Abstract
Acute myeloid leukemia (AML) continues to represent an area of critical unmet need with respect to new and effective targeted therapies. The Bcl-2 family of pro- and antiapoptotic proteins stands at the crossroads of cellular survival and death, and the expression of and interactions between these proteins determine tumor cell fate. Malignant cells, which are often primed for apoptosis, are particularly vulnerable to the simultaneous disruption of cooperative survival signaling pathways. Indeed, the single agent activity of agents such as mammalian target of rapamycin (mTOR) and mitogen-activated protein kinase kinase (MEK) inhibitors in AML has been modest. Much work in recent years has focused on strategies to enhance the therapeutic potential of the bona fide BH3-mimetic, ABT-737, which inhibits B-cell lymphoma 2 (Bcl-2) and Bcl-xL. Most of these strategies target Mcl-1, an antiapoptotic protein not inhibited by ABT-737. The phosphatidylinositol-3-kinase (PI3K)/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways are central to the growth, proliferation, and survival of AML cells, and there is much interest currently in pharmacologically interrupting these pathways. Dual inhibitors of PI3K and mTOR overcome some intrinsic disadvantages of rapamycin and its derivatives, which selectively inhibit mTOR. In this review, we discuss why combining dual PI3K/mTOR blockade with inhibition of Bcl-2 and Bcl-xL, by virtue of allowing coordinate inhibition of three mutually synergistic pathways in AML cells, may be a particularly attractive therapeutic strategy in AML, the success of which may be predicted for by basal Akt activation.
Keywords: AML, PI3K, mTOR, Bcl-2, Bcl-xL
acute myeloid leukemia (AML) is diagnosed in more than 250,000 adults worldwide each year, the majority of whom are elderly (111). Induction chemotherapy with “7+3,” consisting of cytarabine and an anthracycline such as idarubicin or daunorubicin (or the anthracenedione mitoxantrone), first described over 40 years ago (140), has remained the standard approach to initial treatment and is usually followed by consolidation chemotherapy once morphologic remission is achieved, with bone marrow transplantation playing a potentially curative role in selected cases (9, 59). Despite substantial progress in recent years in advancing our understanding of the biology of AML, in large part due to the availability of mutational screening and whole genome/exome sequencing (12, 95), there has been little improvement in therapy, with cure remaining elusive for the large majority of patients (10). The outlook is particularly dismal for elderly patients (29) and those with relapsed/refractory disease (35). The inability of traditional chemotherapeutics to kill the quiescent, self-renewing leukemic stem cell (LSC) population (68) is potentially a major reason why cure has not been consistently achieved. It is believed that novel strategies for targeting AML must target this LSC population (86). Recent insights into the nature of oncogene addiction (115) have also revealed that multiple survival signaling pathways cooperate to promote transformed cell survival (116). A corollary of this concept is that multiple pathways, rationally selected for each tumor type and ideally personalized to an individual patient's cancer, must be coordinately disrupted to optimize transformed cell killing (58, 75). An attempt was recently made (101) to target three such critical and interdependent survival pathways in AML cells [the Bcl-2 (B-cell lymphoma 2) family of antiapoptotic proteins, and the PI3K/Akt/mTOR (phosphatidylinositol-3-kinase/Akt/mammalian target of rapamycin) and MEK/ERK (mitogen-activated protein kinase kinase/extracellular signal-regulated kinase) signaling pathways] in an effort to prime them for apoptosis (132).
Bcl-2 FAMILY OF PRO- AND ANTIAPOPTOTIC PROTEINS
The Bcl-2 family proteins control the central, evolutionarily conserved pathway for regulating programmed cell death, i.e., the “intrinsic,” or mitochondrial pathway of apoptosis (106). In essence, the Bcl-2 family of proteins consists of the “multidomain” antiapoptotic proteins [Bcl-2, Bcl-xL, Bcl-w, Bfl-1 (A1), and Mcl-1 (myeloid cell leukemia-1)] and the apoptosis “effectors” Bax and Bak, and the “activator” (Bim, Bid, and perhaps Puma) or “sensitizer” (Bad, Bik, Noxa, Hrk, Bmf, Puma) “BH3-only” proteins (24). Bax and Bak directly participate in the formation of pores in the mitochondrial outer membrane, triggering mitochondrial outer membrane permeabilization (MOMP), the central event that controls cellular commitment to death via apoptosis, and the activator BH3-only proteins interact with and activate Bax and Bak directly to this end (24). The antiapoptotic Bcl-2 family proteins prevent MOMP by sequestering the apoptosis activators and effectors by binding to their BH3 domain, a phenomenon that is competitively inhibited by the proapoptotic sensitizer BH3-only proteins (24). The Bcl-2 family proteins operate as nodal points at the convergence of multiple pathways (106), and their central role in triggering the final common pathway of apoptosis, characterized by caspase activation, makes them particularly attractive drug targets (67). Multiple studies have implicated the role of Bcl-2 family proteins in AML pathogenesis (20, 42, 50), prognosis (27, 48, 131), and resistance to chemotherapy (11, 66, 69, 118). Small-molecule “BH3-mimetics” that inhibit the antiapoptotic functions of Bcl-2 and Bcl-xL have, therefore, been developed (91, 121, 129). Significantly, however, these drugs do not inhibit Mcl-1, which may be more critical for the development and maintenance of AML than Bcl-2 and Bcl-xL (42), and an essential effector of FLT3-ITD (fms-like tyrosine kinase - internal tandem duplication)-mediated drug resistance (60) and stem cell survival (144) in AML. Not surprisingly, numerous studies have demonstrated Mcl-1, a short-lived protein critically dependent upon active transcription and translation for its maintenance (55), to be a key determinant of resistance to the BH3-mimetic ABT-737 (63, 72, 126, 130, 141). Mcl-1 is an inducible protein (38) that also functions as a stress “sensor” to coordinately regulate both apoptosis and autophagy (41). Another Bcl-2 family protein involved in determining “Bcl-2 dependence” of certain leukemias [e.g., chronic lymphocytic leukemia (CLL), acute lymphoblastic leukemia] and, hence, their sensitivity to ABT-737 is the proapoptotic BH3-only protein, Bim (25, 26). Furthermore, complex interactions between, and not simply expression patterns of Bcl-2 family proteins, particularly those involving Bim, which binds to all three major antiapoptotic proteins (16, 43), have been shown to be critical factors underlying sensitivity or resistance to ABT-737 (18, 87). Several studies have used the aforementioned principles, viz. downregulation of Mcl-1 and upregulation of Bim, to design strategies that synergistically enhance the lethality of ABT-737 and other BH3-mimetics toward AML cells (17, 64, 102, 146), thus simultaneously targeting multiple arms of the apoptotic regulatory machinery (23).
PI3K/AKT/mTOR SIGNALING PATHWAY
The PI3K/AKT/mTOR pathway is a cellular growth, proliferation, motility, and survival signaling axis (13) that represents one of the most frequently dysregulated pathways in cancer (22), including AML (83), where activation of the pathway has been shown to be required for cell survival (138, 139). Three classes of PI3K exist, of which class IA PI3Ks are most clearly implicated in human cancers and targeted by current pharmacologic PI3K inhibitors (31). Class IA PI3Ks are heterodimers that consist of a p85 regulatory and a p110 catalytic subunit (22, 31). In response to extracellular cues [usually growth factor stimulation via receptor tyrosine kinase (RTK) signaling], the p85 regulatory subunit binds to RTKs and/or adaptors, relieving its inhibition of the p110 catalytic subunit and recruiting class IA PI3Ks to the plasma membrane, where their substrate, phosphatidylinositol-4,5-bisphosphate (PIP2), resides (22, 31). PI3K then phosphorylates PIP2 at the 3-hydroxyl position to produce phosphatidylinositol-3,4,5-triphosphate (PIP3), while the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome 10) terminates PI3K-dependent signaling by catalyzing the reverse reaction (22, 31, 112). PIP3 brings two serine/threonine kinases, Akt/protein kinase B and phosphoinositide-dependent kinase 1 (PDK1), into close proximity on the cell membrane, upon which Akt is activated by PDK1 by phosphorylation at Thr308, while full activation requires Ser473 phosphorylation by mTORC2 (mTOR containing protein complex 2) (22, 31). Akt in turn regulates, through [generally inhibitory (13)] phosphorylation, a wide range of target proteins that control cell proliferation, survival, growth, and other processes: these include the proapoptotic Bcl-2 family proteins Bim, Bad, and Bax; forkhead box O (FOXO) transcription factors (which mediate apoptosis by activating the transcription of pro-apoptotic genes such as FasL and Bim); murine double minute 2 homolog, the negative regulator of the tumor suppressor p53; GSK3 (glycogen synthase kinase 3) isoforms (which downregulate cyclin D1 and Myc); procaspase-9; I-kappa B kinase, the negative regulator of the prosurvival transcription factor NF-κB; TSC2 (tuberous sclerosis complex 2) and proline-rich Akt substrate of 40 kDa, critical negative regulators of mTORC1 (mTOR containing complex 1) signaling; the endogenous cyclin-dependent kinase (CDK) inhibitor p27Kip1; and the DNA damage checkpoint kinase Chk1 (74, 78).
RATIONALE FOR DUAL PI3K AND mTOR BLOCKADE IN AML
Dysregulation of the PI3K/Akt/mTOR pathway in AML stems from multiple abnormalities, including PTEN loss (112), RTK activation [e.g., FLT3-ITD (7), KIT (88), Ras, insulin-like growth factor-1 and its receptor (IGF-1/IGF-1R) (113)], or activating mutations or overexpression of PI3K (51) or Akt (83). In AML, Akt activation (phosphorylation at Thr308/Ser473) variably occurs in 50–80% of patients, despite the rarity of activating mutations of Akt or PI3K or of PTEN loss (39, 82, 85, 122). Consequently, there is considerable interest in therapeutically targeting the PI3K/Akt/mTOR pathway in AML (34, 81). Both PI3K (47) and mTOR inhibitors have been shown to inhibit LSC survival (142, 143) and the latter to block Mcl-1 translation (6, 97), making them attractive candidates for AML therapy. Importantly, mTORC1 is a major downstream effector of Akt that is often not only under the control of PI3K/Akt signaling; GSK3 and AMP-activated protein kinase (AMPK), besides the two effector kinases downstream of RAS, ERK and ribosomal protein S6 kinase (RSK), can also phosphorylate TSC2 and thus effectively promote mTORC1 activity (4, 76, 77, 125). This argues for a therapeutic advantage for dual PI3K/mTOR inhibitors over agents that inhibit only PI3K (31). Conversely, currently approved mTOR inhibitors (temsirolimus, everolimus) often lead to feedback activation of PI3K/Akt and MEK/ERK through induction of IRS-1 (insulin receptor substrate 1) and Grb10, a phenomenon that may compromise their antitumor activity (14, 53, 92, 110, 145). This has been demonstrated preclinically to be the case in AML and provides a rationale for therapeutic inhibition of both PI3K/Akt and mTOR (127). Indeed, the dual PI3K/mTOR inhibitors PI-103 (94) and NVP-BEZ235 (15) display substantial activity against AML cell lines and primary AML samples. Finally, through a poorly understood mechanism (5, 30, 61), PI3K inhibitors may disrupt the complementary Ras/Raf/MEK/ERK survival signaling pathway, activated in >80% of AML samples (107), in AML cells (104, 124), in sharp contrast to its activation in other tumor types by PI3K (114) and mTOR inhibitors (14, 62), and by the Akt inhibitor perifosine in AML (103) and multiple myeloma cells (49). The combination of PI3K or mTOR inhibitors with MEK inhibitors to overcome this paradoxical activation is, in fact, a well-described synergistic strategy (32, 45, 117, 120, 134). It appears that, at least in AML cells, dual PI3K/mTOR inhibitors might be able to accomplish the task of cotargeting the PI3K/Akt/mTOR and MEK/ERK survival signaling pathways without the need for a MEK inhibitor.
RATIONALE FOR COMBINING DUAL PI3K/mTOR INHIBITORS WITH BH3-MIMETICS IN AML CELLS
The PI3K/Akt/mTOR and MEK/ERK survival signaling pathways extensively regulate the Bcl-2 family proteins and are thus intimately linked to the apoptotic pathways. Akt upregulates Bcl-2 and Mcl-1 via cyclic adenosine monophosphate response element binding protein (CREB) (80, 98, 133). The disposition of both pro (e.g., Bad, Bim)- and antiapoptotic (e.g., Mcl-1) proteins is regulated not just by Akt but also by ERK1/2 (54, 73, 117). For example, phosphorylation at Ser55/65/100 (by ERK1/2) and Ser87 (by Akt) leads to Bim (-EL) degradation (96, 99). Highly synergistic interactions have been reported between Akt and MEK inhibitors in promoting AML cell death through Bim- and Mcl-1 (but not Bad)-dependent mechanisms (100). Notably, Mcl-1 and Bim expression are tightly regulated by both the PI3K/Akt/mTOR and MEK/ERK pathways. While PI3K drives Mcl-1 transcription through a CREB-dependent process, mTOR regulates its translation, and the Akt/GSK3 axis and the E3 ubiquitin ligase SCF(FBW7) (SKP1-cullin-1-F-box complex that contains FBW7 as the F-box protein) control its posttranslational proteasomal degradation (52, 56, 133, 135). Similarly, ERK may activate Mcl-1 transcription through SRF/Elk-1, while also slowing turnover of the normally rapidly degraded Mcl-1 protein by phosphorylating it at Thr163 (28, 128). As discussed above, regulation of Bim expression and function by the PI3K/Akt and MEK/ERK pathways occurs at the transcriptional level through FOXO3a (33, 123) and particularly at the posttranslational level through phosphorylations by Akt (99) and ERK (46). Finally, it is important to consider the interplay between the MEK/ERK and PI3K/Akt/mTOR pathways: thus, as alluded to earlier, ERK phosphorylation and inactivation of TSC2 remove the latter's inhibitory effects on mTOR signaling, cell proliferation, and oncogenic transformation (76).
It follows from the above discussion that the combination of a dual PI3K/mTOR inhibitor with a BH3-mimetic that antagonizes the antiapoptotic functions of Bcl-2 and Bcl-xL represents, at least theoretically, a particularly potent synergistic strategy against AML cells, especially those that are FLT3-ITD+, including LSCs. First, such a strategy would interrupt two major and complementary signaling pathways (viz., PI3K/Akt/mTOR and Ras/Raf/MEK/ERK) considered critical for AML cell survival while avoiding undesirable feedback activation of PI3K/Akt and ERK1/2. Second, PI3K/mTOR inhibitors would be expected to be particularly effective at downregulating Mcl-1, central to AML development and maintenance and the main determinant of resistance to ABT-737, given that they act at three separate levels (viz., transcriptional, translational, and posttranslational, via GSK3-mediated degradation) to accomplish this. Based on this strong theoretical foundation (Fig. 1), the effects of concomitant PI3K/mTOR and Bcl-2/Bcl-xL inhibition were recently examined in AML cell lines, patient-derived blasts, and xenograft models of AML (101). The findings of these studies are summarized below.
Fig. 1.
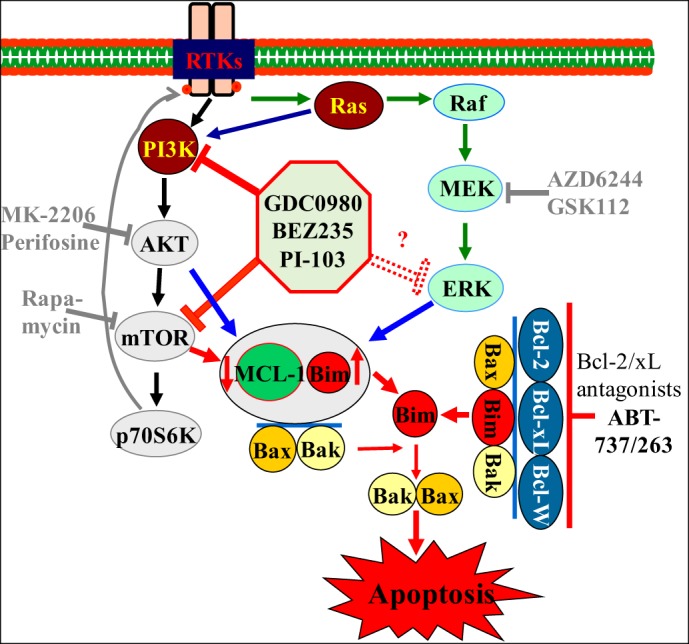
Hypothetical model of interactions between phosphatidylinositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway inhibitors and Bcl-2/-xL antagonists.
Studies conducted on AML cells using tet-inducible short hairpin RNA constructs directed against Akt or Bcl-2 and Bcl-xL individually or together, as well as employing pharmacologic inhibitors (e.g., NVP-BEZ235/PI-103 and ABT-737) revealed that the PI3K/Akt/mTOR pathway and Bcl-2/Bcl-xL play important coordinate roles in protecting leukemia cells from lethality. Specifically, genetic or pharmacologic interventions simultaneously inhibiting the PI3K/Akt/mTOR pathway and disabling Bcl-2/Bcl-xL dramatically enhanced leukemic cell death, with minimal toxicity toward normal CD34+ hematopoietic progenitors. These findings were recapitulated in vivo in an AML xenograft model with little toxicity. Mcl-1 downregulation resulted from PI3K/mTOR inhibition, at least in part through GSK3 activation, which promotes the proteasomal degradation of Mcl-1. Although NVP-BEZ235 alone downregulated Mcl-1, it did not significantly trigger cell death, underscoring the importance of concomitant Bcl-2/Bcl-xL inhibition, as all three major antiapoptotic proteins sequester the apoptosis effectors Bax and Bak. Significantly, release of Bax and Bak from Mcl-1, Bcl-2, and Bcl-xL was observed. Another novel finding was a marked increase in Bim binding to Bcl-2 and Bcl-xL in response to PI3K/mTOR inhibition, a phenomenon effectively abolished by ABT-737. Presumably Bim, liberated from these two antiapoptotic proteins, as well as Mcl-1 (due to downregulation), triggered apoptosis by activating Bax and Bak. A particularly intriguing observation was an apparent correlation between responses to combined treatment with PI3K/mTOR inhibitors and ABT-737 and baseline Akt activation in primary AML blasts. Specifically, four of six specimens analyzed responded to the treatment, and all exhibited basal Akt phosphorylation. In contrast, basal Akt phosphorylation was not detected in nonresponding specimens or in normal CD34+ cells. This raises the possibility that the former cells were addicted to the PI3K/Akt/mTOR pathway and hence vulnerable to its inhibition. Definitive answers to this question will require analysis of a considerably larger number of specimens.
A major challenge with inhibitors of the PI3K/Akt/mTOR pathway has been delineation of their pharmacodynamic effects; i.e., determining whether or not the administration of these agents leads to target inhibition in vivo (109). Biomarkers that have been used to this end have included phosphorylation of Akt and its substrate Akt1S1 (also known as PRAS40), phosphorylation of eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1), and phosphorylation of RPS6, the proliferation marker Ki-67, and the apoptosis marker TUNEL (109). Of note, however, RPS6 and 4EBP1 can also be regulated by Ras/Raf/MEK/ERK/mTORC1 signaling, thereby confounding assessment of the true pharmacodynamic effects of PI3K/Akt/mTOR pathway inhibitors. In the studies discussed above (101), Western blot analysis conducted on excised tumor tissue from treated mice revealed that NVP-BEZ235 alone or in conjunction with ABT-737 markedly decreased Mcl-1 protein levels and Akt phosphorylation, analogous to in vitro observations, thus confirming on-target pharmacodynamic activity of this novel drug combination in vivo in murine xenograft models of AML.
CLINICAL CONSIDERATIONS
Various Bcl-2 family and PI3K/mTOR inhibitors have been studied in clinical trials, including in patients with AML (Table 1), either alone or in combination with standard chemotherapy. To date, however, agents from these classes have not been studied in combination in AML. Among the Bcl-2 family inhibitors, GX15–070 (obatoclax, Teva), a pan-BH-3 mimetic that is no longer in development, was shown to induce apoptosis in AML cells (57, 65). However, significant infusional central nervous system toxicity, including euphoria, somnolence, and ataxia, led to this agent being discontinued (57). G-3139 (oblimersen, Genta), an anti-sense oligonucleotide against Bcl-2 mRNA, failed to obtain regulatory approval despite promising results in the phase III setting in CLL (89, 90). A phase III randomized controlled trial of induction therapy with cytarabine and daunorubicin followed by consolidation therapy with high-dose cytarabine with or without oblimersen in older, previously untreated patients with AML failed to meet any of its endpoints (improvements in complete remission rate, overall survival, disease-free survival from date of complete remission, and event-free survival) (2, 79). Development of ABT-263 (navitoclax), the orally available analog of ABT-737 (129), despite evidence of promising single-agent clinical activity, e.g., in CLL (108), has been discontinued owing to concerns regarding dose-dependent thrombocytopenia (40, 137) secondary to on-target inhibition of Bcl-xL, critical for platelet survival (84). Initial reports on the successor molecule, ABT-199 (GDC-0199), a Bcl-2-specific BH3-mimetic that spares platelets (121), in CLL, a highly Bcl-2 dependent malignancy (25), suggest robust activity (1). In an ongoing phase I trial (1) in patients with CLL/small lymphocytic lymphoma, the most common adverse effects reported were diarrhea (43%), neutropenia (37%), fatigue (33%), upper respiratory tract infection (33%), and cough (22%). A relatively high incidence of tumor lysis syndrome early in the trial was successfully addressed by lowering the dose of this very potent apoptosis inducer and by adopting a more cautious dose-escalation strategy (1). This agent has recently been shown to be remarkably effective in AML cell lines, primary patient samples, and murine primary xenografts (93), and a phase II trial of ABT-199 (GDC-0199) in patients with AML (NCT01994837) is currently recruiting patients.
Table 1.
Dual PI3K/mTOR and Bcl-2 family inhibitors studied in clinical trials involving patients with AML
| Drug | Manufacturer | Class | Phase | Results | References |
|---|---|---|---|---|---|
| BEZ235 | Novartis | dual PI3K and mTOR inhibitor | 1 | information not available | not applicable |
| G3139 (Oblimersen/Genasense/Augmerosen) | Genta | anti-sense oligonucleotide against Bcl-2 | 3 | no differences in CR rate (48 vs. 52%; P = 0.75) or OS (P = 0.83) between groups with or without oblimersen therapy | 79 |
| GX 15-070 (Obatoclax) | Teva | small molecule BH-3 mimetic against Bcl-2, Bcl-xL, Mcl-1, Bcl-w, Bfl-1/A1, and Bcl-B | 2 | no CRs | 105 |
| ABT-199 (GDC-0199) | Abbvie/Genentech | small molecule BH-3 mimetic against Bcl-2 | 2 | information not available | not applicable |
PI3K, phosphatidylinositol-3-kinase; mTOR, mammalian target of rapamycin; Bcl-2, B-cell lymphoma 2; AML, acute myeloid leukemia; CR, complete remission; OS, overall survival.
Of the myriad PI3K/Akt/mTOR pathway inhibitors that are in development, only a few have been tested in AML (37, 109). Of these, NVP-BEZ235 is the only dual PI3K/mTOR inhibitor that is currently under clinical investigation in AML (NCT01756118). Trials in other, predominantly solid, tumor types using inhibitors of the PI3K/Akt/mTOR pathway have shown that toxicities with this group of drugs are generally manageable. Hyperglycemia, hyperlipidemia, skin rashes, cytopenias, infections, gastrointestinal symptoms (nausea, vomiting, anorexia, dyspepsia, and diarrhea), mucositis, and stomatitis have been some of the more commonly reported adverse effects of these agents (109), two of which [the mTOR inhibitors temsirolimus (Torisel, Pfizer) and everolimus (Afinitor, Novartis)] are available commercially. While some of these are “off-target” effects, others have been thought to be directly related to their mechanism of action. In a large, phase II trial, the most frequent adverse effects of idelalisib, the first PI3K (delta isoform-specific) inhibitor likely to be approved (36), were diarrhea, fatigue, nausea, cough, pyrexia, neutropenia, and transaminitis (44). Dual PI3K/mTOR inhibitors cause a wider range of off-target adverse effects, e.g., fatigue, but these, too, have generally been found to be acceptable in clinical trials (109). The toxicity profiles of PI3K/mTOR inhibitors and ABT-199 (GDC-0199) thus do overlap slightly, and carefully designed phase I dose-escalation trials will be necessary to optimize the clinical potential of this novel combination.
CONCLUSIONS
Recent approaches to the targeted therapy of AML have focused upon attempts to inhibit the kinase activity of mutated oncoproteins such as FLT3 (21, 71) and KIT (3, 8, 19) with small molecules. Although the field continues to evolve (70), currently, the number of such “actionable mutations” is small. An inherent problem with such approaches stems from the upstream location of these mutated oncoproteins and the marked redundancy of survival signaling pathways that allows cells to bypass an inhibited kinase. Specifically, kinase inhibition can trigger activation of compensatory prosurvival pathways that relieve the neoplastic cell of its addiction to the kinase. Moreover, kinase mutations conferring resistance to potent inhibitors of FLT3 have already been described (119) and may contribute to the relatively transient nature of responses to this class of antileukemic agents (21, 71). Pending more definitive validation, the above findings with simultaneous PI3K/mTOR and Bcl-2/Bcl-xL blockade suggest novel candidate biomarkers (in this case, basal Akt activation) that might better inform the choice of targeted therapies in AML. It will be of interest to assess the efficacy of ABT-199 (GDC-0199) in combination with agents that downregulate Mcl-1 and to determine whether similar interactions as observed in the case of ABT-737 occur, in light of evidence that proapoptotic Bak is sequestered by and must be displaced from both Mcl-1 and Bcl-xL, but not Bcl-2, by BH3-only proteins, or BH3-mimetics in order to trigger apoptosis (136). Recent data from ex vivo studies in AML cells demonstrate great sensitivity to ABT-199 (GDC-0199), with results comparable to those observed in CLL cells, despite its selectivity for Bcl-2, raising hopes that clinically meaningful efficacy might be able to be achieved even without Bcl-xL blockade in this disease (93). Regardless, rational combinations, such as with dual PI3K/mTOR inhibitors, are likely to prove necessary to further augment the activity of selective Bcl-2 antagonists like ABT-199 (GDC-0199) in AML for maximum therapeutic benefit.
GRANTS
This work was supported by National Cancer Institute Grants RO1 CA-093738-09, RO1 CA-167708-01A1, RO1 CA-100866-09, R21 CA-137823-02, P50 CA-130805-05, P50 CA-142509-04, P30 CA-16059-31 and an award from the Leukemia and Lymphoma Society (to S. Grant).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.V. and P.B. drafted manuscript; P.V., P.B., M.R., and S.G. edited and revised manuscript; P.V., P.B., M.R., and S.G. approved final version of manuscript; M.R. prepared figures.
REFERENCES
- 1.Anonymous. BCL2 inhibitor yields high response in CLL and SLL. Cancer Discov 4: OF5, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Oblimersen: Augmerosen. BCL2 antisense oligonucleotide - Genta, G 3139, GC 3139, oblimersen sodium. Drugs R D 8: 321–334, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Advani AS, Tiu R, Saunthararajah Y, Maciejewski J, Copelan EA, Sobecks R, Sekeres MA, Bates J, Rush ML, Tripp B, Salvado A, Noon E, Howard M, Jin T, Hsi E, Egorin MJ, Lim K, Cotta CV, Price C, Kalaycio M. A phase 1 study of imatinib mesylate in combination with cytarabine and daunorubicin for c-kit positive relapsed acute myeloid leukemia. Leuk Res 34: 1622–1626, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Ballif BA, Roux PP, Gerber SA, MacKeigan JP, Blenis J, Gygi SP. Quantitative phosphorylation profiling of the ERK/p90 ribosomal S6 kinase-signaling cassette and its targets, the tuberous sclerosis tumor suppressors. Proc Natl Acad Sci USA 102: 667–672, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balmanno K, Chell SD, Gillings AS, Hayat S, Cook SJ. Intrinsic resistance to the MEK1/2 inhibitor AZD6244 (ARRY-142886) is associated with weak ERK1/2 signalling and/or strong PI3K signalling in colorectal cancer cell lines. Int J Cancer 125: 2332–2341, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Bhaskar PT, Nogueira V, Patra KC, Jeon SM, Park Y, Robey RB, Hay N. mTORC1 hyperactivity inhibits serum deprivation-induced apoptosis via increased hexokinase II and GLUT1 expression, sustained Mcl-1 expression, and glycogen synthase kinase 3beta inhibition. Mol Cell Biol 29: 5136–5147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandts CH, Sargin B, Rode M, Biermann C, Lindtner B, Schwable J, Buerger H, Muller-Tidow C, Choudhary C, McMahon M, Berdel WE, Serve H. Constitutive activation of Akt by Flt3 internal tandem duplications is necessary for increased survival, proliferation, and myeloid transformation. Cancer Res 65: 9643–9650, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Brandwein JM, Hedley DW, Chow S, Schimmer AD, Yee KW, Schuh AC, Gupta V, Xu W, Kamel-Reid S, Minden MD. A phase I/II study of imatinib plus reinduction therapy for c-kit-positive relapsed/refractory acute myeloid leukemia: inhibition of Akt activation correlates with complete response. Leukemia 25: 945–952, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol 29: 487–494, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Burnett AK. Treatment of acute myeloid leukemia: are we making progress? Hematol Am Soc Hematol Educ Prog 2012: 1–6, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C, Archimbaud E, Magaud JP, Guyotat D. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood 81: 3091–3096, 1993 [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 368: 2059–2074, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantley LC. The phosphoinositide 3-kinase pathway. Science 296: 1655–1657, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest 118: 3065–3074, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapuis N, Tamburini J, Green AS, Vignon C, Bardet V, Neyret A, Pannetier M, Willems L, Park S, Macone A, Maira SM, Ifrah N, Dreyfus F, Herault O, Lacombe C, Mayeux P, Bouscary D. Dual inhibition of PI3K and mTORC1/2 signaling by NVP-BEZ235 as a new therapeutic strategy for acute myeloid leukemia. Clin Cancer Res 16: 5424–5435, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17: 393–403, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res 67: 782–791, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Chen S, Dai Y, Pei XY, Grant S. Bim upregulation by histone deacetylase inhibitors mediates interactions with the Bcl-2 antagonist ABT-737: evidence for distinct roles for Bcl-2, Bcl-xL, and Mcl-1. Mol Cell Biol 29: 6149–6169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chevallier P, Hunault-Berger M, Larosa F, Dauriac C, Garand R, Harousseau JL. A phase II trial of high-dose imatinib mesylate for relapsed or refractory c-kit positive and Bcr-Abl negative acute myeloid leukaemia: the AFR-15 trial. Leuk Res 33: 1124–1126, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Chou FS, Wunderlich M, Griesinger A, Mulloy JC. N-Ras(G12D) induces features of stepwise transformation in preleukemic human umbilical cord blood cultures expressing the AML1-ETO fusion gene. Blood 117: 2237–2240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortes JE, Perl AE, Dombret H, Kayser S, Steffen B, Rousselot P, Martinelli G, Estey EH, Burnett AK, Gammon G, Trone D, Leo E, Levis MJ. Final results of a phase 2 open-label, monotherapy efficacy and safety study of quizartinib (AC220) in patients >= 60 years of age with FLT3 ITD positive or negative relapsed/refractory acute myeloid leukemia. ASH Ann Meet Abstracts 120: 48, 2012 [Google Scholar]
- 22.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol 28: 1075–1083, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai Y, Grant S. Targeting multiple arms of the apoptotic regulatory machinery. Cancer Res 67: 2908–2911, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Davids MS, Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J Clin Oncol 30: 3127–3135, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest 117: 112–121, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood 111: 2300–2309, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Poeta G, Venditti A, Del Principe MI, Maurillo L, Buccisano F, Tamburini A, Cox MC, Franchi A, Bruno A, Mazzone C, Panetta P, Suppo G, Masi M, Amadori S. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood 101: 2125–2131, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Domina AM, Vrana JA, Gregory MA, Hann SR, Craig RW. MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene 23: 5301–5315, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood 119: 34–43, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebi H, Costa C, Faber AC, Nishtala M, Kotani H, Juric D, Della Pelle P, Song Y, Yano S, Mino-Kenudson M, Benes CH, Engelman JA. PI3K regulates MEK/ERK signaling in breast cancer via the Rac-GEF, P-Rex1. Proc Natl Acad Sci USA 110: 21124–21129, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 9: 550–562, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, Maira M, McNamara K, Perera SA, Song Y, Chirieac LR, Kaur R, Lightbown A, Simendinger J, Li T, Padera RF, Garcia-Echeverria C, Weissleder R, Mahmood U, Cantley LC, Wong KK. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med 14: 1351–1356, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Essafi A, Fernandez de Mattos S, Hassen YA, Soeiro I, Mufti GJ, Thomas NS, Medema RH, Lam EW. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene 24: 2317–2329, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Evangelisti C, Evangelisti C, Bressanin D, Buontempo F, Chiarini F, Lonetti A, Soncin M, Sparta A, McCubrey JA, Martelli AM. Targeting phosphatidylinositol 3-kinase signaling in acute myelogenous leukemia. Expert Opin Ther Targets 17: 921–936, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Forman SJ, Rowe JM. The myth of the second remission of acute leukemia in the adult. Blood 121: 1077–1082, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fruman DA, Cantley LC. Idelalisib–a PI3Kdelta inhibitor for B-cell cancers. N Engl J Med 370: 1061–1062, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov 13: 140–156, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukuchi Y, Kizaki M, Yamato K, Kawamura C, Umezawa A, Hata J, Nishihara T, Ikeda Y. Mcl-1, an early-induction molecule, modulates activin A-induced apoptosis and differentiation of CML cells. Oncogene 20: 704–713, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Gallay N, Dos Santos C, Cuzin L, Bousquet M, Simmonet Gouy V, Chaussade C, Attal M, Payrastre B, Demur C, Recher C. The level of AKT phosphorylation on threonine 308 but not on serine 473 is associated with high-risk cytogenetics and predicts poor overall survival in acute myeloid leukaemia. Leukemia 23: 1029–1038, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, Hann CL, McKeegan EM, Litvinovich E, Hemken PM, Dive C, Enschede SH, Nolan C, Chiu YL, Busman T, Xiong H, Krivoshik AP, Humerickhouse R, Shapiro GI, Rudin CM. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol 29: 909–916, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Germain M, Nguyen AP, Le Grand JN, Arbour N, Vanderluit JL, Park DS, Opferman JT, Slack RS. MCL-1 is a stress sensor that regulates autophagy in a developmentally regulated manner. EMBO J 30: 395–407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD, Izon DJ, Zuber J, Rappaport AR, Herold MJ, Alexander WS, Lowe SW, Robb L, Strasser A. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev 26: 120–125, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez-Bougie P, Bataille R, Amiot M. Endogenous association of Bim BH3-only protein with Mcl-1, Bcl-xL and Bcl-2 on mitochondria in human B cells. Eur J Immunol 35: 971–976, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, Flinn IW, Flowers CR, Martin P, Viardot A, Blum KA, Goy AH, Davies AJ, Zinzani PL, Dreyling M, Johnson D, Miller LL, Holes L, Li D, Dansey RD, Godfrey WR, Salles GA. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 370: 1008–1018, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grant S. Cotargeting survival signaling pathways in cancer. J Clin Invest 118: 3003–3006, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harada H, Quearry B, Ruiz-Vela A, Korsmeyer SJ. Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc Natl Acad Sci USA 101: 15313–15317, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassane DC, Sen S, Minhajuddin M, Rossi RM, Corbett CA, Balys M, Wei L, Crooks PA, Guzman ML, Jordan CT. Chemical genomic screening reveals synergism between parthenolide and inhibitors of the PI-3 kinase and mTOR pathways. Blood 116: 5983–5990, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Hess CJ, Berkhof J, Denkers F, Ossenkoppele GJ, Schouten JP, Oudejans JJ, Waisfisz Q, Schuurhuis GJ. Activated intrinsic apoptosis pathway is a key related prognostic parameter in acute myeloid leukemia. J Clin Oncol 25: 1209–1215, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, Podar K, Munshi NC, Chauhan D, Richardson PG, Anderson KC. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood 107: 4053–4062, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hogstrand K, Hejll E, Sander B, Rozell B, Larsson LG, Grandien A. Inhibition of the intrinsic but not the extrinsic apoptosis pathway accelerates and drives MYC-driven tumorigenesis towards acute myeloid leukemia. PLoS One 7: e31366, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horn S, Bergholz U, Jucker M, McCubrey JA, Trumper L, Stocking C, Basecke J. Mutations in the catalytic subunit of class IA PI3K confer leukemogenic potential to hematopoietic cells. Oncogene 27: 4096–4106, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Hsieh AC, Costa M, Zollo O, Davis C, Feldman ME, Testa JR, Meyuhas O, Shokat KM, Ruggero D. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell 17: 249–261, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB, Marto JA, Sabatini DM. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 332: 1317–1322, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang HM, Huang CJ, Yen JJ. Mcl-1 is a common target of stem cell factor and interleukin-5 for apoptosis prevention activity via MEK/MAPK and PI-3K/Akt pathways. Blood 96: 1764–1771, 2000 [PubMed] [Google Scholar]
- 55.Iglesias-Serret D, Pique M, Gil J, Pons G, Lopez JM. Transcriptional and translational control of Mcl-1 during apoptosis. Arch Biochem Biophys 417: 141–152, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, Xiao Y, Christie AL, Aster J, Settleman J, Gygi SP, Kung AL, Look T, Nakayama KI, DePinho RA, Wei W. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 471: 104–109, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joudeh J, Claxton D. Obatoclax mesylate: pharmacology and potential for therapy of hematological neoplasms. Expert Opin Investig Drugs 21: 363–373, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Kaiser J. Combining targeted drugs to stop resistant tumors. Science 331: 1542–1545, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Kantarjian H, O'Brien S, Cortes J, Wierda W, Faderl S, Garcia-Manero G, Issa JP, Estey E, Keating M, Freireich EJ. Therapeutic advances in leukemia and myelodysplastic syndrome over the past 40 years. Cancer 113, 7 Suppl: 1933–1952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasper S, Breitenbuecher F, Heidel F, Hoffarth S, Markova B, Schuler M, Fischer T. Targeting MCL-1 sensitizes FLT3-ITD-positive leukemias to cytotoxic therapies. Blood Cancer J 2: e60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol 17: 4406–4418, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, Sun Y, Ouyang X, Gerald WL, Cordon-Cardo C, Abate-Shen C. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest 118: 3051–3064, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, Verhaegen M, Soengas M, Ruvolo VR, McQueen T, Schober WD, Watt JC, Jiffar T, Ling X, Marini FC, Harris D, Dietrich M, Estrov Z, McCubrey J, May WS, Reed JC, Andreeff M. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 10: 375–388, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Konopleva M, Milella M, Ruvolo P, Watts JC, Ricciardi MR, Korchin B, McQueen T, Bornmann W, Tsao T, Bergamo P, Mak DH, Chen W, McCubrey J, Tafuri A, Andreeff M. MEK inhibition enhances ABT-737-induced leukemia cell apoptosis via prevention of ERK-activated MCL-1 induction and modulation of MCL-1/BIM complex. Leukemia 26: 778–787, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, Bornmann W, Kantarjian H, Viallet J, Samudio I, Andreeff M. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15–070 (obatoclax). Cancer Res 68: 3413–3420, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Konopleva M, Zhao S, Hu W, Jiang S, Snell V, Weidner D, Jackson CE, Zhang X, Champlin R, Estey E, Reed JC, Andreeff M. The anti-apoptotic genes Bcl-X(L) and Bcl-2 are over-expressed and contribute to chemoresistance of non-proliferating leukaemic CD34+ cells. Br J Haematol 118: 521–534, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Labi V, Grespi F, Baumgartner F, Villunger A. Targeting the Bcl-2-regulated apoptosis pathway by BH3 mimetics: a breakthrough in anticancer therapy? Cell Death Differ 15: 977–987, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367: 645–648, 1994 [DOI] [PubMed] [Google Scholar]
- 69.Lauria F, Raspadori D, Rondelli D, Ventura MA, Fiacchini M, Visani G, Forconi F, Tura S. High bcl-2 expression in acute myeloid leukemia cells correlates with CD34 positivity and complete remission rate. Leukemia 11: 2075–2078, 1997 [DOI] [PubMed] [Google Scholar]
- 70.Levis M. Targeting IDH: the next big thing in AML. Blood 122: 2770–2771, 2013 [DOI] [PubMed] [Google Scholar]
- 71.Levis MJ, Perl AE, Dombret H, Dohner H, Steffen B, Rousselot P, Martinelli G, Estey EH, Burnett AK, Gammon G, Trone D, Leo E, Cortes JE. Final results of a phase 2 open-label, monotherapy efficacy and safety study of quizartinib (AC220) in patients with FLT3-ITD positive or negative relapsed/refractory acute myeloid leukemia after second-line chemotherapy or hematopoietic stem cell transplantation. ASH Ann Meet Abstracts 120: 673, 2012 [Google Scholar]
- 72.Lin X, Morgan-Lappe S, Huang X, Li L, Zakula DM, Vernetti LA, Fesik SW, Shen Y. ‘Seed’ analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene 26: 3972–3979, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, Auberger P. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene 22: 6785–6793, 2003 [DOI] [PubMed] [Google Scholar]
- 74.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell 4: 257–262, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136: 823–837, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121: 179–193, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Ma L, Teruya-Feldstein J, Bonner P, Bernardi R, Franz DN, Witte D, Cordon-Cardo C, Pandolfi PP. Identification of S664 TSC2 phosphorylation as a marker for extracellular signal-regulated kinase mediated mTOR activation in tuberous sclerosis and human cancer. Cancer Res 67: 7106–7112, 2007 [DOI] [PubMed] [Google Scholar]
- 78.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marcucci G, Moser B, Blum W, Stock W, Wetzler M, Kolitz JE, Thakuri M, Carter T, Stuart RK, Larson RA. A phase III randomized trial of intensive induction and consolidation chemotherapy ± oblimersen, a pro-apoptotic Bcl-2 antisense oligonucleotide in untreated acute myeloid leukemia patients >60 years old (Abstract). ASCO Meeting Proc 25: 7012, 2007 [Google Scholar]
- 80.Martelli AM, Evangelisti C, Chappell W, Abrams SL, Basecke J, Stivala F, Donia M, Fagone P, Nicoletti F, Libra M, Ruvolo V, Ruvolo P, Kempf CR, Steelman LS, McCubrey JA. Targeting the translational apparatus to improve leukemia therapy: roles of the PI3K/PTEN/Akt/mTOR pathway. Leukemia 25: 1064–1079, 2011 [DOI] [PubMed] [Google Scholar]
- 81.Martelli AM, Evangelisti C, Chiarini F, Grimaldi C, Manzoli L, McCubrey JA. Targeting the PI3K/AKT/mTOR signaling network in acute myelogenous leukemia. Exp Opin Investig Drugs 18: 1333–1349, 2009 [DOI] [PubMed] [Google Scholar]
- 82.Martelli AM, Evangelisti C, Chiarini F, McCubrey JA. The phosphatidylinositol 3-kinase/Akt/mTOR signaling network as a therapeutic target in acute myelogenous leukemia patients. Oncotarget 1: 89–103, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martelli AM, Nyakern M, Tabellini G, Bortul R, Tazzari PL, Evangelisti C, Cocco L. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia 20: 911–928, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW, Huang DC, Kile BT. Programmed anuclear cell death delimits platelet life span. Cell 128: 1173–1186, 2007 [DOI] [PubMed] [Google Scholar]
- 85.Min YH, Eom JI, Cheong JW, Maeng HO, Kim JY, Jeung HK, Lee ST, Lee MH, Hahn JS, Ko YW. Constitutive phosphorylation of Akt/PKB protein in acute myeloid leukemia: its significance as a prognostic variable. Leukemia 17: 995–997, 2003 [DOI] [PubMed] [Google Scholar]
- 86.Misaghian N, Ligresti G, Steelman LS, Bertrand FE, Basecke J, Libra M, Nicoletti F, Stivala F, Milella M, Tafuri A, Cervello M, Martelli AM, McCubrey JA. Targeting the leukemic stem cell: the Holy Grail of leukemia therapy. Leukemia 23: 25–42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morales AA, Kurtoglu M, Matulis SM, Liu J, Siefker D, Gutman DM, Kaufman JL, Lee KP, Lonial S, Boise LH. Distribution of Bim determines Mcl-1 dependence or codependence with Bcl-xL/Bcl-2 in Mcl-1-expressing myeloma cells. Blood 118: 1329–1339, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Munugalavadla V, Sims EC, Borneo J, Chan RJ, Kapur R. Genetic and pharmacologic evidence implicating the p85 alpha, but not p85 beta, regulatory subunit of PI3K and Rac2 GTPase in regulating oncogenic KIT-induced transformation in acute myeloid leukemia and systemic mastocytosis. Blood 110: 1612–1620, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O'Brien S, Moore JO, Boyd TE, Larratt LM, Skotnicki A, Koziner B, Chanan-Khan AA, Seymour JF, Bociek RG, Pavletic S, Rai KR. Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol 25: 1114–1120, 2007 [DOI] [PubMed] [Google Scholar]
- 90.O'Brien S, Moore JO, Boyd TE, Larratt LM, Skotnicki AB, Koziner B, Chanan-Khan AA, Seymour JF, Gribben J, Itri LM, Rai KR. 5-year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. J Clin Oncol 27: 5208–5212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O'Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435: 677–681, 2005 [DOI] [PubMed] [Google Scholar]
- 92.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66: 1500–1508, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, Cortes J, Deangelo DJ, Debose L, Mu H, Dohner H, Gaidzik VI, Galinsky I, Golfman LS, Haferlach T, Harutyunyan KG, Hu J, Leverson JD, Marcucci G, Muschen M, Newman R, Park E, Ruvolo PP, Ruvolo V, Ryan J, Schindela S, Zweidler-McKay P, Stone RM, Kantarjian H, Andreeff M, Konopleva M, Letai AG. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov 4: 362–375, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park S, Chapuis N, Bardet V, Tamburini J, Gallay N, Willems L, Knight ZA, Shokat KM, Azar N, Viguie F, Ifrah N, Dreyfus F, Mayeux P, Lacombe C, Bouscary D. PI-103, a dual inhibitor of Class IA phosphatidylinositide 3-kinase and mTOR, has antileukemic activity in AML. Leukemia 22: 1698–1706, 2008 [DOI] [PubMed] [Google Scholar]
- 95.Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, Van Vlierberghe P, Dolgalev I, Thomas S, Aminova O, Huberman K, Cheng J, Viale A, Socci ND, Heguy A, Cherry A, Vance G, Higgins RR, Ketterling RP, Gallagher RE, Litzow M, van den Brink MR, Lazarus HM, Rowe JM, Luger S, Ferrando A, Paietta E, Tallman MS, Melnick A, Abdel-Wahab O, Levine RL. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 366: 1079–1089, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pei XY, Dai Y, Tenorio S, Lu J, Harada H, Dent P, Grant S. MEK1/2 inhibitors potentiate UCN-01 lethality in human multiple myeloma cells through a Bim-dependent mechanism. Blood 110: 2092–2101, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pradelli LA, Beneteau M, Chauvin C, Jacquin MA, Marchetti S, Munoz-Pinedo C, Auberger P, Pende M, Ricci JE. Glycolysis inhibition sensitizes tumor cells to death receptors-induced apoptosis by AMP kinase activation leading to Mcl-1 block in translation. Oncogene 29: 1641–1652, 2010 [DOI] [PubMed] [Google Scholar]
- 98.Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem 275: 10761–10766, 2000 [DOI] [PubMed] [Google Scholar]
- 99.Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem 281: 813–823, 2006 [DOI] [PubMed] [Google Scholar]
- 100.Rahmani M, Anderson A, Habibi JR, Crabtree TR, Mayo M, Harada H, Ferreira-Gonzalez A, Dent P, Grant S. The BH3-only protein Bim plays a critical role in leukemia cell death triggered by concomitant inhibition of the PI3K/Akt and MEK/ERK1/2 pathways. Blood 114: 4507–4516, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rahmani M, Aust MM, Attkisson E, Williams DC, Jr, Ferreira-Gonzalez A, Grant S. Dual inhibition of Bcl-2 and Bcl-xL strikingly enhances PI3K inhibition-induced apoptosis in human myeloid leukemia cells through a GSK3- and Bim-dependent mechanism. Cancer Res 73: 1340–1351, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rahmani M, Aust MM, Attkisson E, Williams DC, Jr, Ferreira-Gonzalez A, Grant S. Inhibition of Bcl-2 antiapoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood 119: 6089–6098, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rahmani M, Reese E, Dai Y, Bauer C, Payne SG, Dent P, Spiegel S, Grant S. Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and ERK1/2 inactivation and the generation of ceramide and reactive oxygen species. Cancer Res 65: 2422–2432, 2005 [DOI] [PubMed] [Google Scholar]
- 104.Rahmani M, Yu C, Reese E, Ahmed W, Hirsch K, Dent P, Grant S. Inhibition of PI-3 kinase sensitizes human leukemic cells to histone deacetylase inhibitor-mediated apoptosis through p44/42 MAP kinase inactivation and abrogation of p21(CIP1/WAF1) induction rather than AKT inhibition. Oncogene 22: 6231–6242, 2003 [DOI] [PubMed] [Google Scholar]
- 105.Raza A, Galili N, Borthakur G, Carter TH, Claxton DF, Erba HP, DeAngelo DJ, Berger MS, Schimmer A. A safety and schedule seeking trial of Bcl-2 inhibitor obatoclax in previously untreated older patients with acute myeloid leukemia (AML) (Abstract). ASCO Meet Proc 27: 3579, 2009 [Google Scholar]
- 106.Reed JC. Bcl-2-family proteins and hematologic malignancies: history and future prospects. Blood 111: 3322–3330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ricciardi MR, McQueen T, Chism D, Milella M, Estey E, Kaldjian E, Sebolt-Leopold J, Konopleva M, Andreeff M. Quantitative single cell determination of ERK phosphorylation and regulation in relapsed and refractory primary acute myeloid leukemia. Leukemia 19: 1543–1549, 2005 [DOI] [PubMed] [Google Scholar]
- 108.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, Carney DA, He SZ, Huang DC, Xiong H, Cui Y, Busman TA, McKeegan EM, Krivoshik AP, Enschede SH, Humerickhouse R. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol 30: 488–496, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol 10: 143–153, 2013 [DOI] [PubMed] [Google Scholar]
- 110.Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, Baselga J, Guichard S, Rosen N. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov 1: 248–259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rowe JM, Tallman MS. How I treat acute myeloid leukemia. Blood 116: 3147–3156, 2010 [DOI] [PubMed] [Google Scholar]
- 112.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell 133: 403–414, 2008 [DOI] [PubMed] [Google Scholar]
- 113.Scholl C, Gilliland DG, Frohling S. Deregulation of signaling pathways in acute myeloid leukemia. Semin Oncol 35: 336–345, 2008 [DOI] [PubMed] [Google Scholar]
- 114.Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, Markman B, Rodriguez O, Guzman M, Rodriguez S, Gili M, Russillo M, Parra JL, Singh S, Arribas J, Rosen N, Baselga J. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene 30: 2547–2557, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sharma SV, Fischbach MA, Haber DA, Settleman J. “Oncogenic shock”: explaining oncogene addiction through differential signal attenuation. Clin Cancer Res 12: 4392s–4395s, 2006 [DOI] [PubMed] [Google Scholar]
- 116.Sharma SV, Settleman J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev 21: 3214–3231, 2007 [DOI] [PubMed] [Google Scholar]
- 117.She QB, Solit DB, Ye Q, O'Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell 8: 287–297, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smith BD, Bambach BJ, Vala MS, Barber JP, Enger C, Brodsky RA, Burke PJ, Gore SD, Jones RJ. Inhibited apoptosis and drug resistance in acute myeloid leukaemia. Br J Haematol 102: 1042–1049, 1998 [DOI] [PubMed] [Google Scholar]
- 119.Smith CC, Chin J, Lasater E, Paguirigan AL, Lin K, Stewart W, Salerno S, Damon LE, Levis MJ, Perl AE, Travers K, Kasarskis A, Radich JP, Shah N. Constitutively activating mutations at the FLT3 activation loop residue D835 are associated with clinical resistance to AC220. ASH Ann Meet Abstracts 120: 674, 2012 [Google Scholar]
- 120.Sos ML, Fischer S, Ullrich R, Peifer M, Heuckmann JM, Koker M, Heynck S, Stuckrath I, Weiss J, Fischer F, Michel K, Goel A, Regales L, Politi KA, Perera S, Getlik M, Heukamp LC, Ansen S, Zander T, Beroukhim R, Kashkar H, Shokat KM, Sellers WR, Rauh D, Orr C, Hoeflich KP, Friedman L, Wong KK, Pao W, Thomas RK. Identifying genotype-dependent efficacy of single and combined PI3K- and MAPK-pathway inhibition in cancer. Proc Natl Acad Sci USA 106: 18351–18356, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19: 202–208, 2013 [DOI] [PubMed] [Google Scholar]
- 122.Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Basecke J, Libra M, Stivala F, Milella M, Tafuri A, Lunghi P, Bonati A, Martelli AM, McCubrey JA. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia 22: 686–707, 2008 [DOI] [PubMed] [Google Scholar]
- 123.Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH, Coombes RC, Lam EW. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem 278: 49795–49805, 2003 [DOI] [PubMed] [Google Scholar]
- 124.Tabe Y, Jin L, Tsutsumi-Ishii Y, Xu Y, McQueen T, Priebe W, Mills GB, Ohsaka A, Nagaoka I, Andreeff M, Konopleva M. Activation of integrin-linked kinase is a critical prosurvival pathway induced in leukemic cells by bone marrow-derived stromal cells. Cancer Res 67: 684–694, 2007 [DOI] [PubMed] [Google Scholar]
- 125.Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, Martinelli E, Ramon y Cajal S, Jones S, Vidal L, Shand N, Macarulla T, Ramos FJ, Dimitrijevic S, Zoellner U, Tang P, Stumm M, Lane HA, Lebwohl D, Baselga J. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 26: 1603–1610, 2008 [DOI] [PubMed] [Google Scholar]
- 126.Tahir SK, Yang X, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J, Warner RB, Ng SC, Fesik SW, Elmore SW, Rosenberg SH, Tse C. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res 67: 1176–1183, 2007 [DOI] [PubMed] [Google Scholar]
- 127.Tamburini J, Chapuis N, Bardet V, Park S, Sujobert P, Willems L, Ifrah N, Dreyfus F, Mayeux P, Lacombe C, Bouscary D. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood 111: 379–382, 2008 [DOI] [PubMed] [Google Scholar]
- 128.Townsend KJ, Zhou P, Qian L, Bieszczad CK, Lowrey CH, Yen A, Craig RW. Regulation of MCL1 through a serum response factor/Elk-1-mediated mechanism links expression of a viability-promoting member of the BCL2 family to the induction of hematopoietic cell differentiation. J Biol Chem 274: 1801–1813, 1999 [DOI] [PubMed] [Google Scholar]
- 129.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, Roberts L, Tahir SK, Xiao Y, Yang X, Zhang H, Fesik S, Rosenberg SH, Elmore SW. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 68: 3421–3428, 2008 [DOI] [PubMed] [Google Scholar]
- 130.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, Adams JM, Roberts AW, Huang DC. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10: 389–399, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Stijn A, Feller N, Kok A, van der Pol MA, Ossenkoppele GJ, Schuurhuis GJ. Minimal residual disease in acute myeloid leukemia is predicted by an apoptosis-resistant protein profile at diagnosis. Clin Cancer Res 11: 2540–2546, 2005 [DOI] [PubMed] [Google Scholar]
- 132.Villanueva MT. Targeted therapies: priming apoptosis. Nat Rev Clin Oncol 10: 67, 2013 [DOI] [PubMed] [Google Scholar]
- 133.Wang JM, Chao JR, Chen W, Kuo ML, Yen JJ, Yang-Yen HF. The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol 19: 6195–6206, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang X, Hawk N, Yue P, Kauh J, Ramalingam SS, Fu H, Khuri FR, Sun SY. Overcoming mTOR inhibition-induced paradoxical activation of survival signaling pathways enhances mTOR inhibitors' anticancer efficacy. Cancer Biol Ther 7: 1952–1958, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, Belmont LD, Kaminker JS, O'Rourke KM, Pujara K, Kohli PB, Johnson AR, Chiu ML, Lill JR, Jackson PK, Fairbrother WJ, Seshagiri S, Ludlam MJ, Leong KG, Dueber EC, Maecker H, Huang DC, Dixit VM. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 471: 110–114, 2011 [DOI] [PubMed] [Google Scholar]
- 136.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev 19: 1294–1305, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wilson WH, O'Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, Tulpule A, Dunleavy K, Xiong H, Chiu YL, Cui Y, Busman T, Elmore SW, Rosenberg SH, Krivoshik AP, Enschede SH, Humerickhouse RA. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol 11: 1149–1159, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood 102: 972–980, 2003 [DOI] [PubMed] [Google Scholar]
- 139.Xu Q, Thompson JE, Carroll M. mTOR regulates cell survival after etoposide treatment in primary AML cells. Blood 106: 4261–4268, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yates JW, Wallace HJ, Jr, Ellison RR, Holland JF. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep 57: 485–488, 1973 [PubMed] [Google Scholar]
- 141.Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood 115: 3304–3313, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yilmaz OH, Morrison SJ. The PI-3kinase pathway in hematopoietic stem cells and leukemia-initiating cells: a mechanistic difference between normal and cancer stem cells. Blood Cells Mol Dis 41: 73–76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441: 475–482, 2006 [DOI] [PubMed] [Google Scholar]
- 144.Yoshimoto G, Miyamoto T, Jabbarzadeh-Tabrizi S, Iino T, Rocnik JL, Kikushige Y, Mori Y, Shima T, Iwasaki H, Takenaka K, Nagafuji K, Mizuno S, Niiro H, Gilliland GD, Akashi K. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 activation. Blood 114: 5034–5043, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villen J, Kubica N, Hoffman GR, Cantley LC, Gygi SP, Blenis J. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science 332: 1322–1326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang W, Konopleva M, Ruvolo VR, McQueen T, Evans RL, Bornmann WG, McCubrey J, Cortes J, Andreeff M. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia 22: 808–818, 2008 [DOI] [PubMed] [Google Scholar]


