Abstract
Since 1976, the US Food and Drug Administration (FDA) has used the premarket approval (PMA) process to approve high-risk medical devices, including implantable cardioverter defibrillators (ICDs), coronary stents, and artificial heart valves. The PMA process is widely viewed as a rigorous evaluation of device safety and effectiveness, though recent recalls—most notably related to underperforming ICD leads—have raised concerns about whether physicians and patients should sometimes be more wary about devices approved via this pathway. The FDA must utilize a “least burdensome” approach to approve new medical devices, and many widely used device models have been approved as supplements to existing PMA-approved devices with limited clinical testing. A recent Supreme Court ruling has made it difficult for patients harmed by unsafe PMA-approved devices to seek damages in court. Cardiologists who utilize high-risk medical devices should be aware that FDA approval of new devices relies on variable levels of evidence and does not necessarily indicate improved effectiveness over existing models. Clinician and patient engagement in post-market surveillance and comparative effectiveness research remains imperative.
Keywords: Medical devices, Premarket approval, US Food and DrugAdministration, Implantable cardioverter defibrillator, Patient safety, High risk, Clinical cardiology
Introduction
Medical devices are widely employed in medicine and serve a particularly integral role in cardiac care. In fact, the use of many types of cardiac devices, including pacemakers and implantable cardioverter-defibrillators (ICDs), has increased substantially in recent years [1, 2]. Despite the importance and pervasive utilization of medical devices, few cardiologists are aware of the pathways through which new devices become available to US patients and the regulatory systems currently in place to evaluate investigational devices for safety and effectiveness.
The Food and Drug Administration (FDA) regulates all medical devices marketed in the US. High-risk devices, a category that includes ICDs, pacemakers, coronary stents, and artificial heart valves, are reviewed at the FDA via the premarket approval (PMA) process. The PMA process is generally regarded as the most rigorous medical device regulatory review process in the world [3–5]. However, recent studies examining the process have raised concerns about the quality of data that FDA experts consider when they review PMA applications [6–8]. In addition, many high-risk cardiac devices are actually approved as PMA “supplements,” or changes to already-approved device models, often without the use of new clinical data to support the altered device design [9••]. Two recently recalled ICD leads – Medtronic Sprint Fidelis and St. Jude Medical Riata – were both approved via the PMA supplement process, during which the FDA did not require clinical data to support their approval [9••, 10•, 11, 12]. These recalled ICD leads were adopted briskly into clinical practice and implanted in hundreds of thousands of patients prior to their recall [10•, 12]. In addition, Zuckerman et al. found that from 2005–2009, the FDA issued 21 high-risk safety recalls for PMA-approved devices, over half of which were cardiovascular devices [13]. For example, the FDA’s 2013 high-risk recall list included two vascular stents and a device used to deploy an atrial septal defect occlusion device [14]. These recalls underscore the importance of device regulation in protecting patient safety as well as the need for clinicians to understand the evidence supporting FDA approval of specific device technologies.
Cardiologists rely on the FDA’s premarket approval process to provide assurance that high-risk devices are safe and effective, and patients depend on their physicians to choose the safest and most effective device models available. In this article, we review the PMA process used to approve high-risk cardiovascular devices, as well as the five different types of PMA supplements used to approve alterations to device design. We summarize the types of data required to approve new high-risk cardiovascular devices and discuss implications of the PMA process for clinicians and patients.
History of Premarket Approval
The FDA’s Regulatory Power
The FDA first received statutory authority to regulate medical devices as part of the 1976 Medical Device Amendments to the federal Food, Drug, and Cosmetic Act [15]. Until that point, medical devices had no official premarket requirements and were subject to state-level oversight via consumer-protection statutes applicable to all commercial products. However, a public health crisis arose when the Dalkon Shield, an intrauterine device originally marketed in 1970 and used by millions of women in the US, was found to be associated with increased risk of pelvic inflammatory disease, sepsis, miscarriage, and death [16]. As with all medical devices at the time, there was no premarket assessment of the Dalkon Shield’s safety or effectiveness [17]. After the Dalkon Shield was ultimately withdrawn from the market in 1974, the episode inspired Congress to centralize medical device regulatory power in the FDA [16, 17].
The Medical Device Amendments enumerated three different regulatory classes of devices based on their risk to patients. Low-risk, or class I, devices (e.g., bandages, stethoscopes) are generally exempt from FDA review, requiring only registration with the agency and adherence to basic FDA standards about good manufacturing practices. Medium-risk, or class II, devices (e.g., blood pressure cuffs, peripheral vascular catheters) most often gain clearance for widespread use based on a finding of “substantial equivalence” to an existing marketed device [18]. This process is commonly referred to as the 510(k) pathway after the applicable section of the Federal Code. A finding of “substantial equivalence” means that the device shares pertinent characteristics with another marketed device that has been safely used by patients and, therefore, does not require additional clinical testing to ensure its safety and effectiveness. The substantial equivalence standard for class II devices has been criticized in recent years for failing to adequately assure device safety and effectiveness [16, 19]. The recent recall of metal-on-metal hip implants has served as an example of the danger in allowing new devices to gain 510(k) clearance based on similarity to previous versions, not based on evidence of safety and effectiveness [19].
High-risk, or class III, devices — those which support or sustain human life, prevent impairment of human health, or present a potential, unreasonable risk of illness or injury—are generally reviewed via the premarket approval (PMA) process [20]. Historically, the FDA has permitted select categories of high-risk devices to gain clearance through the 510(k) pathway rather than requiring PMA approval, a practice that has been criticized in recent years [13, 19]. The FDA has been working to update device classifications to prevent high-risk devices from gaining approval via the substantial equivalence standard, so fewer such devices should be on the US market in the future [21].
The PMA Process
The Medical Device Amendments established the standard of evidence for PMA review, authorizing the FDA to require “reasonable assurance of safety and effectiveness” for new high-risk devices [15]. Similar to the FDA process for approving new drugs, the PMA process requires manufacturers to performpre-clinical and clinical studies before a device can be marketed (Table 1). The FDA was granted permission to request from a device manufacturer any data it considered relevant to providing reasonable assurance of safety and effectiveness.
Table 1.
Comparing US FDA authorization of drugs and devices
| Drugs | High-risk devices | Medium-risk devices | |
|---|---|---|---|
| Year FDA began mandating proof of safety and efficacy | 1962 | 1976 | N/A |
| Authorization process | New drug application (NDA) | Premarket approval (PMA) | 510(k) clearance |
| Standard of evidence | Substantial evidence that the drug will have the effect it purports or is represented to havea | Reasonable assurance of safety and effectiveness | Substantial equivalence to a predicate device |
| Supporting data | Clinical trials and pre-clinical studies | Clinical trials and pre-clinical studies | Pre-clinical studiesb |
| Process for introducing post-authorization changes | Nonec | PMA supplement pathways | New 510(k) clearance |
| Tort liability claims against manufacturer mostly preempted? | No | Yes | No |
Substantial evidence is defined as “adequate and well-controlled investigations, including clinical investigations”
Clinical trials are rarely required as part of the 510(k) process
Changes to the molecular structure of an active ingredient require their own New Drug Application. In some instances, the FDA may approve new formulations of an approved drug based on bioequivalence studies
In the subsequent decades, Congress continued to amend the Food, Drug, and Cosmetic Act to address concerns that over-burdensome regulatory processes were inefficient and preventing new medical devices from reaching the market in a timely manner. For example, while all new high-risk devices were originally required to be reviewed by an independent panels of experts, Congress amended the law in 1990 to allow the FDA to internally review PMA applications in cases in which such panels were not deemed to be necessary [22].
In the 1997 FDA Modernization Act (FDAMA), Congress required the FDA to work with manufacturers to determine “the least burdensome appropriate means of evaluating device effectiveness that would have a reasonable likelihood of resulting in approval”[23]. The FDA released a guidance document in 2002 that described how it intended to implement this “least burdensome” approach while still assuring device safety and effectiveness prior to marketing [24]. The FDA permitted the use of non-clinical data (bench or animal testing) in place of clinical data in limited circumstances, and, when clinical data was needed, urged manufacturers to consider study designs other than randomized controlled trials and the use of surrogate endpoints to shorten study duration [24]. The FDA also indicated that manufacturers could offer to formally collect safety and effectiveness data in the postmarket period to speed premarket review [24]. Finally, the FDA recommended using “information that is available from earlier versions of the same device or from marketing experience with similar devices” as part of a PMA review [24].
Changes to a Device after Approval
After a high-risk device is approved via PMA, manufacturers may wish to make modifications to integral components of the design, such as improving battery life, upgrading software, or revising connectors to ensure compatibility with other devices. Unlike drugs, where any change to the active ingredient would create a new product requiring its own original FDA authorization, incremental alterations to device design are an accepted part of the product life cycle. The 1976 Medical Device Amendments provided no formal pathway for a device manufacturer to submit changes to a PMA-approved device. However, when the FDA published its first set of regulations related to PMA in 1986, it included the ability to supplement the design of existing devices to account for small technological innovations [25]. Congress then codified the PMA supplement process in FDAMA in 1997 [23].
Supplements are permitted through five distinct pathways, with different data requirements for each type [26]. Manufacturers choose which pathway to use for a given device change, though the FDA maintains the right to override this decision if necessary. The most significant changes (so-called “panel-track” supplements) are reviewed by an independent panel of experts and, like original PMA applications, must be supported by new clinical data. Changes requiring a panel-track supplement include new indications for an existing device, such as using a prosthetic heart valve approved for use in the aortic position to also be used in the mitral position [26]. Major changes to a device’s design are approved via a 180-day review pathway, in which preclinical testing is often sufficient to assure safety and effectiveness with “limited confirmatory clinical data” needed in some cases [26]. Such major changes may include altered design (e.g., a thinner, more flexible ICD lead) or new features (e.g., wireless programming capabilities) [26]. Minor design changes, such as a circuitry modification to correct battery problems, may qualify for “real-time” review, in which the FDA meets with a manufacturer and issues a same-day decision [26]. To qualify for real-time review, a change must not require any new clinical data to assure safety and effectiveness [27].
Changes to a device’s manufacturing that do not alter the device itself (e.g., converting a manual manufacturing process to an automated one) require notice to the FDA [26]. The manufacturer may enact the manufacturing change 30 days after the FDA receives the notice, unless the FDA deems the notice inadequate and requests a more formal 135-day review. Finally, changes to a device’s labeling to address a newly discovered safety concern (e.g., adding a contraindication) can be approved via a “special supplement” process, during which the manufacturer may enact the change prior to formal FDA approval [26].
User Fees
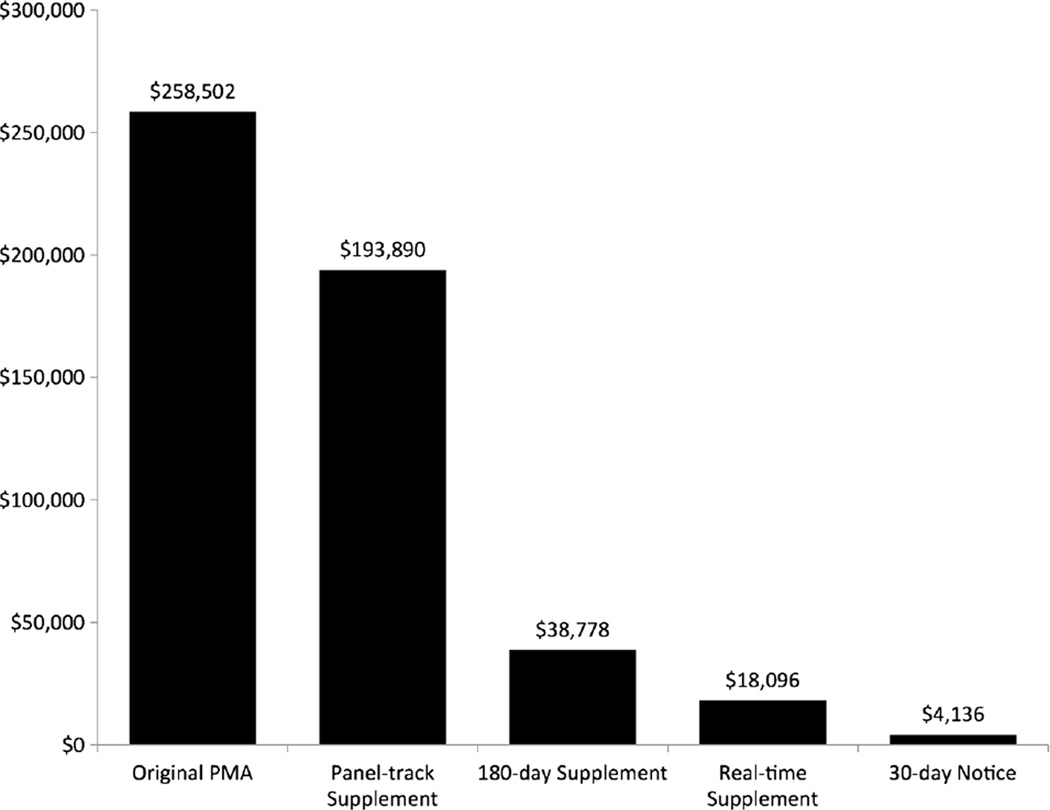
In 2002, Congress acted to increase FDA’s resources by requiring manufacturers to pay user fees for each device approval [28]. The user fee provision, which expires every 5 years, was renewed in 2007 and again in 2012 [29]. Currently, the FDA collects user fees for reviewing original PMAs and all supplement types except special supplements. For the 2014 fiscal year, an original PMA application costs $258,502, a panel-track supplement costs $193,890, and other supplement types cost $38,778 or less (Fig. 1) [30]. Small business may qualify for reduced fees for PMA and supplement applications [30].
Fig. 1.
User fees for premarket approval (PMA) applications, FY2014. Since 2002, manufacturers have to pay a fee for each PMA application reviewed by the FDA. The graph shows the fee for each type of PMA application for the 2014 fiscal year [30]
The PMA Pathway in Practice
Level of Evidence
Several investigators have studied how the FDA has implemented its charge to ensure safety and effectiveness of high-risk devices through the PMA pathway. Dhruva and colleagues found that for high-risk cardiovascular devices approved from 2000–2007, 27 % of premarket studies were randomized and 52 % included control groups [6]. Over a similar time frame, Kramer and colleagues found that 60 % of premarket studies for new cardiovascular devices had clearly defined safety outcomes, with important clinical comorbidities commonly unreported [8]. Chen and colleagues examined devices across multiple specialties approved via PMA and similarly found that 36 % were studied against an active control group [7].
All of the above studies reviewed original PMA applications. In addition, we recently found that among cardiac implantable electronic devices approved from 1979–2012, 77 original PMAs led to 5829 supplement applications [9••]. Of these supplements, over one-third represented a change to a device’s design, and the FDA approved most of these supplements without requiring new clinical data to support safety and effectiveness. Furthermore, PMAs underwent a median 50 supplements over a median period of 15 years, and most cardiac implantable electronic device models currently used by clinicians were approved via a supplement pathways rather than as original PMAs [9••].
The FDA uses pre-clinical testing, including mechanical engineering tests and animal studies, in addition to human trials to determine the safety and effectiveness of high-risk devices. In certain circumstances, pre-clinical studies may be more appropriate than clinical studies for evaluating device safety and effectiveness [31]. For example, new ICD leads studied in animals can be fired intentionally many times to evaluate performance, whereas clinical studies may offer fewer firing events leading to less valuable performance data [31].
The FDA also utilizes the clinical experience and post-market data from existing devices when determining safety and effectiveness for a new device. These existing devices may be previous versions of the device, other devices that perform similar functions, or experience with the device itself if it was previously approved for use in Europe or other international contexts. However, the FDA does not have a mandate to require comparative effectiveness testing, and so studies comparing a new device to existing models are commonly not required for FDA approval [7]. Consequently, FDA approval of a high-risk device does not indicate that the device is superior to its predecessors.
Postmarket Surveillance
The “least burdensome” principle suggests that the FDA should sometimes rely on rigorous postmarket surveillance to complement medical device approval and potentially reduce the premarket testing burden [24]. However, the FDA’s authority to require postmarket studies of medical devices is limited. The FDA can make non-binding recommendations for postmarket studies. Also, in 1990, the FDA was granted the authority to require manufacturers to conduct post-approval studies, named “522 postmarket surveillance studies,” for certain high-risk medical devices [22]. Currently, the FDA may require a 522 study if failure of the device would lead to serious adverse health consequences, if the device is intended to be implanted into the body for more than one year, or if the device is intended to be life-sustaining or life-supporting [32].
FDA-mandated postmarket studies complement data collection through the FDA’s adverse event reporting system, registries organized by the manufacturer or medical societies, and product performance reports from manufacturers [33•]. However, this piecemeal approach to data collection and the limitations of passive adverse event reporting in particular can lead to gaps in the availability of data and inconsistent analysis of data that are available. Hauser and colleagues simulated a prospective postmarket evaluation of the Sprint Fidelis ICD lead using a three-center database, finding that such active investigation may have raised red flags about early lead failure two years before Fidelis was actually taken off the market [34]. In the case of the recalled Riata ICD lead, the FDA did not mandate a postmarket study until after the lead was recalled in 2011 [33•].
In recent years, the FDA has called for improved postmarket surveillance of medical devices as part of integrated strategies to protect public health [35]. To aid in this effort, in September 2013, the FDA ruled that all devices must include a unique device identifier as a first step toward allowing study of patients’ experiences with medical devices in claims data [36].
Transparency of FDA Decisions
Physicians and patients wishing to better understand the data supporting the safety and effectiveness of high-risk medical devices rely on the FDA to provide details about the decision-making process. The FDA publishes information about approved original and supplement PMA applications in an online PMA database [37]. Some proprietary information may be excluded from publication. For each approved original PMA and panel-track supplement, the FDA publishes a structured Summary of Safety and Effectiveness Data (SSED) report that contains information about the clinical and preclinical data reviewed prior to device approval. Starting in 2010, the FDA began publishing abbreviated Review Memos for approved 180-day supplements. For other types of supplements, the PMA database contains a few-sentence description of the approved change. The FDA launched a widespread transparency initiative in 2009 with the hopes of making more data available to the public, including the proposed publication of a PMA clinical trials database [38, 39].
Legal Implications of Device Approval Pathways
Before the 1976 Medical Device Amendments, oversight of medical devices occurred mostly at the state law level. Thus, Congress included a clause in the legislation that ensured federal medical device oversight “preempted” any state statutes regulating medical devices [15]. In 2008, the US Supreme Court ruled in Riegel v. Medtronic that this clause also applied to preempt common-law tort claims brought by individuals against the manufacturer of a faulty device that was approved via the PMA pathway [4]. Essentially, Riegel provides immunity to device manufacturers against most lawsuits for injuries or deaths resulting from PMA-approved devices in which it is alleged that the manufacturer did not design the device properly or sufficiently warn patients about its risks [11].
Writing for an 8-1 majority, Justice Antonin Scalia justified the outcome in Riegel by referring to the PMA as a “rigorous” process in which the “FDA spends an average of 1200 hours reviewing each application” to determine safety and effectiveness [4]. The Court also noted that PMA supplements were “evaluated under largely the same criteria as an initial application”[4]. As a result, courts have applied preemption to faulty devices that were reviewed as PMA supplements. Such was the case in a class action lawsuit against Medtronic for the recalled Sprint Fidelis ICD leads, in which the Court of Appeals for the Eighth Circuit ruled that claims against the manufacturer were preempted because the product was approved as a PMA supplement [40].
The Riegel ruling stands in contrast to the 2009 case Wyeth v. Levine, in which the Supreme Court ruled that the FDA’s regulatory oversight scheme for prescription drugs did not preempt state tort actions against drug manufacturers for faulty design or warnings about their products [41]. Interestingly, in 1996, the Supreme Court ruled in Medtronic v. Lohr that the 510(k) substantial equivalence standard used to clear many medium-risk medical devices did not justify preemption. Thus, only high-risk devices approved via the PMA process are protected by preemption [42].
As a result of Riegel, patients may have difficulty collecting damages from manufacturers of high-risk devices that are approved by the FDA but are eventually found to be excessively dangerous [43]. One limitation to the ruling is that the preemption clause only prevents states from enacting requirements “different from, or in addition to” the regulations imposed by the FDA’s approval process, thereby sparing state regulations that parallel the FDA scheme [43]. To qualify under the “parallel claims” principle, a plaintiff must prove that the device at issue was not manufactured in accordance with its PMA approval order [42, 43]. This is a difficult legal argument to make, and it may require proof from proprietary information that the FDA and manufacturers withhold from the public [44]. The parallel claims approach was not successful in one Medtronic Sprint Fidelis case, in which the Eighth Circuit ruled that the plaintiffs had simply re-packaged traditional liability arguments and called them parallel claims just to avoid preemption under Riegel [40]. However, in other recent cases, parallel claims were permitted [45, 46]. In Stengel v. Medtronic, the Court of Appeals for the Ninth Circuit ruled that the plaintiff’s claims that Medtronic withheld known safety risks from the FDA related to the SynchroMed EL implantable medication pump were parallel to federal regulations and were thereby exempt from preemption [46]. Similarly, in Ramirez v. Medtronic, the District Court of Arizona ruled that the plaintiff established “parallel claims” in arguing that Medtronic had actively promoted off-label use of a liquid bone-graft substitute and obfuscated the risks associated with such use. Consequently, the Court denied in part Medtronic’s motion to dismiss the claims on grounds of preemption [45]. Cases like Stengel and Ramirez have demonstrated that the Riegel ruling does not protect device manufacturers from all legal claims of wrongdoing, but the circumstances necessary to avoid preemption are limited.
After Riegel, members of Congress have introduced legislation on more than one occasion to modify the preemption language to prevent it from blocking liability claims against manufacturers [47]. However, to this point, no proposed legislation has made it out of committee.
Implications for Cardiologists and Patients with Cardiovascular Disease
No regulatory process is perfect and high-risk medical devices will inevitably reach the market with lingering uncertainties regarding safety and effectiveness [10•, 11, 48]. As such, cardiologists who use medical devices as a part of patient care should understand the strengths and weaknesses of the various pathways through which high-risk devices are approved for sale in the US.
Our review of the PMA regulatory process serves as a reminder that the evidence supporting FDA approval of high-risk devices varies considerably. While the FDA strives to assure safety and effectiveness of each new device, devices are often approved based on studies that may have important limitations and are nearly always short-term evaluations. The statutory “least burdensome” requirement has been interpreted to allow pre-clinical studies rather than clinical studies to approve some new devices [24]. Even when clinical studies are used to support a PMA application, recent articles have raised questions about the quality of the study designs [6–8].
Furthermore, most new high-risk devices are approved via PMA supplement pathways, which are associated with smaller fees, have shorter review times, and often are approved without the need to collect premarket clinical data [9••, 30]. These features all encourage manufacturers to implement evolving technologies to create new models of devices that are incrementally different from previously approved versions. This helps facilitate rapid improvements in device technology, but also means that high-risk medical devices can gain PMA approval as supplements without any direct clinical study of the specific change made to the device.
To support device safety, the FDA has recently pushed for improved postmarket surveillance of high-risk medical devices [35]. While postmarket device evaluation is important, studies show that physicians are quick to adopt new device technologies once they gain FDA approval. One recent study of ICD registry data found that nearly two-thirds of ICDs implanted in the US are a manufacturer’s newestmodel [49]. The patchwork device postmarket surveillance system that currently exists means that design flaws may not be identified until well after approval.
The Supreme Court’s 2008 ruling in Riegel v. Medtronic further emphasized the importance of physicians exercising a cautious approach to use of new devices supported by limited data. The Riegel ruling limits the recourse available to patients harmed by devices that turn out to be dangerous and, in doing so, undermines the role that the risk of litigation plays in motivating device manufacturers to pursue timely postmarket device evaluation [50]. Congress has considered revising the preemption clause to permit cases against manufacturers of faulty devices, but no changes have been made. Were Congress to alter the preemption clause, they could eliminate the immunity that the Riegel ruling provides to manufacturers of faulty devices [47].
Practicing cardiologists should recognize that FDA-pproved devices undergo different levels of premarket investigation, and they should be aware of the data used to support a new device even once it gains approval via PMA. The FDA performs a detailed analysis of each approved device to reasonably assure safety and effectiveness. Newer devices may offer improvements over older versions that can benefit patients, such as increased battery life, improved wireless technologies, or smaller design. However, newer models may not have as much evidence to support their safety and effectiveness as older ones, and physicians should not make assumptions about the quantity and quality of evidence for devices that are approved by the FDA.
The FDA has made efforts in recent years to improve transparency, but more needs to be done to provide patients, clinicians, and policymakers with adequate information about all approved device models [38, 51]. Additionally, because PMA supplements allow for approval of new devices that differ only slightly from previous generations, researchers and policymakers should boost efforts to support studies evaluating the comparative effectiveness of medical devices. Such studies, while not part of the FDA’s mandate, would provide valuable information to help physicians and patients when choosing between similar device models.
Conclusion
Medical devices are critically important to the field of cardiology, and physicians who use medical devices should be more aware about the regulatory processes supporting their availability. The FDA reviews high-risk devices for safety and effectiveness, and less premarket testing is required for devices that are modifications to existing devices. Furthermore, FDA approval is not intended to compare new device performance to previous iterations. Cardiologists who utilize devices should ensure that they incorporate these perspectives in helping patients understand the benefits and risks related to use of medical devices.
Acknowledgments
Benjamin N. Rome has received support from a Harvard Medical School fellowship.
Daniel B. Kramer has received grant support from NIH/NIA (Paul B. Beeson career development award to Dr. Kramer [K23AG045963]).
Aaron S. Kesselheim has received the following in grant support: Supported by a Robert Wood Johnson Foundation Investigator Award in Health Policy Research; Supported by a career development award from the Agency for Healthcare Research and Quality (K08HS18465-01); Supported by a grant as a Greenwall Faculty Scholar in Bioethics from the Greenwall Foundation; and Received a contract (2011) from the US FDA Center for Devices and Radiological Health to conduct comparative study of device regulation in US and EU.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Benjamin N. Rome, Daniel B. Kramer, and Aaron S. Kesselheim declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Benjamin N. Rome, Email: Benjamin_Rome@hms.harvard.edu, Program On Regulation, Therapeutics, And Law (PORTAL), Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, 1620 Tremont St., Suite 3030, Boston, MA 02120, USA; Harvard Medical School, 25 Shattuck St., Boston, MA 02215, USA.
Daniel B. Kramer, Email: DanielKramer@hsl.harvard.edu, Program On Regulation, Therapeutics, And Law (PORTAL), Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, 1620 Tremont St., Suite 3030, Boston, MA 02120, USA; Harvard Medical School, 25 Shattuck St., Boston, MA 02215, USA; Beth Israel Deaconess Medical Center, 185 Pilgrim Road, West Campus B4, Boston, MA 02215, USA; Hebrew SeniorLife Institute for Aging Research, Roslindale, MA, USA.
Aaron S. Kesselheim, Email: akesselheim@partners.org, Program On Regulation, Therapeutics, And Law (PORTAL), Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, 1620 Tremont St., Suite 3030, Boston, MA 02120, USA; Harvard Medical School, 25 Shattuck St., Boston, MA 02215, USA.
References
Papers of particular interest, published recently, have been highlighted as:.
• Of importance.
•• Of major importance.
- 1.Kurtz SM, Ochoa JA, Lau E, Shkolnikov Y, Pavri BB, Frisch D, et al. Implantation trends and patient profiles for pacemakers and implantable cardioverter defibrillators in the United States: 1993–2006. Pacing Clin Electrophysiol. 2010;33:705–711. doi: 10.1111/j.1540-8159.2009.02670.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20059714. [DOI] [PubMed] [Google Scholar]
- 2.Greenspon AJ, Patel JD, Lau E, Ochoa Ja, Frisch DR, Ho RT, et al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol. 2012;60:1540–1545. doi: 10.1016/j.jacc.2012.07.017. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22999727. [DOI] [PubMed] [Google Scholar]
- 3.Medtronic, Inc. v. Lohr. 1996. Supreme Court of the United States. [Google Scholar]
- 4.Supreme Court of the United States. Riegel v.Medtronic, Inc. 2008 [Google Scholar]
- 5.Kramer DB, Xu S, Kesselheim AS. Regulation of medical devices in the United States and European Union. N Engl JMed. 2012;366:848–855. doi: 10.1056/NEJMhle1113918. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22332952. [DOI] [PubMed] [Google Scholar]
- 6.Dhruva SS, Bero LA, Redberg RF. Strength of study evidence examined by the FDA in premarket approval of cardiovascular devices. JAMA. 2009;302:2679–2685. doi: 10.1001/jama.2009.1899. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20040556. [DOI] [PubMed] [Google Scholar]
- 7.Chen CE, Dhruva SS, Redberg RF. Inclusion of comparative effectiveness data in high-risk cardiovascular device studies at the time of premarket approval. JAMA. 2012;308:1740–1742. doi: 10.1001/jama.2012.14491. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23117769. [DOI] [PubMed] [Google Scholar]
- 8.Kramer DB, Mallis E, Zuckerman BD, Zimmerman BA, Maisel WH. Premarket clinical evaluation of novel cardiovascular devices: quality analysis of premarket clinical studies submitted to the Food and Drug Administration 2000–2007. Am J Ther. 2010;17:2–7. doi: 10.1097/MJT.0b013e3181ca8105. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20038828. [DOI] [PubMed] [Google Scholar]
- 9. Rome BN, Kramer DB, Kesselheim AS. FDA approval of cardiac implantable electronic devices via original and supplement premarket approval pathways, 1979–2012. JAMA. 2014;311:385–391. doi: 10.1001/jama.2013.284986. The authors studied cardiac implantable electronic devices approved from 1979–2012, finding that 77 original PMAs were the subject of 5,289 supplement PMAs. They found that nearly all devices used today were approved as PMA supplements to older models.
- 10. Hauser RG, Abdelhadi R, McGriff D, Retel LK. Heart Rhythm. Vol. 9. Elsevier Inc.; 2012. Deaths caused by the failure of Riata and Riata ST implantable cardioverter-defibrillator leads; pp. 1227–1235. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22449741. The authors searched the FDA event database and found that failure of the recalled Riata ICD lead caused 22 deaths. Riata was approved via a PMA supplement application without any clinical data, and this study highlights the importance of ensuring adequate premarket testing to prevent unsafe devices from being used in patient care.
- 11.Hauser RG, Almquist AK. Learning from our mistakes? Testing new ICD technology. N Engl J Med. 2008;359:2517–2519. doi: 10.1056/NEJMp0805359. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19073974. [DOI] [PubMed] [Google Scholar]
- 12.Maisel WH. Semper fidelis–consumer protection for patients with implanted medical devices. N Engl J Med. 2008;358:985–987. doi: 10.1056/NEJMp0800495. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18322280. [DOI] [PubMed] [Google Scholar]
- 13.Zuckerman DM, Brown P, Nissen SE. Medical device recalls and the FDA approval process. Arch Intern Med. 2011;171:1006–1011. doi: 10.1001/archinternmed.2011.30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21321283. [DOI] [PubMed] [Google Scholar]
- 14.US Food and Drug Adminstration. List of Device Recalls. 2014 Available from: http://www.fda.gov/MedicalDevices/Safety/ListofRecalls/default.htm.
- 15.Medical Device Amendments of 1976. US: Public Law 94–295. 1976 Available from: http://www.gpo.gov/fdsys/pkg/STATUTE-90/pdf/STATUTE-90-Pg539.pdf.
- 16.Institute of Medicine. Medical devices and the public’s health: The 510(k) clearance process at 35 Years. Washington, DC: The National Acadamies Press; 2011. Available from: http://www.iom.edu/Reports/2011/Medical-Devices-and-the-Publics-Health-The-DA-510k-Clearance-Process-at-35-Years.aspx. [Google Scholar]
- 17.Curfman GD, Morrissey S, Drazen JM. A pivotal medical-device case. N Engl J Med. 2008;358:76–77. doi: 10.1056/NEJMe0708590. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18172178. [DOI] [PubMed] [Google Scholar]
- 18.US Food and Drug Adminstration. Premarket Notification (510k) 2010 Available from: http://www.fda.gov/medicaldevices/deviceregulationandguidance/howtomarketyourdevice/premarketsubmissions/premarketnotification510k/default.htm.
- 19.Ardaugh BM, Graves SE, Redberg RF. The 510(k) ancestry of a metal-on-metal hip implant. N Engl J Med. 2013;368:97–100. doi: 10.1056/NEJMp1211581. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23301729. [DOI] [PubMed] [Google Scholar]
- 20.US Food and Drug Adminstration. Premarket Approval (PMA) 2012 Available from: http://www.fda.gov/medicaldevices/deviceregulationandguidance/howtomarketyourdevice/premarketsubmissions/premarketapprovalpma/.
- 21.US Food and Drug Adminstration. 515 Program Initiative. 2013 Available from: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHTransparency/ucm240310.htm.
- 22.Safe Medical Devices Act of 1990. US: Public Law 101–629. 1990 Available from: http://www.gpo.gov/fdsys/pkg/STATUTE-104/pdf/STATUTE-104-Pg4511.pdf.
- 23.Food and Drug Administration Modernization Act of 1997. 2296 US: 111. 1997 [Google Scholar]
- 24.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health, Office of Device Evaluation, Center for Biologics Evaluation and Research. The Least Burdensome Provisions of the FDA Modernization Act of 1997: Concept and Principles; Final Guidance for FDA and Industry. 2002 [Google Scholar]
- 25.Premarket Approval of Medical Devices. 21 CFR 814. 1986 [Google Scholar]
- 26.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health, Center for Biologics Evaluation and Research. Guidance for Industry and FDA Staff: Modifications to Devices Subject to Premarket Approval (PMA) - The PMA Supplement Decision-Making Process. 2008 Available from: http://www.fda.gov/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm089274.htm.
- 27.US Food and Drug Adminstration. Guidance for Industry and FDA Staff Real-Time Premarket Approval Application (PMA) Supplements. 2006 Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm089602.htm.
- 28.Medical Device User Fee and Modernization Act of 2002. US: Public Law 107–250. 2002 Available from: http://www.gpo.gov/fdsys/pkg/PLAW-107publ250/content-detail.html.
- 29.Kramer DB, Kesselheim AS. User fees and beyond–the FDA Safety and Innovation Act of 2012. N Engl J Med. 2012;367:1277–1279. doi: 10.1056/NEJMp1207800. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23034017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Food and Drug Adminstration. PMA Review Fees. 2013 Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/PremarketSubmissions/PremarketApprovalPMA/ucm048161.htm.
- 31.Shein MJ, Schultz DG. Testing new ICD technology. N. Engl. J. Med. [Internet] 2008 doi: 10.1056/NEJMc086467. [cited 2013 Aug 29];359:2610. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19073982. [DOI] [PubMed]
- 32.US Food and Drug Adminstration. Medical Devices 522 Postmarket Surveillance Studies – Frequently Asked Questions (FAQs) 2013 Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/PostmarketRequirements/PostmarketSurveillance/ucm134497.htm.
- 33. Blake K. Postmarket surveillance of medical devices: current capabilities and future opportunities. J. Interv. Card. Electrophysiol. 2013;36:119–127. doi: 10.1007/s10840-013-9778-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23479089. This review article discusses the current systems for postmarket device surveillance and proposes ways to improve postmarket surveillance to better assure patient safety.
- 34.Hauser RG, Mugglin AS, Friedman PA, Kramer DB, Kallinen L, McGriff D, et al. Early detection of an underperforming implantable cardiovascular device using an automated safety surveillance tool. Circ Cardiovasc Qual Outcomes. 2012;5:189–196. doi: 10.1161/CIRCOUTCOMES.111.962621. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22396584. [DOI] [PubMed] [Google Scholar]
- 35.Normand S-LT, Hatfield L, Drozda J, Resnic FS. Postmarket surveillance for medical devices: America’s new strategy. BMJ. 2012;345:e6848. doi: 10.1136/bmj.e6848. Available from: http://www.bmj.com.ezp-prod1.hul.harvard.edu/content/345/bmj.e6848.pdf+html. [DOI] [PubMed] [Google Scholar]
- 36.Department US. of Health and Human Services Food and Drug Administration. Unique Device Identification System. Fed Regist. 2013;78:58786–58828. Available from: https://www.federalregister.gov/articles/2013/09/24/2013-23059/unique-device-identificationsystem. [Google Scholar]
- 37.US Food and Drug Adminstration. Premarket Approval (PMA) Database. 2012 Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm.
- 38.US Food and Drug Adminstration. FDA Transparency Initiative. 2012 Available from: http://www.fda.gov/AboutFDA/Transparency/TransparencyInitiative/default.htm.
- 39.US Food and Drug Adminstration. Premarket Approval (PMA) Clinical Trials Database. 2011 Available from: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHTransparency/ucm204243.htm.
- 40.Sprint Fidelis Leads Products Liability Litigation. re: Medtronic, Inc.; 2010. United States Court of Appeals for the Eighth Circuit. [Google Scholar]
- 41.Supreme Court of the United States. Wyeth v. Levinen. 2009 [Google Scholar]
- 42.Patsner B. Riegel v. Medtronic, Inc.: revisiting pre-emption for medical devices. J Law Med Ethics. 2009;37:305–317. doi: 10.1111/j.1748-720X.2009.00374.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19493075. [DOI] [PubMed] [Google Scholar]
- 43.Wartman GJ. Life after Riegel: a fresh look at medical device preemption one year after Riegel v. Medtronic, Inc. Food Drug Law J. 2009;64:291–311. Available from: http://www.ncbi.nlm.nih.gov/publimed/19999286. [PubMed] [Google Scholar]
- 44.Feder BJ. Medical Device Ruling Redraws Lines on Lawsuits. New York Times. 2008 Available from: http://www.nytimes.com/2008/02/22/business/22device.html?pagewanted=all. [Google Scholar]
- 45.United States District Court of Arizona. Ramirez v. Medtronic, Inc. 2013 [Google Scholar]
- 46.United States Court of Appeals for the Ninth Circuit. Stengel v. Medtronic, Inc. 2013 [Google Scholar]
- 47.Curfman GD, Morrissey S, Drazen JM. The Medical Device Safety Act of 2009. N Engl J Med. 2009;360:1550–1551. doi: 10.1056/NEJMe0902377. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19297562. [DOI] [PubMed] [Google Scholar]
- 48.Redberg RF, Dhruva SS. Medical device recalls: get it right the first time: Comment on “Medical device recalls and the FDA approval process”. Arch Intern Med. 2011;171:1011–1012. doi: 10.1001/archinternmed.2011.27. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3191575&tool=pmcentrez&rendertype=abstract. [DOI] [PubMed] [Google Scholar]
- 49.Lampert R, Wang Y, Curtis JP. Am. Heart. J. Vol. 165. Mosby, Inc.; 2013. Variation among hospitals in selection of higher-cost, “higher-tech,” implantable cardioverter-defibrillators: data from the National Cardiovascular Data Registry (NCDR) Implantable Cardioverter/Defibrillator (ICD) Registry; pp. 1015–1023. e2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23708175. [DOI] [PubMed] [Google Scholar]
- 50.Kesselheim AS, Avorn J. The role of litigation in defining drug risks. JAMA. 2007;297:308–311. doi: 10.1001/jama.297.3.308. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17227983. [DOI] [PubMed] [Google Scholar]
- 51.Goodman SN, Redberg RF. Opening the FDA Black Box. JAMA. 2014;311:361–363. doi: 10.1001/jama.2013.283946. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24449313. [DOI] [PubMed] [Google Scholar]