Abstract
Aims—(1) To investigate the expression in human derived glioblastoma cell lines of two structurally related genes, novH (nephroblastoma overexpressed gene) and CTGF (connective tissue growth factor), which encode putative insulin-like growth factor binding proteins of a novel type. (2) To investigate whether the same transcription factors regulate CTGF and novH expression.
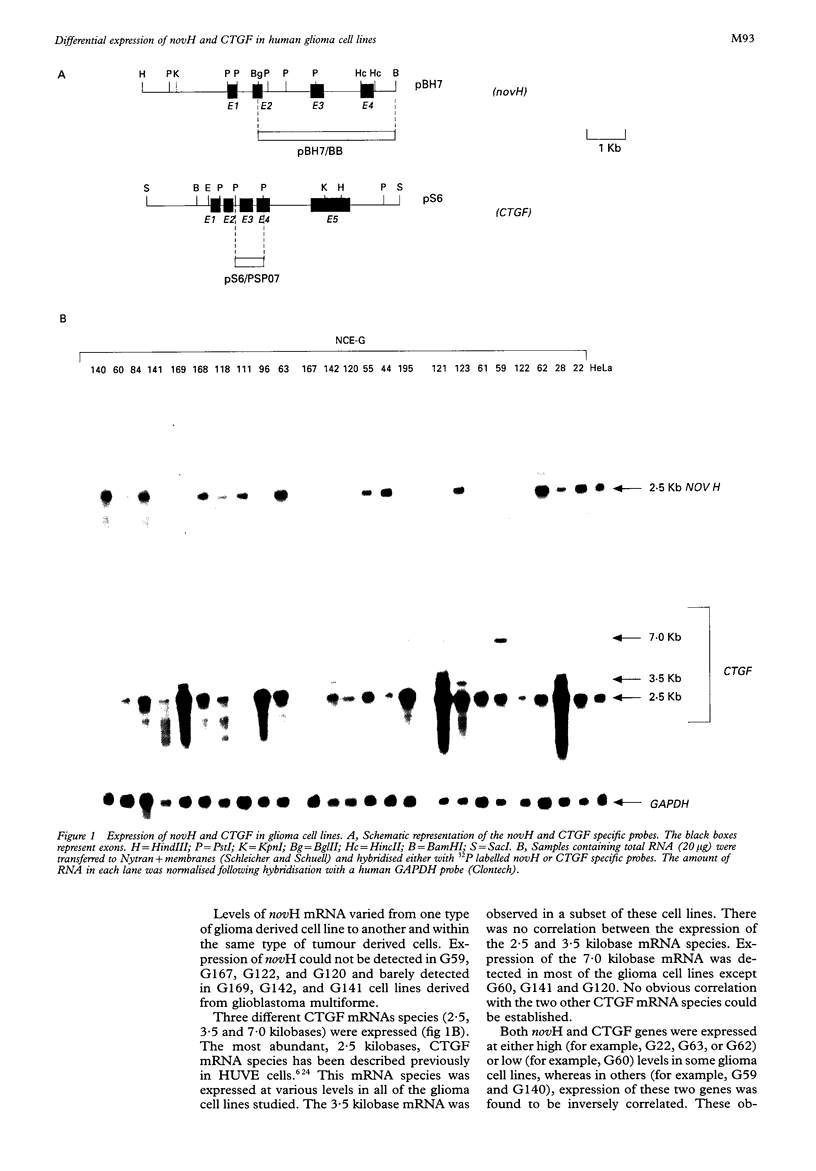
Methods—Expression of novH and CTGF was analysed in 24 glioblastoma derived cell lines by northern blotting. The CTGF promoter region was characterised by nucleotide sequencing, RNase protection experiments, by transient transfections, and CAT assays.
Results—CTGF and novH mRNA levels differed in the glioma cell lines studied. NovH and CTGF genes were not co-expressed in all cell lines. The CTGF promoter region was highly conserved compared with the corresponding region in the mouse (FISP12) and exhibited in vitro transcriptional activity.
Conclusions—Although the coding regions of novH and CTGF are highly homologous, their promoter regions are substantially different, suggesting that these two genes may be regulated by different mechanisms. Considering that novH and CTGF are likely to be, respectively, negative and positive regulators of growth and that some glioma cell lines expressing novH are not tumorigenic, expression of these two genes might represent a key element in determining the stage of differentiation or the malignant potential, or both, of some tumour cell lines.
Keywords: nov
Keywords: CTGF
Keywords: IGF binding proteins
Keywords: gliomas
Keywords: brain tumours
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anker L., Ohgaki H., Ludeke B. I., Herrmann H. D., Kleihues P., Westphal M. p53 protein accumulation and gene mutations in human glioma cell lines. Int J Cancer. 1993 Dec 2;55(6):982–987. doi: 10.1002/ijc.2910550618. [DOI] [PubMed] [Google Scholar]
- Azzarone B., Chaponnier C., Krief P., Mareel M., Suarez H., Macieira-Coelho A. Human fibroblasts from cancer patients: lifespan and transformed phenotype in vitro and role of mesenchyme in vivo. Mutat Res. 1988 Jun;199(2):313–325. doi: 10.1016/0027-5107(88)90211-4. [DOI] [PubMed] [Google Scholar]
- Biol M. C., Pintori S., Mathian B., Louisot P. Dietary regulation of intestinal glycosyl-transferase activities: relation between developmental changes and weaning in rats. J Nutr. 1991 Jan;121(1):114–125. doi: 10.1093/jn/121.1.114. [DOI] [PubMed] [Google Scholar]
- Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993 Jul 26;327(2):125–130. doi: 10.1016/0014-5793(93)80155-n. [DOI] [PubMed] [Google Scholar]
- Bradham D. M., Igarashi A., Potter R. L., Grotendorst G. R. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991 Sep;114(6):1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEINE U., DE THE G., ISHIGURO H., SOMMER J. R., BEARD D., BEARD J. W. Multiplicity of cell response to the BAI strain A (myeloblastosis) avian tumor virus. II. Nephroblastoma (Wilms' tumor): ultrastructure. J Natl Cancer Inst. 1962 Jul;29:41–105. [PubMed] [Google Scholar]
- Hamel W., Westphal M., Shepard H. M. Loss in expression of the retinoblastoma gene product in human gliomas is associated with advanced disease. J Neurooncol. 1993 May;16(2):159–165. doi: 10.1007/BF01324703. [DOI] [PubMed] [Google Scholar]
- Hamel W., Westphal M., Szönyi E., Escandón E., Nikolics K. Neurotrophin gene expression by cell lines derived from human gliomas. J Neurosci Res. 1993 Feb 1;34(2):147–157. doi: 10.1002/jnr.490340202. [DOI] [PubMed] [Google Scholar]
- Igarashi A., Okochi H., Bradham D. M., Grotendorst G. R. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993 Jun;4(6):637–645. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot V., Martinerie C., Dambrine G., Plassiart G., Brisac M., Crochet J., Perbal B. Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol. 1992 Jan;12(1):10–21. doi: 10.1128/mcb.12.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M. C., Ioh R. S., Bauer D. M., Zapf J. Molecular cloning of a new human insulin-like growth factor binding protein. Biochem Biophys Res Commun. 1991 Apr 15;176(1):219–225. doi: 10.1016/0006-291x(91)90912-q. [DOI] [PubMed] [Google Scholar]
- Lebeau J., Le Chalony C., Prosperi M. T., Goubin G. Constitutive overexpression of a 89 kDa heat shock protein gene in the HBL100 human mammary cell line converted to a tumorigenic phenotype by the EJ/T24 Harvey-ras oncogene. Oncogene. 1991 Jul;6(7):1125–1132. [PubMed] [Google Scholar]
- Mar J. H., Ordahl C. P. A conserved CATTCCT motif is required for skeletal muscle-specific activity of the cardiac troponin T gene promoter. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6404–6408. doi: 10.1073/pnas.85.17.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar J. H., Ordahl C. P. M-CAT binding factor, a novel trans-acting factor governing muscle-specific transcription. Mol Cell Biol. 1990 Aug;10(8):4271–4283. doi: 10.1128/mcb.10.8.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinerie C., Huff V., Joubert I., Badzioch M., Saunders G., Strong L., Perbal B. Structural analysis of the human nov proto-oncogene and expression in Wilms tumor. Oncogene. 1994 Sep;9(9):2729–2732. [PubMed] [Google Scholar]
- Martinerie C., Viegas-Pequignot E., Guenard I., Dutrillaux B., Nguyen V. C., Bernheim A., Perbal B. Physical mapping of human loci homologous to the chicken nov proto-oncogene. Oncogene. 1992 Dec;7(12):2529–2534. [PubMed] [Google Scholar]
- McMorris F. A., Smith T. M., DeSalvo S., Furlanetto R. W. Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proc Natl Acad Sci U S A. 1986 Feb;83(3):822–826. doi: 10.1073/pnas.83.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. P., Yang G. P., Sanders L., Lau L. F. Expression of cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol. 1990 Jul;10(7):3569–3577. doi: 10.1128/mcb.10.7.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. Contribution of MAV-1-induced nephroblastoma to the study of genes involved in human Wilms' tumor development. Crit Rev Oncog. 1994;5(6):589–613. [PubMed] [Google Scholar]
- Recio-Pinto E., Rechler M. M., Ishii D. N. Effects of insulin, insulin-like growth factor-II, and nerve growth factor on neurite formation and survival in cultured sympathetic and sensory neurons. J Neurosci. 1986 May;6(5):1211–1219. doi: 10.1523/JNEUROSCI.06-05-01211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Macdonald-Bravo H., Mattéi M. G., Bravo R. Structure, mapping, and expression of fisp-12, a growth factor-inducible gene encoding a secreted cysteine-rich protein. Cell Growth Differ. 1991 May;2(5):225–233. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara V. R., Carlsson-Skwirut C. The role of the insulin-like growth factors in the regulation of brain development. Prog Brain Res. 1988;73:87–99. doi: 10.1016/S0079-6123(08)60499-9. [DOI] [PubMed] [Google Scholar]
- Scholz G., Martinerie C., Perbal B., Hanafusa H. Transcriptional down regulation of the nov proto-oncogene in fibroblasts transformed by p60v-src. Mol Cell Biol. 1996 Feb;16(2):481–486. doi: 10.1128/mcb.16.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Levy D. B., Yannoni Y., Erikson R. L. Identification of a phorbol ester-repressible v-src-inducible gene. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1178–1182. doi: 10.1073/pnas.86.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B., Rahmsdorf H. J., Steffen A., Litfin M., Herrlich P. UV-induced DNA damage is an intermediate step in UV-induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Mol Cell Biol. 1989 Nov;9(11):5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal M., Herrmann H. D. Growth factor biology and oncogene activation in human gliomas and their implications for specific therapeutic concepts. Neurosurgery. 1989 Nov;25(5):681–694. doi: 10.1097/00006123-198911000-00001. [DOI] [PubMed] [Google Scholar]
- Westphal M., Hänsel M., Brunken M., König A., Köppen J. A., Herrmann H. D. Initiation of primary cell cultures from human intracranial tumors on extracellular matrix from bovine corneal endothelial cells. Exp Cell Biol. 1987;55(3):152–163. doi: 10.1159/000163411. [DOI] [PubMed] [Google Scholar]
- Westphal M., Hänsel M., Hamel W., Kunzmann R., Hölzel F. Karyotype analyses of 20 human glioma cell lines. Acta Neurochir (Wien) 1994;126(1):17–26. doi: 10.1007/BF01476489. [DOI] [PubMed] [Google Scholar]






