Abstract
There are 190,600 cases of acute lung injury/acute respiratory distress syndrome (ALI/ARDS) each year in the United States, and the incidence and mortality of ALI/ARDS increase dramatically with age. Patients with ALI/ARDS have alveolar epithelial injury, which may be worsened by high-pressure mechanical ventilation. Alveolar type II (ATII) cells are the progenitor cells for the alveolar epithelium and are required to reestablish the alveolar epithelium during the recovery process from ALI/ARDS. Lung fibroblasts (FBs) migrate and proliferate early after lung injury and likely are an important source of growth factors for epithelial repair. However, how lung FBs affect epithelial wound healing in the human adult lung has not been investigated in detail. Hepatocyte growth factor (HGF) is known to be released mainly from FBs and to stimulate both migration and proliferation of primary rat ATII cells. HGF is also increased in lung tissue, bronchoalveolar lavage fluid, and serum in patients with ALI/ARDS. Therefore, we hypothesized that HGF secreted by FBs would enhance wound closure in alveolar epithelial cells (AECs). Wound closure was measured using a scratch wound-healing assay in primary human AEC monolayers and in a coculture system with FBs. We found that wound closure was accelerated by FBs mainly through HGF/c-Met signaling. HGF also restored impaired wound healing in AECs from the elderly subjects and after exposure to cyclic stretch. We conclude that HGF is the critical factor released from FBs to close wounds in human AEC monolayers and suggest that HGF is a potential strategy for hastening alveolar repair in patients with ALI/ARDS.
Keywords: alveolar epithelial cells, lung fibroblasts, hepatocyte growth factor, wound closure
acute lung injury/acute respiratory distress syndrome (ALI/ARDS) is a severe form of diffuse lung disease that imposes a substantial health burden all over the world. The incidence and mortality of ALI/ARDS increase with age (50, 52). ALI/ARDS is associated with alveolar epithelial injury and increased epithelial permeability resulting in alveolar edema, severe hypoxemia, and a decrease in lung compliance, which usually requires intubation and mechanical ventilation. Mechanical ventilation is a life-saving supportive measure because it allows use of positive end expiratory pressure and allows delivery of high levels of inspired oxygen. However, extensive animal data have demonstrated that it also can contribute to lung injury in a process termed ventilator-induced lung injury (VILI) (52). Although many approaches have been undertaken to improve the outcome of ARDS pharmacologically, the results have been disappointing. One area that has not received a great deal of attention is a focus on epithelial repair.
The alveolar epithelium is comprised of two cell types, alveolar type I (ATI) cells and alveolar type II (ATII) cells. ATI cells cover about 95% of the alveolar surface and participate in transepithelial fluid movement and innate immunity but are sensitive to injury. ATII cells produce pulmonary surfactant and also participate in transepithelial fluid movement and innate immunity but are more resistant to injury, perhaps because of their limited apical surface exposure to the alveolus and their high-volume density of organelles (55). ATII cells are thought to be the main progenitor cells for restoring the alveolar epithelium after injury (3, 12, 15). However, recently other progenitor cells have also been shown to be important after catastrophic injury to type II cells (4, 30, 59). After epithelial injury in the skin, the cornea, the trachea, or the lung, the initial response is for the surviving epithelial cells at the edge of the wound to dedifferentiate, spread, migrate, then proliferate, and finally redifferentiate to restore the epithelium (20, 28, 38, 65).
During this process the epithelial cells may receive signals from underlying mesenchymal cells. Mesenchymal cells are critical for epithelial development in the fetal lung, and lipofibroblasts are thought to be important in the repair of the alveolar epithelium in adult animals (3). Fibroblasts (FBs) have been shown to control proliferation and differentiation of alveolar epithelial cells (AECs) through direct contact (39, 53), as well as by secretion of soluble mediators such as hepatocyte growth factor (HGF) and keratinocyte growth factor (KGF) (44, 60, 63).
In this report, we studied wound repair in primary human AECs and how lung FBs accelerate epithelial wound healing. We hypothesized that HGF secreted by lung FBs would promote wound closure in human AECs. HGF, originally named scatter factor, is thought to be important in lung injury (41) and is known to stimulate the migration of epithelial cells (16, 47, 62). In addition, we examined whether wound closure by human AECs differed with age and/or mechanical stretch, which mimics the mechanical ventilation in vivo. Age is well known to be an important prognosis factor in ARDS but has not been studied in detail. This research contributes to our understanding of how lung FBs augment alveolar epithelial repair and the importance of HGF for accelerating wound repair by human AECs.
MATERIALS AND METHODS
Donor information.
To isolate human primary ATII cells, alveolar macrophages (AMs), and FBs, we obtained human lungs from deidentified organ donors whose lungs were not suitable for transplantation and donated for medical research through the National Disease Research Interchange (Philadelphia, PA) and the International Institute for the Advancement of Medicine (Edison, NJ). The Committee for the Protection of Human Subjects at National Jewish Health deemed this research as not human subject research. We selected donors with reasonable lung function with a PaO2/FiO2 ratio of >225, no history of clinical lung disease, a chest radiograph that indicated no infection, and a time on the ventilator of less than 5 days. The sex, age, race, and smoking history were variable and were not selection criteria. For the aging study, we defined young donors as below 40 yr of age and aged donors as over 65 yr of age, and we used cells only from nonsmokers.
Isolation and culture of human ATII cells.
We modified the human type II cell isolation method published by Fang and coworkers (13, 58). Briefly, the middle lobe was perfused, lavaged, and then instilled with elastase (Worthington, Lakewood, NJ) for 40 min at 37°C. The lung was minced, and the cells were isolated by filtration and partially purified by centrifugation on a discontinuous density gradient made of Optiprep (Accurate Chemical Scientific, Westbury, NY) with densities of 1.080 and 1.040 and by positive selection with MACS MicroBeads human CD326 (EpCAM) (Miltenyi Biotech, Bergisch Gladbach, Germany). Cell preparations were made to assess cell purity by staining for cytokeratin (CAM 5.2; Dako Cytomation, Carpenteria, CA) and surfactant protein A (antibody; a gift from Yoshio Kuroki, Sapporo, Japan). The isolated cells were resuspended in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 2.5 μg/ml amphotericin B, 100 μg/ml streptomycin, 100 μg/ml penicillin G (GIBCO, Life Technologies, Rockville, MD), and 10 μg/ml gentamicin (Sigma-Aldrich, St. Louis, MO). One million cells per square centimeter were plated on either 12- or 24-well cell culture plates (Costar, Corning, NY) that had been previously coated with rat-tail collagen (RTC) (in house) in DMEM with 10% FBS. We changed the culture medium every other day. Crisscross scratch wounds were made with p10 pipette tips when the monolayer was completely formed, usually 5–6 days after plating, and we switched the medium to DMEM without serum overnight before making the wounds.
Wound closure assay in AEC monolayer cocultured with human AMs.
AMs were isolated from deidentified donor lungs described above. The lung was lavaged sequentially with HEPES-buffered saline and then with HEPES-buffered saline containing 2 mM EDTA, and the lavage fluid was centrifuged at 4°C for 10 min. The resulting pellet was resuspended and washed, and the cells were counted and then frozen in 90% FBS and 10% DMSO. Frozen AMs were thawed and plated on BD 12-well cell culture inserts (pore size 0.4 mm, 1.0 million cells/cm2) (BD Bioscience, Franklin Lakes, NJ) in DMEM including 10% FBS and cultured at 37°C in 10% CO2 for 2 days. Cells were then washed and cultured for 24 h in DMEM without serum overnight. The following day, crisscross scratch wounds were made in the AEC monolayer cultured in a 12-well plate, and the inserts with the AMs were placed over the scratched AEC monolayer (apical medium DMEM without serum 0.2 ml, basal medium DMEM without serum 1.0 ml). We measured the wound area immediately after wounding (at 0 h) and at 24 h, calculated Δwound area (wound area at 0 h − wound area at 24 h) using phase-contrast pictures of wounds and Image J software (National Institute of Health, Bethesda, MD), and compared the results as relative Δwound area with AMs to Δwound area of the control, which is shown as relative degree of wound closure to control in the y-axis of the figures (Δwound area of the control is designated as 1.00).
Wound closure assay in AEC monolayer cocultured with human lung FBs.
FBs were isolated from deidentified donor lungs described above. Human lung FBs were prepared by explanting minced human lungs into 100-mm tissue-culture dishes. The medium consisted of DMEM supplemented with 10% FBS, 2 mM glutamine, 100 U/ml penicillin G, 100 μg/ml streptomycin, 2.5 μg/ml amphotericin B, and 10 μg/ml gentamicin. After ∼3 days, FBs grew out from the edge of the explant, the medium and remaining explant tissue fragments were removed, and the adherent cells were grown to 50% confluence. The FBs were then passaged and grown to near confluence. These cells (passage 2) were used for all experiments. We confirmed FB purity with immunostaining of vimentin (+), thy 1 (CD90) (+), and cytokeratin (-). FBs were plated on 12-well BD Bioscience cell culture inserts (pore size 0.4 μm, 0.5 million cells/cm2) (BD Bioscience) in DMEM including 10% FBS and cultured at 37°C in 10% CO2 for 2 days. The cells were then washed and cultured for another day in DMEM without serum overnight. The following day, crisscross scratch wounds were made in AEC monolayers in the 12-well plates, an insert with FBs was placed over the scratched AEC monolayers (apical medium DMEM without serum 0.2 ml, basal medium DMEM without serum 1.0 ml), and phase-contrast pictures of wounds in AEC monolayers were taken at 0 and 24 h after wounding. Wound areas were measured using Image J software and normalized by comparing the experimental wells to the control wells whose area was designated as 1.00 at specified times.
To evaluate the effect of HGF and activation of its receptor c-Met on wound closure in the FB coculture system, we added 500 nM PHA 665752 (TOCRIS Bioscience, Bristol, UK) (a phospho-c-Met inhibitor), 100 nM AG1478 (BioVision, Milpitas, CA) (an EGFR inhibitor), or 500 ng/ml anti-human HGF antibody (R&D Systems, Minneapolis, MN).
Quantitative measurement of 40 human growth factors using culture medium from human lung FBs.
Five million FBs were plated on a 100-mm tissue-culture dish in DMEM with 10% FBS. When the cells became confluent, they were washed once and cultured with DMEM without serum overnight. The following day, the cells were incubated either with or without 100 nM adenosine 5′-[γ-thio] triphosphate tetralithium salt (ATP-γ-S) (Sigma), 10 ng/ml recombinant human IL-1α, or IL-1β (R&D Systems). After 48 h, we collected the culture media from the four different conditions (control, ATP-γ-S, IL-1α, and IL-1β), which were stored at −80°C. We measured 40 different human growth factors in these samples using the Quantibody Human Growth Factor Array 1 (RayBiotech, Norcross, GA). Briefly, this array used a multiplexed sandwich ELISA-based technology. The target growth factors were trapped on the glass surface by capturing antibodies, then biotin-labeled detection antibodies were added, which recognized the target growth factors, and finally the growth factor-antibody-biotin complex was visualized through the addition of the streptavidin-labeled Cy3 equivalent dye and measured with a laser scanner (Agilent Technologies G2505B Micro Array Scanner; Agilent Technologies, Santa Clara, CA). After the detection, we analyzed the data with the Human Growth Factor Q-Analyzer (RayBiotech) as recommended by the supplier.
Wound closure assay in AEC monolayer with recombinant human growth factors.
As described above, when cultured AECs formed a monolayer, the cells were washed and cultured overnight in DMEM without serum. The following day, we made crisscross scratch wounds in the AEC monolayers made on wells in either 24-well or 12-well plates. To compare the effect of growth factors on wound closure in AEC monolayers, we added 10 ng/ml of each recombinant human growth factor in DMEM without serum to the scratched AEC monolayers, took phase-contrast pictures of wounds in AEC monolayers under the microscope at 0 and 24 h after wounding and adding growth factors, measured wound areas using Image J software, and analyzed the degree of wound closure based on the area of control condition as 1.00. Human recombinant HGF and KGF were obtained from Amgen (Thousand Oaks, CA), and human recombinant basic FB growth factor (bFGF), FGF4, growth differentiation factor (GDF)-15, insulin growth factor (IGF)-1, bone morphogenetic protein 5 (BMP-5), osteoproteogrin (OPG), and vascular endothelial growth factor (VEGF) were purchased from R&D Systems.
Real-time RT-PCR.
For real-time RT-PCR, the expression levels of genes were expressed as a ratio to the expression of the constitutive probe Cyclophilin B. The specific primers and probes for HGF were purchased from Applied Biosystems (Foster City, CA).
HGF ELISA assay and Western blotting.
To measure the concentration of HGF in the culture medium, we used a human HGF ELISA (R&D Systems) according to the instruction manual.
For the Western blotting analysis, polyacrylamide gradient gels (8–16%; Invitrogen) were run in Tris glycine buffer to separate the proteins. Proteins were run in the reduced state. Protein loading was normalized to GAPDH. The primary antibodies were anti-Met (c-Met) antibody (Abcam, Cambridge, MA), anti-phospho-Met (Cell Signaling Technology, Danvers, MA), anti-phospho-focal adhesion kinase (FAK) (Y397) (Life Technologies, Grand Island, NY), anti-phospho-Src (Try416) (Cell Signaling Technology), anti-phospho-ERK (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-JNK (Abcam), and anti-phospho-Akt (Cell Signaling Technology).
Cell motility assay.
Human ATII cells were plated and cultured on RTC-coated 35-mm glass-bottom dishes (MatTek, Ashland, MA) until the AECs formed a monolayer. Crisscross wounds were made with p10 pipette tips and cultured with or without 50 ng/ml HGF. Cells were then mounted on a culture dish heater (DH-35; Warner Instruments, Hamden, CT) fitted with a TC-344B dual automatic temperature controller (Warner Instruments). Cells were imaged at 37°C on an inverted microscope (Zeiss Axiovert 200M; Carl Zeiss, Jena, Germany) using a ×63 oil-immersion lens. Images were acquired and analyzed using Intelligent Imaging Innovations 3D software. Time-lapse images (exposure 500 ms) were taken every 20 min. For each series, 54 images were taken. To quantitate cell motility, over 30 cells were randomly chosen, the center of the nucleus was used as a reference point, and travel distance was measured for 18 h. The cell motility was then expressed as the distance the cell moved in microns in 18 h (26).
Immunofluorescence staining and measurement of lamellipodia area.
For immunofluorescence microscopy, cells were fixed with 4% paraformaldehyde and then permeabilized in phosphate-buffered saline containing 0.2% Triton X-100. Cells were stained with phalloidin-tetramethylrhodamine B isothiocyanate (Sigma), anti-vinculin antibody (Sigma), and DAPI (Vector Laboratories, Burlingame, CA) by standard immunofluorescence procedures and imaged with an inverted Zeiss Axiovert 200M deconvolution microscope (Carl Zeiss). Images were acquired and processed using Slidebook 5.0 software (Intelligent Imaging Innovations, Denver, CO). Lamellipodia areas were analyzed from leading edge at the four corners of crisscross wounds using Slidebook 5.0 software by tracing the shape of lamellipodia stained by phalloidin-tetramethylrhodamine B isothiocyanate.
Cyclic stretch experiment.
We plated primary human ATII cells at a density of 4.0 million cells/well on BioFlex Collagen Type I-coated culture plates for the cyclic stretch (CS) system (Flexcell strain unit Flex Central FX-400 tension plus; Flexcell International, Hillsborough, NC). After the AEC monolayer was formed, cells were preincubated with or without 50 ng/ml HGF for 30 min, and wounds were made with P10 pipette tips. The plates were then placed on the Flexcell tension system (10% elongation, 6 cycles/min). To examine the effect of CS and HGF on wound closure, phase-contrast pictures were taken at 0 and 48 h after wounding.
Statistical analysis.
All data are presented as means ± SE. One-way ANOVA was used to compare the difference between two or more groups. The post hoc Bonferonni/Dune test was used for multiple comparisons. Statistical significance was set at P < 0.05.
RESULTS
FBs promote wound closure in human primary AEC monolayers.
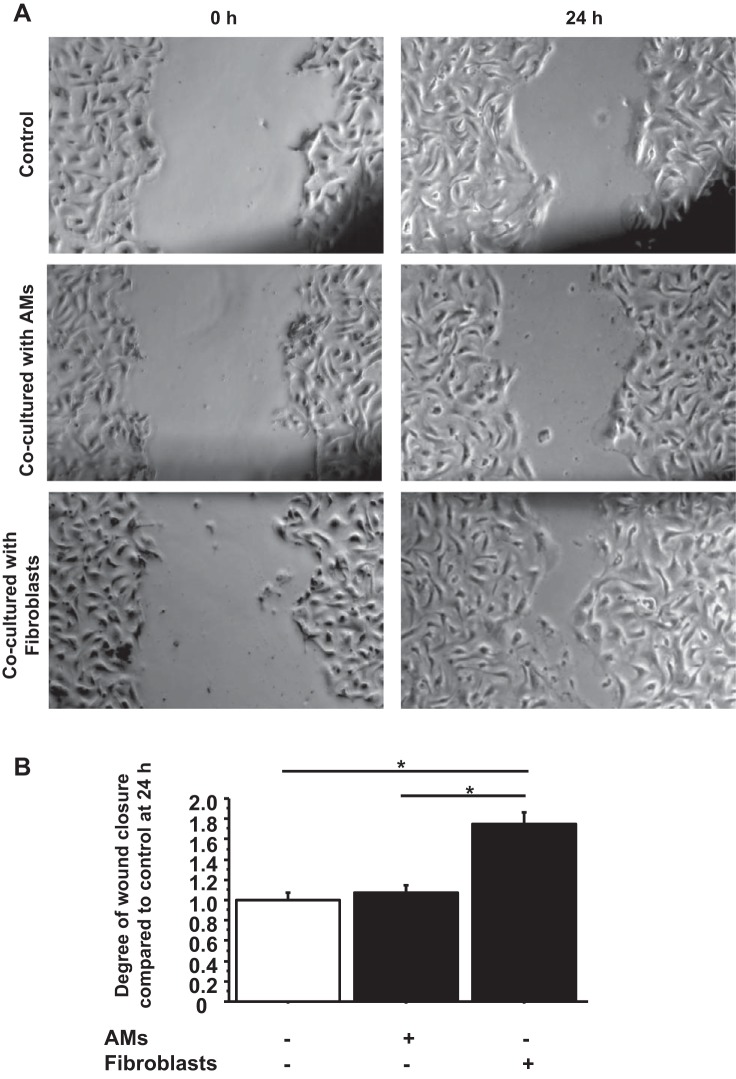
To investigate whether FBs and/or AMs accelerated wound closure in scratched wounds in AEC monolayers, primary human ATII cells were plated on 12-well culture plates coated with rat tail collagen. When a confluent monolayer was visible, scratch wounds were made with p10 pipette tips, and wound closure was observed with or without FBs or AMs cultured on the 12-well inserts (Fig. 1A). FBs accelerated wound closure in AEC monolayers 1.73 times the extent compared with cultures without FBs (P < 0.0001) at 24 h after wounding (Fig. 1B). However, inserts containing AMs did not alter the extent of wound closure.
Fig. 1.
Fibroblasts (FBs) promote wound closure in primary human alveolar epithelial cell (AEC) monolayers. A: phase-contrast pictures were taken of wound areas at 0 and 24 h after wounding to compare wound closure between controls (wounded AEC monolayers without coculture), wounded AEC monolayers cocultured with alveolar macrophages (AMs), or wounded AEC monolayers cocultured with FBs. B: wound closure was analyzed at 24 h after wounding. Each condition has 8 different marked wounds in each well that were analyzed for the degree of wound closure. Values were means ± SE for 3 independent experiments. *P < 0.05.
Screening for growth factors released by human primary FBs.
To examine which growth factors secreted by FBs were likely responsible for the accelerated wound closure in AECs, we ran the Quantibody Human Growth Factor Array 1 (Raybiotech) to screen for 40 growth factors. We used medium from FBs derived from two individuals cultured with or without ATP-γ-S, IL-1α, or IL-1β. Previous reports indicated that scratch wounding released ATP and that the ATP analog ATP-γ-S induced rapid and sustained EGFR activation dependent on human bronchial EGF shedding and enhanced wound closure in epithelial cell lines (64). IL-1β has been reported to be the main bronchoalveolar lavage fluid (BALF) mediator involved in HGF secretion from lung FBs (46). In this experiment, we were determining which growth factors were produced by primary human lung FBs, and we found that either control or stimulated FBs secreted measurable levels of bFGF, BMP-5, FGF-4, KGF (FGF7), GDF-15, HGF, IGF-1, OPG, IGF binding proteins, and VEGF. IL-1 induced BMP-5, HGF, and IGF-1, whereas ATP-γ-S did not consistently stimulate any growth factors (data not shown).
HGF promotes wound closure in human AEC monolayers.
To determine which growth factor/factors released by the FBs accelerate wound closure in AEC monolayers, we added 10 ng/ml recombinant human bFGF, BMP-5, FGF-4, KGF, GDF-15, HGF, IGF-1, OPG, or VEGF to the scratch wounds in human primary AEC monolayer and compared the extent of wound closure to controls. We excluded IGF binding proteins from this experiment because they are binding proteins for IGFs. We tested these growth factors at a concentration of 10 ng/ml according to published papers (1, 22, 25, 27, 29, 42, 51). Of the nine growth factors tested, only HGF accelerated wound closure in AEC monolayers, which was 1.96 times the extent compared with controls without any growth factors at 24 h after wounding (P < 0.001) (Fig. 2). In other experiments, we evaluated FGF10, which was not on the Quantibody Human Growth Factor Array 1 (RayBiotech), but has been shown to be secreted by FBs and known to be a mitogen for rat ATII cells (14, 63). Neither of these factors stimulated wound closure in human AEC monolayers. These results suggest that HGF was the major factor secreted by FBs that accelerated wound closure in AEC monolayers.
Fig. 2.
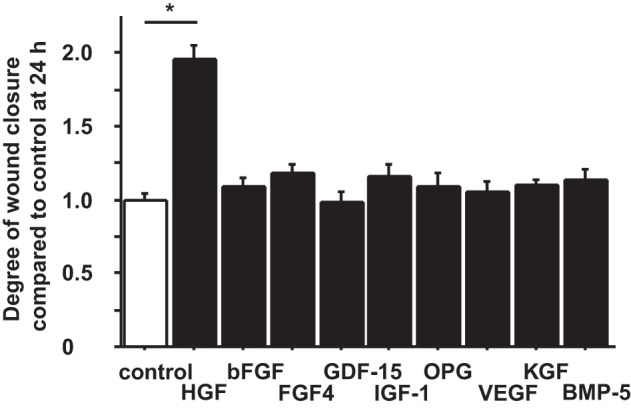
Hepatocyte growth factor (HGF) promotes wound closure in human AEC monolayers. Phase-contrast pictures were taken of marked wound areas at 0 and 24 h after wounding to compare the degree of wound closure in controls (wounded AEC monolayer without additives) and with 9 different growth factors [HGF, human recombinant basic FB growth factor (bFGF), FGF4, growth differentiation factor (GDF)-15, insulin growth factor (IGF)-1, osteoproteogrin (OPG), vascular endothelial growth factor (VEGF), keratinocyte growth factor (KGF), and bone morphogenetic protein 5 (BMP-5)]. The degree of wound closure was analyzed at 24 h after wounding. Each condition has 8 different marked wound areas in each well to analyze for the degree of wound closure. Values were means ± SE for 5 independent experiments. *P < 0.05.
FBs promote wound repair in AECs through HGF/c-Met signaling.
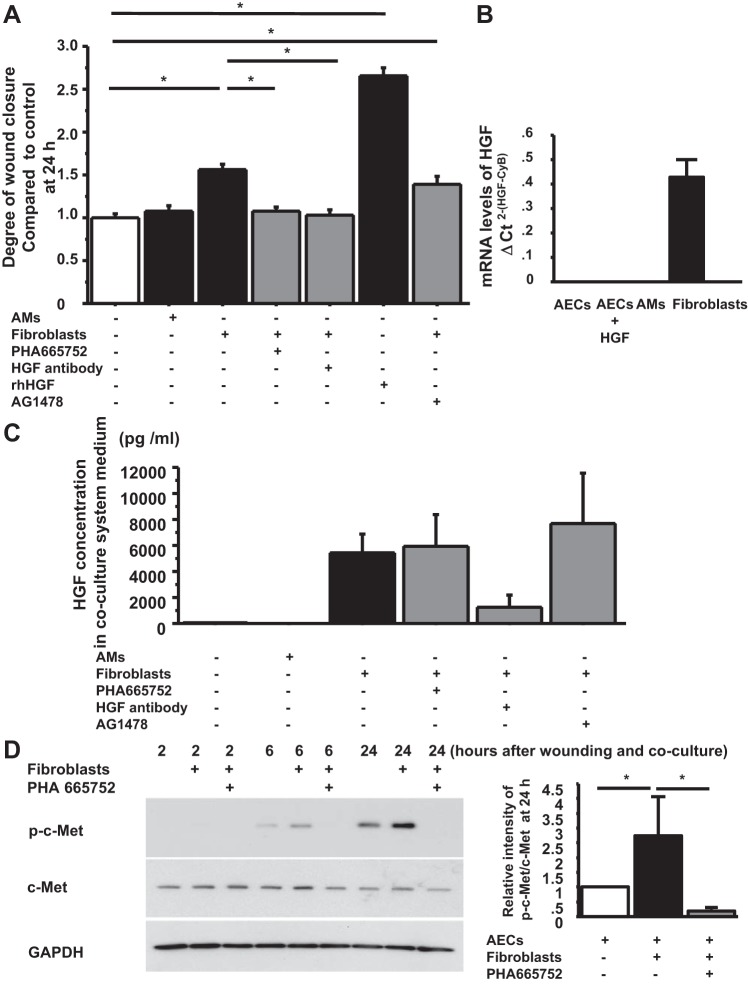
To verify that the HGF/c-Met signaling pathway was responsible for the enhanced wound closure in AECs by FBs, we added 500 nM PHA 665752, a potent, selective inhibitor of Met kinase, (TOCRIS Bioscience), and 500 ng/ml of a neutralizing antibody to HGF (R&D Systems) to the FB coculture system with scratched wounds in AECs. PHA 665752 and the anti-HGF antibody diminished the accelerating effect of FBs on wound closure in AEC monolayers at 24 h after wounding (1.079 ± 0.047 P < 0.0001 vs. wound + FBs, 1.035 ± 0.055 P < 0.0001 vs. wound + FBs, respectively) (Fig. 3A). Because we observed that recombinant HGF also activated EGFR in human AEC monolayers (unpublished data), we added 100 nM AG1478, a potent and highly selective inhibitor of EGF receptor kinase, to scratch wounds in AEC monolayers cocultured with FBs. However, AG1478 did not affect the accelerated wound closure by FBs (1.387 ± 0.100 P = 0.1360 vs. wound + FBs) (Fig. 3A). HGF has been reported to be expressed by rodent AMs and FBs. To better define potential sources of HGF in the human lung, we measured mRNA levels and protein levels of HGF from human AECs, AMs, FBs, and medium from coculture systems. The mRNA levels of HGF were detected only in FBs and not in AECs or AMs (Fig. 3B). The protein levels of HGF in the medium were significantly higher in coculture systems with FBs compared with cultures with AMs or without either AMs or FBs (control, 55 ± 31 pg/ml; wound with AMs, 20 ± 10 pg/ml; wound with FBs, 5,470 ± 1,380 pg/ml; wound with FBs + PHA 665752, 6,060 ± 2,400 pg/ml; wound with FBs + AG 1478, 7,680 ± 3,860 pg/ml) (Fig. 3C). We also confirmed c-Met phosphorylation in the wounded AEC monolayer at 6 and 24 h after coculturing with FBs, which was inhibited by PHA 665752 (Fig. 3D). Hence, these results suggest that FBs promote wound closure in AECs through HGF/c-Met signaling.
Fig. 3.
FBs promote wound closure though HGF/c-Met signaling. A: phase-contrast pictures were taken from marked wound areas at 0 and 24 h after wounding to compare the degree of wound closure among treatment groups: wounded AEC monolayer without coculture, wounded AEC monolayer cocultured with AMs, with FBs, with FBs + PHA 665752, with FBs + anti-HGF antibody, with HGF (as a positive control) and with FBs + AG 1478. The degree of wound closure was analyzed at 24 h after wounding. Values were means ± SE for 6 independent experiments. *P < 0.05. B: mRNA levels of HGF were measured by real-time PCR. These levels were normalized to the constitutive probe cyclophilin B (CyB). Values are means ± SE. C: HGF protein concentration in coculture media at 48 h after wounding was measured by ELISA. Values were means ± SE for 6 independent experiments. D, left: human AEC monolayers with wounds were cultured without FBs, with FBs, and with FBs + PHA 665752. The AECs were harvested at 2, 6, and 24 h after wounding, and protein levels of phospho-c-Met and c-Met normalized by GAPDH were measured by immunoblotting. This is a representative blot of 3 reproducible experiments. Right: relative intensity of p-c-Met/c-Met at 24 h by Western blotting analyzed by Image J from 3 independent experiments. Values are means ± SE for 3 independent experiments. *P < 0.05.
Additionally, we compared HGF secretion by FBs between coculture with unwounded AEC monolayers and wounded AEC monolayers. However, we did not observe a difference between these two conditions, which indicates that wounding was not necessary for HGF secretion by FBs in our system (data not shown).
Recombinant human HGF promotes cell motility through phosphorylation of c-Met in primary human AEC monolayers.
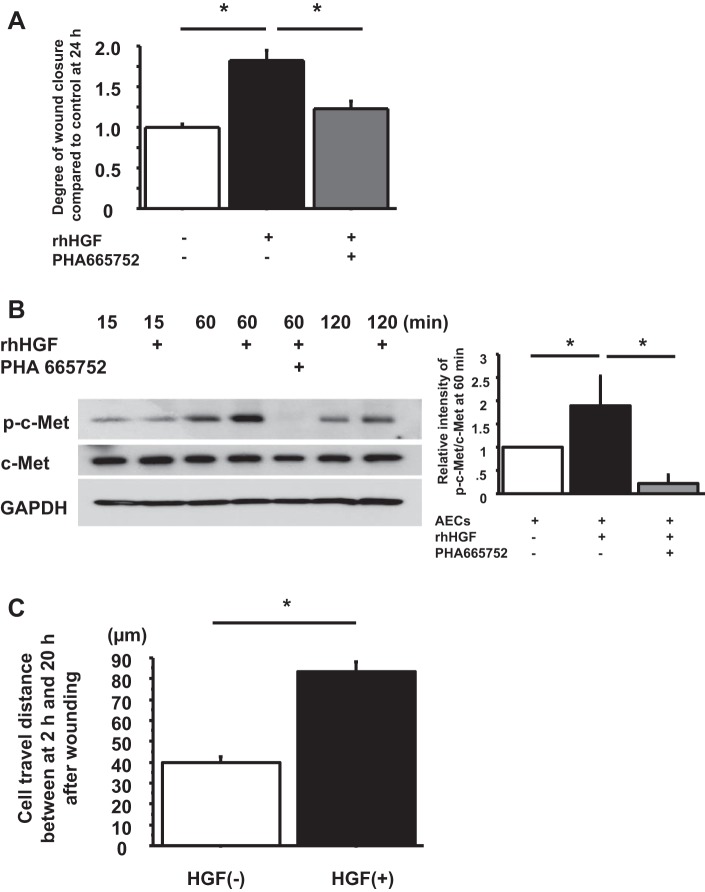
To determine whether recombinant human HGF promoted wound closure through phosphorylation of c-Met in primary human AECs, wound closure was compared with HGF, without HGF, and with HGF + 500 nM PHA 665752. Wound closure was accelerated by HGF, and the effect was inhibited by PHA 665752 at 24 h after wounding (control, 1.00 ± 0.04; wound + HGF, 1.82 ± 0.12 P < 0.0001 vs control; wound + HGF + PHA 665752, 1.23 ± 0.10 P < 0.0001 vs. wound + HGF) (Fig. 4A). Phosphorylation of c-Met in AEC monolayer was observed by recombinant HGF at 60 and 120 min after wounding, which was diminished by PHA 665752 (Fig. 4B). Hence, HGF promotes wound closure in AEC monolayers through activation of c-Met.
Fig. 4.
Recombinant HGF promotes cell motility through phosphorylation of c-Met in primary human AEC monolayers. A: phase-contrast pictures were taken from marked wound areas at 0 and 24 h after wounding to compare wound closure among wounded AEC monolayer without HGF, wounded AEC monolayer with HGF, and wounded AEC monolayer with HGF + PHA 665752. The degree of wound closure was analyzed at 24 h after wounding. Values were means ± SE for 3 independent experiments. *P < 0.05. B, left: human AEC monolayers with wounds were cultured without HGF, with HGF, and with HGF + PHA 665752. The AECs were harvested at 15, 60, and 120 min after wounding, and protein levels of phospho-c-Met and c-Met normalized by GAPDH were measured by immunoblotting. This blot is representative of 1 of 4 independent but reproducible experiments. Right: relative intensity of p-c-Met/c-Met at 60 min by Western blotting analyzed by Image J from 4 independent experiments. Values are means ± SE for 3 independent experiments. *P < 0.05. C: cell motility at wound edge in human AEC monolayers with and without HGF was measured by time-lapse microscopy between at 2 h and 20 h after wounding. Values were means ± SE for 3 independent experiments. *P < 0.05.
HGF increases AEC motility.
To examine whether the effect of HGF on wound closure in human AEC monolayers was attributable to cell motility and/or cell proliferation, cell travel distance was measured by time-lapse microscopy from 2 h to 20 h after wounding, and cell proliferation was determined by bromodeoxyuridine (BrdU) incorporation and immunostaining at 24 and 48 h after wounding. Three independent experiments were done, and over 30 cells per each sample were counted for cell travel distance. We observed that AECs moved as an epithelial cell sheet to close the wound. AECs treated with HGF showed longer cell travel distance between 2 h and 20 h after wounding under time-lapse microscopy, which indicated that HGF promoted cell motility [HGF (-), 39.8 ± 2.9 μm; HGF (+), 83.5 ± 4.7 μm, P < 0.0001] (Fig. 4C). By BrdU incorporation and immunostaining, there was no epithelial proliferation at the wound edge during wound closure at 24 h and 48 h either with or without recombinant HGF (data not shown). Hence, at 24 h, HGF promoted cell motility in AECs but not cell proliferation.
HGF promotes lamellipodia formation and activates FAK, ERK1/2, Akt, and JNK, but not Src.
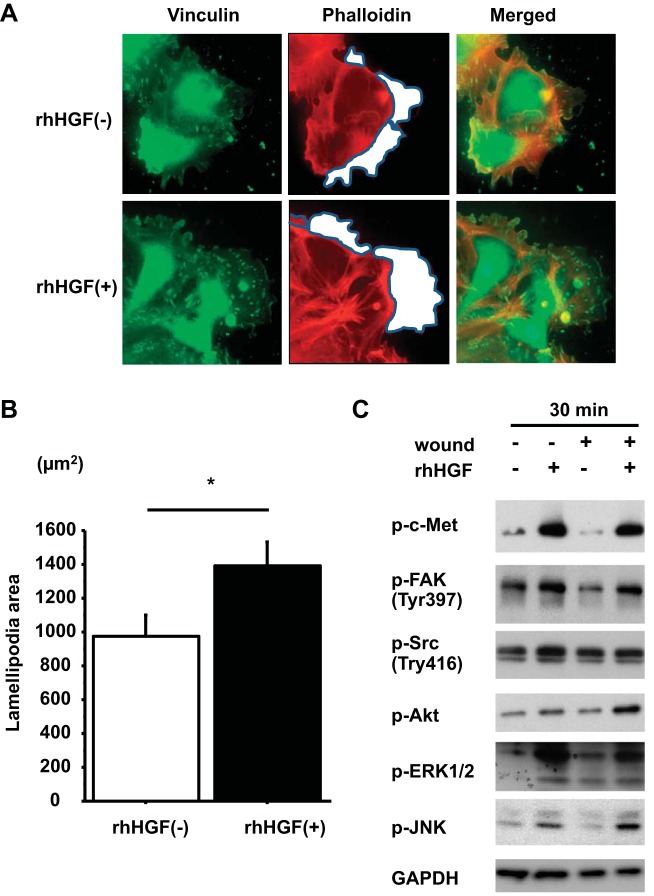
To define how HGF promoted cell motility, we compared the areas of lamellipodia with and without HGF at 2 h after wounding. The areas of lamellipodia at the four corners of crisscross scratch wounds were significantly increased by HGF [HGF (−), 977 ± 119 μm2; HGF (+), 1,388 ± 142 μm2, P = 0.0340 from 4 independent experiments] (Fig. 5, A and B). We also measured phosphorylation of the cell migration regulatory kinases FAK and Src by western blotting. FAK was activated by HGF at 30 min, whereas Src was not (Fig. 5C). We also showed the activation of other signaling kinases downstream from c-Met. FAK, Akt, ERK1/2, and JNK were all activated by HGF at 30 min from all donors (Fig. 5C). We also examined whether wounding alone would activate c-Met and these downstream kinases. We made multiple wounds in the AEC monolayers, but wounding alone did not activate c-Met, FAK, Src, Akt, ERK1/2, or JNK in our system.
Fig. 5.
HGF promotes lamellipodia formation and phosphorylation of focal adhesion kinase (FAK). A: human AEC monolayers with wounds were cultured with or without HGF. Immunostaining of scratched AEC monolayer at 2 h after wounding. Red, phalloidin; green, vinculin; blue, DAPI. White area encircled with gray lines indicates lamellipodia area. Magnification: ×50. B: area of lamellipodia at 2 h after wounding was analyzed with Slidebook 5.0 software. Values are means ± SE for 4 independent experiments. *P < 0.05. C: unwounded and wounded AEC monolayers cultured either with or without HGF were harvested at 30 min after wounding and addition of HGF. Protein levels of phospho-c-Met, phospho-FAK, phospho-Src, phospho-Akt, phospho-ERK1/2, and phospho-JNK were normalized by GAPDH measured by immunoblotting (representative blot of 3 independent experiments).
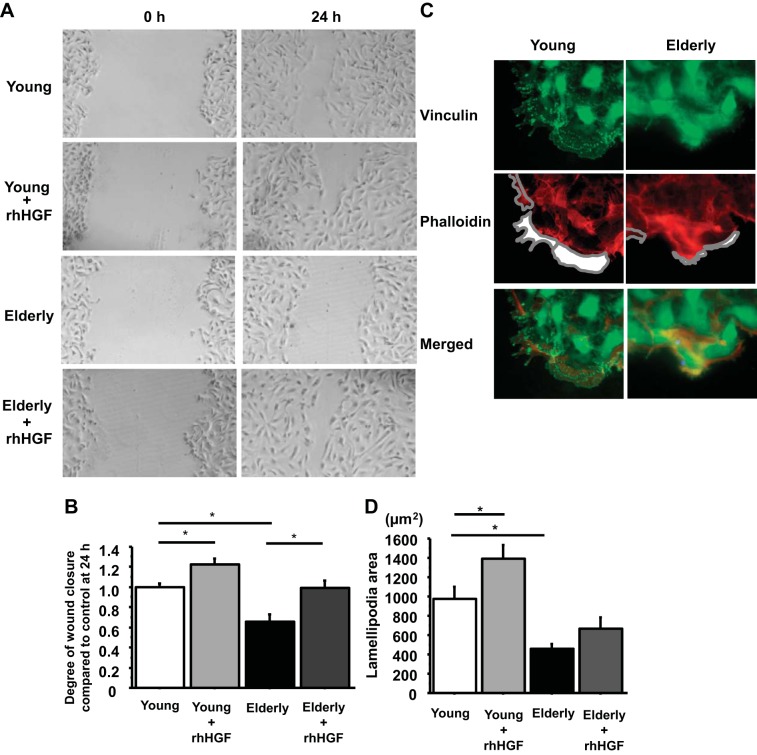
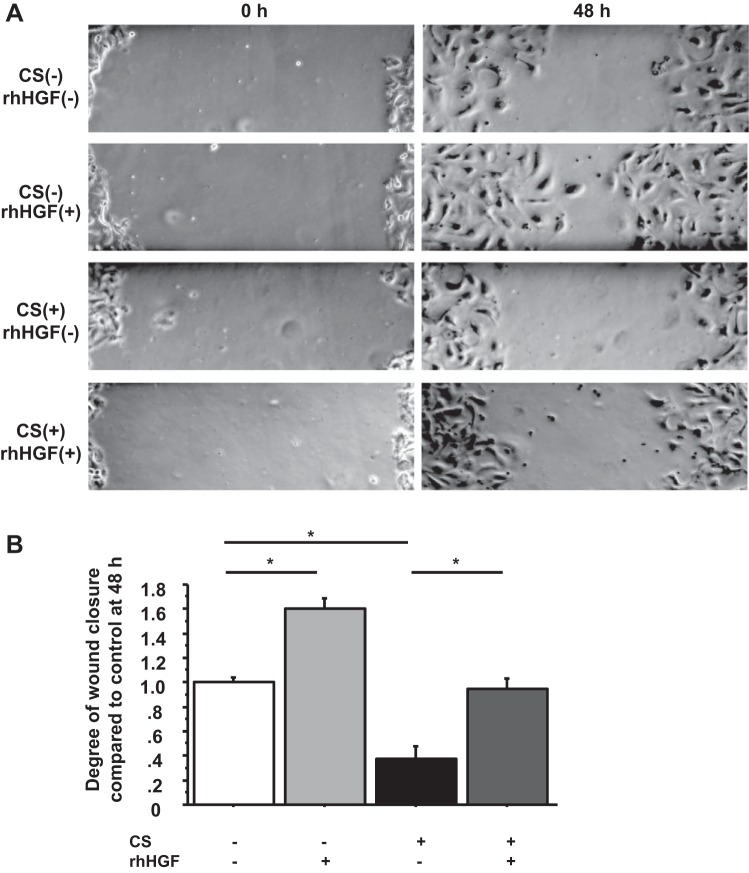
HGF restores the delayed wound closure due to age and CS.
Alveolar epithelial injury is a critical feature of ALI/ARDS, and recovery requires alveolar epithelial repair. The incidence and mortality of ALI/ARDS increase dramatically with age, and high-pressure mechanical ventilation is almost uniformly required for treatment of ARDS. Therefore, we sought to determine whether the wound closure in the AEC monolayer was delayed with age and/or CS and whether HGF restored the delay. Primary human ATII cells from young donors (20–39 yr old, n = 4) and aged donors (over 65 yr old, n = 4) were plated on 12-well culture plates coated with RTC. When a complete monolayer was visible, scratch wounds were made with P10 pipette tips, and wound closure was observed with or without HGF. The wound closure in AEC monolayers from aged donors was significantly delayed and was restored by HGF (young without HGF, 1.00 ± 0.04; young with HGF, 1.22 ± 0.06 P = 0.006 vs. young without HGF; elderly without HGF, 0.66 ± 0.07 P < 0.0001 vs. young without HGF; elderly with HGF, 1.00 ± 0.07 P = 0.0002 vs. elderly without HGF) (Fig. 6, A and B). Lamellipodia formation was also significantly decreased in the elderly [young 977 ± 119 μm2 and elderly 457 ± 48 μm2, P = 0.0003 young donors (n = 4) and elderly donors (n = 4)] (Fig. 6, C and D). However, we were not able to demonstrate that HGF enhanced the lamellipodia formation in AEC monolayers from older organ donors (Fig. 6D). To determine the effect of CS on wound closure in AEC monolayer, primary human ATII cells were plated on BioFlex Collagen Type I-coated culture plates for CS system (Flexcell tension system). When a complete monolayer was visible, 50 ng/ml HGF was added 30 min before wounding and starting CS, scratch wounds were made with P10 pipette tips, and then the cell culture plates were put on the CS system (10% elongation, 6 cycles/min). At 48 h after wounding and starting CS, CS impaired wound closure and HGF restored the impairment (no CS without HGF, 1.00 ± 0.04; no CS with HGF, 1.60 ± 0.08 P < 0.0001 vs. no CS without HGF; CS without HGF, 0.37 ± 0.10 P < 0.0001 vs. no CS without HGF; CS with HGF, 0.94 ± 0.09 P < 0.0002 vs. CS without HGF) (Fig. 7, A and B).
Fig. 6.
HGF restores the delayed wound closure in the elderly. A: phase-contrast pictures were taken from marked wound areas at 0 and 24 h after wounding to compare wound closure in young controls (wounded AEC monolayer from young donors without HGF, young with HGF, elderly controls, and elderly with HGF) (young donors n = 4, elderly donors n = 4). B: wound closure was analyzed at 24 h after wounding. Values are means ± SE for comparison between wounds in AEC monolayers from 4 young and 4 elderly donors. *P < 0.05. C: representative immunostaining of vinculin: green, vinculin; red, phalloidin; and DAPI, blue at the wound edge in scratched AEC monolayer from young (19-yr-old nonsmoker) and elderly (65-yr-old nonsmoker) at 2 h after wounding. White area encircled with gray lines indicates lamellipodia area. Magnification: × 50 (4 different donors/each group). D: areas of lamellipodia at 2 h after wounding were analyzed with Slidebook 5.0 software. Values are means ± SE for comparison between wounds in AEC monolayers from 4 young and 4 elderly donors. *P < 0.05.
Fig. 7.
HGF restores the delayed wound closure due to cyclic stretch (CS). A: phase-contrast pictures were taken from marked wound areas at 0 and 48 h after wounding to compare the degree of wound closure among 4 different groups (wounded AEC monolayers without HGF, with HGF, on CS without HGF, and on CS with HGF). B: degree of wound closure was analyzed at 48 h after wounding. Values are means ± SE for 4 independent experiments. *P < 0.05.
DISCUSSION
Using a monolayer scratch assay with human AECs, we demonstrated that lung FBs accelerated wound closure through the HGF/c-Met pathway and that HGF induced cell motility in AEC monolayers. We also found that wound closure in human AEC monolayer was delayed in the elderly and by CS. In both of these conditions, wound closure was accelerated by HGF.
The confluent monolayer scratch assay used in this study provides an in vitro model of cell migration, which can be used to identify factors that may be important in the in vivo setting. However, in vivo wound healing is much more complex and involves coagulation, granulation, inflammation, reepithelialization, tissue remodeling. and the interaction of many other cell types. Although any in vitro model will not be able to address all these processes simultaneously, they do allow specific factors to be investigated with individual cell types in a controlled environment (1, 16, 28, 47). We used monolayers of primary human ATII cells plated on RTC-coated plastic wells. The phenotype of these AECs may be similar to what is found in an injured lung, as these cells have lost some surfactant protein expression, acquired some type I cell markers, and morphologically became flatter and more spread but still contain residual lamellar bodies (58). ATI cells are the most susceptible to injury, presumably attributable to their large surface area and relative paucity of cellular organelles. ATII cells are responsible for covering the denuded alveolar surface and restoring the epithelium (15, 33, 56). Our study is the first in vitro wound-healing assay using primary human AECs.
Epithelial-mesenchymal interactions are important for normal lung growth, development, and the restoration of normal lung architecture after lung injury (39, 53). Proliferation and/or constitutive activation of prosurvival signaling in mesenchymal cells at the early stage of ARDS are associated with poor prognosis of patients with ARDS, which leads to fibrosis (5, 21, 32). On the other hand, epithelial growth and survival factors such as HGF and KGF (FGF-7) (44) found in BALF and produced by FBs in patients with ARDS likely lead to healing (46, 54, 57). Lung FB migration and proliferation occur early after lung injury and are suggested to be important in lung healing. In this study, we have shown that lung FBs promote wound closure in human primary AECs and that HGF is the critical factor for the wound repair in this system. A surprising finding was that among all the factors tested, only HGF significantly accelerated the wound closure in human AEC monolayers. Other factors, such as bFGF, BMP-5, FGF-4, KGF, FGF-10, GDF-15, IGF-1, OPG, and VEGF, which are known to be associated with cell proliferation, wound closure, and/or inflammation in the cells from the different organs, had no apparent effect on wound closure (1, 19, 22, 25, 27, 29, 42, 51). These soluble factors are likely important in other organs or species, which could have different levels of receptors and receptor activation. In addition to finding high levels of HGF in the FB-conditioned media, we also confirm the importance of the HGF/c-Met pathway in this system by using phospho-c-Met inhibitor, PHA 665752, and an antibody to human HGF. Recently, Myerburg et al. (39) also showed that FBs promoted the closure of mechanically scraped wounds in human bronchial epithelial (HBE) cells through HGF secreted by FBs, which supports our conclusions (39). However, as shown in a previous report (35), we cannot rule out the possibility that activation and release of HGF from the extracellular matrix by proteases enhance wound closure in AEC monolayers. Hence, this consideration may be important in vivo. In addition, in the malignancy, epithelial cells might be a source of HGF and could be stimulated by a paracrine factor released by FBs. However, under our experimental conditions, we did not observe any HGF secretion by AECs, and they did not express the mRNA for HGF (Fig. 3B).
We previously reported that coculturing rat AMs with rat ATII cells stimulated DNA synthesis in the epithelial cells (31). However, in our current study, we observed that human AMs did not alter wound closure in a human AEC monolayer in our cocultures. A likely reason for this difference is because HGF mRNA and protein were not detected in human AMs, whereas HGF mRNA is expressed in rat AMs (34). Hence we believe that there is a significant species difference in expression of HGF in AMs.
The HGF/c-Met pathway plays important roles in the embryonic development of the liver and the placenta, in the migration of myogenic precursor cells, and in epithelial morphogenesis (40). HGF is a mitogen for rat ATII cells in vitro (34), and HGF stimulates both proliferation and migration of immortalized rat ATII cells (SV40-T2 cells) (18). HGF increases cell migration but not proliferation during human nasal epithelial repair in vitro (66). These results suggest that epithelial cells from different species and locations display different responses to HGF (9). Because the effect of HGF on primary human AECs has not been reported, in this study, we examined whether human primary AECs, which were in the middle of transdifferentiation from ATII to ATI-like cells, could migrate and/or proliferate during the wound closure. We have found that HGF enhances cell motility but not proliferation at the wound edge at 24 h and 48 h after wounding. However, HGF may stimulate proliferation at later time points.
A complex interplay between the actin cytoskeleton and cell adhesion sites is required for the generation of membrane protrusions such as lamellipodia (7, 37). Focal adhesion sites are complex and include structural proteins such as talin, paxillin, and vinculin and signaling proteins such as Src and FAK. HGF increases phosphorylation of Src and FAK and enhanced expression level of vinculin in the human prostate epithelial cell line PNT1A (45). Activation of HGF receptor (c-Met) in epithelial cells also results in lamellipodia protrusion, spreading, migration, and tubular formation through ERK, FAK, and Rac activation (23). We found that lamellipodia formation was enhanced by HGF in young subjects and were able to demonstrate increased phosphorylation of FAK and ERK. However, we did not observe activation of Src (Fig. 5C) and did not observe enhanced expression of vinculin (data not shown) by HGF. Therefore, HGF is likely to enhance lamellipodia formation in the human AEC monolayer through FAK and ERK, but the details of how HGF enhances lamellipodia formation in human AEC monolayers remain to be determined.
The incidence rates and mortality of ALI/ARDS increase dramatically with age (50, 52), and animal models of ALI showed higher mortality and increased severity with age (24, 36, 48, 49). Although the importance of the ATII cells as progenitor cells for restoring the alveolar epithelium after alveolar damage is well documented, alterations of ATII cell function with age have not been studied. In this study. we showed that wound closure was delayed and the size of lamellipodia was decreased in AECs from elderly subjects. We also observed less peripheral distribution of vinculin in human AECs from the elderly (Fig. 6B). These results are similar to those reported for tendon FBs from elderly subjects (2). HGF restored the delay in wound closure in the elderly without a detectable change in lamellipodia formation (Fig. 6D). Arnesen et al. (2) demonstrated that Achilles tendon FBs from old mice have lower motility and proliferation, a disorganized actin cytoskeleton, and a different localization of key focal adhesion proteins such as FAK, talin, and paxillin compared with the same cells from young mice. In addition, FBs from the old mice had increased expression of an endoplasmic reticulum (ER) stress marker, GADD153 (2). Aged satellite cells, which carry out repair and maintenance of skeletal muscle as a resident stem cell population, migrate at less than half the speed of young cells (6). In this report, although HGF increased the rate of aged satellite cell movement, it did not alter the size of blebs on old satellite cells, which are similar to lamellipodia. Therefore, these reports support our results in human AECs. However, we did not observe increased ER stress markers such as GADD153, Bip, and ATF6, decreased phosphorylation of FAK and Src, or downregulated protein levels of vinculin and talin from whole cell lysates in the elderly (data not shown). Additional studies will be needed to define the mechanism of delayed cell motility in the elderly and how HGF restores the delayed wound repair without enhancement of lamellipodia formation. We observed cell sheet movement to close wound in AECs when we measured cell travel distance (cell motility) by time-lapse imaging. Fenteany et al. (17) have shown two distinct mechanisms to account for wound closure in epithelial cell sheets. One mechanism of epithelial sheet wound closure is the protrusion of filopodia and ruffling lamellae, which occurs at the edge of wound (the first row of cells at the wound edge), resembling the crawling behavior of the free cells. Another mechanism is the contribution of cells behind the wound margin, which are called submarginal cells, push in, and close the wound. Thus, although we could not observe the detectable change in the size of lamellipodia by HGF in the elderly, HGF might activate submarginal cells, move cells deeper in the monolayer, and lead to the restoration of the delayed wound closure in the elderly.
Mechanical ventilation in ALI/ARDS is lifesaving but also can contribute to lung injury in a process termed VILI (43, 52). Previously, Crosby et al. (8,9) reported that mechanical stretch by CS slowed wound repair and caused apoptosis and gap formation in rat AEC monolayers. KGF has been known to accelerate wound closure in airway epithelial cell lines during cyclic mechanical strain (61). In our study, we have confirmed that CS delayed wound closure and that HGF restored the delay in human AEC monolayers. Previously, ATII cells isolated from rats exposed to hyperoxia and high-tidal-volume mechanical ventilation exhibited significantly decreased cell adhesion and reduced phosphorylation of FAK and the protein levels of paxillin compared with control rats (10). CS also decreased cell migration in human airway cell line 16HBE by inhibiting PI3 kinase and FAK-mediated JNK activation (11). Although wound closure was delayed by CS, we did not observe decreased phosphorylation of FAK and Src or decreased protein levels of vinculin and talin by CS (data not shown). One reason for this variance could be because we used a lower level of CS (10%, 6 cycle/min) compared with the previous report (20%, 30 cycle/min), as higher levels of CS caused our cells to detach from the membrane.
In summary, we have shown that FBs enhanced wound closure in the human AEC monolayer through the HGF/c-Met pathway and that HGF promoted wound closure by accelerating cell motility but not proliferation. We have also found that wound closure was delayed with age and CS, which were restored by HGF. These findings suggest that HGF would be a potential treatment for repairing the denuded areas of alveolar epithelium during acute lung injury. Additional studies are required to determine the mechanism of how HGF enhances the wound closure in the elderly and during CS.
GRANTS
This work was supported by grants from the National Institutes of Health (HL 106112), the ExxonMobil Foundation, and National Jewish Health Basic Science Section Microfunding grants awarded in 2011 and 2012.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.I., J.H.F., and R.J.M. conception and design of research; Y.I., K.C., and J.A.S. performed experiments; Y.I. and K.C. analyzed data; Y.I., J.H.F., R.P., and R.J.M. interpreted results of experiments; Y.I. prepared figures; Y.I. drafted manuscript; Y.I., K.C., J.A.S., J.H.F., R.P., and R.J.M. edited and revised manuscript; Y.I., K.C., J.A.S., J.H.F., R.P., and R.J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Karen E. Edeen, Beata Kosmider, Elise Messier, and Jieru Wang for assistance with human ATII isolations, Elizabeth Redente for assistance with human FBs isolations, Daniel LaFlamme for assistance with analysis of Quantibody Human Growth Factor Array, and Jeffrey Kern for discussion of this project. Finally, we thank Teneke M. Warren and Sarah Murrell for assistance with manuscript preparation.
REFERENCES
- 1.Adams DH, McIntosh D, Wormald PJ, Cowin AJ. Differential effects of insulin-like growth factors on scratch wound repair in respiratory epithelial cells. Am J Rhinol 20: 652–657, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Arnesen SM, Lawson MA. Age-related changes in focal adhesions lead to altered cell behavior in tendon fibroblasts. Mech Ageing Dev 127: 726–732, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123: 3025–3036, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, Vu TH. Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest 121: 2855–2862, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chesnutt AN, Kheradmand F, Folkesson HG, Alberts M, Matthay MA. Soluble transforming growth factor-alpha is present in the pulmonary edema fluid of patients with acute lung injury. Chest 111: 652–656, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Collins-Hooper H, Woolley TE, Dyson L, Patel A, Potter P, Baker RE, Gaffney EA, Maini PK, Dash PR, Patel K. Age-related changes in speed and mechanism of adult skeletal muscle stem cell migration. Stem Cells 30: 1182–1195, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Craig SW, Chen H. Lamellipodia protrusion: moving interactions of vinculin and Arp2/3. Curr Biol 13: R236–R238, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Crosby LM, Luellen C, Zhang Z, Tague LL, Sinclair SE, Waters CM. Balance of life and death in alveolar epithelial type II cells: proliferation, apoptosis, and the effects of cyclic stretch on wound healing. Am J Physiol Lung Cell Mol Physiol 301: L536–L546, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 298: L715–L731, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai LP, Sinclair SE, Chapman KE, Hassid A, Waters CM. High tidal volume mechanical ventilation with hyperoxia alters alveolar type II cell adhesion. Am J Physiol Lung Cell Mol Physiol 293: L769–L778, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Desai LP, White SR, Waters CM. Cyclic mechanical stretch decreases cell migration by inhibiting phosphatidylinositol 3-kinase- and focal adhesion kinase-mediated JNK1 activation. J Biol Chem 285: 4511–4519, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am J Pathol 70: 175–198, 1973 [PMC free article] [PubMed] [Google Scholar]
- 13.Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol 290: L242–L249, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Farkas L, Farkas D, Gauldie J, Warburton D, Shi W, Kolb M. Transient overexpression of Gremlin results in epithelial activation and reversible fibrosis in rat lungs. Am J Respir Cell Mol Biol 44: 870–878, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res 2: 33–46, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felder M, Sallin P, Barbe L, Haenni B, Gazdhar A, Geiser T, Guenat O. Microfluidic wound-healing assay to assess the regenerative effect of HGF on wounded alveolar epithelium. Lab Chip 12: 640–646, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Fenteany G, Janmey PA, Stossel TP. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr Biol 10: 831–838, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Furuyama A, Mochitate K. Hepatocyte growth factor inhibits the formation of the basement membrane of alveolar epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 286: L939–L946, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Galiacy S, Planus E, Lepetit H, Fereol S, Laurent V, Ware L, Isabey D, Matthay M, Harf A, d'Ortho MP. Keratinocyte growth factor promotes cell motility during alveolar epithelial repair in vitro. Exp Cell Res 283: 215–229, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Gorissen SH, Hristova M, Habibovic A, Sipsey LM, Spiess PC, Janssen-Heininger YM, van der Vliet A. Dual oxidase-1 is required for airway epithelial cell migration and bronchiolar reepithelialization after injury. Am J Respir Cell Mol Biol 48: 337–345, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horowitz JC, Cui Z, Moore TA, Meier TR, Reddy RC, Toews GB, Standiford TJ, Thannickal VJ. Constitutive activation of prosurvival signaling in alveolar mesenchymal cells isolated from patients with nonresolving acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 290: L415–L425, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu C, Ding Y, Chen J, Liu D, Zhang Y, Ding M, Wang G. Basic fibroblast growth factor stimulates epithelial cell growth and epithelial wound healing in canine corneas. Vet Ophthalmol 12: 170–175, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Ishibe S, Joly D, Liu ZX, Cantley LG. Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol Cell 16: 257–267, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Ito Y, Betsuyaku T, Nagai K, Nasuhara Y, Nishimura M. Expression of pulmonary VEGF family declines with age and is further down-regulated in lipopolysaccharide (LPS)-induced lung injury. Exp Gerontol 40: 315–323, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA 99: 11946–11950, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jing J, Tarbutton E, Wilson G, Prekeris R. Rab11-FIP3 is a Rab11-binding protein that regulates breast cancer cell motility by modulating the actin cytoskeleton. Eur J Cell Biol 88: 325–341, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kangsamaksin T, Morris RJ. Bone morphogenetic protein 5 regulates the number of keratinocyte stem cells from the skin of mice. J Invest Dermatol 131: 580–585, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Kheradmand F, Folkesson HG, Shum L, Derynk R, Pytela R, Matthay MA. Transforming growth factor-α enhances alveolar epithelial cell repair in a new in vitro model. Am J Physiol Lung Cell Mol Physiol 267: L728–L738, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Kosaka N, Kodama M, Sasaki H, Yamamoto Y, Takeshita F, Takahama Y, Sakamoto H, Kato T, Terada M, Ochiya T. FGF-4 regulates neural progenitor cell proliferation and neuronal differentiation. FASEB J 20: 1484–1485, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, Wang de Y, Lim B, Chow VT, Crum CP, Xian W, McKeon F. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147: 525–538, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leslie CC, McCormick-Shannon K, Cook JL, Mason RJ. Macrophages stimulate DNA synthesis in rat alveolar type II cells. Am Rev Respir Dis 132: 1246–1252, 1985 [DOI] [PubMed] [Google Scholar]
- 32.Marshall RP, Bellingan G, Webb S, Puddicombe A, Goldsack N, McAnulty RJ, Laurent GJ. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med 162: 1783–1788, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Mason RJ. Biology of alveolar type II cells. Respirology 11, Suppl: S12–S15, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Mason RJ, Leslie CC, McCormick-Shannon K, Deterding RR, Nakamura T, Rubin JS, Shannon JM. Hepatocyte growth factor is a growth factor for rat alveolar type II cells. Am J Respir Cell Mol Biol 11: 561–567, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Matsuoka H, Sisson TH, Nishiuma T, Simon RH. Plasminogen-mediated activation and release of hepatocyte growth factor from extracellular matrix. Am J Respir Cell Mol Biol 35: 705–713, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McConnell KW, Fox AC, Clark AT, Chang NY, Dominguez JA, Farris AB, Buchman TG, Hunt CR, Coopersmith CM. The role of heat shock protein 70 in mediating age-dependent mortality in sepsis. J Immunol 186: 3718–3725, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6: 56–68, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Moura J, da Silva L, Cruz MT, Carvalho E. Molecular and cellular mechanisms of bone morphogenetic proteins and activins in the skin: potential benefits for wound healing. Arch Dermatol Res 305: 557–569, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Myerburg MM, Latoche JD, McKenna EE, Stabile LP, Siegfried JS, Feghali-Bostwick CA, Pilewski JM. Hepatocyte growth factor and other fibroblast secretions modulate the phenotype of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 292: L1352–L1360, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Nakamura T, Sakai K, Matsumoto K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J Gastroenterol Hepatol 26, Suppl 1: 188–202, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Naldini L, Weidner KM, Vigna E, Gaudino G, Bardelli A, Ponzetto C, Narsimhan RP, Hartmann G, Zarnegar R, Michalopoulos GK. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J 10: 2867–2878, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nickel N, Jonigk D, Kempf T, Bockmeyer CL, Maegel L, Rische J, Laenger F, Lehmann U, Sauer C, Greer M, Welte T, Hoeper MM, Golpon HA. GDF-15 is abundantly expressed in plexiform lesions in patients with pulmonary arterial hypertension and affects proliferation and apoptosis of pulmonary endothelial cells. Respir Res 12: 62, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nin N, Lorente JA, De Paula M, Fernandez-Segoviano P, Penuelas O, Sanchez-Ferrer A, Martinez-Caro L, Esteban A. Aging increases the susceptibility to injurious mechanical ventilation. Intensive Care Med 34: 923–931, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Panos RJ, Rubin JS, Csaky KG, Aaronson SA, Mason RJ. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J Clin Invest 92: 969–977, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavone LM, Cattaneo F, Rea S, De Pasquale V, Spina A, Sauchelli E, Mastellone V, Ammendola R. Intracellular signaling cascades triggered by the NK1 fragment of hepatocyte growth factor in human prostate epithelial cell line PNT1A. Cell Signal 23: 1961–1971, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Quesnel C, Marchand-Adam S, Fabre A, Marchal-Somme J, Philip I, Lasocki S, Lecon V, Crestani B, Dehoux M. Regulation of hepatocyte growth factor secretion by fibroblasts in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 294: L334–L343, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Radtke S, Milanovic M, Rosse C, De Rycker M, Lachmann S, Hibbert A, Kermorgant S, Parker PJ. ERK2 but not ERK1 mediates HGF-induced motility in non-small cell lung carcinoma cell lines. J Cell Sci 126: 2381–2391, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Roberts A, Paddock C, Vogel L, Butler E, Zaki S, Subbarao K. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J Virol 79: 5833–5838, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rockx B, Baas T, Zornetzer GA, Haagmans B, Sheahan T, Frieman M, Dyer MD, Teal TH, Proll S, van den Brand J, Baric R, Katze MG. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J Virol 83: 7062–7074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89: 309–319, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Siner JM, Pisani MA. Mechanical ventilation and acute respiratory distress syndrome in older patients. Clin Chest Med 28: 783–791, vii, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Sirianni FE, Chu FS, Walker DC. Human alveolar wall fibroblasts directly link epithelial type 2 cells to capillary endothelium. Am J Respir Crit Care Med 168: 1532–1537, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Stern JB, Fierobe L, Paugam C, Rolland C, Dehoux M, Petiet A, Dombret MC, Mantz J, Aubier M, Crestani B. Keratinocyte growth factor and hepatocyte growth factor in bronchoalveolar lavage fluid in acute respiratory distress syndrome patients. Crit Care Med 28: 2326–2333, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol 6: 235–243, 1992 [DOI] [PubMed] [Google Scholar]
- 56.Tsushima K, King LS, Aggarwal NR, De Gorordo A, D'Alessio FR, Kubo K. Acute lung injury review. Intern Med 48: 621–630, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Verghese GM, McCormick-Shannon K, Mason RJ, Matthay MA. Hepatocyte growth factor and keratinocyte growth factor in the pulmonary edema fluid of patients with acute lung injury. Biologic and clinical significance. Am J Respir Crit Care Med 158: 386–394, 1998 [DOI] [PubMed] [Google Scholar]
- 58.Wang J, Edeen K, Manzer R, Chang Y, Wang S, Chen X, Funk CJ, Cosgrove GP, Fang X, Mason RJ. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am J Respir Cell Mol Biol 36: 661–668, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wansleeben C, Barkauskas CE, Rock JR, Hogan BL. Stem cells of the adult lung: their development and role in homeostasis, regeneration, and disease. Wiley Interdiscip Rev Dev Biol 2: 131–148, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol 282: L924–L940, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Waters CM, Savla U. Keratinocyte growth factor accelerates wound closure in airway epithelium during cyclic mechanical strain. J Cell Physiol 181: 424–432, 1999 [DOI] [PubMed] [Google Scholar]
- 62.Weidner KM, Arakaki N, Hartmann G, Vandekerckhove J, Weingart S, Rieder H, Fonatsch C, Tsubouchi H, Hishida T, Daikuhara Y, et al. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci USA 88: 7001–7005, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yano T, Mason RJ, Pan T, Deterding RR, Nielsen LD, Shannon JM. KGF regulates pulmonary epithelial proliferation and surfactant protein gene expression in adult rat lung. Am J Physiol Lung Cell Mol Physiol 279: L1146–L1158, 2000 [DOI] [PubMed] [Google Scholar]
- 64.Yin J, Xu K, Zhang J, Kumar A, Yu FS. Wound-induced ATP release and EGF receptor activation in epithelial cells. J Cell Sci 120: 815–825, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin J, Yu FS. ERK1/2 mediate wounding- and G-protein-coupled receptor ligands-induced EGFR activation via regulating ADAM17 and HB-EGF shedding. Invest Ophthalmol Vis Sci 50: 132–139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zahm JM, Debordeaux C, Raby B, Klossek JM, Bonnet N, Puchelle E. Motogenic effect of recombinant HGF on airway epithelial cells during the in vitro wound repair of the respiratory epithelium. J Cell Physiol 185: 447–453, 2000 [DOI] [PubMed] [Google Scholar]