Abstract
Human airway smooth muscle (HASM) contraction plays a central role in regulating airway resistance in both healthy and asthmatic bronchioles. In vitro studies that investigate the intricate mechanisms that regulate this contractile process are predominantly conducted on tissue culture plastic, a rigid, 2D geometry, unlike the 3D microenvironment smooth muscle cells are exposed to in situ. It is increasingly apparent that cellular characteristics and responses are altered between cells cultured on 2D substrates compared with 3D topographies. Electrospinning is an attractive method to produce 3D topographies for cell culturing as the fibers produced have dimensions within the nanometer range, similar to cells' natural environment. We have developed an electrospun scaffold using the nondegradable, nontoxic, polymer polyethylene terephthalate (PET) composed of uniaxially orientated nanofibers and have evaluated this topography's effect on HASM cell adhesion, alignment, and morphology. The fibers orientation provided contact guidance enabling the formation of fully aligned sheets of smooth muscle. Moreover, smooth muscle cells cultured on the scaffold present an elongated cell phenotype with altered contractile protein levels and distribution. HASM cells cultured on this scaffold responded to the bronchoconstrictor bradykinin. The platform presented provides a novel in vitro model that promotes airway smooth muscle cell development toward a more in vivo-like phenotype while providing topological cues to ensure full cell alignment.
Keywords: airway smooth muscle, tissue engineering, aligned fibers, electrospinning, in vitro model
within the airway bronchiole, human airway smooth muscle (HASM) exists as an aligned population within bundles that wrap around the bronchiole in a helical fashion (25). Smooth muscle is the key effector cell regulating airway tone; with the smooth muscle contractile state directly controlling the luminal capacity of the bronchiole. Dysfunction to the regulation of smooth muscle contraction can alter the airway tone, causing both airway hyperresponsiveness (10, 44) and airway remodeling; increased smooth muscle mass is evident in both mild and severe asthmatic airways (7, 18, 30). To uncover the mechanisms controlling these physiological and pathophysiological roles attributed to HASM cells, researchers have developed numerous experimental techniques including in vivo, ex vivo, and in vitro platforms. The relative advantages and disadvantages in utilizing these models have been discussed extensively elsewhere (3, 5, 50).
Monitoring functional properties of in vitro cultures of primary HASM cells using different analytical techniques has provided better understanding into the direct effects of bronchoconstrictors and inflammatory agonists on smooth muscle responses. One such technique is the real-time imaging of intracellular signaling molecules that are mobilized when muscle contraction is initiated, such as the increase in intracellular calcium concentration ([Ca2+]i). Many important bronchoconstrictors mediate their effects through G protein-coupled receptors that cause downstream activation of PLC, leading to the generation of the second messengers inositol 1,4,5-triphosphate and diacylglycerol and a subsequent increase in [Ca2+]i from intracellular sarcoplasmic reticulum stores and/or Ca2+ influx through voltage-dependent L-type Ca2+ channels in the plasma membrane (4, 5). Bradykinin (BK) is a powerful bronchoconstrictor whose effects are mediated through Gαq/11-coupled receptors present on HASM cells (21), which show an increase in [Ca2+]i upon stimulation (31). These, and most in vitro studies, have been conducted on glass or on tissue culture plastic (TCP), both 2D, planar surfaces routinely used for cell culture. It is increasingly apparent that the topography and stiffness of cell culture substrate can greatly alter cell phenotype and function, hence the substantial interest in developing more biomimetic cell culture systems (13, 14, 52).
Within a lung context, collagen gels can provide a simple 3D matrix for HASM cell culture (8, 32, 46), with cells residing in random planes of the gel matrix opposed to an aligned population of smooth muscle cells. Advancements in tissue engineering have provided new fabrication techniques offering precise control of a substrate's nanoscale topography (29). Model studies have shown multiple cell types can be aligned over extended areas by using different topological references such as plasma/extracellular matrix deposition (38), nanoimprinting (12, 26, 54), or aligned electrospun fibers (11, 51). Electrospinning produces fibrous, porous, 3D mats that closely resemble the natural extracellular matrix: The desired polymer is dissolved in an appropriate solvent and this solution is passed through a syringe with a negative electrical charge applied to the needle tip, causing the electrically charged solution to become attracted to a grounded collector plate. As the polymer jet passes through the air the solvent evaporates depositing a continuous, nonwoven mesh of fibers on the collector plate. Alterations to intrinsic parameters including (but not exhaustive) polymer concentration, electrical field strength, or collector plate aspects allow precise control over scaffold characteristics including shape, porosity, tensile strength, and fiber diameter (16, 43, 47). Electrospun fibers can be manipulated to form uniaxially aligned scaffolds through alterations to fiber collection methods such as employing a rotating collector or parallel electrodes (47). This technology has been applied in culturing cells requiring directional growth in oriented tissues such as neurons (9, 37, 41, 53), ligaments (42), and smooth muscle (2, 19, 51) and has recently been shown to influence stem cell differentiation (40).
In the present study we investigated the influence of fiber alignment and diameter on HASM cell characteristics using the nondegradable, nontoxic, polymer polyethylene terephthalate (PET). Although randomly aligned PET fibers have been employed for the culture of aortic smooth muscle (34), and other polymers have been electrospun into aligned scaffolds for the culture of vascular (51), and bladder (2) smooth muscle, to our knowledge this is the first time HASM has been cultured on aligned electrospun fibers. The effects of this 3D aligned topography on HASM cell orientation, morphology, and contractile characteristics were investigated and compared with HASM cells cultured on rigid 2D surfaces or within in situ airway tissue.
MATERIALS AND METHODS
Materials.
All materials were purchased from Sigma-Aldrich (Dorset, UK) unless stated otherwise. The Novex protein separation kit, nitrocellulose membrane, alamarBlue solution, Fluo-4 AM, anti-mouse and anti-rabbit FITC- and rhodamine-conjugated secondary antibodies, and the nuclear stain Hoechst were all obtained from Invitrogen Life Technologies (Paisley, UK). Rabbit polyclonal antibodies raised against Calponin and SM22α were purchased from AbCam (Cambridge, UK). The Protease Inhibitor Cocktail Set III was purchased from Merk Millipore (Nottingham, UK).
Scaffold fabrication.
Electrospinning procedures were conducted at room temperature within in a vented chemical fume hood. PET scaffolds were produced by dissolving PET (food-grade drinking bottles) in a 1:1 trifluoroacetic acid-dichloromethane (Fisher Chemicals, Loughborough, UK) solution to create a 10% (wt/vol) or a 20% (wt/vol) PET polymer solution. The PET polymer solution was loaded into a syringe attached to a blunt 18-gauge needle (BD Falcon, Oxford, UK) and placed in a syringe pump driver (Harvard Apparatus, Kent, UK) set to a solution feed rate of 0.5 or 1.5 ml/h for the 10 or 20% scaffolds, respectively. A 14-kV voltage charge was applied to the needle tip with fibers being collected on a stainless steel grounded cylindrical mandrel positioned 15 cm from the needle tip. The mandrel was rotated at 2,000 revolutions per minute (equivalent to 600 m/min) to establish fiber alignment. Individual electrospinning parameters are summarized in Table 1.
Table 1.
Characteristics of aligned PET scaffolds
| PET, wt/vol | Solvent, DCM:TFA | Flow Rate, ml/h | Needle Gauge | Voltage, kV | Total Volume, ml | Working Distance, cm | Mandrel Speed, m/min | Fiber Diameter, μm±SE | % Fibers <10° mean fiber angle | Young's Modulus, MPa | Thickness, μm±SE | Porosity, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10% | 1:1 | 0.5 | 18 | 14 | 1.5 | 15 | 600 | 0.22 ± 0.002 | 91.7 | 211.20 ± 18.63 | 24.4 ± 2.4 | 84.15 ± 0.78 |
| 20% | 1:1 | 1.5 | 18 | 14 | 2.0 | 15 | 600 | 1.1 ± 0.01 | 92.6 | 290.30 ± 40.67 | 107.2 ± 5.1 | 83.82 ± 0.47 |
The electrospinning parameters used to create polyethylene terephthalate (PET) scaffolds consisting of uniaxially orientated fibers of different diameters are listed. The table also shows the average intrascaffold fiber diameters, the percentage of fibers orientated within ±10° mean fiber angle, tensile strength, scaffold thickness, and scaffold porosity as described in material and methods. Data are means ± SE from 3 independently electrospun scaffolds.
Scaffold characterization.
Each scaffold type was electrospun independently three times. Scaffolds were punctured and mounted on carbon discs prior to coating with a thin layer of gold (Balzers Union SCD 030, Balzers, Liechtenstein) and imaged on a scanning electron microscope (JEOL JMS-6060 LV, JEOL, Welwyn Garden City, Hertfordshire, UK). The fiber diameters and fiber orientation within the scaffolds were determined through image analysis from two samples taken from each scaffold. Four scanning electron microscope images were taken of each sample, and 20 fiber measurements were analyzed from each individual image (480 fiber measurements per scaffold type).
Fiber diameter measurements were calculated with the software package measureIT 5.1 (Olympus Soft Imaging solutions, Münster, Germany). To determine the degree of angle uniformity within the scaffolds, the mean fiber angle of an individual scaffold was determined by using ImageJ, with the individual fiber deviation from this mean angle calculated.
Scaffold thickness was measured with a digital thickness gauge (accurate to 10 μm) (Mitutoyo, Coventry, UK). Scaffold mass was calculated with a top-pan balance (accurate to 0.1 mg) (Mettler Toledo, Leicester, UK). The mass and scaffold thickness determined the apparent density of the scaffold and the porosity calculated as porosity (%) = 1 − (ad/bd) × 100 where ad is the scaffold's apparent density and bd is the density of pure amorphous PET (1.38 g/cm3). Scaffold porosity was calculated by using 8-mm diameter scaffold samples.
Uniaxial tensile tests were performed on three samples from three independently electrospun scaffolds (n = 9). Samples (30 mm length, 3 mm width) were loaded with fibers running parallel to the applied load direction on a 5969 Universal testing system (Instron, High Wycombe, UK), with a 50 N load cell operating with an extension rate of 5 mm/min. The Young's moduli of the samples were calculated from the resultant stress/strain curves generated by using an Imetrum VideoGauge.
Cell culture on aligned scaffolds.
Primary HASM cells from nonasthmatic individuals were isolated from bronchial biopsies at the Glenfield Hospital (Leicester, UK) as described previously (28). The research was approved by the Leicestershire Ethics Committee, and patients gave their written, informed consent. HASM cells (passage 3–6) were grown in DMEM supplemented with 10% (vol/vol) fetal calf serum, 2 mM l-glutamine solution, 1% (vol/vol) antibiotic/antimycotic solution (10,000 units/ml penicillin G, 100 mg/ml streptomycin sulfate, and 25 μg/ml amphotericin B). Prior to cell seeding, scaffolds were sterilized by UV irradiation for 30 min on both scaffold surfaces (60 min total). Scaffolds were subsequently soaked in a 20% (vol/vol) antibiotic/antimycotic solution (200,000 units/ml penicillin G, 2,000 mg/ml streptomycin sulfate, and 500 μg/ml amphotericin B) overnight at 37°C before washing in media prior to cell seeding.
Immunocytochemistry.
Samples were fixed in 3.8% (wt/vol) paraformaldehyde before permeabilization in a 0.5% (vol/vol) Triton X-100 PBS solution. Nonspecific antibody binding was reduced by incubation in 3% (wt/vol) BSA solution proceeded by a 10% (vol/vol) goat serum solution incubation. Samples were incubated with primary antibody overnight at 4°C. Protein expression was visualized with species-appropriate secondary antibodies and nuclei were visualized by Hoechst staining. Samples were viewed on a Leica TCS SP2 laser scanning confocal inverted microscope (Leica Microsystems, Milton Keynes, UK) with postvisualization image modification performed with Volocity (Perkin Elmer, Cambridge, UK).
Cell elongation and height were analyzed by using Volocity to determine individual cell's dimensions: XY images determined the long (l), and short (s) axes (length and width), with the elongation factor (EF) determined as EF = (l/s)−1. XZ images were used to determine cell height (h) through cross-sectional images. Cell alignment in relation to fiber orientation was determined using HASM cell nuclei angles from four or more images for each time point and using three HASM cell donors (n = 3). Nuclei angle deviation from the fiber angle orientation was calculated and expressed as a percentage of cells orientated within 10° incremental steps from the mean fiber angle.
Histological staining.
Airway biopsies were fixed in PMSF-acetone solution before washing in water-free acetone. Samples were incubated in methyl benzoate followed by incubating in 5% methyl benzoate/glycol methacrylate (GMA) processing solution in three 2-h incubations at 4°C. Biopsies were embedded in GMA after polymerization for 48 h at 4°C. Samples were cut into 2-μm sections and placed onto superfrost slides (Thermo Scientific, Surrey, UK). Samples were serially rehydrated through a descending ethanol (in water) concentration to 0% (vol/vol) ethanol before staining in Harris hematoxylin. Samples were serially dehydrated through an ascending ethanol concentration to 90% (vol/vol) ethanol before staining in eosin and further dehydration in 100% ethanol and xylene. Samples were air dried and mounted in DPX mountant prior to imaging.
Western blotting.
Airway tissue was freeze-snap dried in liquid nitrogen and stored at −80°C until use, and 30 mg of tissue was homogenized by using a manual homogenizer (Thermo Scientific) in lysis buffer (in mM: 20 HEPES, 200 NaCl, 10 EDTA, 0.1% Triton X-100, and 0.5% Protease Inhibitor Set Cocktail III, pH 7.4). Homogenized tissue was agitated for 2 h on an orbital shaker at 4°C before centrifugation (16,000 g, 20 min 4°C), and samples were stored at −20°C until use. HASM cells cultured in 2D or 3D were maintained for 14 days prior to incubation in lysis buffer. The Novex protein separation kit was used to measure protein content in HASM cells. Lysates (20 μg/lane) were separated by SDS-PAGE before transfer onto nitrocellulose membrane. Membranes were blocked in 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween before incubation with primary antibody overnight at 4°C. Membranes were incubated with horseradish peroxidase-conjugated secondary antibody and visualized by enhanced chemiluminescence (GE Healthcare, Buckinghamshire, UK). Images were captured on a LAS 4000 Luminescent Image Analyzer (Fujifilm, Düsseldorf, Germany). Quantitative signals were derived by densitometric analysis using Advanced Image Data Analyzer software, with total protein content corrected to GAPDH protein levels.
Calcium signaling.
HASM cells were cultured for 10 days prior to loading with the Ca2+-sensitive dye Fluo-4 AM (3 μM, 60 min). Scaffolds were transferred to an imaging unit where cells were maintained at 37°C in Krebs-Henseleit buffer (in mM: 134 NaCl, 6 KCl, 1 MgCl2, 1.2 KH2PO4, 10 glucose, 10 HEPES, 1.3 CaCl2, pH 7.4). Real-time images were taken with an epifluorescence Nikon Eclipse TE200 microscope (Nikon, Tokyo, Japan) (×40 objective). Cells were excited at 488 nm and emission collected between 505 and 560 nm. BK (100 nM) was applied to cells, and the fluorescence emission was measured from regions of interest within the cells' cytosol. [Ca2+]i changes are displayed as the fluorescence emission relative to basal (F/F0).
Data and statistical analysis.
Data are presented for cells obtained from at least three individual HASM cell donors cultured on aligned scaffolds electrospun independently at least three times. Data are expressed as means ± SE. Data have been analyzed (GraphPad Prism, San Diego CA) by t-test or one-way ANOVA and appropriate post hoc testing as indicated.
RESULTS
Scaffold characteristics controlled through alterations to electrospinning parameters and fiber collection protocols.
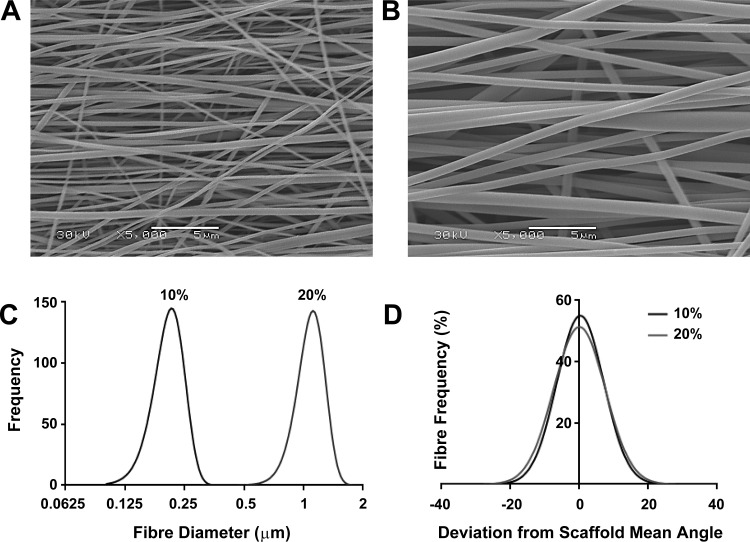
To investigate whether HASM cell characteristics were affected by the size and orientation of scaffold fibers, we electrospun a randomly aligned 10% (wt/vol) PET solution onto a collector plate (data not shown), or a 10% (wt/vol) or 20% (wt/vol) PET solution onto a rotating mandrel to produce aligned fibers with diameters in either the nanometer or micrometer range. Representative scanning electron microscope images of the 10 and 20% scaffolds are shown in Fig. 1, A and B, respectively. The 10% (wt/vol) PET solution produced fiber diameters of 0.2 ± 0.002 μm (mean ± SE), and the 20% (wt/vol) produced fiber diameters of 1.1 ± 0.001 μm (Fig. 1C). Both the 10 and 20% scaffolds had >90% fibers orientated within 10° of the mean fiber angle and >50% of fibers orientated within 5° of the mean fiber angle (Fig. 1D). Both scaffolds had a porosity >80%, whereas the 20% aligned scaffold both was thicker than the 10% aligned scaffold (107 vs. 24 μm) and had a greater tensile strength (290 vs. 211 MPa). All the electrospinning parameters and individual scaffold properties are summarized in Table 1.
Fig. 1.
Polyethylene terephthalate (PET) electrospun scaffolds can be manipulated to form aligned nano- and microfiber scaffolds. PET [either 10 or 20% (wt/vol)] was electrospun onto a rotating mandrel (600 m/min) to produce aligned fibers with diameters in the nano- or micrometer range. Representative scanning electron microscope images of the 10% (nano) and 20% (micro) scaffolds are shown in A and B, respectively. Intrascaffold fiber diameter distributions are shown in C (n = 480 from 3 separate scaffolds), and intrascaffold fiber alignments are shown in D (deviation of individual fiber angle from scaffold's mean fiber angle, n = 480 from 3 separate scaffolds).
HASM cells show altered phenotypic characteristics when cultured on aligned electrospun scaffolds.
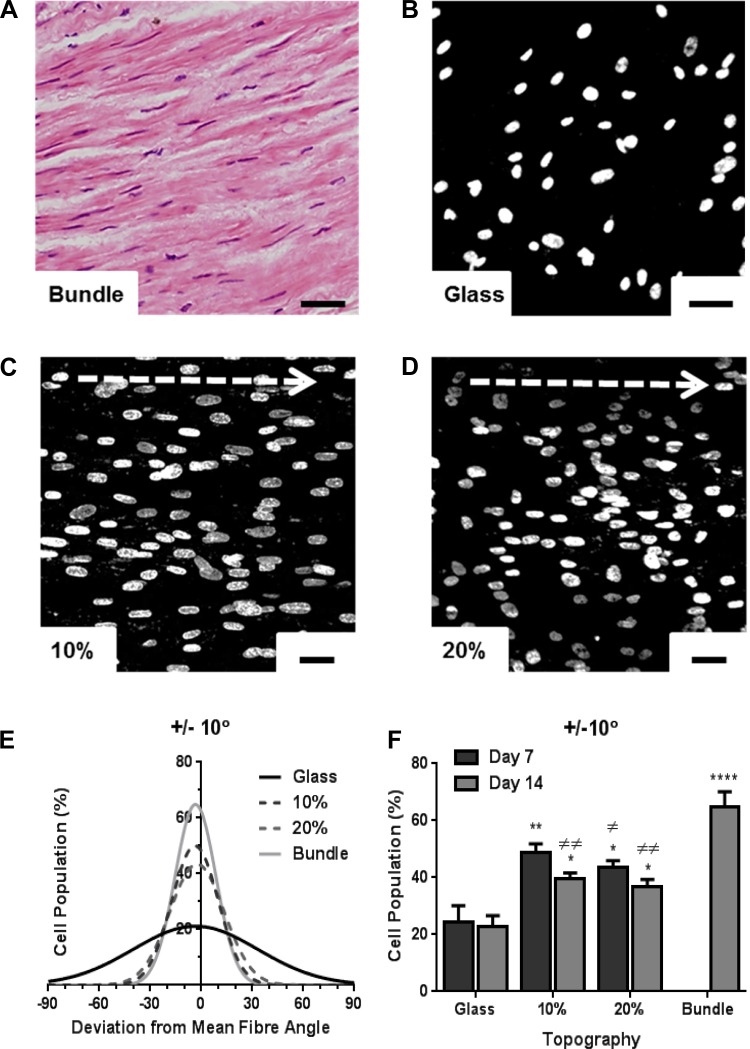
Airway bronchiole sections displaying longitudinal HASM cell populations (Fig. 2A) were used to quantify in situ HASM cell alignment by referencing the angle of each cell's nucleus to indicate cellular direction, with >60% cells orientated within 10° of the mean bundle angle (Fig. 2, E and F). HASM cells were also cultured on 22-mm glass coverslips (Fig. 2B), randomly aligned scaffold, 10% (Fig. 2C) or 20% (Fig. 2D) aligned scaffolds (cut to 22-mm-diameter dimensions). Cell alignment was measured in three separate areas of the same sample to quantify cell alignment over extended distances. Cell alignment to the underlying fiber orientation was determined by using nuclei angles to indicate cell direction at day 7 and day 14. With no topological references, cells grown on 2D glass coverslips (Fig. 2B) or 3D randomly aligned scaffolds (data not shown) showed little orientation as a cell population over extended distances, although cells showed local alignment upon confluency by day 14 (Fig. 5, A and C). Cells showed consistent orientation along the 10% (Fig. 2C), and 20% aligned scaffold (Fig. 2D), with >40% cells orientated within 10° of the mean fiber angle on both days 7 and 14 on either scaffold, compared with <25% cells showing alignment when cultured on glass (Fig. 2, E and F).
Fig. 2.
Human airway smooth muscle (HASM) cells orientate along scaffold fibers. Hematoxylin and eosin-stained immunohistological sections from airway smooth muscle (ASM) bundles were used to quantify cell directionality in situ (A). HASM cells (1.5 × 105) were cultured on glass coverslips, the 10%, or 20% aligned scaffolds for 7 or 14 days prior to fixation. Nuclei were visualized by Hoechst staining (blue) and nuclei angles were used as reference to cell directionality. Deviation of individual cell nuclei from average fiber orientation was determined, and % cell population range was plotted. Representative images of HASM nuclei cultured on glass, 10%, or 20% aligned scaffolds at day 7 are shown in B, C, and D, respectively. E shows distribution of cell alignment on all 3 in vitro topographies and in ASM bundles. F shows % cell population within ±10° fiber orientation at day 7 and day 14 (means ± SE, HASM cells cultured on 3 independently electrospun scaffolds). Statistical significance is indicated as *P < 0.05, **P < 0.01, and ****P < 0.0001 (vs. glass), or ≠P < 0.05, and ≠≠P < 0.01 (vs. bundle), one-way ANOVA, Tukey's posttest. Scale bar indicates 40 μm. Arrow indicates orientation of scaffold fibers.
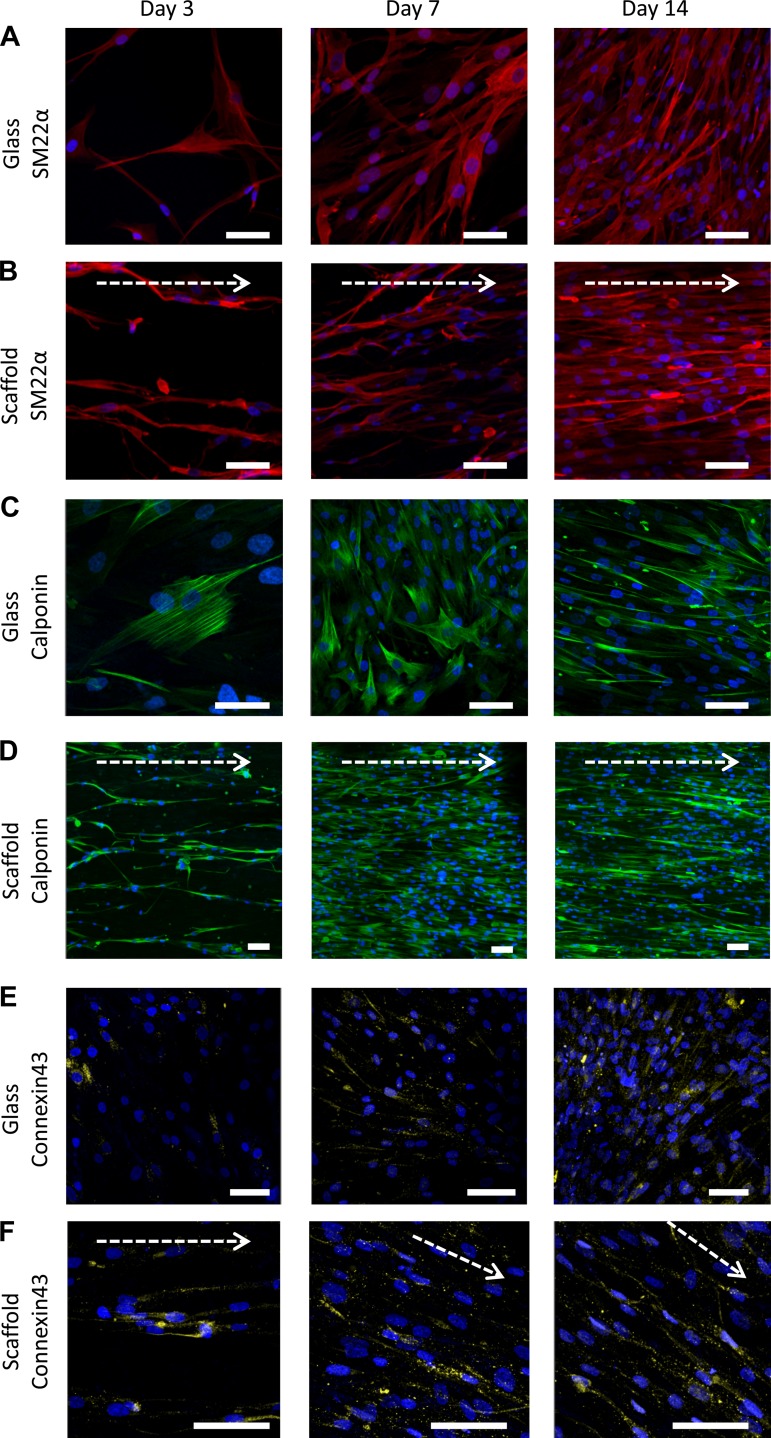
Fig. 5.
Smooth muscle specific protein expression in HASM cells cultured over 14 days on 2D or aligned fiber topography. HASM cells (1.0 × 105) were cultured on glass coverslips (A, C, and E) or 10% aligned scaffolds (B, D, and F) for 3, 7, or 14 days prior to fixation. Cells were immunostained for the smooth muscle-specific contractile proteins SM22α (A and B, red) or calponin (C and D, green), and the gap junction protein connexin-43 (E and F, yellow), with nuclei stained with Hoechst (blue). Scale bar indicates 70 μm. Arrow indicates orientation of scaffold fibers.
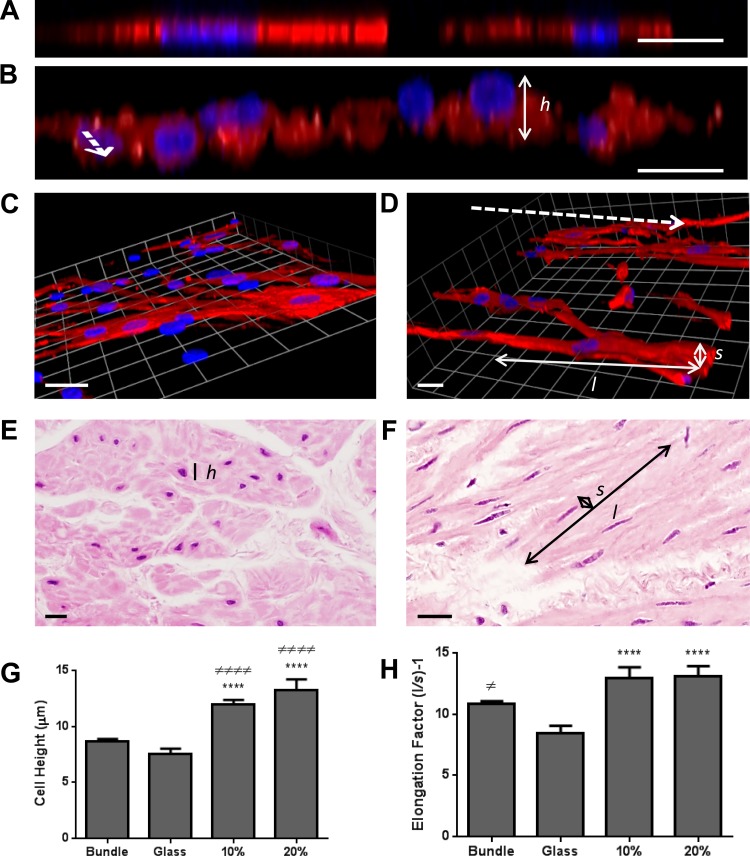
The effect of a 3D aligned topography on cellular dimensions was compared with 2D and 3D randomly aligned surfaces by culturing HASM cells for 7 days prior to fixation and immunostaining for SM22α. Airway bronchiole sections displaying longitudinal and cross-sectional HASM cell populations were used to quantify in situ HASM cellular dimensions: XY images determined individual cell's long- and short-axis measurements to calculate their elongation factor, while XZ cross-sectional images determined cell height. The heights of HASM cells cultured on 2D substrates or residing within smooth muscle bundles were significantly less than cells cultured on 3D aligned scaffolds (Fig. 3, A, B, E, and G). Cells showed an approximate doubling in elongation when cultured on a 3D aligned surface compared with 2D culturing (Fig. 3, C, D, and H), mainly due to a reduction in cell width opposed to an increase in cell length. Although cells were significantly more elongated within smooth muscle bundles compared with 2D cultured cells, there was no significant difference in elongation compared with HASM cells cultured on aligned scaffolds (Fig. 3, F and H). Cells cultured on 3D randomly aligned scaffolds had a similar elongation factor to cells cultured in 2D but showed a significant increase in cell height compared with 2D culture (data not shown). Since there was no significant variation in cell characteristics between cells cultured on the 10 or 20% aligned scaffolds, the 10% scaffold was used in further investigations because of the nanofibrous nature of the scaffold.
Fig. 3.
HASM cell morphology is altered when cultured on aligned fiber topography. HASM cells (1.5 × 105) were cultured on glass coverslips, 10%, or 20% aligned scaffolds for 7 days. Cells were fixed and immunostained for the contractile protein SM22α (red) with nuclei stained with Hoechst (blue). Representative XZ images of HASM cells grown on glass or 10% aligned scaffolds are shown in A and B, respectively. Representative 3D opacity images of HASM cells grown on glass or 10% aligned scaffolds are shown in C and D, respectively. Representative hematoxylin and eosin-stained immunohistological sections from ASM bundles sectioned cross-sectionally and longitudinally are shown in E and F, respectively. Dashed arrows indicate electrospun-fiber orientation (B and D). Scale bars represent 20 μm. The heights (h) of individual cells were calculated from XZ images through cells. Individual cells' long (l) and short (s) axes were calculated from XY images, and cell elongation factors determined. Cumulative cell height data are shown in G (n = 20–82 individual cells grown on 3 independently electrospun scaffolds and 3 separate airway tissue donors). Cumulative cell elongation data is shown in H (n = 42–73 individual cells grown on 3 independently electrospun scaffolds and 3 separate airway tissue donors). Statistical significance is indicated as ****P < 0.0001 (glass vs. scaffold), ≠P < 0.05 (ASM bundle vs. glass), ≠≠≠≠P < 0.001 (ASM bundle vs. scaffold) 1-way ANOVA, Tukey's posttest.
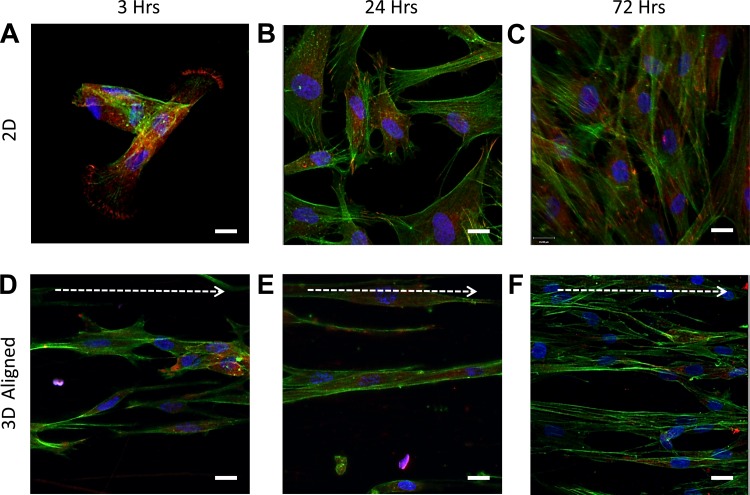
Where cells attach to the extracellular environment, focal adhesion complexes link the cell's actin cytoskeleton to the surrounding matrix (49). To determine how the underlying topological references affected focal adhesion complex formation and spatial distribution, cells cultured on a 3D aligned topography were compared with those cultured upon a 2D surface. Postseeding, cells were fixed after 3, 24, or 72 h before immunostaining for the focal adhesion protein vinculin and costained for F-actin. On glass, HASM cell F-actin organized into clear stress fibers throughout the cells with vinculin clusters at each cell's distal ends (Fig. 4A). This intracellular F-actin and vinculin expression was maintained at 24 (Fig. 4B) and 72 h (Fig. 4C), with cells also displaying a flattened cell phenotype. Cells cultured on the aligned scaffolds showed little evidence of vinculin clusters at any of the time points (with localized staining apparent around the nuclei), and F-actin was not organized into stress fibers throughout individual cells (Fig. 4D). Vinculin staining became less apparent after 3 h, and cells started to exhibit an elongated shape that aligned along the fiber orientation (Fig. 3, E and F).
Fig. 4.
HASM cells show a reduction in intracellular-stress fibers when cultured on aligned fiber topographies. HASM cells (1.5 × 105) were cultured on glass coverslips or 10% aligned scaffolds for 3, 24, or 72 h prior to fixation. Cells were immunostained for the focal adhesion protein vinculin (red), with F-actin stained with phalloidin (green) and nuclei with Hoechst (blue). Representative images of HASM cultured on glass coverslips for 3, 24, and 72 h, and 10% aligned scaffolds for 3, 24, or 72 h are shown in A, B, C, and D, E, F, respectively. Scale bar indicates 20 μm. Arrow indicates orientation of scaffold fibers.
Over a prolonged time period (3, 7, and 14 days), HASM cells cultured on 2D glass coverslips or aligned fibers were fixed and immunostained for the smooth muscle specific proteins SM22α (Fig. 5, A and B) and calponin (Fig. 5, C and D). Contractile protein distribution in HASM cells cultured on glass showed distinct stress fiber organization, maintained a flat phenotype, and displayed increasing local cell alignment upon cell confluency at day 14 (Fig. 5, A and C). Conversely, HASM cells cultured on aligned scaffolds showed little evidence of contractile protein localization into stress fibers, while maintaining an elongated phenotype and aligned cell distribution at every time point (Fig. 5, B and D). The presence of gap junctions was visualized by immunostaining for connexin-43 with qualitatively greater localized connexin-43 expression seen between adjacent HASM cells cultured on aligned scaffolds than cells cultured on glass (Fig. 5, F and E, respectively).
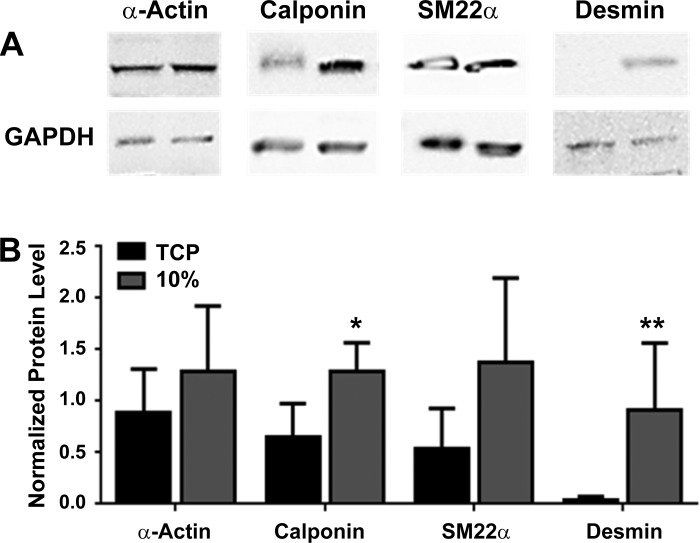
HASM cells cultured on TCP or aligned scaffolds were lysed, and protein levels were semiquantitatively compared after protein levels were normalized to levels of GAPDH protein expression (Fig. 6A). Cumulative data (Fig. 6B) found that levels of the smooth muscle-specific proteins smooth muscle α-actin (0.9 ± 0.2 vs. 1.2 ± 0.3), calponin (0.7 ± 0.1 vs. 1.3 ± 0.1), SM22α (0.5 ± 0.2 vs. 1.3 ± 0.4), and desmin (0.02 ± 0.01 vs. 0.9 ± 0.3) (n = 5–6, means ± SE) all increased upon aligned scaffold culture, with levels of calponin and desmin increasing significantly, in contrast to previous studies in which the expression of smooth muscle specific proteins is often reduced/lost upon prolonged cell culture (23, 39). Protein levels from freshly isolated airway tissue were determined separately, with the relative levels of smooth muscle-specific proteins expressed as a ratio to the level of smooth-muscle α-actin. The ratio of smooth muscle α-actin-calponin-SM22α-desmin for airway tissue was 1.0:0.9 ± 0.04:0.7 ± 0.1:1.2 ± 0.3, HASM cultured on aligned scaffold was 1.0:1.1 ± 0.2:1.0 ± 0.2:1.0 ± 0.3, and HASM cultured on 2D TCP was 1.0:0.7 ± 0.1:0.7 ± 0.3:0.03 ± 0.02 (n = 3–6, means ± SE).
Fig. 6.
Relative HASM cells protein levels are altered when cultured on either 2D or 3D aligned topography. HASM cells (1.0 × 105) were cultured on tissue culture plastic (TCP) or 10% aligned scaffolds for 14 days. Cells were lysed and 20 μg protein was loaded for SDS-PAGE separation and immunoblotting. Representative immunoblots for the smooth muscle-specific proteins calponin, SM22α, smooth muscle specific α-actin, desmin, and GAPDH controls are shown in A. Cumulative densitometric data showing protein levels normalized to GAPDH protein expression are shown in B (data shown are means ± SE, n = 5–6). Statistical significance is indicated as *P < 0.05 and **P < 0.01 (TCP vs. scaffold) unpaired t-test.
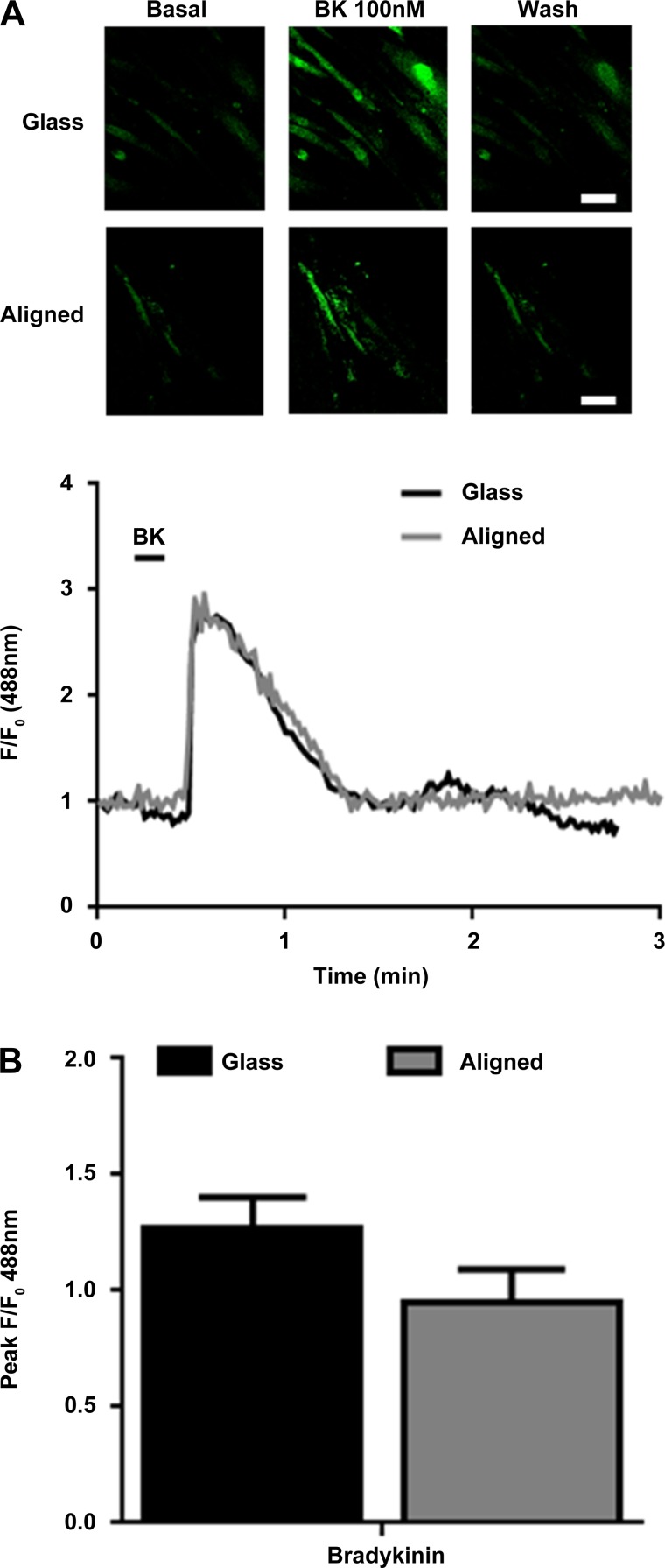
To investigate HASM cell responses to bronchoconstrictor application, HASM cells cultured either on 2D glass or aligned scaffolds were loaded with the Ca2+ sensitive dye Fluo-4 AM and stimulated with the Gαq/11-agonist BK (100 nM), causing a biphasic increase in [Ca2+]i, with an initial transient increase in Ca2+ concentration followed by a reduction to lower steady-state Ca2+ concentration (Fig. 7A). HASM cells cultured on glass or the aligned scaffold showed similar levels of peak Ca2+ release (Fig. 7B).
Fig. 7.
Primary HASM cells respond to bradykinin (BK) when cultured on aligned scaffolds. HASM cells (1.5 × 105) were cultured on glass coverslips or aligned scaffolds for 10 days. Cells were loaded with the calcium-sensitive dye Fluo-4 AM and stimulated with BK (100 nM). Representative images showing Ca2+ changes, and representative Ca2+ traces from HASM cells cultured on glass (black trace) or aligned scaffold (gray trace) and stimulated with BK (100 nM) are shown in A. B shows cumulative peak Ca2+ release from HASM cells stimulated with BK (100 nM) when cultured on glass (black bar) or aligned scaffold (gray bar). Data are means ± SE (30–60 cells, n = 3 separate HASM donors).
DISCUSSION
Advancements in biomaterial design allow better control to fabricate 3D matrices that are specifically tailored to individual cell-types opposed to the “one rigid 2D substrate fits all” approach offered by TCP. Smooth muscle exists as an aligned population of cells, and this organization ensures a coordinated contractile response. To maintain these characteristics in vitro, we electrospun uniaxially orientated fibers to attempt to direct the HASM cell alignment. One previous study has described the alignment of PET fibers by electrospinning onto a rotating mandrel (22). However, the rotational velocity employed was much lower than applied here (36 vs. 600 m/min, respectively). This large increase in velocity produced thinner nanofibers (0.2 μm vs. 0.7 μm) at similar polymer concentrations (10 vs. 9% wt/vol). Comparable micrometer-diameter fibers were produced by using a 20% wt/vol polymer solution compared with the 12.5% wt/vol solution (1 μm vs. 1.7 μm fibers). Qualitatively, we demonstrated more consistent alignment of fibers throughout the scaffolds and greater tensile strength (211 vs. 90 MPa) with respect to aligned nanofiber scaffolds.
When HASM cells were cultured on either a 2D or 3D surface with no orientation reference, only transitory cell alignments were seen over short distances upon contact inhibition. We measured local cell alignment from separate regions of the same sample to investigate cell population alignment over extended distances. The contact guidance provided by fiber orientation ensured good mediation of cell alignment from 3 h postseeding, an effect maintained over 14 days. Cells seeded on both aligned scaffolds (10 and 20% polymer solutions) showed consistently good alignment to the fiber orientations (>70% cells within 20° of the scaffold mean fiber angle using either aligned scaffold), although this was still less than cell alignment within in situ smooth muscle bundles (>90% cells within 20° of the mean fiber angle). Aligned nanofibers have shown previously to enhance vascular smooth muscle alignment over an immediate time course of 24 h (51), whereas here we show that this effect can be maintained over an extended time period under static conditions. Other studies have achieved similar results with no underlying topological reference through the application of mechanical strain to HASM cells within collagen gels (20, 45) or pulsed tubes (33).
HASM cells cultured in 2D showed clear vinculin localization within defined focal adhesions at the distal ends of the cells, with F-actin and smooth muscle-specific contractile proteins organized into stress fibers throughout the cells, an effect most likely caused by the rigidity of the substrate [TCP ∼2–4 GPa (6)]. On the aligned scaffolds, HASM cells showed a reduction in vinculin localization within focal adhesion complexes and a more even distribution of intracellular F-actin, calponin, and SM22α protein expression. Similar reductions in focal adhesions and cytoskeletal organization have been noted in HASM cells (15, 24), and fibroblasts (1, 27) cultured on 2D surfaces coated with specific extracellular matrix proteins, although HASM cells cultured on these substrates still maintained a flattened cell shape (15, 24). HASM cells cultured in 2D also showed a reduction in elongation compared with those cultured on aligned scaffolds where cells elongated along individual fibers and displayed a more spindlelike morphology than the short, flat, and fat morphology that can occur upon 2D culture. Taken together these data suggest the topography encountered by the smooth muscle may play a dominant role in the cytoskeletal protein organization opposed to the relative rigidity of the substrate, and the cues provided by the aligned fibers ensure HASM cells orientate, and elongate along individual fibers opposed to interacting with multiple fibers as seen when cultured on randomly aligned scaffolds.
Levels of contractile proteins are known to rapidly decrease when HASM cells are passaged upon isolation from fresh tissue (23, 39), with levels of desmin virtually abolished within 7 days postisolation (23). The culturing of HASM cells on a 3D aligned scaffold was sufficient to significantly increase calponin and desmin protein levels compared with 2D culture, with desmin expression upregulated from negligible levels (2D culture) to consistent expression (3D aligned culture). These expression levels were compared with freshly isolated airway tissue. Relative protein levels, when normalized to smooth muscle α-actin levels, displayed similar expression patterns between airway tissue and 3D-aligned cultured cells, with smooth muscle α-actin, calponin, and desmin all showing a 1:1 ratio whereas 3D-aligned cultured cells showed a relative enhancement in calponin expression (1.0:0.6). 2D cultured cells showed a relative reduction in calponin expression and a significant reduction in desmin expression as reported elsewhere (23). Although expression patterns are comparable between airway tissue and aligned scaffold cultured cells, the magnitude in protein expression is likely to be underestimated within this study owing to the heterogeneous airway cell population used to determine levels of specific smooth muscle proteins within tissue opposed to a homogenous smooth muscle population. Alterations to smooth muscle-specific contractile proteins have also been observed in 3D cultures of HASM cells when cocultured with fibroblasts (48), or fibroblasts and epithelial cells under pulsatile conditions, albeit over an extended time course of 28 days (33). Mechanical strain causes similar alterations to HASM cell contractile protein mRNA and protein levels, including desmin and calponin (2, 20, 45, 55), suggesting similar SMC phenotypic modulations (SMC alignment and contractile protein levels) can be induced through mechanical stimulation, or exposure to a 3D aligned topography. These enhancements in the underlying contractile machinery of the HASM cells could help more accurately model smooth muscle functionality within an in vitro setting.
Smooth muscle contraction can be studied by a variety of techniques. Measuring changes in key second-messenger signaling molecules such as [Ca2+]i have previously been employed in airway (17) and vascular smooth muscle (35, 36) contractile profiling. When the bronchoconstrictor BK was applied to HASM cells cultured on 2D or aligned scaffolds, peak Ca2+ levels and Ca2+ release profiles were comparable. Given the pronounced alterations in cellular characteristics and increased contractile and gap junction protein expression when HASM cells were cultured on aligned scaffolds compared with 2D culture, one may have expected an increase in Ca2+ responses. There is a need for greater assessment of the functional cell-cell coupling of cells cultured on the aligned scaffold in the future since the present data are suggestive but inconclusive. Other studies employing alternative outputs to measure smooth muscle contraction have noted an increase in the comparative shortening of asthmatic smooth muscle compared with healthy smooth muscle (32, 46). A novel multicell microtissue culture model coculturing HASM cells with 3T3 fibroblast cells showed alterations in contractile profiles in response to some agonists (such as histamine), but not others (such as KCl) (48). These suggest that underlying differences in Ca2+ responses may be uncovered if diseased cells were employed in the present model.
In conclusion, we have developed a fully aligned nanofibrous scaffold, whose orientation guidance is sufficient to produce fully aligned HASM cell sheets that can be cultured for prolonged periods while maintaining more in vivo-like characteristics.
GRANTS
This work was funded by the National Centre for the Replacement, Refinement, and Reduction of Animals in Research (NC3Rs) and Asthma UK. J. C. Bridge was funded by the EPSRC DTC in Regenerative Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.E.M., A.J.K., J.W.A., C.E.B., A.M.G., and F.R.A.J.R. conception and design of research; G.E.M. and J.C.B. performed experiments; G.E.M., J.C.B., and F.R.A.J.R. analyzed data; G.E.M., J.C.B., A.J.K., J.W.A., C.E.B., and A.M.G. interpreted results of experiments; G.E.M. prepared figures; G.E.M. drafted manuscript; G.E.M., A.J.K., J.W.A., C.E.B., A.M.G., and F.R.A.J.R. edited and revised manuscript; G.E.M., J.C.B., A.J.K., J.W.A., C.E.B., A.M.G., and F.R.A.J.R. approved final version of manuscript.
REFERENCES
- 1.Ahmed I, Ponery AS, Nur EKA, Kamal J, Meshel AS, Sheetz MP, Schindler M, Meiners S. Morphology, cytoskeletal organization, and myosin dynamics of mouse embryonic fibroblasts cultured on nanofibrillar surfaces. Mol Cell Biochem 301: 241–249, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Ahvaz HH, Soleimani M, Mobasheri H, Bakhshandeh B, Shakhssalim N, Soudi S, Hafizi M, Vasei M, Dodel M. Effective combination of hydrostatic pressure and aligned nanofibrous scaffolds on human bladder smooth muscle cells: implication for bladder tissue engineering. J Mater Sci Mater Med 23: 2281–2290, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Beamish JA, He P, Kottke-Marchant K, Marchant RE. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev 16: 467–491, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berair R, Hollins F, Brightling C. Airway smooth muscle hypercontractility in asthma. J Allergy (Cairo) 2013: 185971, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billington CK, Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res 4: 2, 2003. [PMC free article] [PubMed] [Google Scholar]
- 6.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer 9: 108–122, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis 147: 405–410, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Ceresa CC, Knox AJ, Johnson SR. Use of a three-dimensional cell culture model to study airway smooth muscle-mast cell interactions in airway remodeling. Am J Physiol Lung Cell Mol Physiol 296: L1059–L1066, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Chew SY, Mi R, Hoke A, Leong KW. The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials 29: 653–661, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cockcroft DW. Direct challenge tests: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest 138: 18S–24S, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Corey JM, Gertz CC, Wang BS, Birrell LK, Johnson SL, Martin DC, Feldman EL. The design of electrospun PLLA nanofiber scaffolds compatible with serum-free growth of primary motor and sensory neurons. Acta Biomater 4: 863–875, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crouch AS, Miller D, Luebke KJ, Hu W. Correlation of anisotropic cell behaviors with topographic aspect ratio. Biomaterials 30: 1560–1567, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science 294: 1708–1712, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol 14: 633–639, 2002. [DOI] [PubMed] [Google Scholar]
- 15.D'Antoni ML, Risse PA, Ferraro P, Martin JG, Ludwig MS. Effects of decorin and biglycan on human airway smooth muscle cell adhesion. Matrix Biol 31: 101–112, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Dalton PD, Vaquette C, Farrugia BL, Dargaville TR, Brown TD, Hutmacher DW. Electrospinning and additive manufacturing: converging technologies. Biomater Sci 1: 171–185, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Deshpande DA, Dogan S, Walseth TF, Miller SM, Amrani Y, Panettieri RA, Kannan MS. Modulation of calcium signaling by interleukin-13 in human airway smooth muscle: role of CD38/cyclic adenosine diphosphate ribose pathway. Am J Respir Cell Mol Biol 31: 36–42, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Dunnill MS, Massarella GR, Anderson JA. A comparison of the quantitative anatomy of the bronchi in normal subjects, in status asthmaticus, in chronic bronchitis, and in emphysema. Thorax 24: 176–179, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott JT, Woodward JT, Langenbach KJ, Tona A, Jones PL, Plant AL. Vascular smooth muscle cell response on thin films of collagen. Matrix Biol 24: 489–502, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Fairbank NJ, Connolly SC, Mackinnon JD, Wehry K, Deng L, Maksym GN. Airway smooth muscle cell tone amplifies contractile function in the presence of chronic cyclic strain. Am J Physiol Lung Cell Mol Physiol 295: L479–L488, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Farmer SG, Ensor JE, Burch RM. Evidence that cultured airway smooth muscle cells contain bradykinin B2 and B3 receptors. Am J Respir Cell Mol Biol 4: 273–277, 1991. [DOI] [PubMed] [Google Scholar]
- 22.Hadjizadeh A, Ajji A, Bureau MN. Nano/micro electro-spun polyethylene terephthalate fibrous mat preparation and characterization. J Mech Behav Biomed Mater 4: 340–351, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Halayko AJ, Salari H, Ma X, Stephens NL. Markers of airway smooth muscle cell phenotype. Am J Physiol Lung Cell Mol Physiol 270: L1040–L1051, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Hirst SJ, Twort CH, Lee TH. Differential effects of extracellular matrix proteins on human airway smooth muscle cell proliferation and phenotype. Am J Respir Cell Mol Biol 23: 335–344, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med 164: S28–S38, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Johansson F, Carlberg P, Danielsen N, Montelius L, Kanje M. Axonal outgrowth on nano-imprinted patterns. Biomaterials 27: 1251–1258, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Kapoor A, Sen S. Synergistic modulation of cellular contractility by mixed extracellular matrices. Int J Cell Biol 2012: 471591, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaur D, Saunders R, Berger P, Siddiqui S, Woodman L, Wardlaw A, Bradding P, Brightling CE. Airway smooth muscle and mast cell-derived CC chemokine ligand 19 mediate airway smooth muscle migration in asthma. Am J Respir Crit Care Med 174: 1179–1188, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Kim DH, Provenzano PP, Smith CL, Levchenko A. Matrix nanotopography as a regulator of cell function. J Cell Biol 197: 351–360, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert RK, Wiggs BR, Kuwano K, Hogg JC, Pare PD. Functional significance of increased airway smooth muscle in asthma and COPD. J Appl Physiol 74: 2771–2781, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Marsh KA, Hill SJ. Characteristics of the bradykinin-induced changes in intracellular calcium ion concentration of single bovine tracheal smooth muscle cells. Br J Pharmacol 110: 29–35, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto H, Moir LM, Oliver BG, Burgess JK, Roth M, Black JL, McParland BE. Comparison of gel contraction mediated by airway smooth muscle cells from patients with and without asthma. Thorax 62: 848–854, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller C, George S, Niklason L. Developing a tissue-engineered model of the human bronchiole. J Tissue Eng Regen Med 4: 619–627, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Moreno MJ, Ajji A, Mohebbi-Kalhori D, Rukhlova M, Hadjizadeh A, Bureau MN. Development of a compliant and cytocompatible micro-fibrous polyethylene terephthalate vascular scaffold. J Biomed Mater Res B Appl Biomater 97: 201–214, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Morris GE, Nelson CP, Everitt D, Brighton PJ, Standen NB, Challiss RA, Willets JM. G protein-coupled receptor kinase 2 and arrestin2 regulate arterial smooth muscle P2Y-purinoceptor signalling. Cardiovasc Res 89: 193–203, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris GE, Nelson CP, Standen NB, Challiss RA, Willets JM. Endothelin signalling in arterial smooth muscle is tightly regulated by G protein-coupled receptor kinase 2. Cardiovasc Res 85: 424–433, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray-Dunning C, McArthur SL, Sun T, McKean R, Ryan AJ, Haycock JW. Three-dimensional alignment of schwann cells using hydrolysable microfiber scaffolds: strategies for peripheral nerve repair. Methods Mol Biol 695: 155–166, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Paik I, Scurr DJ, Morris B, Hall G, Denning C, Alexander MR, Shakesheff KM, Dixon JE. Rapid micropatterning of cell lines and human pluripotent stem cells on elastomeric membranes. Biotechnol Bioeng 109: 2630–2641, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol Cell Physiol 256: C329–C335, 1989. [DOI] [PubMed] [Google Scholar]
- 40.Parrag IC, Zandstra PW, Woodhouse KA. Fiber alignment and coculture with fibroblasts improves the differentiated phenotype of murine embryonic stem cell-derived cardiomyocytes for cardiac tissue engineering. Biotechnol Bioeng 109: 813–822, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Schnell E, Klinkhammer K, Balzer S, Brook G, Klee D, Dalton P, Mey J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly epsilon-caprolactone and a collagen/poly epsilon-caprolactone blend. Biomaterials 28: 3012–3025, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Shang S, Yang F, Cheng X, Walboomers XF, Jansen JA. The effect of electrospun fiber alignment on the behaviour of rat periodontal ligament cells. Eur Cell Mater 19: 180–192, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29: 1989–2006, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest 96: 2393–2403, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith PG, Moreno R, Ikebe M. Strain increases airway smooth muscle contractile and cytoskeletal proteins in vitro. Am J Physiol Lung Cell Mol Physiol 272: L20–L27, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Sutcliffe A, Hollins F, Gomez E, Saunders R, Doe C, Cooke M, Challiss RA, Brightling CE. Increased nicotinamide adenine dinucleotide phosphate oxidase 4 expression mediates intrinsic airway smooth muscle hypercontractility in asthma. Am J Respir Crit Care Med 185: 267–274, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teo WE, Ramakrishna S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 17: R89–R106, 2006. [DOI] [PubMed] [Google Scholar]
- 48.West AR, Zaman N, Cole DJ, Walker MJ, Legant WR, Boudou T, Chen CS, Favreau JT, Gaudette GR, Cowley EA, Maksym GN. Development and characterization of a 3D multicell microtissue culture model of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 304: L4–L16, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta 1692: 103–119, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Wright D, Sharma P, Ryu MH, Risse PA, Ngo M, Maarsingh H, Koziol-White C, Jha A, Halayko AJ, West AR. Models to study airway smooth muscle contraction in vivo, ex vivo and in vitro: implications in understanding asthma. Pulm Pharmacol Ther 26: 24–36, 2013. [DOI] [PubMed] [Google Scholar]
- 51.Xu CY, Inai R, Kotaki M, Ramakrishna S. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials 25: 877–886, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell 130: 601–610, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(l-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 26: 2603–2610, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Yim EK, Reano RM, Pang SW, Yee AF, Chen CS, Leong KW. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials 26: 5405–5413, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Wang X, Keshav V, Wang X, Johanas JT, Leisk GG, Kaplan DL. Dynamic culture conditions to generate silk-based tissue-engineered vascular grafts. Biomaterials 30: 3213–3223, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]