC under photoinhibitory conditions (Hideg and Vass 1995, Rutherford et al. 2012), but there is evidence for their formation in PSII which is related to the very high oxidizing potential of P680+, which is required for water oxidation to occur. The β-carotene in the RC has been shown to be bound well beyond van der Waals distance from the Chls of the RC cofactor cluster (Fig. 2; Loll et al. 2005) and so cannot be invoked to quench directly any 3P680 which might be formed. There is strong evidence that both under high light (van Mieghem et al. 1989, Vass et al. 1992) and also under very low light 3P680 is formed via the radical pair recombination pathway (Keren et al. 1997). The significance of this mechanism for formation of the primary donor triplet state is that P680+ must be formed first (Equations 3–5) and as it has such a high redox potential any Car bound close enough to quench a triplet would have been oxidized previously by the cationic P680.
It was not only the very high sensitivity of the isolated D1D2 complex to light in the presence of molecular oxygen which suggested that 1O2 was being formed at a high yield. The lifetime of the 3P680 was lengthened dramatically under anaerobic conditions (from ∼30 µs to 1 ms) and the consequent inactivation of P680, loss of the red-most absorbance due to P680 and degradation of the D1 and D2 proteins were ascribed to the damaging effect of 1O2 (Durrant et al. 1990, Barber and Archer 2001). Note that during the early 1990s it became clear that 1O2 formation by the isolated D1D2 complex occurs but it had only been detected by indirect methods.
Direct Detection of 1O2 Formed by Isolated PSII RCs
It was in the early 1990s that we began using the techniques used by experimentalists studying photosensitization of 1O2 by dyes to be used in photodynamic therapy (PDT) (Allison 2004) to look for direct evidence for 1O2 formation by the D1D2 complex. The dramatic change in the lifetime of the P680 triplet and the fact that the Chl triplet yield was very high, about 0.3, whereas that of the Car triplet was very low (0.03) all suggested that the yield of 1O2 should also be high (Durrant et al. 1990).
The first technique we used was to look for the very weak luminescence at 1,270 nm from 1O2. This is so weak that it is only detected using a 77 K cooled photomultiplier (Macpherson et al. 1993). Here we showed steady-state emission of luminescence at 1,270 nm from the isolated D1D2 complex on illumination under aerobic conditions. The luminescence (L1,270) was partially quenched by azide, a known quencher of 1O2. The azide-quenchable part of the signal (30–50%) was concluded to be due to 1O2 and the remaining part to infrared phosphorescence from the Chls in the PSII RC. Note that it was necessary to exchange the RCs into a D2O medium as water itself is a very good quencher of 1O2, shortening its lifetime from ∼70 µs to ∼3 µs and hence reducing the size of the steady-state emission signal until it was undetectable (Gorman and Rogers 1989, Wilkinson et al. 1995). As concluded by Telfer et al. (1994a), this was probably the first direct observation of 1O2 luminescence sensitized by an intrinsically bound chromophore in a defined biological system as opposed to a sensitizer-doped biological material (e.g. Firey and Rogers 1998).
Complementary to the L1,270 method, we also used a chemical trapping technique to estimate the yield of 1O2 formed on illumination of the isolated D1D2 complex (Telfer et al. 1994a). The uptake of molecular oxygen due to the reaction of 1O2 with histidine or imidazole was measured using an oxygen electrode, and the yield was compared with similar experiments using 1O2 sensitizing dyes such as mesotetra-(4-sulfonatophenyl)porphine (TPPS) and aluminum phthalocyanine disulfonate (AlPcS) for which the 1O2 yield is already known. We found that the yield of 1O2 was about 0.16 whereas the yield of 3P680 in the complex is 0.3 (Durrant et al. 1990). The lower yield of 1O2 as compared with that of the Chl triplet is to be expected as some 1O2 will be quenched rapidly by the protein and pigments within the RC complex before it escapes into the medium.
We also used the dye bleaching technique of Kraljic and El Mohsni (1978) to detect 1O2. This technique is based on the bleaching of p-nitrosodimethylaniline (RNO) to the nitro form caused by the trans-annular peroxide product of the reaction of 1O2 with either histidine or imidazole. We measured the bleaching of the dye due to 1O2 simultaneously with the bleaching of Chl associated with the inactivation of the sample by this ROS (Telfer et al. 1994a).
All these techniques indicated that under illumination and aerobic conditions, the D1D2 complex produces a large amount of 1O2. It escapes from the complex into the aqueous medium, and we conclude that it was quenched or detected there as there was no protection against bleaching of the Chl by added quenchers such as azide or histidine or by water vs. D2O (Telfer et al. 1994a). This effect had been noted by Macpherson et al. (1993) where the bleaching of Chl was not prevented by the addition of azide, although the L1,270 was quenched, leading us to conclude that the 1O2 detected as emission at 1,270 nm is in a different environment (accessible to quenchers) from that giving rise to the beaching of Chl. In essence the 1O2 is formed within the D1D2 complex on the Chl of P680 and then it diffuses out of the complex into the aqueous medium where not only is it accessible to water-soluble quenchers such as azide but its lifetime is lengthened by the presence of D2O as compared with H2O. In experiments where H2O and D2O buffers were compared, there was no stimulation of the inactivation of P680 in the latter medium compared with H2O medium. This indicates that several rounds of buffer exchange of the complex (using Millipore concentrator tubes; see Macpherson et al. 1993), which was originally isolated in an H2O-based medium, into a D2O-based medium either does not exchange the water molecules within the complex or that there are no water molecules close enough to P680 to quench the 1O2. The latest structure of the PSII core complex, which is at 1.9 Å resolution, shows that the majority of the very many water molecules in the structure are located in two layers on the surfaces of the stromal and lumenal sides (Umena et al. 2011). Of the few water molecules found in the interior of the complex, most of them serve as ligands to Chls. Note that the magnesium of the accessory Chl on D1, which is thought to be where the 3P680 is located, is liganded by a water molecule as is that of accessory ChlD2.
This evidence that the site of 1O2 formation is deep within the D1D2 complex was confirmed by Telfer et al. (1994a) in RNO bleaching experiments. Absorption difference spectra show clearly that although the quencher, azide, prevents the RNO bleaching it does not stop the loss of absorption of P680, which we concluded is caused by 1O2 before it escapes from the complex, i.e. internal intrinsic quenching mechanisms compete very effectively with externally added water-soluble quenchers. However, the presence of D2O in place of water increased the rate of RNO bleaching approximately 3-fold, which is consistent with the increase in the lifetime of 1O2 in the external medium when it is present in place of H2O.
Telfer et al. (1994a) also carried out a number of experiments to show that it was 1O2 causing the inactivation of P680 and that it was not due to any other ROS. As Foote (1990) warned, ‘detection of a species does not indicate its intermediacy in a process’, and in PDT it has been difficult to demonstrate that it is actually 1O2 causing cell death. We definitively showed that it is 1O2 that brings about Chl bleaching and inactivation of P680 in the isolated D1D2 complex (Telfer et al. 1994a).
Correlation of P680 Triplet Decay and L1,270 Signal Rise Rates in the Isolated PSII RC
We also measured time-resolved 1,270 nm luminescence of 1O2, formed on illumination of the D1D2 complex in the presence of D2O when suspended in air-saturated medium (Macpherson et al. 1993, Telfer et al. 1994b), and later correlated the rise time of the L1,270 signal with the decay of the 3P680 absorption decrease at 680 nm (Telfer et al. 1999). Here we showed the similarity in the triplet decay rate and L1,270 rise times and the dependence on the molecular oxygen concentration for the rate of quenching of the triplet and rise of the L1,270 which indicated that 1O2 is formed directly by quenching of 3P680. Li et al. (2012) carried out similar experiments, measuring time-resolved L1,270, on isolated PSII RCs in aqueous media and concluded that the lifetime of the 1O2 would be so short (<0.5 µs) that determining the 1O2 rate constants in chloroplasts suspended in aqueous medium, i.e. in vivo conditions, would be ‘a tall order’, i.e. they imply it would be impossible.
Role of β-Carotene in Protection against Photodamage
As shown in Fig. 2, the β-carotene bound to the PSII RC can act as an admittedly relatively inefficient electron donor to P680+ (De Las Rivas et al. 1993). This occurs if the lifetime of the oxidized donor is prolonged by the addition of an artificial electron acceptor, e.g. silicomolybdate and dibromothymoquinone, which are able to accept electrons directly from the pheophytin primary electron acceptor which is bound to the D1 protein of the RC complex. In addition to this role of rereduction of P680+ (if it is not reduced rapidly by the tyrosine electron donor, YZ, and then by electrons from water), the Car should also quench 1O2, diffusing within the Chl protein complex, directly. The idea, as discussed already, is that the Car cannot be bound closely enough to quench 3P680 directly as it would be oxidized first by P680+ which has to be formed prior to the formation of the triplet by the radical pair mechanism (Telfer 2002). Indeed the crystal structure of PSII by Loll et al. (2005) shows that the closest approach of the two β-carotenes is 13.2 Å for CarD2 to ChlD2 and 19.9 Å for CarD1 to ChlD1 (Fig. 2). Using the Moser and Dutton rule (1992), both distances are far too great to allow either rapid quenching of 3P680 or rapid electron transfer directly to P680+.
The question arises as to what is the role of the Cars bound to the PSII RC aside from rereduction of any stabilized P680+. As discussed earlier, it is inevitable that some 3P680 will be formed during turnover of the PSII RC (van Mieghem et al. 1989, Keren et al. 1997) and, because there will always be molecular oxygen around, 1O2 will be formed. It was known that carotenoids can quench 1O2 directly (Hirayama et al. 1994) and so we tested the proposition that this is another role for the Cars in the RC. The β-carotene level of isolated PSII RCs was lowered by extensive washing of the preparatory anion exchange column with low salt buffer before elution with high salt, and thus we prepared complexes with various levels of Car. We were then able to show an inverse correlation between the size of the L1,270 signal and the Car level and the rate of irreversible bleaching of Chl, indicating that when the normal two Cars were present the complex was less susceptible to inactivation on illumination in the presence of molecular oxygen (Telfer et al. 1994b). The fact that when two Cars were present [i.e. as seen in the native structure, Loll et al. (2005) and see Fig. 2], they could not quench all of the 1O2 formed is due to the fact that the 1O2 formed at P680 can diffuse in all directions within the complex and, because of the distance of the Cars from the source of the 1O2, a certain amount of damage will be done by 1O2 not scavenged by them (Telfer 2002).
Conclusions
3P680 is inevitably formed within the PSII RC when operating in oxygenic organisms, which are also continuously evolving molecular oxygen, both at low light intensities and under high light, i.e. photoinhibitory, conditions. The P680 triplet thus forms 1O2 as the RC Cars are unable to quench the triplet before its reaction with ground state 3O2. 1O2 scavenging mechanisms are in place, including the two β-carotene molecules bound to the D1 and D2 RC polypeptides and, in vivo, some of the other Cars bound near the interface between the inner antenna polypeptides CP43 and CP47 close to the D1 and the D2 polypeptides, respectively (Loll et al. 2005), may well also scavenge some 1O2.
1O2 scavengers
1O2 that is not quenched by Car and hence escapes from the PSII core complex into the membrane will be quenched by tocopherol (Kruk et al. 2005) and plastoquinone (Kruk and Trebst 2008, Yadav et al. 2010), which is present in the thylakoid lipid membranes. Tocopherol has been implicated in protection against 1O2 damage to the membrane lipids (Kruk et al. 2005, Krieger-Liszkay and Trebst 2006). However, this is a sacrificial reaction and depends on resynthesis, using ascorbate, to restore depleted stocks of tocopherol. Inevitably some 1O2 will escape quenchers and may exit into the aqueous thylakoid lumen or stroma where it may damage proteins and nucleic acids. In the stroma, ascorbate is a good scavenger (Bisby et al. 1999) and it is usually present at high levels. It is replenished using glutathione and NADPH (Smirnoff 2000). For a discussion on scavenger effectiveness in photosynthetic systems. see Li et al. (2012).
Relevance in vivo—photoinhibition and retrograde signaling
After the initial demonstration of formation of 1O2 by D1D2 complexes (Macpherson et al. 1993), 1O2 formation by isolated thylakoid membranes (TMs) after a high light treatment (photoinhibition) was demonstrated by Hideg et al. (1994), using a spin trapping technique, using the spin label 2,2,6,6,tetramethylpiperidine (TEMP) and EPR spectroscopy. These techniques have subsequently been used to demonstrate formation of 1O2 in PSII-enriched particles subjected to high light conditions (Krieger et al. 1998, Fufezan et al. 2002) though use of this technique in leaves is not possible (Fischer et al. 2013).
Since our initial demonstrations, during the early 1990s, of 1O2 detection in PSII RCs the techniques have been expanded greatly not only to show its involvement in photoinhibition in cells (see reviews by Krieger-Liskay et al. 2008, Fischer et al. 2013) but also it has been invoked in retrograde signaling (from the chloroplast to the nucleus) inducing cellular responses to environmental stresses including high light (op den Camp et al. 2003, Apel and colleagues; Fischer et al. 2004, Trianphylidès et al. 2009, Reinbothe et al. 2010).
Some of the new techniques employed recently, to detect 1O2, are changes in fluorescence or luminescence of 1O2-specific probes (such as DanePy and singlet oxygen sensor green, SOSG) along with imaging (for further information on the success and problems with these techniques, see Fischer et al. 2013). Fischer et al. (2007) used the DanePy technique to detect 1O2 in the cytoplasm of Chlamydomonas reinhardtii cells after very high intensity light stress. The significant result they found was that treatment with herbicides, which change the redox potential of QA (Rutherford and Krieger-Liszkay 2001), had the expected effect on the size of the 1O2 signal, indicating the PSII origin of the 1O2. SOSG was also used with confocal microscopy to image 1O2 formation by Synechocystis sp. PCC 6803 cells (Sinha et al. 2012). Additionally histidine-catalyzed molecular oxygen uptake has been demonstrated in high light-stressed Synechocystis sp. PCC 6803 cells (Rehman et al. 2013), and exogenously formed 1O2 (Rose Bengal sensitized) has been shown to stimulate gene expression in Arabidopsis thaliana (see Fischer et al. 2013).
As discussed already there are so many quenching processes going on that it was thought that it was not possible for 1O2 to get far enough to act as a signal to activate protein synthesis in the nucleus, and the debate rages as to whether 1O2 can travel that far and induce gene expression directly or whether the 1O2 detected in the cytoplasm is produced by secondary reactions. It is more likely that lipid peroxidation side products, i.e. peroxyl radicals, regenerate 1O2 by the Russell mechanism (Miyamoto et al. 2007). Recent evidence for this mechanism comes from Pospíšil and colleagues in response to both heat (Chan et al. 2012) and high light (Yadav and Pospíšil 2012).
Many calculations have been carried out to try and work out the distance 1O2 might be able to travel in the chloroplast and cell— and the projected distance is getting longer and longer. According to Moan (1990), it should only travel <70 nm before being quenched or decaying, but a more recent estimate in liposomes was >100 nm in 10 µs (Ehrenberg et al. 1998). However, it is likely that this would be much less in TMs as they contain, in addition to ascorbate, a lot of unsaturated lipids and proteins which would act as physical quenchers of 1O2, as pointed out by Ehrenberg et al. (1998). This will also be the case in the highly dense stroma and cytoplasm. More recently, Skovsen et al. (2005) showed that dye-sensitized 1O2 can travel ∼270 nm in 6 µs in the aqueous region of cells. However, this was measured in rat neurons and, as already mentioned, plant cells have high concentrations of antioxidants such as ascorbate in their cytoplasm (Bisby et al. 1999) which would reduce this distance considerably.
There is also the question of how much 1O2 is formed. The greater the amount, the more chance that a few molecules will travel some distance before meeting a quencher. Fischer et al. (2007) showed that it required very high light intensities to produce detectable levels of 1O2 in the cytoplasm of C. reinhardtii cells. However, it should be noted that in leaves TMs come very close to the chloroplast envelope and some chloroplasts are very close to the nucleus, although distances will still be greater than 270 nm. There is also a question of whether 1O2 originating from PSII can carry out retrograde signaling directly or whether it activates a signal transduction pathway directed to the nucleus to activate gene expression in which secondarily produced 1O2 may well play a part (Laloi et al. 2007, Baruah et al. 2009). The low light stress that also results in formation of some 1O2 in the PSII RC (Keren et al. 1997) probably only yields levels that are sufficient to be involved in photoinhibition and triggering of the turnover of the D1 protein, and not for retrograde signaling.
Funding
This study was supported by the Agricultural and Food Research Council (AFRC) and the Biotechnology and Biological Sciences Research Council (BBSRC) [funding to Professor Jim Barber] and the Science and Engineering Research Council (SERC) and the Engineering and Physical Sciences Research Council (EPSRC) [funding to Professor David Phillips].
Acknowledgments
I am extremely grateful to all my colleagues who were involved in the detection of singlet oxygen in the 1990s. I would also like to thank Anja Krieger-Liszkay for discussions and critical advice about the manuscript. I am especially grateful to Professor Jim Barber (Biochemistry Department) and to Professor David Phillips (Chemistry Department) in Imperial College London and members of their research groups at the time.
Glossary
Abbreviations
- Car
carotenoid
- EPR
electron paramagnetic resonance
- L1,270
luminescence at 1,270 nm
- LHC
light-harvesting complex
- PDT
photodynamic therapy
- 1O2
singlet oxygen, 3O2, triplet oxygen
- P680
primary electron donor in PSII
- Pheo
primary electron acceptor in PSII
- RC
reaction center
- QA and QB
secondary electron acceptors in PSII
- RC
reaction center
- RNO
p-nitrosodimethylaniline
- ROS
reactive oxygen species
- RP
radical pair
- SOSG
singlet oxygen sensor green
- TM
thylakoid membranes
- Yz
tyrosine electron donor to P680+
Disclosures
The author has no conflicts of interest to declare.
References
- Adir N, Zer H, Shochat S, Ohad I. Photoinhibition—a historical perspective. Photosynth. Res. 2003;76:343–370. doi: 10.1023/A:1024969518145. [DOI] [PubMed] [Google Scholar]
- Allison RR, Downie GH, Cuenca R, Hu X-H, Childs CJH, Sibata CH. Photosensitizers in clinical PDT. Photodiagn. Photodyn. Ther. 2004;1:27–42. doi: 10.1016/S1572-1000(04)00007-9. [DOI] [PubMed] [Google Scholar]
- Aro EM, Virgin I, Andersson B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Barber J, Andersson B. Too much of a good thing: light can be bad for photosynthesis. Trends Biochem. Sci. 1992;17:61–66. doi: 10.1016/0968-0004(92)90503-2. [DOI] [PubMed] [Google Scholar]
- Barber J, Archer MD. P680, the primary electron donor of photosystem II. J. Photochem. Photobiol. A: Chem. 2001;142:97–106. [Google Scholar]
- Barber J, Chapman DJ, Telfer A. Characterisation of a PS II reaction centre isolated from the chloroplasts of Pisum sativum. FEBS Lett. 1987;220:67–73. [Google Scholar]
- Baruah A, Simkova K, Apel K, Laloi C. Arabidopsis mutants reveal multiple singlet oxygen signaling pathways involved in stress response and development. Plant Mol. Biol. 2009;70:547–563. doi: 10.1007/s11103-009-9491-0. [DOI] [PubMed] [Google Scholar]
- Bisby RH, Morgan CG, Hamblett I, Gorman AA. Quenching of singlet oxygen by Trolox C, ascorbate, and amino acids: effects of pH and temperature. J. Phys. Chem. A. 1999;103:7454–7459. [Google Scholar]
- Blankenship RE. Molecular Mechanisms of Photosynthesis. 2nd edn. Oxford: Wiley-Blackwell; 2014. [Google Scholar]
- Chan T, Shimizu Y, Pospíšil P, Nijo N, Fujiwara A, Taninaka Y, et al. Quality control of photosystem II: lipid peroxidation accelerates photoinhibition under excessive illumination. PLoS One. 2012;7:e52100. doi: 10.1371/journal.pone.0052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Las Rivas J, Telfer A, Barber J. Two coupled β-carotene molecules protect P680 from photodamage in isolated photosystem II reaction centres. Biochim. Biophys. Acta. 1993;1142:155–164. [Google Scholar]
- Durrant JR, Giorgi LB, Barber J, Klug DR, Porter G. Characterization of triplet-states in isolated photosystem-II reaction centres—oxygen quenching as a mechanism for photodamage. Biochim. Biophys. Acta. 1990;1017:167–175. [Google Scholar]
- Ehrenberg B, Anderson JL, Foote CS. Kinetics and yield of singlet oxygen photosensitized by hypericin in organic and biological media. Photochem. Photobiol. 1998;68:135–140. [PubMed] [Google Scholar]
- Firey PA, Rodgers MAJ. Photochemical properties of ethrythrocyte ghosts containing porphyrins. Photochem. Photobiol. 1988;47:615–619. doi: 10.1111/j.1751-1097.1988.tb02756.x. [DOI] [PubMed] [Google Scholar]
- Fischer BB, Hideg E, Krieger-Liszkay A. Production, detection and redox signaling of singlet oxygen in photosynthetic organisms. Antioxidants Redox Signal. 2013;18:2145–2162. doi: 10.1089/ars.2012.5124. [DOI] [PubMed] [Google Scholar]
- Fischer BB, Krieger-Liszkay A, Eggen RIL. Photosensitizers neutral red (type I) and rose bengal (type II) cause light dependent toxicity in Chlamydomonas reinhardtii and induce the Gpxh gene via increased singlet oxygen formation. Environ. Sci. Technol. 2004;38:6307–6313. doi: 10.1021/es049673y. [DOI] [PubMed] [Google Scholar]
- Fischer BB, Krieger-Liszkay A, Hideg E, Snyrychova I, Wiesendanger M, Eggen RIL. Role of singlet oxygen in chloroplast retrograde signalling in Chlamydomonas reinhardtii. FEBS Lett. 2005;581:5555–5560. doi: 10.1016/j.febslet.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Foote, C. S. (1990) Future Directions and Applications in Photodynamic Therapy, SPIE Institute Series Vol. IS 6, pp. 115–126. SPIE Optical Engineering Press.
- Frank HA, Cogdell RJ. Carotenoids in photosynthesis. Photochem. Photobiol. 1996;63:257–264. doi: 10.1111/j.1751-1097.1996.tb03022.x. [DOI] [PubMed] [Google Scholar]
- Fufezan C, Rutherford AW, Krieger-Liszkay A. Singlet oxygen production in herbicide-treated photosystem II. FEBS Lett. 2002;532:407–410. doi: 10.1016/s0014-5793(02)03724-9. [DOI] [PubMed] [Google Scholar]
- Gorman AA, Rodgers MAJ. Singlet oxygen. In: Scaiano JC, editor. The Handbook of Organic Photochemistry. Vol. 2. Boca Raton, FL: CRC Press; 1989. pp. 229–247. [Google Scholar]
- Hideg E, Spetea C, Vass I. Singlet oxygen production in thylakoid membranes during photoinhibition as detected by EPR spectroscopy. Photosynth. Res. 1994;39:191–199. doi: 10.1007/BF00029386. [DOI] [PubMed] [Google Scholar]
- Hideg E, Vass I. Singlet oxygen is not produced in photosystem I under photoinhibitory conditions. Photochem. Photobiol. 1995;62:949–952. [Google Scholar]
- Hirayama O, Nakamura K, Hamada S, Kobayasi Y. Singlet oxygen quenching ability of naturally occurring carotenoids. Lipids. 1994;29:149–150. doi: 10.1007/BF02537155. [DOI] [PubMed] [Google Scholar]
- Keren N, Berg A, van Kan PJM, Levanon H, Ohad I. Mechanism of photosystem II photoinactivation and D1 protein degradation at low light: the role of back electron flow. Proc. Natl Acad. Sci. USA. 1997;19:1579–1584. doi: 10.1073/pnas.94.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraljic I, El Mohsni S. A new method for detection of singlet oxygen in aqueous solutions. Photochem. Photobiol. 1978;28:577–581. [Google Scholar]
- Krieger A, Rutherford AW, Vass I, Hideg E. Relationship between activity, D1 loss and Mn binding in photoinhibition of photosystem II. Biochemistry. 1998;37:16262–16269. doi: 10.1021/bi981243v. [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A, Fufezan C, Trebst A. Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 2008;98:551–564. doi: 10.1007/s11120-008-9349-3. [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A, Rutherford AW. Influence of herbicide binding on the redox potential of the quinone acceptors in photosystem II: relevance to photodamage and phytotoxicity. Biochemistry. 1998;37:17339–17344. doi: 10.1021/bi9822628. [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A, Trebst A. Tocopherol is the scavenger of singlet oxygen produced by the triplet states of chlorophyll in the PSII reaction centre. J Exp. Bot. 2006;57:1677–1684. doi: 10.1093/jxb/erl002. [DOI] [PubMed] [Google Scholar]
- Kruk J, Holländer-Czytko H, Oettmeier W, Trebst A. Tocopherol as a singlet oxygen scavenger in Photosystem II. J. Plant Physiol. 2005;162:749–757. doi: 10.1016/j.jplph.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Kruk J, Trebst A. Plastoquinol as a singlet oxygen scavenger in photosystem II. J Exp. Bot. . 2008;1777:154–162. doi: 10.1016/j.bbabio.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Laloi C, Stachowiak M, Pers-Kamczyc E, Warzych E, Murgia I, Apel K. Cross-talk between singlet oxygen and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA. 2007;104:672–677. doi: 10.1073/pnas.0609063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Melo TB, Arellano JB, Naqvi KR. Temporal profile of the singlet oxygen emission endogenously produced by photosystem II reaction centres in an aqueous buffer. Photosynth. Res. 2012;112:75–79. doi: 10.1007/s11120-012-9739-4. [DOI] [PubMed] [Google Scholar]
- Loll B, Kern J, Saenger W, Zouni A, Biesiadka J. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature. 2005;438:1040–1044. doi: 10.1038/nature04224. [DOI] [PubMed] [Google Scholar]
- Macpherson AN, Telfer A, Truscott TG, Barber J. Direct detection of singlet oxygen from isolated photosystem two reaction centres. Biochim. Biophys. Acta. 1993;1143:301–309. [Google Scholar]
- Miyamoto S, Ronsein GE, Prado FM, Uemi M, Correa TC, Toma IN, et al. Biological hydroperoxides and singlet molecular oxygen generation. IUBMB Life. 2007;59:322–331. doi: 10.1080/15216540701242508. [DOI] [PubMed] [Google Scholar]
- Moan J. On the diffusion length of singlet oxygen in cells and tissues. J. Photochem. Photobiol. B: Biol. 1990;6:343–347. [Google Scholar]
- Moser CC, Dutton PL. Engineering protein structure for electron transfer function in photosynthetic reaction centers. Biochim. Biophys. Acta. 1992;1101:171–176. [PubMed] [Google Scholar]
- Nanba O, Satoh K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc. Natl Acad. Sci. USA. 1987;84:109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilby PR. Singlet oxygen: there is indeed something new under the sun. Chem. Soc. Rev. 2010;39:3181–3209. doi: 10.1039/b926014p. [DOI] [PubMed] [Google Scholar]
- Okamura MY, Satoh K, Isaacson RA, Feher G. Evidence of the primary charge separation in theD1/D2 complex of photosystem II from spinach: Epr of the triplet state. In: Biggins J, editor. Progress in Photosynthesis Research. Vol. 1. Dordrecht: Martinus Nijhoff; 1987. pp. 379–381. [Google Scholar]
- op den Camp RGL, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, et al. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell. 2003;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasil O, Adir N, Ohad I. Dynamics of photosystem II: mechanism of photoinhibition and recovery process. In: Barber J, editor. Topics in Photosynthesis. The Photosystems: Structure, Function and Molecular Biology. Vol. 11. Amsterdam: Elsevier; 1992. pp. 220–250. [Google Scholar]
- Rehman AU, Cser K, Sass L, Vass I. Characterization of singlet oxygen production and its involvement in photodamage of Photosystem II in the cyanobacterium Synechocystis PCC 6803 by histidine-mediated chemical trapping. Biochim. Biophys. Acta. 2013;1827:689–698. doi: 10.1016/j.bbabio.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Reinbothe C, Pollmann S, Reinbothe S. Singlet oxygen signalling links photosynthesis to translation and plant growth. Trends Plant Sci. 2010;15:499–506. doi: 10.1016/j.tplants.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Rutherford AW, Krieger-Liszkay A. Herbicide-induced oxidative stress in photosystem II. Trends Biochem. Sci. 2001;26:648–653. doi: 10.1016/s0968-0004(01)01953-3. [DOI] [PubMed] [Google Scholar]
- Rutherford AW, Osyczka A, Rappaport F. Back-reactions, short circuits, leaks and other energy wasteful reactions in biological electron transfer: redox tuning to survive life in O2. FEBS Lett. 2012;586:603–616. doi: 10.1016/j.febslet.2011.12.039. [DOI] [PubMed] [Google Scholar]
- Schödel R, Irrgang KD, Voigt J, Renger G. Rate of carotenoid triplet formation in solubilized light-harvesting complex II (LHCII) from spinach. Biophys J. 1998;75:3143–3153. doi: 10.1016/S0006-3495(98)77756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha RK, Komenda J, Knoppova J, Sedlarova M, Pospíšil P. Small CAB-like proteins prevent formation of singlet oxygen in the damaged photosystem II complex of the cyanobacterium Synechocystis sp. PCC 6803. Plant, Cell Environ. 2012;35:806–818. doi: 10.1111/j.1365-3040.2011.02454.x. [DOI] [PubMed] [Google Scholar]
- Skovsen E, Snyder JW, Lambert JDC, Ogilby PR. Lifetime and diffusion of singlet oxygen in a cell. J. Phys. Chem. B. 2005;109:8570–8573. doi: 10.1021/jp051163i. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. Ascorbate biosynthesis and function in photoprotection. Philos. Trans. R. Soc. B: Biol. Sci. 2000;355:1455–1464. doi: 10.1098/rstb.2000.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Hansson Ö, Mathis P, Satoh K. Primary radical pair in the photosystem II reaction centre. Biochim. Biophys. Acta. 1987;893:49–59. [Google Scholar]
- Telfer A. What is β-carotene doing in the photosystem II reaction centre? Philos. Trans. R. Soc. B: Biol. Sci. 2002;357:1431–1440. doi: 10.1098/rstb.2002.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer A, Bishop SM, Phillips D, Barber J. Isolated photosynthetic reaction center of photosystem two as a sensitizer for the formation of singlet oxygen. J. Biol. Chem. 1994a;269:13244–13253. [PubMed] [Google Scholar]
- Telfer A, Dhami S, Bishop SM, Phillips D, Barber J. β-Carotene quenches singlet oxygen formed by isolated photosystem II reaction centers. Biochemistry. 1994b;33:14469–14474. doi: 10.1021/bi00252a013. [DOI] [PubMed] [Google Scholar]
- Telfer A, Oldham TC, Phillips D, Barber J. Singlet oxygen formation detected by near-infrared emission from isolated photosystem II reaction centres: direct correlation between P680 triplet decay and luminescence rise kinetics and its consequences for photoinhibition. J. Photochem. Photobiol. B: Biol. 1999;48:89–96. [Google Scholar]
- Triantaphylidès C, Havaux M. Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci. 2009;14:219–228. doi: 10.1016/j.tplants.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Umena Y, Kawakami K, Shen J-R, Kamiya N. Crystal structure of oxygen evolving photosystem II at a resolution of 1.9 Å. Nature. 2011;473:55–61. doi: 10.1038/nature09913. [DOI] [PubMed] [Google Scholar]
- van Mieghem FJE, Nitschke W, Mathis P, Rutherford AW. The influence of the quinone–iron electron acceptor complex on the reaction centre photochemistry of photosystem II. Biochim. Biophys. Acta. 1989;977:207–214. [Google Scholar]
- van Mieghem FJE, Satoh K, Rutherford AW. A chlorophyll tilted 30° relative to the membrane in the photosystem II reaction centre. Biochim. Biophys. Acta. 1991;1058:379–385. [Google Scholar]
- Vass I, Styring S, Hundal T, Koivuniemi A, Aro EM, Andersson B. Reversible and irreversible intermediates during photoinhibition of photosystem II: stable reduced QA species promote chlorophyll triplet formation. Proc. Natl Acad. Sci. USA. 1992;89:1408–1412. doi: 10.1073/pnas.89.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walles B. Plastid structures of carotenoid-deficient mutants of sunflower (Helianthus annus L.) I. The white mutant. Hereditas. 1965;53:247–256. [Google Scholar]
- Wilkinson F, Helman WP, Ross AB. Rate constants for the decay and reactions of the lowest electronically excited singlet-state of molecular-oxygen in solution—an expanded and revised compilation. J. Phys. Chem. Ref. Data. 1995;24:663–1021. [Google Scholar]
- Witt HT. Coupling of quanta, electrons, fields, ions and phosphorylation in the functional membrane of photosynthesis. Q. Rev. Biophys. 1971;4:365–477. doi: 10.1017/s0033583500000834. [DOI] [PubMed] [Google Scholar]
- Wolff C, Witt HT. On metastable states of carotenoids in primary events of photosynthesis. Registration by repetitive ultra-short-flash photometry. Z. Naturforsch. 1969;24B:1031–1037. doi: 10.1515/znb-1969-0818. [DOI] [PubMed] [Google Scholar]
- Yadav DK, Kruk J, Sinha RK, Pospíšil P. Singlet oxygen scavenging activity of plastoquinol in photosystem II of higher plants: electron paramagnetic resonance spin-trapping study. Biochim. Biophys. Acta. 2010;1797:1807–1811. doi: 10.1016/j.bbabio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Yadav DK, Pospíšil P. Evidence on the formation of singlet oxygen in the donor side photoinhibition of photosystem II: EPR spin-trapping study. PLoS One. 2012;7:e45883. doi: 10.1371/journal.pone.0045883. [DOI] [PMC free article] [PubMed] [Google Scholar]