Abstract
Background and aim
Serum calprotectin is elevated in patients with inflammatory bowel disease (IBD). Whether it correlates other markers of disease activity is unknown. The aim of this study was to correlate serum calprotectin with biochemical and histological measures of intestinal inflammation.
Materials and methods
TNBS colitis was induced in wistar rats, and serial blood samples were collected at 0, 3, and 12 days. Animals were subsequently sacrificed for pathological evaluation at day 12. Serum calprotectin and cytokines were measured by ELISA. Pathologic changes were classified at the macroscopic and microscopic levels.
Results
TNBS colitis induced elevated serum calprotectin, TNF and IL-6 within 24 h. Levels of serum calprotectin remained elevated in parallel to persistence of loose stool and weight loss to day 12. Serum calprotectin levels correlated with serum levels of TNF-α and IL6 (p < 0.001), but not CRP. Animals with liquid stool had significantly higher levels of serum calprotectin than control animals. There was a correlation between macroscopic colitis scores, and levels of serum calprotectin.
Conclusion
Serum calprotectin levels correlate with biochemical and histological markers of inflammation in TNBS colitis. This biomarker may have potential for diagnostic use in patients with IBD.
Keywords: TNBS, C-reactive protein, Inflammatory marker, Mucosal scores, Serum calprotectin
1. Introduction
Inflammatory bowel disease (IBD) is characterized by mucosal inflammation in the intestinal tract and this process is strongly associated with an individual’s disease course and risk of complications [1,2]. Patients who obtain mucosal healing have been associated with lower relapse rates and lower complication rates within the IBD population [2,3]. Colonoscopy with tissue biopsy is currently the gold-standard to investigate a patient’s level or extent of mucosal inflammation, especially in measuring response to therapy. However, colonoscopies cannot always be performed in a timely manner, and colonoscopies are expensive, invasive, and require a pathologist for biopsy analyses [4]. Other methods such as CT, MRI or capsule endoscopy also carry significant costs, and are associated with risks such as radiation exposure, reactions to contrast, or obstruction [5–9].
Serum biomarkers that correlate with intestinal inflammation have been sought-after as a minimally invasive alternative to these tests. C-reactive protein levels have been used as a marker of active Crohn’s, and a return to a normal levels can indicate mucosal healing [10–12]. Although this test is widely used, studies have shown that the level of this protein does not always parallel disease activity, as a number of other conditions can lead to elevated CRP levels in patients with inactive IBD [13]. Use of CRP as threshold for entry into clinical trials of therapies for IBD has not significantly reduced the placebo rates, suggesting elevated levels alone are not a sensitive marker of intestinal inflammation [13,14].
Calprotectin is a marker of neutrophil activation that has been reported to be elevated in the blood and feces of patients with active IBD [15,16]. In particular, fecal calprotectin has been studied as a potential biomarker for IBD due to its non-invasive nature and low cost [17,18]. In contrast, little has been published regarding levels of serum calprotectin in colitis, and whether they correlate with other markers of inflammation. The optimal biomarker in IBD would be performed at the time of the medical visit and clearly reflect the expression of inflammatory markers and the state of the bowel mucosa. There is increasing interest in such a biomarker in IBD, particularly to identify patients with active inflammation, versus those with mucosal healing [19]. It is unknown whether serum calprotectin also exhibits these properties.
The purpose of this study was to determine whether serum calprotectin could be used as a marker of intestinal inflammation, both microscopic and macroscopic, in an animal model of colitis. We also sought to compare serum calprotectin to other markers of intestinal inflammation, such as CRP, TNF and IL-6.
2. Materials and methods
Colitis was induced with TNBS in male Wistar rats weighing 240 to 280 g according to the model proposed by Morris et al. [20]. The animals were allowed to adjust to the laboratory, given water and food, and subjected to a 12-h sleep-wake cycle. Three days later, the animals were subjected to TNBS-induced colitis (n = 5). The control group was administered a saline solution and followed in parallel (n = 5).
2.1. Induction of experimental colitis
Colitis was induced after the animals were anesthetized with ketamine (100 mg/mL and 0.3 mL/animal), and the animals in the TNBS group were given 2,4,6-nitrobenzenesulfonic acid (Sigma) via intracolonic instillation at a concentration of 25 mg TNBS (0.6 mL) dissolved in 0.25 mL of 50% ethanol, which was injected into the colon 8 cm from the anal margin using PE-50 tubing. Next, the animals were placed on a 90-degree plank for approximately 20 min. The control group was administered a physiological saline solution via the same method that was used for the TNBS group.
2.2. Assessment of inflammatory markers
The inflammatory (TNF-α and IL-6) and activity (serum calprotectin and C-reactive protein) markers were assessed 1 h after the administration of TNBS or saline (day 0). The serum samples were collected on day 3 and day 12, and the animals were sacrificed on day 12.
To determine the serum concentrations of IL-6, TNF-α and calprotectin, specific ELISA kits were used. These included the IL-6 rat ELISA kit from BD Pharmingen (sensitivity level, 3.8 pg/mL), the TNF-α rat ELISA kit from BD Pharmingen (sensitivity, 13 pg/mL), and the calprotectin ELISA kit from Immunodiagnosis SA (1009/SA1009/Sa 1008 kit 6935; sensitivity, 0.25 ng/mL). The C-reactive protein level was measured using an immunoturbidimetry method and the ultra-sensitive C-reactive protein AU600 kit from Olympus (sensitivity level, 0.53 to 18.08 mg/dL).
2.3. Macroscopic and microscopic assessment
The animals were sacrificed on day 12 after the serum samples had been collected. The colons were removed, opened, and stained with Evans blue. Three different observers established the macroscopic score by using a magnifying glass, and these evaluations are described in Fig. 1.
Fig. 1.
TNBS colitis leads to systemic evidence of inflammation in treated animals. (A) Percent change in mean body weight between day 0 and day 12 in saline (clear column) and TNBS-treated (colored column) animals. *p > 0.05 by two-sided t test. (B) Microscopy image on H&E-stained section of rat intestine from TNBS-treated animal (40×). (C) Mean serum TNF (13 pg/ml) in saline-treated (squares) and TNBS-treated (triangles) animals at various time-points after treatment. *p > 0.05 by two-sided t test. (D) Mean serum IL-6 (3.8 pg/ml) in saline-treated (squares) and TNBS-treated (triangles) animals at various time-points after treatment. *p > 0.05 by two-sided t test.
The most heavily damaged tissue sites were selected for staining. These samples were fixed in 10% formalin and then subjected to paraffin and hematoxylin-eosin staining. Two different pathologists examined and scored the samples, as described in Fig. 2.
Fig. 2.
Serum calprotectin levels correlate with biochemical markers of colitis. (A) Mean serum calprotectin (0.25 ng/ml) in saline-treated (squares) and TNBS-treated (triangles) animals at various time-points after treatment. *p > 0.05 by two-sided t test. (B) Correlation between serum calprotectin (x-axis) and serum TNF (Y-axis) in animals treated with saline or TNBS. Straight line represents linear regression slope for correlation of x and y variables. (C) Correlation between serum calprotectin (x-axis) and serum IL-6 (Y-axis) in animals treated with saline or TNBS. Straight line represents linear regression slope for correlation of x and y variables. (D) Correlation between serum calprotectin (x-axis) and serum CRP (Y-axis) in animals treated with saline or TNBS. Straight line represents linear regression slope for correlation of x and y variables.
2.4. Statistical methods
The data presented are summarised in frequency tables or as means, standard deviations, ranges, or proportions, as indicated. The baseline data are expressed as the means ± standard deviations. The follow-up measurements of the continuous variables are summarised as absolute values and absolute and percent changes, which are given with respect to the baseline. The dichotomous variables were summarised in frequency tables or as means and proportions.
For the repeated measurements, an ANOVA was used to compare the outcome levels of interest between the time-points, and p-values of <0.05 were considered to be significant. For this purpose, Fisher’s test of least-squares differences was used when it was allowed by the F-test. Student’s t-test was used for pairwise comparisons with Bonferroni corrections. The data not normally distributed were analysed using non-parametric tests. For these tests, p-values of <0.05 were considered to be significant. The chi-squared test or Fisher’s exact test was used to analyse the categorical data. The data were analysed using JMP software (SAS, Cary, NC).
2.5. Ethics committee
This study protocol (number 2444) was approved by the ethics committee of the Federal University of São Paulo (UNIFESP) on June 17, 2009. Each of the research stages followed the Guide for the Care and Use of Laboratory Animals from the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council (National Academy Press, Washington, DC, 1996).
3. Results
Introduction of TNBS led to significant weight loss (mean 10% loss in body weight) and development of liquid bloody stools in all exposed animals, whereas control (saline) animals remained healthy and gained weight (mean 30% gain over 12 days) (Fig. 1A). At the time of sacrifice on Day 12, all TNBS-treated animals have microscopic and macroscopic evidence of colitis (Fig. 1B). In the serum compartment, levels of TNF and IL-6 were significantly higher in TNBS-treated than control animals 24 h after induction of TNBS colitis, but declined by days 3 and 12 (Fig. 1C and D).
In contrast, serum calprotectin levels were significantly elevated after TNBS, and remained elevated on day 3 and day 12, in parallel to objective evidence of disease in the animals (Fig. 2A). Levels were threefold higher (mean 21,786 ng/mL than those measured in saline-treated animals (mean 4032). A linear correlation was observed between the serum levels of calprotectin and those of TNF-α (r2 0.7, p < 0.001) and IL-6 (r2 0.79, p < 0.0001) in saline and TNBS-treated animals on Day 3 (Fig. 2B and C). There was no correlation between serum calprotectin and CRP (r2 0.1, p = 0.06) (Fig. 2D).
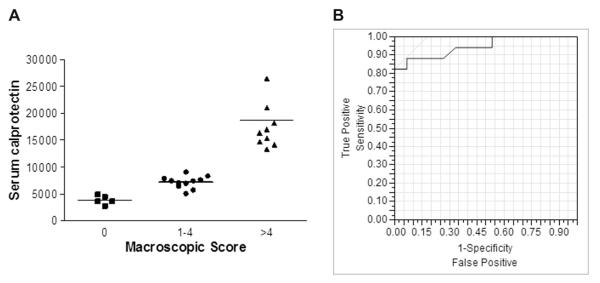
When grouped according to clinical signs of colitis, animals with liquid stool had significantly higher serum calprotectin levels than those with normal stool consistency (Fig. 3A). Similarly, animals with intestinal macroscopic damage scores >4 had higher mean serum calprotectin levels than animals with no damage (saline) or mild damage (scores 1–4) (Fig. 3B). When an ROC curve was plotted for serum calprotectin for macroscopic damage (score >2), the area under the curve value was 0.94, with a serum level of 9500 being the optimal cut-off value in TNBS colitis (Fig. 3).
Fig. 3.
Serum calprotectin reflects clinical and endoscopic markers of colitis. (A) Serum calprotectin levels grouped according to stool score on Day 3 (0 = normal, 1 = liquid) in animals treated with saline or TNBS (horizontal line = mean). (B) Serum calprotectin levels in individual animals grouped according to stool score on Day 3 (0 = normal, 1 = liquid) in animals treated with saline or TNBS (horizontal line = mean). ROC curve for characteristics of serum calprotectin to identify macroscopic inflammation score > 2 in TNBS-treated animals.
4. Discussion
This study has demonstrated that TNBS-induced colitis provokes elevations in serum calprotectin that correlate closely to biochemical and endoscopic measures of colitis. Unlike TNF and IL-6, which declined after the acute phase, this marker remained elevated with persistent inflammation in the intestinal tract.
Calprotectin is a Toll-like receptor-4 (TLR4) ligand expressed by neutrophils, monocytes, and early differentiated macrophages [21]. These cell populations are all elevated in the peripheral blood of patients with ulcerative colitis (UC), secrete pro-inflammatory cytokines, and correlate with levels of intestinal inflammation [22,23]. Serum levels of calprotectin are elevated in some inflammatory conditions, and selective removal of peripheral blood activated granulocytes and monocytes/macrophages leads to a decline in fecal calprotectin levels in patients with UC [24,25]. Some studies have demonstrated that calprotectin in the stool can be a good marker to evaluate the activity of the disease as well as predict its severity [25,26]. This study has demonstrated that measuring calprotectin in serum may provide a more practical means for measuring this biomarker in practice. Not only did it correlate with stool output, and serum biochemistry, it also exhibited high correlation with endoscopic scores in this model. This is a key characteristic in future markers for patients with IBD, where markers of mucosal healing will be required [27]. Future studies in this model would require a comparison between serum and fecal calprotectin levels to determine which correlates highly with intestinal inflammation.
In recent years, TNF-α, IL-1, and IL-6, have been attributed increasingly important roles in the physiopathology of inflammatory diseases [28]. It is believed that increased levels and excessive synthesis of these mediators result in a loss of bowel homeostasis, which leads to significant disequilibrium and directly contributes to disease development. The results of this study have confirmed the participation of TNF-α and IL-6 in TNBS-induced inflammation, which has been previously described [29]. However, we have demonstrated that both cytokines decline by day 12 in this model, despite on-going evidence of histologic and endoscopic inflammation. This may explain why neither have been adopted as markers of disease activity in patients with IBD, as many Crohn’s disease patients exhibit normal serum levels of these mediators [30,31].
C-reactive protein (CRP) is also used in clinical practice as a marker of intestinal inflammation in IBD. However, no correlation was found when comparing the serum calprotectin and C-reactive protein levels in our studies (Fig. 3). These findings confirm what other groups have found with respect to CRP and inflammation in patients with ulcerative colitis [32]. This may have been due to the fact that the C-reactive protein levels peak at the beginning of the disease process and then decrease rapidly. Alternatively, this may have been due to genetic polymorphism, which has been repeatedly described in humans [33,34] the microscopic and macroscopic disease scores were very closely related to the serum calprotectin levels, which suggest that this protein reflects the state of the bowel mucosa. In medical practice, a real-time assessment of disease activity during a clinical visit has paramount importance for deciding which therapeutic strategy or diagnostic imaging technique to employ (e.g., colonoscopy). Our results indicate that the serum levels of calprotectin could be measured during routine laboratory tests, as a marker of underlying intestinal inflammation. This measurement has also been shown to be related to the stages of inflammatory bowel disease, based on the expression of the inflammatory markers IL-6 and TNF- α and the state of the bowel mucosa. Further study in humans with IBD would be required to confirm this data.
5. Conclusion
Serum calprotectin levels exhibited a direct correlation with the levels of the chemical mediators of inflammation but did not correlate with the levels of C-reactive protein. Serum calprotectin levels were also shown to have a direct relationship with the macroscopic and microscopic disease scores.
Acknowledgments
The authors would like to thank Ms. Fatima Lombardi and Professor Dr Marcelo S Cury.
ACM is supported by NIH Grant K23DK084338.
References
- [1].Froslie KF, Jahnsen J, Moum BA, Vatn MH. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology [Multicenter Study Research Support, Non-U.S. Gov’t] 2007 Aug;133(2):412–422. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- [2].Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology [Multicenter Study Research Support, Non-U.S. Gov’t] 2010 Feb;138(2):463–468. doi: 10.1053/j.gastro.2009.09.056. quiz e10–e11. [DOI] [PubMed] [Google Scholar]
- [3].Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm. Bowel Dis. [Research Support, Non-U.S. Gov’t] 2009 Sep;15(9):1295–1301. doi: 10.1002/ibd.20927. [DOI] [PubMed] [Google Scholar]
- [4].Loftus EV., Jr. Clinical perspectives in Crohn’s disease. Objective measures of disease activity: alternatives to symptom indices. Rev. Gastroenterol. Disord. 2007;7(Suppl. 2):S8–S16. [Review] [PubMed] [Google Scholar]
- [5].Friedrich C, Fajfar A, Pawlik M, Hoffstetter P, Rennert J, Agha A, et al. Magnetic resonance enterography with and without biphasic contrast agent enema compared to conventional ileocolonoscopy in patients with Crohn’s disease. Inflamm. Bowel Dis. 2012 Jan 9;:1842–1848. doi: 10.1002/ibd.22843. [DOI] [PubMed] [Google Scholar]
- [6].Girlich C, Schacherer D, Jung EM, Klebl F, Huber E. Comparison between quantitative assessment of bowel wall vascularization by contrast-enhanced ultrasound and results of histopathological scoring in ulcerative colitis. Int. J. Colorectal Dis. [Clinical Trial Comparative Study] 2012 Feb;27(2):193–198. doi: 10.1007/s00384-011-1300-y. [DOI] [PubMed] [Google Scholar]
- [7].Koulaouzidis A, Douglas S, Plevris JN. Lewis score correlates more closely with fecal calprotectin than capsule endoscopy Crohn’s disease activity index. Dig. Dis. Sci. 2012 Apr;57(4):987–993. doi: 10.1007/s10620-011-1956-8. [DOI] [PubMed] [Google Scholar]
- [8].Feuerbach S. MRI enterography: the future of small bowel diagnostics? Dig Dis. 2010;28(3):433–438. doi: 10.1159/000320399. [DOI] [PubMed] [Google Scholar]
- [9].Ordas I, Rimola J, Rodriguez S, Gallego M, Ricart E, Panes J. Imaging of the colon in inflammatory bowel disease: ready for prime time? Curr. Drug Targets. 2012 May 28;:1252–1260. doi: 10.2174/138945012802429714. [DOI] [PubMed] [Google Scholar]
- [10].Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm. Bowel Dis. 2004 Sep;10(5):661–665. doi: 10.1097/00054725-200409000-00026. [Review] [DOI] [PubMed] [Google Scholar]
- [11].Schoepfer AM, Vavricka S, Zahnd-Straumann N, Straumann A, Beglinger C. Monitoring inflammatory bowel disease activity: clinical activity is judged to be more relevant than endoscopic severity or biomarkers. J. Crohns Colitis [Research Support, Non-U.S. Gov’t] 2012 May;6(4):412–418. doi: 10.1016/j.crohns.2011.09.008. [DOI] [PubMed] [Google Scholar]
- [12].Card T. Commentary: is CRP ready for use to indicate response or remission with infliximab in Crohn’s? Aliment Pharmacol Ther. [Comment Research Support, Non-U.S. Gov’t] 2012 Apr;35(8):960–961. doi: 10.1111/j.1365-2036.2012.05039.x. [discussion 1–2] [DOI] [PubMed] [Google Scholar]
- [13].Denis MA, Reenaers C, Fontaine F, Belaiche J, Louis E. Assessment of endoscopic activity index and biological inflammatory markers in clinically active Crohn’s disease with normal C-reactive protein serum level. Inflamm. Bowel Dis. [Research Support, Non-U.S. Gov’t] 2007 Sep;13(9):1100–1105. doi: 10.1002/ibd.20178. [DOI] [PubMed] [Google Scholar]
- [14].Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006 Mar;55(3):426–431. doi: 10.1136/gut.2005.069476. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fagerberg UL, Loof L, Lindholm J, Hansson LO, Finkel Y. Fecal calprotectin: a quantitative marker of colonic inflammation in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. [Research Support, Non-U.S. Gov’t Validation Studies] 2007 Oct;45(4):414–420. doi: 10.1097/MPG.0b013e31810e75a9. [DOI] [PubMed] [Google Scholar]
- [16].Sipponen T, Karkkainen P, Savilahti E, Kolho KL, Nuutinen H, Turunen U, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol. Ther. [Research Support, Non-U.S. Gov’t] 2008 Nov 15;28(10):1221–1229. doi: 10.1111/j.1365-2036.2008.03835.x. [DOI] [PubMed] [Google Scholar]
- [17].Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology [Research Support, N.I.H., Extramural Review] 2011 May;140(6):1817–1826. doi: 10.1053/j.gastro.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ricanek P, Brackmann S, Perminow G, Lyckander LG, Sponheim J, Holme O, et al. Evaluation of disease activity in IBD at the time of diagnosis by the use of clinical, biochemical, and fecal markers. Scand. J. Gastroenterol. [Research Support, Non-U.S. Gov’t] 2011 Sep;46(9):1081–1091. doi: 10.3109/00365521.2011.584897. [DOI] [PubMed] [Google Scholar]
- [19].Foell D, Wittkowski H, Roth J. Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut [Research Support, Non-U.S. Gov’t Review] 2009 Jun;58(6):859–868. doi: 10.1136/gut.2008.170019. [DOI] [PubMed] [Google Scholar]
- [20].Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology [Comparative Study Research Support, Non-U.S. Gov’t] 1989 Mar;96(3):795–803. [PubMed] [Google Scholar]
- [21].Edgeworth J, Freemont P, Hogg N. Ionomycin-regulated phosphorylation of the myeloid calcium-binding protein p14. Nature. 1989 Nov 9;342(6246):189–192. doi: 10.1038/342189a0. [DOI] [PubMed] [Google Scholar]
- [22].Nikolaus S, Bauditz J, Gionchetti P, Witt C, Lochs H, Schreiber S. Increased secretion of pro-inflammatory cytokines by circulating polymorphonuclear neutrophils and regulation by interleukin 10 during intestinal inflammation. Gut. 1998 Apr;42(4):470–476. doi: 10.1136/gut.42.4.470. (PubMed PMID: 9616306. Pubmed Central PMCID: 1727082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hanai H, Takeuchi K, Iida T, Kashiwagi N, Saniabadi AR, Matsushita I, et al. Relationship between fecal calprotectin, intestinal inflammation, and peripheral blood neutrophils in patients with active ulcerative colitis. Dig. Dis. Sci. 2004 Sep;49(9):1438–1443. doi: 10.1023/b:ddas.0000042243.47279.87. [DOI] [PubMed] [Google Scholar]
- [24].Andres Cerezo L, Mann H, Pecha O, Plestilova L, Pavelka K, Vencovsky J, et al. Decreases in serum levels of S100A8/9 (calprotectin) correlate with improvements in total swollen joint count in patients with recent-onset rheumatoid arthritis. Arthritis Res. Ther. 2011;13(4):R122. doi: 10.1186/ar3426. [PubMed PMID: 21791097. Pubmed Central PMCID: 3239361] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology [Comparative Study] 2000 Jul;119(1):15–22. doi: 10.1053/gast.2000.8523. [DOI] [PubMed] [Google Scholar]
- [26].Schoepfer AM, Beglinger C, Straumann A, Trummler M, Vavricka SR, Bruegger LE, et al. Fecal calprotectin correlates more closely with the simple endoscopic score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am. J. Gastroenterol. [Research Support, Non-U.S. Gov’t] 2010 Jan;105(1):162–169. doi: 10.1038/ajg.2009.545. [DOI] [PubMed] [Google Scholar]
- [27].Peyrin-Biroulet L, Ferrante M, Magro F, Campbell S, Franchimont D, Fidder H, et al. Results from the 2nd Scientific Workshop of the ECCO. I: Impact of mucosal healing on the course of inflammatory bowel disease. J. Crohns Colitis [Consensus, Development Conference] 2011 Oct;5(5):477–483. doi: 10.1016/j.crohns.2011.06.009. [DOI] [PubMed] [Google Scholar]
- [28].Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011 May;140(6):1756–1767. doi: 10.1053/j.gastro.2011.02.016. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis, Inflamm. Bowel Dis. [Comparative Study Research Support, N.I.H. Extramural Research Support, Non-U.S. Gov’t] 2009 Mar;15(3):341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gustot T, Lemmers A, Louis E, Nicaise C, Quertinmont E, Belaiche J, et al. Profile of soluble cytokine receptors in Crohn’s disease. Gut [Research Support, Non-U.S. Gov’t] 2005 Apr;54(4):488–495. doi: 10.1136/gut.2004.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hyams JS, Treem WR, Eddy E, Wyzga N, Moore RE. Tumor necrosis factor-alpha is not elevated in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 1991 Feb;12(2):233–236. doi: 10.1097/00005176-199102000-00016. [DOI] [PubMed] [Google Scholar]
- [32].Schoepfer AM, Beglinger C, Straumann A, Safroneeva E, Romero Y, Armstrong D, et al. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm. Bowel Dis. 2013 Feb;19(2):332–341. doi: 10.1097/MIB.0b013e3182810066. [DOI] [PubMed] [Google Scholar]
- [33].Thalmaier D, Dambacher J, Seiderer J, Konrad A, Schachinger V, Pfennig S, et al. The +1059G/C polymorphism in the C-reactive protein (CRP) gene is associated with involvement of the terminal ileum and decreased serum CRP levels in patients with Crohn’s disease. Aliment Pharmacol. Ther. [Research Support, Non-U.S. Gov’t] 2006 Oct 1;24(7):1105–1115. doi: 10.1111/j.1365-2036.2006.03093.x. [DOI] [PubMed] [Google Scholar]
- [34].Souza MM, Nascimento JE, Dock-Nascimento DB. Effects of budesonide and probiotics enemas on the systemic inflammatory response of rats with experimental colitis. Acta Cir. Bras. 2007 Mar-Apr;22(Suppl 1):40–45. doi: 10.1590/s0102-86502007000700009. [DOI] [PubMed] [Google Scholar]