Abstract
Mindfulness meditation (MM) is a stress-reduction technique that may have real biological effects on hemodynamics but has never previously been tested in chronic kidney disease (CKD) patients. In addition, the mechanisms underlying the potential blood pressure (BP)-lowering effects of MM are unknown. We sought to determine whether MM acutely lowers BP in CKD patients, and whether these hemodynamic changes are mediated by a reduction in sympathetic nerve activity. In 15 hypertensive African-American (AA) males with CKD, we conducted a randomized, crossover study in which participants underwent 14 min of MM or 14 min of BP education (control intervention) during two separate random-order study visits. Muscle sympathetic nerve activity (MSNA), beat-to-beat arterial BP, heart rate (HR), and respiratory rate (RR) were continuously measured at baseline and during each intervention. A subset had a third study visit to undergo controlled breathing (CB) to determine whether a reduction in RR alone was sufficient in exacting hemodynamic changes. We observed a significantly greater reduction in systolic BP, diastolic BP, mean arterial pressure, and HR, as well as a significantly greater reduction in MSNA, during MM compared with the control intervention. Participants had a significantly lower RR during MM; however, in contrast to MM, CB alone did not reduce BP, HR, or MSNA. MM acutely lowers BP and HR in AA males with hypertensive CKD, and these hemodynamic effects may be mediated by a reduction in sympathetic nerve activity. RR is significantly lower during MM, but CB alone without concomitant meditation does not acutely alter hemodynamics or sympathetic activity in CKD.
Keywords: muscle sympathetic nerve activity, mindfulness meditation, chronic renal insufficiency
chronic kidney disease (CKD) is highly prevalent among African-Americans (AA); AA are almost four times as likely as caucasians to develop kidney disease (39, 53). Although AA comprise approximately 13% of the general population, AA account for 32% of all CKD patients in the United States (39). Moreover, reduced renal function is an independent risk factor for cardiovascular (CV) disease and mortality (22, 50) and has a more pronounced deleterious effect on CV risk and all-cause mortality among AA compared with caucasians (56) Since hypertension and male gender are also independent risk factors for CV disease, AA males with hypertensive CKD are a highly prevalent and growing patient population at substantially higher CV risk.
One contributing factor underlying increased CV risk in this population is chronic overactivation of the sympathetic nervous system (SNS). Multiple studies have shown that SNS activity is substantially and chronically elevated in patients with both end-stage renal disease (ESRD) as well as mild CKD (18, 25, 33, 42, 45). In addition, AA males also have chronically elevated SNS activity compared with AA women and caucasians (1). Such elevations in SNS activation not only lead to elevated blood pressure (BP) by increasing total peripheral resistance but also increase cardiac risk directly, via cardiac and vascular inflammation, arrhythmogenicity, oxidative stress, and fibrosis (12, 35, 48). In addition, chronic elevation of SNS activity accelerates renal sclerosis and leads to insulin resistance and cardiac hypertrophy, independent of its effects on BP (2, 29, 46). Thus chronic elevation of SNS activity is a major contributing factor leading to increased CV risk and progressive decline in renal function in patients with CKD.
Current strategies to counteract SNS overactivation and lower BP in patients with renal disease include the use of antihypertensive medications, such as β-blockers and central α-agonists, which act as peripheral and central sympatholytics. However, the use of such agents, alone or in combination with other antihypertensives, is often fraught with adverse side effects, and in the case of β-blockers, may lead to long-term metabolic derangements including insulin resistance and hyperlipidemia (4, 14). Moreover, there is no clear evidence that β-blocker therapy improves mortality risk in CKD patients without heart disease (3, 5). Thus, although sympatholytic drugs should be considered in the treatment of hypertension in patients with renal disease, given their limitations, there is a need to investigate safe and effective adjunctive or alternative therapies that may ameliorate SNS overactivation in patients with CKD.
One such potential adjunctive therapy that is noninvasive, safe, and may have real biological effects on BP and SNS activation is mindfulness meditation (MM). MM is a stress-reduction technique involving focused awareness on internal and external sensory stimuli in the present moment without judgment or cognitive elaboration (8, 9). MM has been shown to lower BP in both normotensive and hypertensive patient groups, in some (7, 17, 27, 32) but not all (13), prior studies. However, the effect of MM on hemodynamics in CKD patients, or in AA males, in whom baseline SNS activity is significantly elevated, had not been previously tested. In addition, the mechanisms underlying the BP-lowering effect of MM, and in particular, its potential modulation of SNS activation, is unknown. We conducted a randomized controlled crossover study to determine the effects of MM on BP and SNS activity acutely in hypertensive AA males with CKD. We measured continuous hemodynamics and performed the first direct intraneural recordings of muscle sympathetic nerve activity (MSNA) using microneurography during MM to determine the potential benefits of MM acutely on hemodynamics and autonomic function in CKD patients.
METHODS
Study Population
The study population consisted of 15 total participants (age range 51–66 yr) with hypertension and CKD Stage III, defined as an estimated glomerular filtration rate (eGFR) between 30 and 59 ml·min−1·1.73 m−2 as calculated by the modified Modification of Diet in Renal Disease (MDRD) equation (34). All participants had stable renal function defined as no greater than a 5% fluctuation in eGFR within the prior 3 mo and hypertension controlled on 1–4 medications. Study participants were African-American male veterans that were recruited from clinics at the Atlanta Veterans Affairs Medical Center. None of the participants practiced any form of meditation or exercised regularly. Exclusion criteria for all participants included illicit drug use within the past 12 mo and any major comorbid conditions including diabetes, neuropathy, vascular disease, uncontrolled anemia, or any clinical evidence of heart failure or heart disease determined by electrocardiogram, echocardiogram, stress test, and/or history. This study was approved by the Emory University Institutional Review Board, and the Atlanta Veterans Affairs Medical Center Research and Development Committee. A written informed consent was obtained from each study participant.
Measurements and Procedures
Blood pressure.
Baseline BP was measured with an automated sphygmomanometer (Dinamap PRO Series) while the participant was seated, after 5 min of quiet rest, using standard technique. Baseline BP was the mean of three consecutive readings separated by 3 min. Beat-to-beat arterial BP was measured continuously during the experimental protocols using a noninvasive monitoring device that detects digital blood flow via finger cuffs and translates blood flow oscillations into continuous pulse pressure waveforms and beat-to-beat values of BP (CNAP, CNSystems). Absolute values of BP were internally calibrated using a concomitant upper arm BP reading and were calibrated at the start and every 15 min throughout the study. This device has been validated to reflect accurate absolute BP values as well as accurate beat-to-beat changes in BP as measured via an intra-arterial catheter (28, 30).
Muscle sympathetic nerve activity.
Multiunit postganglionic sympathetic nerve activity directed to muscle (MSNA) was recorded directly from the peroneal nerve by microneurography as previously described (54, 55). A tungsten microelectrode (tip diameter 5–15 μm) (Bioengineering, University of Iowa) was inserted into the nerve, and a reference microelectrode was inserted subcutaneously 1–2 cm from the recording electrode. The signals were amplified (total gain 50,000–100,000), filtered (700–2,000 Hz), rectified, and integrated (time constant 0.1 s) to obtain a mean voltage display of sympathetic nerve activity (model 662C-4, Nerve Traffic Analyzer, University of Iowa, Bioengineering) that was recorded by the LabChart 7 Program (PowerLab 16sp, ADInstruments). Continuous electrocardiogram (ECG) was recorded simultaneously with the neurogram using a bioamp system. All MSNA recordings met previously established criteria (19, 20, 38).
Mindfulness meditation.
During one study visit, participants underwent guided MM by listening to 14 min of prerecorded guided MM using an MP3 player and headphones. The standard guided meditation recording included several basic components of mindfulness: breathing awareness, mini body scan, and brief self-compassion. This particular meditation was used by our coinvestigator in a prior study that demonstrated acute improvements in psychological and physiological parameters (including anxiety, pain, heart rate, and respiratory rate) in a different patient population with chronic disease (bone marrow transplant recipients) (9). The goal of MM is to foster focused, nonjudgmental awareness of one's present-moment experience (i.e., sensations of breathing and other sensory stimuli) with an attitude of receptivity and acceptance. In breathing awareness, participants are asked to notice the sensations of breathing without trying to manipulate the respiratory rate. During body scan, participants are asked to notice sensations in different parts of their bodies. With the self-compassion (or “loving kindness” toward self) portion, participants are directed to have an open and positive attitude about themselves and asked to repeat phrases such as “May I be loving, kind, and gentle to myself.” Continuous beat-to-beat arterial BP and MSNA were recorded throughout the MM and control interventions.
Blood pressure education (control).
As a control intervention for comparison, during a separate study visit, participants underwent 14 min of BP education by listening to a recording on hypertension diagnosis and treatment, using the same MP3 player and headphones. The order of the study visits (MM vs. control intervention) was randomized. The lights were dimmed during both the MM and control interventions.
Controlled breathing.
To determine whether a slowed respiratory rate (RR) alone to low-normal rates was responsible for the observed physiological changes, a subset of participants returned for a third study visit to undergo controlled breathing (CB). The participant's RR was guided to maintain a steady rate of 12 breaths/min, by directing the participant to inhale for 2 s and exhale for 3 s continuously for a total of 10 min. Because reductions in RR, BP, and MSNA occurred within the first 5 min and plateaued by 10 min during MM, 10 min of CB was performed. Data obtained during CB were compared with data obtained during the first 10 min of MM during statistical analysis.
Experimental Protocol
All 15 participants were studied a total of two to three times during separate study visits in a randomized controlled crossover design. For each study visit, participants were studied in the early morning, after abstaining from food, caffeine, smoking, and alcohol for at least 12 h, and exercise for at least 24 h. The participants were instructed to take their antihypertensive medications at exactly the same time before each study, and no medication changes were allowed between studies. The study room was quiet, semidark, and temperate (∼21°C). Blood was drawn for a basic metabolic panel, and a urine sample was collected for urine microalbumin and creatinine concentrations. Participants were placed in a supine position on a comfortable stretcher. Finger cuffs were fitted and placed on the fingers of the dominant arm for continuous beat-to-beat arterial BP measurements, and an upper arm cuff was placed for intermittent automatic calibrations with the finger cuffs. ECG patch electrodes were placed for continuous heart rate (HR) recordings. The leg was positioned for microneurography, and the tungsten microelectrode was inserted and manipulated to obtain a satisfactory nerve recording. After 10 min of rest, baseline BP, HR, RR, and MSNA were recorded continuously for 10 min.
During the study visit, MSNA, ECG, hemodynamics, and RR were continuously measured at baseline and during either: 1) 14 min of MM; or 2) 14 min of BP education (control intervention). The participants were directly observed by the principal investigator during the entire study procedure. None of the participants exhibited signs of falling asleep such as heavy, loud breathing, or snoring. Either MM or BP education was performed during separate study visits to eliminate any carryover effects. The order of the visits was randomized. A subset of participants (N = 6) presented for a third study visit to undergo CB. This was done to determine whether a reduction in RR alone, without concomitant meditation, would lower BP and MSNA. All three visits were completed within a 3-mo time span, and all participants were instructed not to change their medications, diet, or exercise habits during the study period.
Data Analysis
Muscle sympathetic nerve activity.
MSNA and ECG data were exported from the Labchart data acquisition system to WinCPRS (Absolute Aliens, Turku, Finland) for analysis. R-waves were detected and marked from the continuous ECG recording. MSNA bursts were automatically detected by the program using the following criteria: 3:1 burst-to-noise ratio within a 0.5-s search window, with an average latency in burst occurrence of 1.2–1.4 s from the previous R-wave. After automatic detection, the ECG and MSNA neurograms were visually inspected for accuracy of detection by a single investigator (J. Park), without knowledge of the experimental status as control or intervention. MSNA was expressed as burst frequency (bursts/min), burst incidence (bursts/100 heartbeats), and total activity (arbitrary units/min). MSNA burst amplitudes were normalized to the largest burst under resting conditions, which was assigned a value of 1,000 arbitrary units. MSNA total activity takes into account both the frequency and amplitude of bursts and was defined as the average normalized burst amplitude multiplied by burst frequency.
Statistical analysis.
Statistical analysis was performed using the SAS 9.3 program (SAS Institutes). Baseline characteristics were compared using standard two-sided two-sample t-tests. The following mixed effects linear model was fitted to the difference data (i.e., each observation representing the difference between the current observation and the baseline observation):
In this model, Yij represents the difference relative to baseline at minute j (j = 2, 4, 6, 8, 10, 12, 14) for the ith subject, and the variable “Intervent” is an indicator variable taking the value 0 if under the control condition, and 1 if under the MM condition. The inclusion of this indicator variable allows us to model separate average linear trajectories for the difference measures over time under the two experimental conditions. The use of (minute 14) in the model makes the intercept α0 interpretable as the mean difference relative to baseline under the control condition at minute 14. Also it makes α1 interpretable as the discrepancy in that mean difference for the MM condition relative to the control condition at minute 14. β0 is the rate of change of the difference from baseline over time under the control condition, whereas β1 is the discrepancy in that rate of change for the MM condition relative to the control condition. Thus the test of whether β1 = 0 will assess whether the change in the average difference from baseline over time is different under MM versus under control. The mixed model assumes that the random effects ai, bi, and ϵij are all normally distributed with 0 means and was fit in such a way that the variances of these terms are allowed to vary according to whether subjects were under the control or the MM condition. The model allows for correlations among observations from the same subject within a condition (i.e., either control or MM), but it assumes that observations from the same subject are uncorrelated if they are taken under different conditions.
For the variable RR, a slightly generalized version of a standard two-way ANOVA model was fit to the RR difference (from baseline) data. This model treats condition (with levels “control,” “MM,” “CB”) and time (levels “Mid” and “End”) as the two factors and accounts for within-subject correlation via a random intercept. A test to assess presence or absence of a condition by time interaction was followed by a test of the main effect of condition, with Bonferroni-adjusted stepdown tests to compare mean RR under both MM and CB versus mean RR under control conditions. Finally, a similarly generalized version of a one-way ANOVA model was fit to assess variation in mean differences (after 10 min, relative to baseline) in MSNA (bursts/min) across the three conditions. Corresponding figures expressing results in terms of means ± SE were constructed.
RESULTS
Baseline Characteristics
Table 1 depicts the baseline characteristics, hemodynamics, and MSNA values for study participants. All participants were African-American, male veterans with CKD Stage III (mean eGFR of 46 ± 1.5 ml·min·1.73m−2) and microalbuminuria. Most participants were obese, with a mean body mass index (BMI) of >30 kg/m2. One participant had comorbid posttraumatic stress disorder (PTSD) and three participants were smokers. All participants had hypertension controlled on an average of 2.4 medications (range 1–4 medications). Table 2 describes the antihypertensive medication regimen of the study population. The majority of participants were treated with angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin receptor blockers (ARB), β-blockers, and dihydropyridine calcium channel blockers. Adequate MSNA nerve recordings were obtained during both the MM study visit and during the control study visit in 11 of 15 participants.
Table 1.
Baseline characteristics, hemodynamics, and sympathetic activity
| Age, yr | 58.7 ± 1.4 |
| Body mass index, kg/m2 | 32.4 ± 1.4 |
| Systolic BP, mmHg | 132 ± 4 |
| Diastolic BP, mmHg | 81 ± 3 |
| Mean arterial pressure, mmHg | 98 ± 3 |
| Heart rate, beats/min | 59 ± 3 |
| Respiratory rate, breaths/min | 17.0 ± 0.4 |
| Mean number of BP medications | 2.4 ± 0.3 |
| Serum Creatinine, mg/dl | 1.66 ± 0.08 |
| eGFR, ml·min−1·1.73 m−2 | 46 ± 1.5 |
| Urinary microalbumin:creatinine, mg/g | 158.6 ± 54.8 |
| Current smoker | 3/15 |
| Comorbid posttraumatic stress disorder | 1/15 |
| MSNA, bursts/min | 40.0 ± 3.5 |
| MSNA, bursts/100 heartbeats | 68.2 ± 4.5 |
| MSNA, arbitrary units/min | 175.7 ± 37.0 |
Data are expressed as means ± SE, or number of participants with that condition out of 15 total participants. Baseline measurements in study participants. BP, blood pressure; MSNA, muscle sympathetic nerve activity. Estimated glomerular filtration rate (eGFR) was quantified using the 4-variable modified Modified Diet in Renal Disease (MDRD) equation (34).
Table 2.
Antihypertensive medications taken by study participants
| Medications | n |
|---|---|
| ACE-I or ARB | 11 |
| β-Blockers | 8 |
| Dihydropyridine calcium channel blockers | 8 |
| Loop or thiazide diuretics | 5 |
| Aldosterone receptor blockers | 1 |
| Α-Blockers | 1 |
ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers.
Mindfulness Meditation
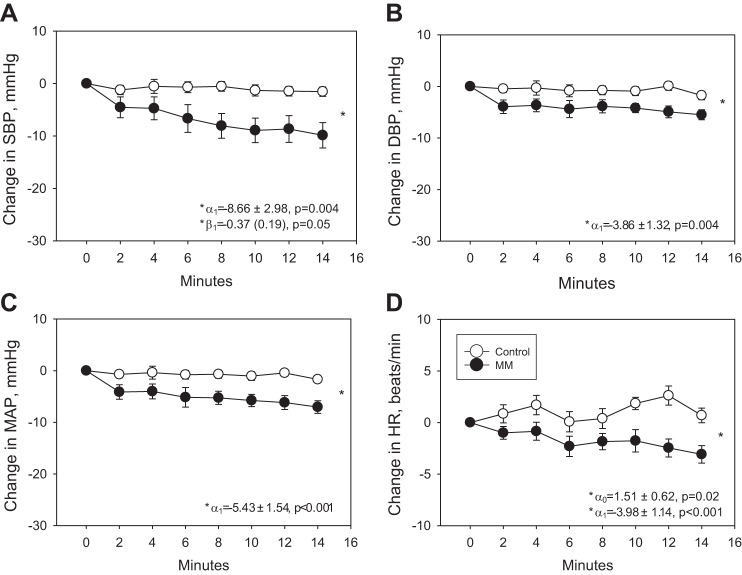
From Table 3, the significance of the α1 parameter indicates that the average change (relative to baseline) at minute 14 for the outcomes systolic BP (SBP), diastolic BP (DBP), mean arterial pressure (MAP), HR, and MSNA quantified as burst frequency (bursts/min) and as total activity [TA, arbitrary units (AU)/min)] is lower under MM than under control conditions. The significance of the β1 parameter for the outcomes SBP and MSNA indicate that the rate of change of the difference over time is faster under MM than under the control condition. Results were similar when MSNA was also quantified as burst incidence (bursts/100 heartbeats, data not shown). The lack of significance of any of the estimated α0 and β0 parameters (with the exception of α0 for HR) suggests that under control conditions, there is no noteworthy change in the mean of the outcomes relative to baseline during followup. For HR, the model estimates a small but significant mean increase in HR of 1.51 under control conditions from baseline to minute 14 but a strong decrease under the MM condition.
Table 3.
Results from mixed linear effects modeling for each outcome
| Outcome Variable (Difference from Baseline)*,† |
||||||
|---|---|---|---|---|---|---|
| Parameter | SBP (n = 13; N = 182) | DBP (n = 13; N = 181) | MAP (n = 13; N = 181) | HR (n = 12; N = 167) | MSNA (n = 11; N = 154) | MSNATA (n = 9; N = 126) |
| α0 | −1.41 (0.77), P = 0.08 | −1.06 (0.76), P = 0.17 | −1.18 (0.64), P = 0.08 | 1.51 (0.62), P = 0.02 | −1.02 (1.37), P = 0.46 | −6.93 (8.00), P = 0.40 |
| α1 | −8.66 (2.98), P = 0.004 | −3.86 (1.32), P = 0.004 | −5.43 (1.54), P < 0.001 | −3.98 (1.14), P < 0.001 | −10.55 (1.87), P < 0.001 | −52.05 (14.78), P < 0.001 |
| β0 | −0.06 (0.09), P = 0.48 | −0.06 (0.06), P = 0.38 | −0.06 (0.05), P = 0.29 | 0.04 (0.07), P = 0.55 | 0.08 (0.08), P = 0.35 | 0.22 (0.59), P = 0.71 |
| β1 | −0.37 (0.19), P = 0.05 | −0.05 (0.11), P = 0.65 | −0.15 (0.11), P = 0.18 | −0.18 (0.09), P = 0.06 | −0.82 (0.16), P < 0.001 | −3.44 (1.09), P = 0.002 |
Available sample sizes given for each outcome (n = no. of subjects; N = no. of observations);
Table provides estimates and standard errors (in parenthese) for parameters of interest (α0, α1, β0, and β1). α0 is the mean difference relative to baseline under the control condition at minute 14, and α1 is the discrepancy in that mean difference for mindfulness meditation (MM) relative to control condition at minute 14. β0 is the rate of change of the difference from baseline over time under the control condition, whereas β1 is the discrepancy in that rate of change for MM relative to the control condition.
These data suggest that there was a significantly greater reduction in SBP, DBP, MAP, and HR, during the 14 min of MM compared with the control intervention (Fig. 1). Similarly, there was a significantly greater reduction in MSNA during MM (by a mean of −10.6 bursts/min at 14 min) compared with the control intervention (Fig. 2A). Results were similar when MSNA was quantified as total activity normalized to the largest burst (AU/min, Fig. 2B) and burst incidence (bursts/100 heart beats, data not shown). Results are also similar in patients treated with inhibitors of the renin angiotensin system (ACE-I and ARBs) versus those not treated with these agents and when the participant with PTSD and smokers were removed from the analysis.
Fig. 1.
Change in systolic blood pressure (SBP, A), diastolic blood pressure (DBP, B), mean arterial pressure (MAP, C), and heart rate (HR, D), during 14 min of mindfulness meditation (MM) versus control intervention. The model equation is given in the text. α0 represents the mean difference relative to baseline under the control condition at minute 14, and α1 is the discrepancy in that mean difference for the MM condition relative to the control condition at minute 14. β0 is the rate of change of the difference from baseline over time under the control condition, whereas β1 is the discrepancy in that rate of change for the MM condition relative to the control condition. *Significant P values <0.05.
Fig. 2.

Change in muscle sympathetic nerve activity (MSNA) quantified in burst frequency (bursts/min, A) and total activity [arbitrary units (AU)/min, B]. The model equation is given in the text. α1 represents the discrepancy in the mean difference for the MM condition relative to the control condition at minute 14. β1 is the discrepancy in the rate of change for the MM condition relative to the control condition. *Significant P values <0.05.
Fig. 3.

SBP, DBP, MAP, and HR (A) and MSNA (B) at baseline and during the 5-min recovery period after a session of MM. *P value <0.05 compared with baseline.
Data from the recovery period immediately following the MM intervention were analyzed from seven participants. We observed that the BP and MSNA-lowering effect of MM was maintained during 5 min of recovery time following MM. SBP, DBP, MAP, but not HR, were significantly lower during the recovery period compared with baseline (Fig. 3A). MSNA burst frequency was also significantly lower during the recovery period compared with baseline (Fig. 3B).
Controlled Breathing
There was no significant condition by time interaction effect upon mean differences (from baseline) in RR (P = 0.64). However, the mean differences showed highly significant variation across the control, MM, and CB conditions (P < 0.001). In particular, the mean difference in RR from baseline was significantly lower under the MM and CB conditions as opposed to control (Fig. 4). During CB, breathing was guided to the same RR observed during MM but without concomitant meditation; there was no significant difference in RR between the MM and slow breathing interventions. The overall difference in mean change from baseline in MSNA burst frequency at 10 min was significantly different across the three conditions (P = 0.012). Whereas there was a highly significant difference for MM compared with the control intervention, there was no significant difference in change in MSNA during CB compared with the control intervention (Fig. 5). We also found no significant difference in change from baseline in SBP, DBP, MAP, or HR between the CB intervention and the control intervention (data not shown).
Fig. 4.

Respiratory rate (RR) at baseline (BL), midway (mid), and at the end of 14 min of MM (MM), control intervention, and controlled breathing (CB). *P value <0.05 for MM vs. control intervention at that time point. †ANOVA F-test was significant for a difference between MM vs. control interventions.
Fig. 5.

Change in MSNA from baseline to minute 14 during control intervention, MM, and CB. *P value <0.05 compared with control intervention.
DISCUSSION
The major new findings of this study are the following: 1) a single session of guided MM lowers BP and HR acutely in hypertensive patients with CKD Stage III; 2) CKD patients had a significantly greater reduction in MSNA acutely during MM, suggesting that MM may modulate central sympathetic output, resulting in lower BP; 3) reductions in BP and MSNA, but not HR, were maintained during the immediate recovery period following MM; 4) participants had a lower RR during MM compared with the control intervention; and 5) during CB, in which RR was lowered without concomitant meditation, BP and MSNA were not lower compared with the control intervention, suggesting that CB alone is not sufficient to acutely modulate central SNS output.
Adjunctive or alternative therapeutic interventions that are safe and free from adverse side effects are needed to lower SNS activity in patients with renal disease and thereby potentially mitigate the long-term adverse consequences of SNS overactivation, as well as lower BP. In this study, we investigated the biological effects of one such potential adjunctive therapy: MM. MM is noninvasive, safe, and may have real beneficial effects on BP, as well as other physiological measures in CKD patients. MM is a stress-reduction technique involving focused awareness on internal and external sensory stimuli in the present moment without judgment or cognitive elaboration (8, 9). Prior studies have shown that MM, and other forms of meditation, reduce anxiety, inflammation, and lower BP in a variety of patient groups. MM has been shown to reduce BP, HR, inflammation, anxiety, and improve quality of life in normotensive individuals (15, 17, 23, 57). In addition, other forms of meditation have been shown to lead to significant reductions in systolic and diastolic BP, HR (43), and reduced resting BP, BP reactivity to stress, and left ventricular mass (6).
Many, but not all (13), prior studies have shown that the BP-lowering effects of meditation are more pronounced in patients with high BP. In a study of 52 pharmacologically untreated patients with hypertension, those randomized to contemplative meditation for 8 wk had a significant reduction in office SBP (by −15 mmHg vs. +3 mmHg in controls), 24-h ambulatory BP profiles, and a reduction in rise of SBP during mental stress by 11 mmHg (37). Breathing awareness meditation, a major component of MM, significantly lowered daytime and nighttime SBP (by nearly 5 mmHg), HR, and overnight urinary sodium excretion, in a group of prehypertensive AA adolescents (7). Similarly, in a group of prehypertensive adults, a 4.9-mmHg reduction in clinic SBP and 1.9 mmHg drop in DBP was observed among the mindfulness-based stress reduction (MBSR) group (23). In a large randomized controlled trial of 103 participants, meditation improved SBP, insulin resistance, and HR variability in patients with stable coronary heart disease (47). MBSR led to sustained improvements in clinic BP persisting up to 1 yr (15), as well as a 23% decrease in all-cause mortality at a mean followup of 7.6 yr in a group of hypertensive elderly patients (51).
We observed a significantly greater reduction in SBP (by −10 mmHg), DBP (by −6 mmHg), MAP (by −7 mmHg), and HR (by −3 beats/min) during MM compared with the control intervention, among patients with CKD. The degree of BP reduction, particularly SBP, in our study population was relatively large compared with prior studies (7, 17, 27, 32), the majority of which report a reduction in SBP of around 5 mmHg, with a range in reduction of around 5–15 mmHg with meditation. There are several potential key differences that may account for the relatively greater reductions in BP during MM observed in our cohort. First, whereas most prior studies reported effects of MM in prehypertensive and hypertensive groups, our patient population was specifically composed of AA male patients with hypertension as well as CKD. AA and those with reduced renal function have chronic elevations in sympathetic activity and often times have higher BP that is more difficult to control (1, 18, 33, 50). In addition, all study participants were male veterans at high risk for PTSD, another disorder associated with high sympathetic tone (10), although we observed no difference in hemodynamic changes during MM when the PTSD patient was removed from the analyses in the current study. Nonetheless, patient characteristics that are associated with higher sympathetic activation may account for the differences in responsiveness in hemodynamics to the MM intervention in AA male CKD patients versus other patient groups. Finally, we report acute changes in hemodynamics during a single session of MM rather than changes in baseline hemodynamics after chronic MM therapy. Certainly, the sustained changes in baseline hemodynamics with time, and while the patients are not actively undergoing MM, may not be as robust or may be nonexistent in CKD and should be investigated in future studies.
One potential mechanism by which MM may lower BP is by reducing neural sympathetic output. However, direct intraneural measurements of sympathetic nerve activity during meditation had never previously been performed. We performed the first microneurographic measurements of MSNA during MM in CKD patients with hypertension and found significant reductions in MSNA during MM compared with the control intervention, suggesting that the BP- and HR-lowering effects of MM may be mediated by reductions in sympathetic nerve activity. The mechanisms by which MM lowers SNS activity are unclear, but one potential mechanism is via reduction in inflammation. Patients with CKD have high levels of inflammatory biomarkers and oxidative stress (26), arterial baroreflex dysfunction (52), and SNS overactivity (18, 21, 25, 33, 41, 45). Chronic inflammation, in turn, can contribute to chronic SNS overactivation by impairing arterial baroreflex sensitivity (16) or through direct central activation (40). Prior studies have shown that MM and mindfulness-based stress reduction may lower inflammatory markers such as C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), and interleukin 8 (IL-8) (36, 49). Whether MM reduces inflammation or improves arterial baroreflex sensitivy in CKD patients is unknown and should be investigated in future studies.
In the current study, we also observed that RRs were significantly slower during MM compared with the control intervention. Although MM does not specifically guide breathing, RR was spontaneously lower during MM compared with the control intervention. In this study, CB, in which the participant's RR was guided to a low-normal rate (to the same degree as during the MM intervention) but without concomitant meditation, was performed to determine whether the BP and MSNA-lowering effects of MM were due to the reduced RR alone. Whereas BP and MSNA were significantly lower during MM, we did not observe any change in BP and MSNA during CB without concomitant MM, suggesting that a lower breathing rate alone is not sufficient in exacting the hemodynamic and SNS changes. Prior studies have shown that slowing breathing to subphysiological rates acutely reduces arterial BP in patients with hypertension (31, 44) and chronic heart failure (11). The mechanisms underlying the acute BP-lowering effect of slow breathing in these prior studies is unclear but may include modulation of the autonomic nervous system. Slow breathing in prior studies has been shown to acutely lower MSNA (24, 44), suggesting that slow breathing rates may lower central SNS output.
In contrast, we did not observe reductions in either BP or MSNA during CB without concomitant MM. The differences between our observations in CB and prior studies on slow breathing may be due to differences in methodology and degree of reduction in RR. In prior studies, RR was lowered to subphysiological levels (<10 breaths/min) (24, 44), whereas in the present study, RR was lowered to low-normal rates of 12 breaths/min during CB to match the RR observed during MM. Breathing at subphysiological rates is thought to lower BP by resulting in increased tidal volume, which activates cardiopulmonary mechanoreceptors to reflexively inhibit central SNS outflow (31). Reducing RR to low-normal levels in CB, as opposed to lower subphysiological rates, may not be sufficient to increase tidal volume and modulate cardiopulmonary mechanoreceptors. In addition, we performed CB by guiding the participant to inhale for 2 s and exhale for 3 s to achieve a RR of 12 breaths/min. Lowering RR via other methods, such as device-guided slow breathing or yoga slow-breathing, may have different effects on hemodynamics and autonomic function in CKD patients.
Limitations
We recognize several limitations to our study. First, the study population included only African-American male veterans with CKD Stage III and without diabetes; therefore, the results may not be generalizable to women, the non-veteran population, those with more severe renal failure, or those with comorbid diabetes, which is the most common cause of kidney disease in the United States. Second, because the study population had hypertension, CKD, and was overweight or obese, the resting baseline MSNA was relatively high (around 40 bursts/min); it is unclear if similar reductions in SNS activity and BP would be observed among a healthier population with lower levels of baseline MSNA. Third, only SNS activity directed to the muscle (MSNA) was measured during each intervention. The effects of MM on sympathetic innervation to other organs such as the heart and kidneys, as well as parasympathetic activity, remain unknown. Finally, we investigated the acute changes in hemodynamics during a single session of MM. Whether long-term MM intervention leads to sustained improvements in hemodynamics and SNS activity in patients with CKD remains to be tested.
Perspectives and Significance
AA males with hypertensive CKD suffer from excessive CV risk and chronically elevated SNS activity. Adjunctive therapies that may ameliorate SNS activity and reduce BP in this high-risk patient population are needed. MM is a noninvasive, safe intervention, without side effects that may have real biological effects on SNS activity and hemodynamics. In this study, we demonstrate that MM acutely reduces MSNA and BP in AA males with hypertension and CKD, and that these reductions are sustained for at least several minutes post-MM. These findings warrant future investigations testing the long-term effects of several weeks of daily MM on baseline hemodynamics and SNS activity, to determine the clinical utility and therapeutic potential of this intervention.
GRANTS
This work was supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Studies Center, Decatur, GA; the Atlanta Research and Education Foundation; and PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health (NIH), National Center for Research Resources. J. Park was supported by NIH Grant K23 HL-098744, the Emory University Research Council, and Satellite healthcare, a not-for-profit renal care provider.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.P. and S.B.-W. conception and design of research; J.P. performed experiments; J.P. and R.H.L. analyzed data; J.P. interpreted results of experiments; J.P. prepared figures; J.P. drafted manuscript; J.P., R.H.L., and S.B.-W. edited and revised manuscript; J.P., R.H.L., and S.B.-W. approved final version of manuscript.
REFERENCES
- 1.Abate NI, Mansour YH, Tuncel M, Arbique D, Chavoshan B, Kizilbash A, Howell-Stampley T, Vongpatanasin W, Victor RG. Overweight and sympathetic overactivity in black Americans. Hypertension 38: 379–383, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Amann K, Rump LC, Simonaviciene A, Oberhauser V, Wessels S, Orth SR, Gross ML, Koch A, Bielenberg GW, Van Kats JP, Ehmke H, Mall G, Ritz E. Effects of low dose sympathetic inhibition on glomerulosclerosis and albuminuria in subtotally nephrectomized rats. J Am Soc Nephrol 11: 1469–1478, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Badve SV, Roberts MA, Hawley CM, Cass A, Garg AX, Krum H, Tonkin A, Perkovic V. Effects of beta-adrenergic antagonists in patients with chronic kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol 58: 1152–1161, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH, Phillips RA, Raskin P, Wright JT, Jr, Oakes R, Lukas MA, Anderson KM, Bell DS. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. J Am Med Assoc 292: 2227–2236, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Bakris GL, Hart P, Ritz E. Beta blockers in the management of chronic kidney disease. Kidney Int 70: 1905–1913, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Barnes VA, Orme-Johnson DW. Prevention and treatment of cardiovascular disease in adolescents and adults through the transcendental meditation((R)) program: A research review update. Current Hypertens Rev 8: 227–242, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes VA, Pendergrast RA, Harshfield GA, Treiber FA. Impact of breathing awareness meditation on ambulatory blood pressure and sodium handling in prehypertensive African American adolescents. Ethn Dis 18: 1–5, 2008 [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer-Wu S. Mindfulness meditation. Oncology (Williston Park) 24: 36–40, 2010 [PubMed] [Google Scholar]
- 9.Bauer-Wu S, Sullivan AM, Rosenbaum E, Ott MJ, Powell M, McLoughlin M, Healey MW. Facing the challenges of hematopoietic stem cell transplantation with mindfulness meditation: a pilot study. Integrat Cancer Therap 7: 62–69, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Bedi US, Arora R. Cardiovascular manifestations of posttraumatic stress disorder. J Natl Med Assoc 99: 642–649, 2007 [PMC free article] [PubMed] [Google Scholar]
- 11.Bernardi L, Porta C, Spicuzza L, Bellwon J, Spadacini G, Frey AW, Yeung LY, Sanderson JE, Pedretti R, Tramarin R. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 105: 143–145, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Bleeke T, Zhang H, Madamanchi N, Patterson C, Faber JE. Catecholamine-induced vascular wall growth is dependent on generation of reactive oxygen species. Circ Res 94: 37–45, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Blom K, Baker B, How M, Dai M, Irvine J, Abbey S, Abramson BL, Myers MG, Kiss A, Perkins NJ, Tobe SW. Hypertension analysis of stress reduction using mindfulness meditation and yoga: results from the Harmony Randomized Controlled Trial. Am J Hypertens 2013 [DOI] [PubMed] [Google Scholar]
- 14.Carella AM, Antonucci G, Conte M, Di Pumpo M, Giancola A, Antonucci E. Antihypertensive treatment with beta-blockers in the metabolic syndrome: a review. Curr Diabetes Rev 6: 215–221, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun 21: 1038–1049, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Chapleau MW, Li Z, Meyrelles SS, Ma X, Abboud FM. Mechanisms determining sensitivity of baroreceptor afferents in health and disease. Annal NY Acad Sci 940: 1–19, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Yang X, Wang L, Zhang X. A randomized controlled trial of the effects of brief mindfulness meditation on anxiety symptoms and systolic blood pressure in Chinese nursing students. Nurse Education Today 33: 1166–1172, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Converse RL, Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 327: 1912–1918, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 84: 65–81, 1972 [DOI] [PubMed] [Google Scholar]
- 20.Delius W, Hongell A, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand 84: 82–94, 1972 [DOI] [PubMed] [Google Scholar]
- 21.Elias AN, Vaziri ND, Maksy M. Plasma norepinephrine, epinephrine, and dopamine levels in end-stage renal disease. Effect of hemodialysis. Arch Intern Med 145: 1013–1015, 1985 [PubMed] [Google Scholar]
- 22.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Goldstein CM, Josephson R, Xie S, Hughes JW. Current perspectives on the use of meditation to reduce blood pressure. Intl J Hypertens 2012: 578397, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goso Y, Asanoi H, Ishise H, Kameyama T, Hirai T, Nozawa T, Takashima S, Umeno K, Inoue H. Respiratory modulation of muscle sympathetic nerve activity in patients with chronic heart failure. Circulation 104: 418–423, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Grassi G, Quarti-Trevano F, Seravalle G, Arenare F, Volpe M, Furiani S, Dell'Oro R, Mancia G. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension 57: 846–851, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Guarnieri G, Grassi G, Barazzoni R, Zanetti M, Biolo G. The impact of inflammation on metabolic regulation in chronic kidney disease: a review. J Ren Nutr 15: 121–124, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Hughes JW, Fresco DM, Myerscough R, MHMvD, Carlson LE, Josephson R. Randomized controlled trial of mindfulness-based stress reduction for prehypertension. Psychosomatic Med 75: 721–728, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilies C, Bauer M, Berg P, Rosenberg J, Hedderich J, Bein B, Hinz J, Hanss R. Investigation of the agreement of a continuous non-invasive arterial pressure device in comparison with invasive radial artery measurement. Br J Anaesth 2011 [DOI] [PubMed] [Google Scholar]
- 29.Jamerson KA, Julius S, Gudbrandsson T, Andersson O, Brant DO. Reflex sympathetic activation induces acute insulin resistance in the human forearm. Hypertension 21: 618–623, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Jeleazcov C, Krajinovic L, Munster T, Birkholz T, Fried R, Schuttler J, Fechner J. Precision and accuracy of a new device (CNAPTM) for continuous non-invasive arterial pressure monitoring: assessment during general anaesthesia. Br J Anaesth 105: 264–272, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Joseph CN, Porta C, Casucci G, Casiraghi N, Maffeis M, Rossi M, Bernardi L. Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension 46: 714–718, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Kingston J, Chadwick P, Meron D, Skinner TC. A pilot randomized control trial investigating the effect of mindfulness practice on pain tolerance, psychological well-being, and physiological activity. J Psychosomatic Res 62: 297–300, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Klein IH, Ligtenberg G, Neumann J, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic nerve activity is inappropriately increased in chronic renal disease. J Am Soc Nephrol 14: 3239–3244, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Levick SP, Murray DB, Janicki JS, Brower GL. Sympathetic nervous system modulation of inflammation and remodeling in the hypertensive heart. Hypertension 55: 270–276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malarkey WB, Jarjoura D, Klatt M. Workplace based mindfulness practice and inflammation: a randomized trial. Brain Behav Immun 27: 145–154, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manikonda JP, Stork S, Togel S, Lobmuller A, Grunberg I, Bedel S, Schardt F, Angermann CE, Jahns R, Voelker W. Contemplative meditation reduces ambulatory blood pressure and stress-induced hypertension: a randomized pilot trial. J Hum Hypertens 22: 138–140, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Mano T, Iwase S, Toma S. Microneurography as a tool in clinical neurophysiology to investigate peripheral neural traffic in humans. Clin Neurophysiol 117: 2357–2384, 2006 [DOI] [PubMed] [Google Scholar]
- 39.McClellan W, Warnock DG, McClure L, Campbell RC, Newsome BB, Howard V, Cushman M, Howard G. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol 17: 1710–1715, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation 119: 978–986, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Neumann J, Ligtenberg G, Klein II, Koomans HA, Blankestijn PJ. Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int 65: 1568–1576, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Neumann J, Ligtenberg G, Oey L, Koomans HA, Blankestijn PJ. Moxonidine normalizes sympathetic hyperactivity in patients with eprosartan-treated chronic renal failure. J Am Soc Nephrol 15: 2902–2907, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Nidich SI, Rainforth MV, Haaga DA, Hagelin J, Salerno JW, Travis F, Tanner M, Gaylord-King C, Grosswald S, Schneider RH. A randomized controlled trial on effects of the Transcendental Meditation program on blood pressure, psychological distress, and coping in young adults. Am J Hypertens 22: 1326–1331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oneda B, Ortega KC, Gusmao JL, Araujo TG, Mion D., Jr Sympathetic nerve activity is decreased during device-guided slow breathing. Hypertens Res 33: 708–712, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Park J, Campese VM, Nobakht N, Middlekauff HR. Differential distribution of muscle and skin sympathetic nerve activity in patients with end-stage renal disease. J Appl Physiol 105: 1873–1876, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel MB, Stewart JM, Loud AV, Anversa P, Wang J, Fiegel L, Hintze TH. Altered function and structure of the heart in dogs with chronic elevation in plasma norepinephrine. Circulation 84: 2091–2100, 1991 [DOI] [PubMed] [Google Scholar]
- 47.Paul-Labrador M, Polk D, Dwyer JH, Velasquez I, Nidich S, Rainforth M, Schneider R, Merz CN. Effects of a randomized controlled trial of transcendental meditation on components of the metabolic syndrome in subjects with coronary heart disease. Arch Intern Med 166: 1218–1224, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Perlini S, Palladini G, Ferrero I, Tozzi R, Fallarini S, Facoetti A, Nano R, Clari F, Busca G, Fogari R, Ferrari AU. Sympathectomy or doxazosin, but not propranolol, blunt myocardial interstitial fibrosis in pressure-overload hypertrophy. Hypertension 46: 1213–1218, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Rosenkranz MA, Davidson RJ, Maccoon DG, Sheridan JF, Kalin NH, Lutz A. A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behavior Immun 27: 174–184, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 42: 1050–1065, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Schneider RH, Alexander CN, Staggers F, Rainforth M, Salerno JW, Hartz A, Arndt S, Barnes VA, Nidich SI. Long-term effects of stress reduction on mortality in persons > or = 55 years of age with systemic hypertension. Am J Cardiol 95: 1060–1064, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Studinger P, Lenard Z, Mersich B, Reusz GS, Kollai M. Determinants of baroreflex function in juvenile end-stage renal disease. Kidney Int 69: 2236–2242, 2006 [DOI] [PubMed] [Google Scholar]
- 53.System URD. USRDS 2010 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases 2012 [Google Scholar]
- 54.Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979 [DOI] [PubMed] [Google Scholar]
- 55.Wallin BG, Fagius J. Peripheral sympathetic neural activity in conscious humans. Annu Rev Physiol 50: 565–576, 1988 [DOI] [PubMed] [Google Scholar]
- 56.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Zeidan F, Johnson SK, Gordon NS, Goolkasian P. Effects of brief and sham mindfulness meditation on mood and cardiovascular variables. J Altern Complement Med 16: 867–873, 2010 [DOI] [PubMed] [Google Scholar]