Abstract
Interstitial cystitis (IC) is a chronic disorder characterized by bladder discomfort and urinary urgency in the absence of identifiable infection. Despite the expanding use in treatment of IC and other chronic conditions, the effects of Elmiron® treatment on immune system remain unknown. Therefore, female B6C3F1/N mice were orally administered Elmiron® daily for 28-days at doses of 63, 125, 250, 500 or 1000 mg/kg to evaluate its immunomodulatory effects. Mice treated with Elmiron® had a significant increase in absolute numbers of splenic macrophages (63, 500 and 1000 mg/kg) and natural killer (NK) cells (250 and 1000 mg/kg). Elmiron® treatment did not affect the humoral immune response or T cell proliferative response. However, innate immune responses such as phagocytosis by liver macrophages (1000 mg/kg) and NK cell activity were enhanced (500 and 1000 mg/kg). Further analysis using a disease resistance model showed that Elmiron® -treated mice demonstrated significantly increased anti-tumor activity against B16F10 melanoma cells at the 500 and 1000 mg/kg doses. Collectively, we conclude that Elmiron® administration stimulates the immune system, increasing numbers of specific cell populations and enhancing macrophage phagocytosis and NK cell activity in female B6C3F1/N mice. This augmentation may have largely contributed to the reduced number of B16F10 melanoma tumors.
Keywords: Immunotoxicity, Orphan drug, sodium pentosan polysulfate, interstitial cystitis
1. Introduction
Elmiron® (sodium pentosan polysulfate) is a low-molecular weight semisynthetic pentose polysaccharide (Vij et al. 2012). Currently, it is the only oral drug available for treatment of a chronic and debilitating disease; interstitial cystitis (IC) also called the painful bladder syndrome (French and Bhambore 2011). IC is characterized by chronic suprapubic pain, excessive urgency and frequency of urination, in the absence of bacterial infection. It is estimated that 0.3% – 12.6% of individuals may suffer from IC in countries such as Japan, Finland and the United States (Elliott and Payne 2012). IC has been shown to be more prevalent in women (Dunlap et. al. 2013). The US FDA classifies Elmiron® as an orphan drug because of its clinical use in the treatment of a relatively rare disease. However, the clinical use of Elmiron® seems to be gradually expanding to include other pathological conditions such as osteoarthritis (Kumagai et al. 2010), prion diseases (Dealler and Rainov 2003), glomerulosclerosis (Striker et al. 1997) and mucopolysaccharidoses (Schuchman et al. 2013). Although its specific mechanism of action is not known, Elmiron® is thought to alleviate pain associated with IC by its ability to form a protective lining in the urinary bladder wall and by reducing potassium leakage into the bladder muscle (Vij et al. 2012).
There are very little data available addressing adverse effects of chronic consumption of Elmiron® by patients undergoing therapy. Despite the low oral bioavailability of Elmiron, cases of bleeding complications such as increases in gastrointestinal bleeding, activated partial thromboplastin time and hematuria have been reported in human cancer patients (Gill et al. 2002; Marshall et al. 1997; Swain et al. 1995). In a Phase I clinical trial in advanced cancer patients, dose limiting toxicity observed included rectal bleeding and ulceration. No other significant toxicities were seen (Marshall et al. 1997). Also, a few cases of thrombocytopenia and venous thrombosis have been reported in patients taking pentosan polysulfate (Tardy-Poncet et al. 1994). These incidences of bleeding were mainly attributed to Elmiron®’s structural and functional similarity to the anticoagulant, heparin (Jerebtsova et al. 2007; Marshall et al. 1997). Interestingly, a few cases of treatment-induced thrombocytopenia were suggested to be due to immune-mediated responses however the exact immune mechanisms are unknown (Bollaert et al. 1986; Gironell et al. 1996). Apart from the few human case reports describing adverse effects of Elmiron® treatment, the animal data investigating the health effects associated with Elmiron® consumption are scarce. To address the lack of adequate toxicological information on chronic Elmiron® use, the NTP conducted 2-week, 3-month and 2-year toxicity studies in F344/N rats and B6C3F1/N mice ((NTP) 2004; Abdo et al. 2003).
Results from the 3-month NTP toxicity studies demonstrated that Elmiron® treatment could potentially perturb the immune system in treated mice. Daily Elmiron® treatment caused an increase in absolute (11% to 32 %) and relative (11% to 34%) spleen weights in both male (≥ 250 mg/kg) and female rats (1000 mg/kg). Absolute (21%) and relative (18%) weights of spleen were also significantly increased in male mice at 1000 mg/kg. Treatment-related histopathological changes were observed in male and female B6C3F1/N mice and were characterized by the presence of macrophages with increased number of vacuoles in the cytoplasm of rectum, liver, spleen and mandibular and mesenteric lymph nodes ((NTP) 2004; Nyska et al. 2002). Increased numbers of total peripheral white blood cells and lymphocytes were also observed in mice and rats in the 500 and 1000 mg/kg dose groups. Other treatment-related histopathological lesions such as ulcer and inflammation were found in organs including the rectum, liver, mesenteric and mandibular lymph node (mice and rats), as well as in the lungs (rats) and kidneys (rats). Transmission electron microscopy examination showed accumulation of foamy macrophages with cytoplasmic membranes which were indicative of a drug-induced lysosomal storage disorder (Nyska et al. 2002). Results from 2-year toxicity studies showed a similar increase in vacuolated histiocytes in the mesenteric lymph nodes (mice and rats) and spleen (mice) ((NTP) 2004).
Lysosomal storage disorder has been associated with many altered immune responses including immunosuppression, immune potentiation and autoimmune responses (Castaneda et al. 2008). Therefore, potential immune perturbations associated with Elmiron® treatment were a concern. Based on the prolonged use of Elmiron® by IC patients, the observed increases in the leukocyte differentials, and the effects on the secondary lymphoid organs (spleen and lymph nodes) in the NTP subchronic and chronic toxicity studies, the potential for Elmiron® to modulate immune function was investigated in female B6C3F1/N mice treated daily for 28 days by oral gavage with 0, 63.5, 125, 250, 500 or 1000 mg/kg of Elmiron®.
2. Materials and Methods
2.1 Animals
This study was conducted at the Virginia Commonwealth University (VCU) and the study protocols were approved by VCU’s Institutional Animal Care and Use Committee. Female B6C3F1/N mice were obtained from Taconic Farms (Germantown, NY) at 4–8 weeks of age and maintained in facilities at VCU. Upon arrival mice were quarantined for 5 days prior to use. Mice used in these studies were between 8 and 10 weeks of age at the beginning of the studies. Mice were housed 4 per cage in plastic shoebox cages with hardwood chip bedding. The animals were maintained on NTP-2000 diet (Zeigler Brothers, Inc.) and were provided tap water ad libitum. Room temperatures were maintained at 18 –26°C and the relative humidity between 30 and 70% with a 12-hr light/dark cycle. Mouse cages were cleaned and sanitized two times per week.
2.2. Test Article and Controls
Elmiron®, sodium xylan (pentosan) polysulfate (CAS No. 37319-17-8, approximately 96% pure), was obtained from Baker Norton Pharmaceuticals (Miami, FL) in a white powder form. Elmiron® dosing solutions were prepared weekly in USP pharmaceutical grade sterile water (Baxter Healthcare, Deerfield, IL), which was used as vehicle control for these studies. Cyclophosphamide (CP; Sigma Chemical Co., St. Louis, MO) was utilized as the positive control for all assays, unless otherwise indicated and was administered daily at 50 mg/kg by intraperitoneal (i.p.) injection to positive control animals during the last 4 days of the exposure period. In the B16F10 melanoma host resistance assay, CP was administered at 200 mg/kg in a single i.p. injection one day prior to inoculation with the melanoma. For the natural killer (NK) cell assay, rabbit anti-asialo GM1 (AAGM1, Wako BioProducts, Richmond, VA) antibody, diluted in sterile physiological saline was used as a positive control and was administered via intravenous (i.v.) injection of 0.2 ml at a 1: 10 dilution 24 hr prior to evaluation of NK cell activity. Maleic vinyl ether (MVE), prepared in PBS solution was used as a positive control in the mononuclear phagocytic system (MPS) assay and was administered to the animals at 50 mg/kg by intravenous (i.v.) injection approximately 24 hr prior to evaluation of the MPS activity.
2.3. Experimental Protocol
Female B6C3F1/N mice were administered Elmiron® at doses of 63, 125, 250, 500 or 1000 mg/kg in USP grade sterile deionized water by oral gavage once daily. Vehicle control animals were administered deionized water as a negative control. Eight animals were included in each treatment group, with the exception of the B16F10 melanoma assay. For the B16F10 host resistance study, the Elmiron® doses administered were 0, 250, 500 or 1000 mg/kg and each treatment group consisted of 12 animals. The doses, vehicle, and the route of exposure were selected based on the dose levels of the 3-month NTP toxicity studies and to minimize the potential for overt toxicity that could confound immunologic evaluation. Body weights were obtained weekly, and dosing volumes were adjusted accordingly.
2.4. General Toxicology Endpoints
Whole body weights of mice were obtained on days 1, 8, 15, 22 and 29 of the study. On day 29, animals were euthanized by carbon dioxide (CO2) inhalation. Organs including liver, spleen, lungs, thymus, and kidneys with adrenals were removed and weighed. Blood was collected from the retro-orbital sinus into the EDTA tubes. The number of erythrocytes and leukocytes, hemoglobin, hematocrit, mean corpuscular volume (MCV), mean cell hemoglobin (MCH) and mean cell hemoglobin concentration (MCHC) was assessed. Hematological parameters were evaluated using a K-1000 hematology analyzer (Sysmex America, Inc., Mundelein, IL). The number of reticulocytes was measured using a Retic-Count reagent stain (Thiazole Orange, Beckton Dickinson, Mountain View, CA) followed by flow cytometry analysis. Blood smears were prepared, and air-dried. After fixation with methanol and staining with Wright-Giemsa (Fischer Scientific, Pittsburg, PA) the smear was used to determine leukocyte differentials in blood.
2.5. Immunophenotyping in Spleen
Following euthanasia, spleens were collected and placed in 3 ml Earle’s balanced salt solution (EBSS) with HEPES (GibcoBRL, Grand Island, NY) and single cell suspensions were prepared. The number of total B cells, total T cells, T cell subpopulations, macrophages and NK cells was enumerated using flow cytometry as described previously (Auttachoat et al. 2009). Antibodies (BD Pharmingen, San Diego, CA, USA) utilized were fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse Ig, phycoerythrin (PE) conjugated anti-mouse CD4, FITC conjugated CD8a monoclonal antibody FITC conjugated Mac3 antibody and PE conjugated NK1.1 antibody. Isotype-matched antibodies were used as the controls. After a 30 min incubation at 4°C with the antibodies, the cells were washed twice and propidium iodide (PI) was added. Cell acquisition and analysis was performed on a Becton Dickinson FACScan flow cytometer in which log fluorescence intensity was read by setting a live gate on red fluorescence PI to eliminate non-viable cells. Five thousand cells were counted for each sample. The data were analyzed using Cellquest Software Version 3.2.1 (Becton Dickinson, San Jose, CA). The percentage of B cells (Ig+), T cells (CD3+) and macrophages (Mac3+) were calculated following gating. Also, cells that were NK1.1+CD3− were identified as NK cells, CD4−CD8+ cells were identified as cytotoxic T lymphocytes (TCTL), CD4+CD8− cells were identified as T-helper (TH) lymphocytes and CD4+CD8+ cells were identified as immature T lymphocytes.
2.6. NK cell activity
NK cell activity was assessed as previously described (Auttachoat et al. 2009). Briefly, single cell suspensions from control and treated spleens were adjusted to concentrations of 2×107, 1×107, 5×106, 2.5×106, 1.25× 106, and 0.625 × 106 cells/ml. A 100 µl aliquot of these effector cells were added in duplicate at each concentration in 96-well round bottom microtiter plates. The target cells (YAC-1cells) were adjusted to a concentration of 107 cells/ml and labeled by incubating with 200 µCi of 51Cr (as Na2CrO4; Perkin Elmer, Wellesley, MA) for 90 min in a 37°C incubator with frequent agitation. Following the incubation, the target cells were washed in balanced salt solution plus 25mM, HEPES, counted, and adjusted to 105 cells/ml. The target cells were added to a 96-well plate to obtain 104 YAC-1 cells/well to yield final ratios of 200:1, 100:1, 50:1, 25:1, 12.5:1, and 6.25:1. After 4 hr of incubation, the supernatants from each well were counted using a γ-counter to quantify the 51Cr released by the cells. The percent cytotoxicity at each effector concentration was calculated using the following formula: Percent cytotoxicity= (CPM exp−CPMspon)/(CPMtotal− CPMspon)× 100; where, CPMexp= counts per minute in experimental wells, CPMspon= spontaneous release, and CPM total= total release upon addition of 0.1% Triton X-100.
2.7. Functional activity of fixed tissue macrophages in spleen, liver and lung
This assay was conducted as described previously (White et al. 1985). Briefly on day 29, animals were injected i.v. with 51Cr- sheep red blood cells (SRBC). Clearance of 51Cr-SRBC from the blood was determined over the first 30 minutes by taking 5 µl blood samples from the tail vein of each animal. The time points were set at 3, 6, 9, 12, 15 and 30 minutes after 51Cr-SRBC injection for vehicle control animals and those receiving Elmiron®. The radioactivity in the blood was used to determine the vascular half-life of 51Cr-SRBC. Due to delayed clearance in animals treated with the positive control (MVE), blood samples from these animals were collected at 5, 10, 15, 20, 30 and 60 minutes after 51Cr-SRBC injection. After 60 minutes all animals were euthanized. Liver, lung, spleen, thymus and kidneys were isolated, weighed and the radioactivity of these tissues was determined using an LKB gamma counter to determine distribution of 51Cr-SRBC to major organs of the MPS. The data were expressed as percent uptake of the total 51Cr-SRBC cells injected and as CPM/mg of tissue (Specific Activity).
2.8. Spleen IgM antibody forming cell (AFC) response
The primary IgM AFC response to SRBC was evaluated using a modified hemolytic plaque assay (Jerne and Nordin 1963; White et al. 2010). Briefly, on day 25 of the study, mice were immunized with 7.5 ×10 7 SRBC by i.v. injection. On day 29, the animals were euthanized and the spleen was isolated from each mouse, prepared into single cells suspensions and an aliquot of spleen cells were combined with guinea pig complement, SRBC and warm agar. The mixture was plated in a petri dish, covered with a microscope cover slip, and incubated at 37°C for 3 hr. The plaques formed were counted using a Bellco plaque viewer. The number of cells/ spleen, the specific activity (AFC/106 splenocytes) and the total spleen activity (AFC/spleen) were determined. Serum titers of SRBC-specific IgM from the same animals were also determined by using an enzyme-linked immunoabsorbent assay (ELISA). Briefly, SRBC membrane antigen was prepared at a 1:100 dilution of SRBC membrane preparation in PBS and 100 µl per well of the high salt release antigen were incubated in Immulon 2 (Thermo-Fischer Scientific, Pittsburg, PA) microtiter plates overnight at 4°C. Following an initial wash to remove unbound antigen, serially diluted serum samples were added. Intermittent washes with PBS in 0.05% Tween 20 were performed and the plates were incubated with the secondary antibody, HRP- conjugated goat anti-mouse IgM diluted in assay buffer (Auttachoat et al. 2009; Temple et al. 1993). The color in each well was read at 405 nm on a Molecular Devices plate reader after 45 min incubation. Results were obtained using SoftMax (Version 2.32, Molecular Devices Corp.). Titers for each sample were determined using multipoint analysis and were defined to be the reciprocal of the dilution corresponding to an optical density (O.D.) of 0.5 (Kawabata et al. 1995).
2.9. Mixed Leukocyte response (MLR) to DBA/2 spleen cells
The MLR assay was conducted as described previously (Guo et al. 2000). On day 29, the control and Elmiron®-treated mice were euthanized with CO2 and the spleens were isolated from each mouse. Responder spleen cells from control and Elmiron®-treated animals were plated at 1× 105 cells/well. DBA/2 stimulator cells were prepared by incubation at 37°C for 45 min with mitomycin C (50 µg/ 2×107 cells) to render them unable to proliferate. The stimulator cells were added to wells of a 96-well plate in quadruplicate at a concentration of 4 × 105 cells /well resulting in a ratio of stimulators to responders of 4:1. After 5 days in culture, 1 µCi of [3H]-thymidine was added to each well and the plates were harvested and counted 18–24 hr later. The incorporation of [3H]-thymidine into proliferating cells was used as the endpoint of the assay and expressed as CPM/105 cells.
2.10. Anti CD3-mediated spleen cell proliferative response
The anti-CD3 antibody mediated proliferative response was measured as described previously (Smith et al. 2010). On day 29, the mice were euthanized and single cell suspensions of splenocytes prepared and incubated in flat-bottom 96-well microtiter plates at a concentration of 2×105 cells /well. The media for this proliferative assay was RPMI 1640 supplemented with 10 % FBS and 5×10−5 2-mercaptoethanol. T cell activation plates (BD Biosciences, San Jose, CA) were coated with 100 µl of a 1: 500 dilution of anti-CD3 antibody (0.5 mg/ml stock; BD Pharmingen, San Diego, CA) in PBS added to each well and incubated at 2–8 C overnight prior to use in the assay. Splenocytes from Elmiron®-treated animals were cultured in either control or anti-CD3 coated wells for 3 days. For the last 18–24 hours, the cells were incubated with [3H]-thymidine (1 microCurie/well) and the incorporation of [3H]-thymidine was measured by counting using an LKB liquid scintillation counter. The data was expressed as CPM/2×105 cells.
2.11. B16F10 melanoma assay
Host resistance to the B16F10 melanoma tumor was assessed as described previously (Guo et al. 2010). Briefly, on day 29 of Elmiron® treatment, the mice were injected i.v. with B16F10 melanoma cells at one of two challenge levels; 1 × 105 or 3 × 105 cells per mouse. Sentinel mice were used to monitor the tumor burden in order to select the appropriate day for study termination. Mice given 1 ×105 tumor cells (low challenge level) were sacrificed 19 days following tumor cell challenge and those receiving 3 ×10 5 tumor cells (high challenge level) were sacrificed after 13 days. One day prior to sacrifice, the mice were administered [125I]- UdR by i.v. injection. Twenty-four hours later, the mice were euthanized and their lungs removed and placed in Bouin’s solution (Sigma, St. Louis, MO) and radioassayed (CPM/lung). Tumor burden was also determined by manually counting the number of nodules (Nodules/Lung).
2.12. Statistical Analysis
The data obtained in this study was first tested for homogeneity of variances using the Bartlett’s test (Bartlett, 1937) to select the type of analysis to be conducted. Homogenous data were analyzed using a one-way analysis of variance (ANOVA). When significant results were obtained the Dunnett’s test (Dunnett 1955) was used to determine differences between the experimental groups and the control group. Non-homogenous data were analyzed using a non-parametric ANOVA (Kruskal and Wallis 1952; Wilson 1956), and the Wilcoxon Rank Test (Wilcoxon 1945). The student’s t-test was used (Sokal and Rohlf, 1981) to compare the vehicle and the positive control groups. The Jonckheere’s test (Jonckheere 1954) was used to test for dose-related trends. In all evaluations, p<0.005 indicated statistically significant differences. P values ≤ 0.05 or less were considered statistically significant.
3. Results
3.1. Body weights, organ weights and hematology parameters
No signs of overt toxicity were observed in the Elmiron® treated animals. No significant treatment-related effects were observed in mice with respect to body weights except a significant increase (40%) in body weight gain in the 250 mg/kg dose group. The absolute liver weights were increased at the 500 and 1000 mg/kg doses (13% and 23%, respectively) and the relative liver weight was increased at 1000 mg/kg (17%). Treatment-related effects on absolute or relative weights of thymus, spleen, lung or kidney were not observed. Elmiron® treatment resulted in statistically significant increases in the percentage of reticulocytes in the peripheral blood in the 125, 500, and 1000 mg/kg treatment groups (23%, 19% and 29%, respectively). Erythrocyte numbers, differential leukocyte counts, hemoglobin, hematocrit, MCV, and platelet concentration were not affected.
3.2. Flow cytometry analysis of splenocyte populations
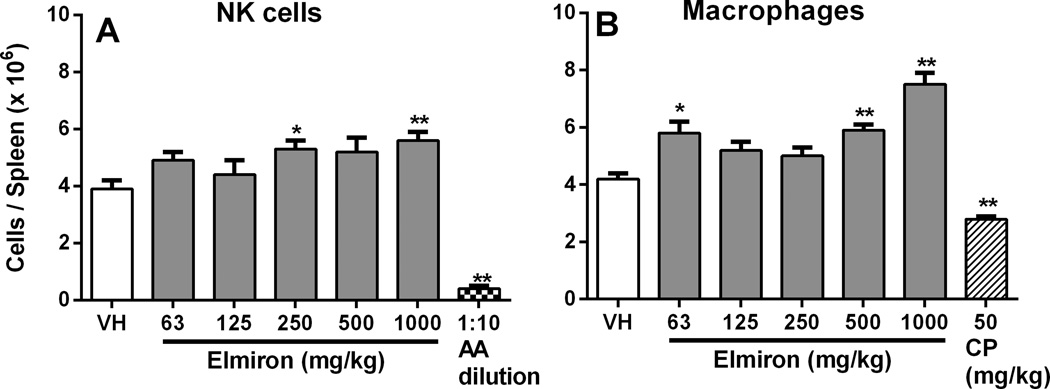
The effect of Elmiron® treatment on immune cell populations in the spleen was evaluated by flow cytometry. Total numbers of splenocytes were significantly increased in the 63 and 1000 mg/kg dose group (data not shown). Elmiron® induced significant increases in the absolute numbers of splenic NK cells and macrophages. The increase in the number of NK cells reached the level of statistical significance at the 250 and 1000 mg/kg dose levels, where increases of 36% and 44% were observed (Figure 1A). Absolute numbers of splenic macrophages were increased in the 63, 500, and 1000 mg/kg Elmiron® dose groups (Figure 1B). At these dose levels, increases of 38%, 40%, and 79% were observed, respectively. Absolute numbers of splenic B cells were increased 22% in the 1000 mg/kg treatment group (data not shown). Total T cell and absolute TCTL numbers were not significantly affected by Elmiron® treatment. Absolute numbers of TH cell numbers were significantly increased 24% at the 63 mg/kg dose only.
Figure 1. Increase in the total number of splenic NK cells and macrophages in Elmiron® treated mice.
Significant increases in absolute numbers of splenic (A) NK cells (B) Macrophages were observed following Elmiron® treatment. AAGM1 rabbit antibody and CP was used as positive control for NK cells and macrophages, respectively. Data are presented as Mean ± SEM (n=8). *p≤ 0.05, ** p≤ 0.01 as compared to the vehicle control
3.3. NK cell activity increased in Elmiron® treated mice
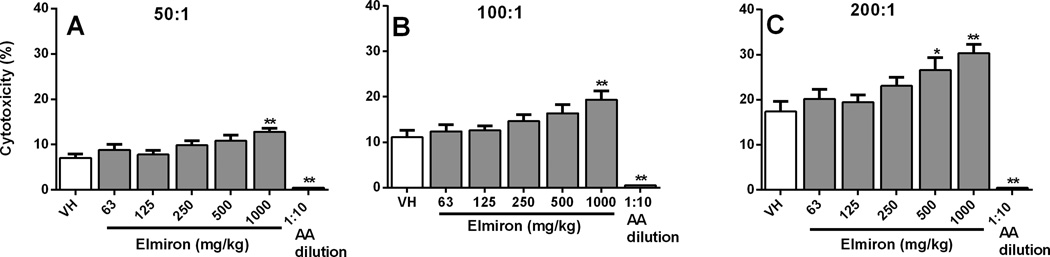
The effect of Elmiron® treatment on NK cell activity was measured ex vivo by culturing spleen cells of treated and control animals at six effector:target ratios with the YAC-1 tumor cell line, which is known to be sensitive to NK cell mediated cytotoxicity. NK cell activity in Elmiron® treated mice was significantly increased at the 1000 mg/kg dose group at the 200:1, 100:1 and 50:1 effector:target ratios (Figure 2 A–C). NK cell activity was also significantly increased at 500 mg/kg at the 200:1 effector:target ratio (Figure 2A). The increase in NK cell activity ranged from 53% and 80% and dose-dependent increasing trends were observed various effector:target ratios. As anticipated the positive control, AAGM1 antibody, significantly decreased NK cell activity.
Figure 2. Increase in NK cell activity in Elmiron® treated mice.
Splenocytes from Elmiron®-treated and control mice were co-cultured with 51Cr-labeled YAC-1 tumor cells for 4 hr at different effector:target ratios (A) 50:1 (B) 100:1 and (C) 200:1. Significant increases in NK cell cytotoxicity were observed at 500 and 1000 mg/kg dose groups. Results are expressed as percent (%) cytotoxicity. Anti-Asialo (AA) GM1 rabbit antibody was used as positive control. Data are represented as Mean ± SEM (n=8). * p≤ 0.05, ** p≤ 0.01 as compared to the vehicle control
3.4. Enhanced phagocytic response of liver macrophages in Elmiron® treated mice
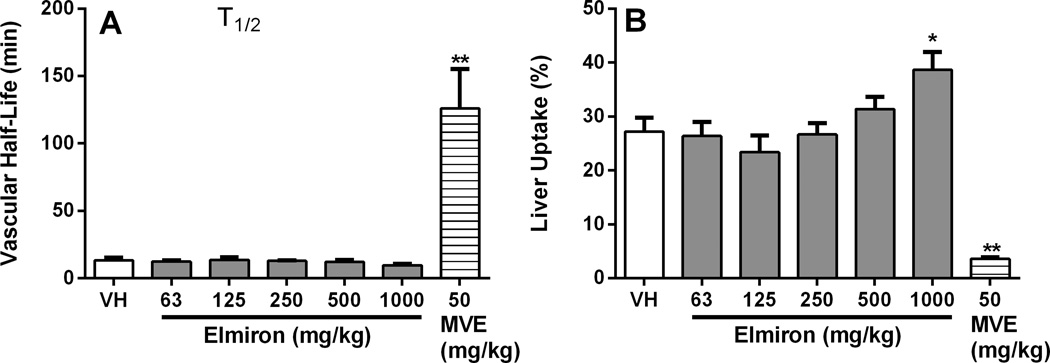
In order to evaluate the effect of Elmiron® treatment on the functional activity of the reticuloendothelial system, vascular clearance and organ distribution following injection of 51Cr-SRBC were measured. Liver, spleen, lung, thymus and kidney uptake of 51Cr-SRBC were determined in control and Elmiron® treated mice. Liver macrophages from the 1000 mg/kg Elmiron® dose group showed significantly increased (42%) uptake of 51Cr-SRBC (Figure 3B). No change in uptake of 51Cr-SRBC was observed in the other tissues evaluated including spleen, lung, thymus and kidney. Elmiron® treatment did not affect the vascular clearance rate of 51Cr-SRBC in the mice (Figure 3A). The positive control MVE significantly decreased the phagocytosis of 51Cr-SRBC and increased vascular half life, as expected (Figure 3A–B).
Figure 3. Enhanced phagocytic activity of liver macrophages in Elmiron® treated mice.
Elmiron®-treated and control mice were treated were injected i.v. with 51Cr-SRBC on day 29 of the study. (A) Vascular clearance of 51Cr-SRBC was monitored by sampling the peripheral blood from the mice. (B) Significant increase in the uptake of 51Cr-SRBC by the liver macrophages was observed in the 1000 mg/kg dose group. No significant differences in 51Cr-SRBC uptake were observed in lungs, spleen, thymus or kidney. The positive control, maleic vinyl ether (MVE), was administered i.v. on day 29 of the study. Data are represented as Mean ± SEM (n=7–8). * p≤ 0.05, ** p≤ 0.01 as compared to the vehicle control
3.5. Elmiron® treatment did not affect humoral immune responses
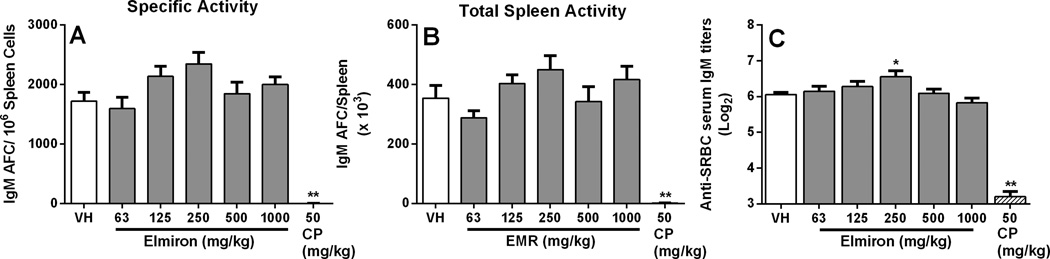
Fully functional antigen presenting cells, T lymphocytes and B lymphocytes are required for responding to a diverse array of T-dependent antigens (eg. SRBC, KLH). To evaluate whether treatment with Elmiron® altered the ability to trigger the AFC response and to mount a robust T-dependent antibody response, mice were treated with Elmiron® daily for 28 days and immunized with SRBC on day 25. There were no detectable effects on the AFC response following Elmiron® treatment when the data were expressed as specific activity (Figure 4A) or total spleen activity (Figure 4B). The positive control CP significantly inhibited the AFC response. Concurrent with the AFC response, no effect of Elmiron® treatment was observed on serum IgM antibody titers to SRBC. Although, a 16% increase in serum anti-SRBC IgM antibody was noted in the 250 mg/kg dose group, this increase was not considered biologically relevant due to the lack of a increase in the corresponding AFC response and the lack of a dose-response across treatment groups.
Figure 4. No effect on the humoral immune response in Elmiron® treated mice.
Elmiron®-treated and control mice were immunized with 7.5 × 107 SRBC by i.v. injection on day 25 of the study. No changes in humoral response following Elmiron® treatment was observed when the data was expressed as (A) Specific Activity (B) Total Spleen Activity. In addition, (C) no differences between serum levels of anti-SRBC IgM antibodies from control and Elmiron®-treated animals were observed. The positive control CP was administered i.p on days 25–28. Data are represented as Mean ± SEM (n=7–8). * p≤ 0.05, ** p≤ 0.01 as compared to the vehicle control
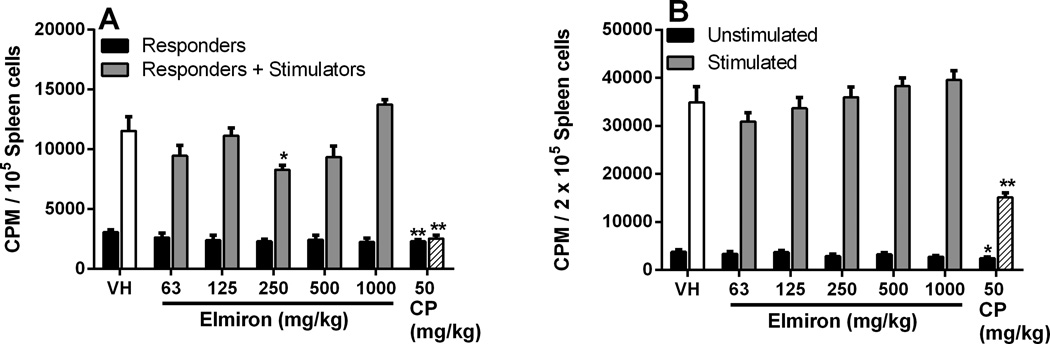
3.6. Elmiron® exposure did not modulate the T cell proliferative response
Two methods were used to examine the effects of Elmiron® on the cell-mediated immunity; proliferation following ligation of the T cell receptor by antibody to CD3 (Figure 5A) and the MLR (Figure 5B). Twenty-eight days after exposure to Elmiron®, no effects were observed on the anti-CD3-driven T cell proliferative response at any of the tested doses (Figure 5A). Similarly, no dose-dependent difference in proliferative response against allogeneic cells was observed between spleen cells from the Elmiron® -treated and control mice. At the 250 mg/kg dose, proliferation in response to allogeneic leukocytes was decreased by 28% when compared to the controls. This decrease was not considered treatment-related since there was no dose-response observed. No effects were observed in unstimulated responders at any dose level following Elmiron® exposure (Figure 5B).
Figure 5. No effect on the T cell proliferative response in Elmiron® treated mice.
(A) Anti-CD3 mediated proliferation: Splenocytes were cultured in the presence or absence of anti-CD3 antibody for 3 days. There were no significant differences in the ability of Elmiron®-treated T cells to proliferate in response to signaling through the T cell receptor initiated by anti-CD3 antibody. (B) MLR assay: Splenocytes were cultured for 5 days in the presence of mitomycin C-treated DBA/2 allogenic stimulator cells. No consistent changes in the ability of host T cells (responders) to recognize and proliferate in response to allogeneic stimulator cells were observed. The positive control CP was administered i.p. on days 25–28. CPM=counts per minute. Data are represented as Mean ± SEM (n=7–8). * p≤ 0.05, ** p≤ 0.01 as compared to the vehicle control.
3.7. Resistance to tumor cell challenge
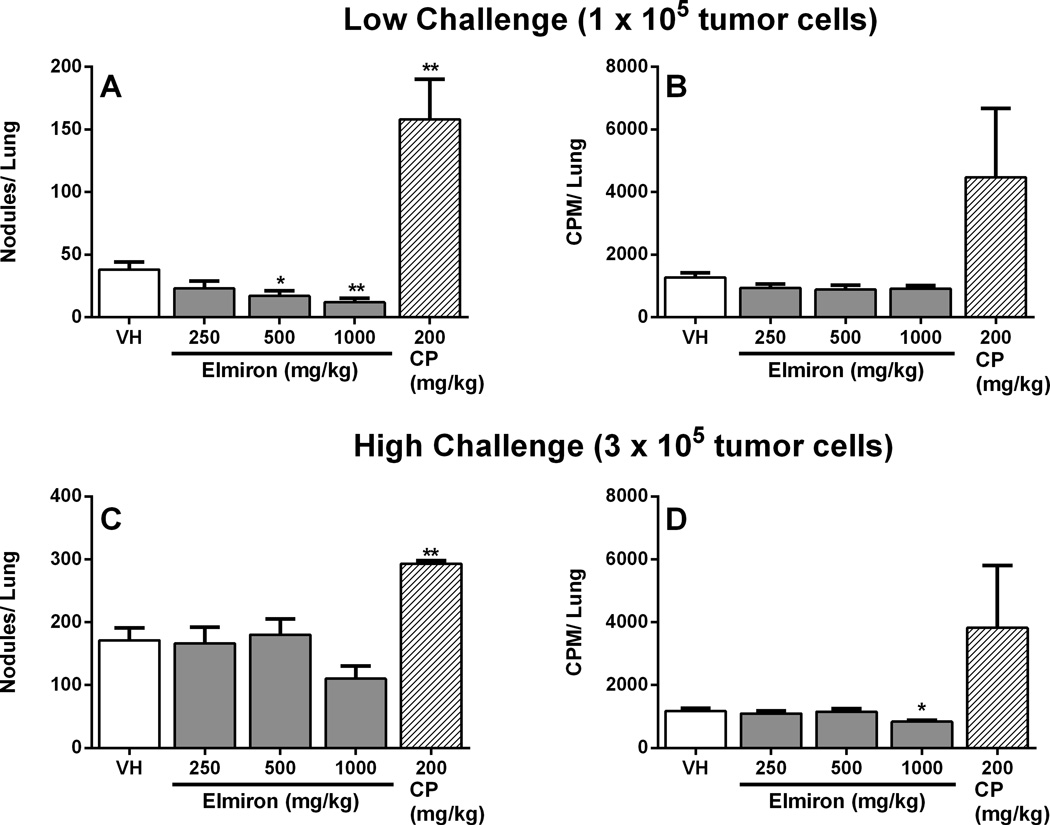
As treatment with Elmiron® significantly enhanced both cell numbers and functional measures associated with innate immunity, host resistance to the B16F10 melanoma was evaluated. B16F10 is an artificial metastatic model used for immunotoxicological evaluation because of the propensity of tumor cells to migrate from sites of injection and localize in the lungs. A dose-dependent decrease in the number of nodules per lung was observed in animals challenged with 1×105 tumor cells following treatment with Elmiron® at doses of 250, 500 or 100 mg/kg. This augmentation in tumor resistance was significant at the 500 and 1000 mg/kg dose levels; 55% and 68% respectively (Figure 6A). Dose-dependent decreases in number of tumor nodules were observed at the two highest dose levels. However, there were no differences in the level of 125IudR incorporated by proliferating tumor cells between the treated mice and controls (Figure 6B). In contrast, when challenged with a higher number of B16F10 cells (3×105), a slight decrease in the number of tumor nodules was observed in the 1000 mg/kg dose group but statistical significance was not reached (Figure 6C). However, 125IudR uptake was significantly diminished (28%) at 1000 mg/kg dose in the 3×105 challenge group (Figure 6D). Treatment with the positive control, CP, resulted in anticipated increases in both number of nodules and tumor proliferation measured by 125IudR uptake.
Figure 6. Enhanced host resistance to B16F10 melanoma in Elmiron® treated mice.
Elmiron®-treated and control mice were injected i.v. with two different concentrations of tumor cells on day 29 of the study. (A and B) Low challenge level (1× 105 tumor cells) animals were evaluated for lung tumor burden on day 19 post-challenge. Dose-dependent decreases in the number of tumor nodules/lung were observed in low challenge level animals. (C and D) High challenge level (3× 105 tumor cells) animals were assessed for lung tumor burden on day 13 postchallenge. A decrease in the number of nodules/lung was observed at 1000 mg/kg. CP was administered as positive control. Data are represented as Mean ± SEM (n=12). * p≤ 0.05, ** p≤ 0.01 as compared to the vehicle control.
4. Discussion
The FDA approved oral medication, Elmiron® is used for treatment of the chronic condition – ‘interstitial cystitis’, associated with bladder pain and urinary discomfort. As an off-label prescription, it has been used to treat osteoarthritis patients (Kumagai et al. 2010). In addition, its use as a potential treatment option for mucopolysaccharidoses is currently being investigated (Schuchman et al. 2013). With the expanding therapeutic use of Elmiron®, and with an increasing need to evaluate its potential toxicity and carcinogenicity, NTP conducted 2-week, 3-month and 2-year toxicology studies. These standard toxicology study results identified a need to conduct an additional evaluation of the potential immunotoxicity of Elmiron®. Hence, NTP conducted a 28-day immunotoxicity study in female B6C3F1/N mice for Elmiron® administered daily by oral gavage. According to the International Conference on Harmonisation (ICH) guidelines, a 28-day repeat dose study is an acceptable study design for immunotoxicological evaluation for pharmaceutical drugs.
On a body weight basis, the doses used in the current study were greater than that used for humans by 10 fold to 117 fold for female mice in the 28-day study. Elmiron® treatment had no significant effect on body weight and the 40% increase in body weight at the 250 mg/kg was considered sporadic. The observed liver weight increases in the current study were similar to the liver weight changes in the 3-month toxicity study in mice and rats. However, unlike the 3-month NTP studies, weights of a secondary immune organ (spleen) showed sporadic increases only at 63 and 125 mg/kg of Elmiron® treatment. Hence, these spleen weight changes were not considered biologically relevant in the current study. Additional evaluation of spleen cell phenotypes showed increases in total number of spleen cells and certain populations of innate immune cells, specifically NK cells and macrophages. These increases in innate immune cell components were parallel to the increases observed in peripheral leukocyte cell numbers in the 3-month standard toxicology studies ((NTP) 2004; Nyska et al. 2002). The sporadic increase in TH cell numbers in the 63 mg/kg dose was not considered biologically relevant.
A dose-dependent increase in ex vivo NK cell cytolytic activity and increase in phagocytic activity of the liver macrophages clearly demonstrated enhanced innate immune functions in Elmiron® treated mice. Previous NTP studies showed that longer term administration of Elmiron® at matching doses (0, 63, 125, 250, 500 or 1000 mg/kg) produced multiple organ histiocytic infiltration characterized by accumulation of foamy macrophages resembling a lysosomal storage disorder ((NTP) 2004; Nyska et al. 2002). Although histopathological evaluations of organs were not performed in the current 28-day study, early signs of immune perturbations were evident from increased NK cell and macrophage numbers and functionality. Both NK cells and macrophages are critical effectors of the immune system and provide early defense against viral infection and tumors. Based on these facts, enhanced innate immune function would appear to be a favorable response. However, unintended and uncontrolled activation of immune function by a drug may lead to adverse effects on immune homeostasis. For example, chronic inflammation, hypersensitivity or autoimmune disease can result from unintended immunostimulation, particularly in individuals that are genetically predisposed to inflammatory diseases.
Host resistance models are often used to evaluate treatment related effects on several immune effector mechanisms. In the metastatic B16F10 tumor model, host defense against tumor development is primarily mediated by NK cells, macrophages and CD8+ T cells. Results from the present study demonstrated that Elmiron®-treated mice caused significant increases in antitumor immunity against the B16F10 melanoma tumor. The number of tumor nodules per lung decreased with increasing concentrations of Elmiron® at the lower tumor challenge level. At the higher challenge level, the Elmiron® induced ability to resist tumor development seemed to be less effective. This could be merely due to the overwhelming of the anti-tumor immune responses due to challenge with a larger number of B16F10 tumor cells. Nevertheless, at both low and high tumor challenge levels, Elmiron® treatment enhanced resistance to B16F10 tumor development and growth. NK cells and macrophages may largely mediate Elmiron®-induced increased resistance to B16F10 tumor development, given that enhanced NK cell and macrophage functionalities were observed in the Elmiron®-treated mice. The consistency between the numbers of NK cells and macrophages, the increases in innate immune function and the B16F10 data suggests that this is a true biological effect. Elmiron®- treatment had no effect on splenic T cell numbers (both CD4+ and CD8+ T cells) and activity in mice. Additionally, previous investigative studies on anti-tumor agents such as Rituximab (an IgG1 chimeric monoclonal antibody) and thalidomide derivatives have reported similar enhancement of NK cell numbers and cytotoxic activity as one of their primary mechanisms of anti-tumor effects (Hernandez-Ilizaliturri et al. 2005; Veeramani et al. 2011).
Although the exact mechanisms for Elmiron®-induced NK cell activity is not clear, it could be speculated that Elmiron® may modulate differential immune components and effector mechanisms. No measurements of Elmiron®-induced cytokine production were conducted in the current study due to the primary focus on hazard-identification. However stimulating cytokines such as IFN-γ, IL-12, IL-18, IL-2 and IL-15 have been shown to contribute to enhancement of NK cell activity and may, in part, contribute to the effects observed in the present study. Previous studies have established that along with the cytokine microenvironment, NK cell interaction with other immune cells such as T cells and macrophages also significantly influence the cytotoxic activity of NK cells (Long 2007; Walzer et al. 2005). Elmiron® may also be acting directly on the NK cell proteins that are involved in target cell lysis, such as perforin and granzymes. These possible mechanisms remain to be explored and the current study has identified more research needs on mechanistic effects of Elmiron® treatment on NK cells and macrophages.
In contrast to the innate immune enhancing effects of Elmiron® treatment observed in the present study, a few other reports have described anti-inflammatory effects of Elmiron® in animal models of diseases such as aging mice with diabetic nephropathy (Wu et al. 2011), hyperlipidemia (Lupia et al. 2012) and inflammatory arthritis (Smith et al. 1994). Elmiron® (25 mg/kg/day) administered in drinking water has been reported to decrease macrophage infiltration in kidneys of aging streptozocin-induced diabetic C57Bl/6 mice (Wu et al. 2011). The apparent variance between the results from our study and the previously published studies could be due to the differences in the animal models used for evaluating the immunomodulatory effects of Elmiron®. The current immunotoxicity evaluation is the only study that has been conducted in healthy adult animals, while the previously published studies were conducted in disease prone animal models. Other factors could also contribute to differential responses between the studies such as doses, route of administration and treatment conditions. On the other hand, it is also likely that the Elmiron®-induced NK cell activity and release of anti-inflammatory cytokines from activated macrophages may have potentially contributed to the overall anti-inflammatory effect observed in the different animal models of disease. Overall, the current study and previous reports underscore the immunomodulatory properties of Elmiron® treatment in different animal models.
In summary, the current study demonstrated that 28-day Elmiron® treatment enhances the innate immune responses in healthy female B6C3F1/N mice by specifically increasing macrophage and NK cell number, NK cell activity and macrophage phagocytosis. Consistent with their reported function in host defense, the increases in NK cell and macrophage activity appeared to increase resistance to B16F10 tumor development in mice. The identified immune potentiating properties of Elmiron® indicates that long term Elmiron® treatment should be used with caution in patients with genetic disposition to development of inflammatory disorders such as autoimmune diseases due to potential exacerbation of innate immune responses. Additional studies identifying the mechanisms involved in upregulation of NK cell and macrophage activity would enhance our understanding of immune perturbations caused by Elmiron® treatment and suggest additional therapeutic applications.
Supplementary Material
Highlights.
Elmiron administration increases the numbers of splenic macrophages and NK cells in female mice.
Elmiron administration increased the macrophage phagocytosis and NK cell activity in female mice
Elmiron administration at 500 mg/kg or 1000 mg/kg caused decreases in growth of B16F10 melanoma tumors in female mice
Acknowledgements
This research was supported by the NIH, National Institute of Environmental Health Sciences. The authors would like to thank William M. Gwinn, Ph.D (DNTP/NIEHS) and Kymberly M. Gowdy, Ph.D. (DIR/NIEHS) for their critical and editorial review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Statement
The authors declare that there is no conflict of interest.
REFERENCES
- Abdo KM, Johnson JD, Nyska A. Toxicity and carcinogenicity of Elmiron in F344/N rats and B6C3F1 mice following 2 years of gavage administration. Arch. Toxicol. 2003;77:702–711. doi: 10.1007/s00204-003-0472-9. [DOI] [PubMed] [Google Scholar]
- Auttachoat W, Germolec DR, Collins BJ, Luebke RW, White KL, Jr, Guo TL. Immunotoxicological profile of chloroform in female B6C3F1 mice when administered in drinking water. Drug Chemical. Toxicol. 2009;32:77–87. doi: 10.1080/01480540802433880. [DOI] [PubMed] [Google Scholar]
- Bartlett MS. Properties of Sufficiency and Statistical Tests. Proceedings of the Royal Society of London Sereies A-Mathematical and Physical Sciences. 1937;160(A901):0268–0282. [Google Scholar]
- Bollaert PE, Laprevote-Heully MC, Maurizi M, Lambert H, Larcan A. Severe thrombopenia associated with the treatment with pentosan polysulfate. Ann. Fr. Anesth. Reanim. 1986;5(5):539–541. doi: 10.1016/s0750-7658(86)80044-2. [DOI] [PubMed] [Google Scholar]
- Castaneda JA, Lim MJ, Cooper JD, Pearce DA. Immune system irregularities in lysosomal storage disorders. Acta neuropathol. 2008;115:159–174. doi: 10.1007/s00401-007-0296-4. [DOI] [PubMed] [Google Scholar]
- Dealler S, Rainov NG. Pentosan polysulfate as a prophylactic and therapeutic agent against prion disease. IDrugs. 2003;6:470–478. [PubMed] [Google Scholar]
- Dunlap C, Enos E, Thom D, Zwickey H. An integrative approach to interstitial cystitis. Explore. 2013;9:48–52. doi: 10.1016/j.explore.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Dunnett CW. A Multiple Comparison Procedure for Comparing Several Treatments with a Control. J. Am. Stat.Assoc. 1955;50:1096–1121. [Google Scholar]
- Elliott CS, Payne CK. Interstitial cystitis and the overlap with overactive bladder. Curr. Urol. Rep. 2012;13:319–326. doi: 10.1007/s11934-012-0264-y. [DOI] [PubMed] [Google Scholar]
- French LM, Bhambore N. Interstitial cystitis/painful bladder syndrome. Am. Fam. Physician. 2011;83:1175–1181. [PubMed] [Google Scholar]
- Gill S, Naiman SC, Jamal A, Vickars LM. Massive bleeding on a bladder protectant: a case report of pentosan polysulfate sodium-induced coagulopathy. Arch. Intern. Med. 2002;162:1644–1645. doi: 10.1001/archinte.162.14.1644. [DOI] [PubMed] [Google Scholar]
- Gironell A, Altes A, Arboix A, Fontcuberta J, Munoz C, Marti-Vilalta JL. Pentosan polysulfate-induced thrombocytopenia: a case diagnosed with an ELISA test used for heparin-induced thrombocytopenia. Ann. Hematol. 1996;73(1):51–52. doi: 10.1007/s002770050202. [DOI] [PubMed] [Google Scholar]
- Guo TL, Germolec DR, Roesh DM, White KL., Jr Immunomodulation in female B(6)C(3)F(1) mice following treatment with the HIV protease inhibitor saquinavir for 28 days by gavage. J. Immunotoxicol. 2010;7:289–297. doi: 10.3109/1547691X.2010.495097. [DOI] [PubMed] [Google Scholar]
- Guo TL, McCay JA, Brown RD, Musgrove DL, Butterworth L, Munson AE, Germolec DR, White KL., Jr Glycidol modulation of the immune responses in female B6C3F1 mice. Drug Chem. Toxicol. 2000;23:433–457. doi: 10.1081/dct-100100127. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ilizaliturri FJ, Reddy N, Holkova B, Ottman E, Czuczman MS. Immunomodulatory drug CC-5013 or CC-4047 and rituximab enhance antitumor activity in a severe combined immunodeficient mouse lymphoma model. Clin. Cancer Res. 2005;11:5984–5992. doi: 10.1158/1078-0432.CCR-05-0577. [DOI] [PubMed] [Google Scholar]
- Jerebtsova M, Wong E, Przygodzki R, Tang P, Ray PE. A novel role of fibroblast growth factor-2 and pentosan polysulfate in the pathogenesis of intestinal bleeding in mice. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H743–H750. doi: 10.1152/ajpheart.00969.2006. [DOI] [PubMed] [Google Scholar]
- Jerne NK, Nordin AA. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963;140:405. [PubMed] [Google Scholar]
- Jonckheere AR. A Distribution-Free Kappa-Sample Test against Ordered Alternatives. Biometrika. 1954;41:133–145. [Google Scholar]
- Kawabata TT, Babcock LS, Gauggel DL, Asquith TN, Fletcher ER, Horn PA, Ratajczak HV, Graziano FM. Optimization and validation of an ELISA to measure specific guinea pig IgG1 antibody as an alternative to the in vivo passive cutaneous anaphylaxis assay. Fundam. Appl. Toxicol. 1995;24:238–246. doi: 10.1006/faat.1995.1027. [DOI] [PubMed] [Google Scholar]
- Kruskal WH, Wallis WA. Use of Ranks in One-Criterion Variance Analysis. J Am Stat Assoc. 1952;47:583–621. [Google Scholar]
- Kumagai K, Shirabe S, Miyata N, Murata M, Yamauchi A, Kataoka Y, Niwa M. Sodium pentosan polysulfate resulted in cartilage improvement in knee osteoarthritis--an open clinical trial. BMC Clin. Pharmacol. 2010;10:7. doi: 10.1186/1472-6904-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long EO. Ready for prime time: NK cell priming by dendritic cells. Immunity. 2007;26:385–387. doi: 10.1016/j.immuni.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Lupia E, Zheng F, Grosjean F, Tack I, Doublier S, Elliot SJ, Vlassara H, Striker GE. Pentosan polysulfate inhibits atherosclerosis in Watanabe heritable hyperlipidemic rabbits: differential modulation of metalloproteinase-2 and -9. Lab. Invest. 2012;92:236–245. doi: 10.1038/labinvest.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JL, Wellstein A, Rae J, DeLap RJ, Phipps K, Hanfelt J, Yunmbam MK, Sun JX, Duchin KL, Hawkins MJ. Phase I trial of orally administered pentosan polysulfate in patients with advanced cancer. Clin. Cancer Res. 1997;3:2347–2354. [PubMed] [Google Scholar]
- National Toxicology Program (NTP) NTP technical report on the toxicology and carcinogenesis studies of Elmiron (Cas No. 37319-17-8) in F344/N rats and B6C3F1 mice (Gavage Studies) National Toxicology Program technical report series, 7-289. 2004 [PubMed] [Google Scholar]
- Nyska A, Nold JB, Johnson JD, Abdo K. Lysosomal-storage disorder induced by elmiron following 90-days gavage administration in rats and mice. Toxicol. Pathol. 2002;30:178–187. doi: 10.1080/019262302753559515. [DOI] [PubMed] [Google Scholar]
- Schuchman EH, Ge Y, Lai A, Borisov Y, Faillace M, Eliyahu E, He X, Iatridis J, Vlassara H, Striker G, Simonaro CM. Pentosan polysulfate: a novel therapy for the mucopolysaccharidoses. PloS One. 2013;8:e54459. doi: 10.1371/journal.pone.0054459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Germolec DR, Luebke RW, Sheth CM, Auttachoat W, Guo TL, White KL., Jr Immunotoxicity of dibromoacetic acid administered via drinking water to female B(6)C(3)F(1) mice. J. Immunotoxicol. 2010;7:333–343. doi: 10.3109/1547691X.2010.519744. [DOI] [PubMed] [Google Scholar]
- Smith MM, Ghosh P, Numata Y, Bansal MK. The effects of orally administered calcium pentosan polysulfate on inflammation and cartilage degradation produced in rabbit joints by intraarticular injection of a hyaluronate-polylysine complex. Arthritis Rheum. 1994;37:125–136. doi: 10.1002/art.1780370118. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: The principles and practice of statistics in biological research, second ed. SanFrancisco: W.H. Freeman and Company; 1981. pp. 226–231. [Google Scholar]
- Striker GE, Lupia E, Elliot S, Zheng F, McQuinn C, Blagg C, Selim S, Vilar J, Striker LJ. Glomerulosclerosis, arteriosclerosis, and vascular graft stenosis: treatment with oral heparinoids. Kidney Inter. Suppl. 1997;63:S120–S123. [PubMed] [Google Scholar]
- Swain SM, Parker B, Wellstein A, Lippman ME, Steakley C, DeLap R. Phase I trial of pentosan polysulfate. Invest. New Drugs. 1995;13:55–62. doi: 10.1007/BF02614221. [DOI] [PubMed] [Google Scholar]
- Tardy-Poncet B, Tardy B, Grelac F, Reynaud J, Mismetti P, Bertrand JC, Guyotat D. Pentosan polysulfate-induced thrombocytopenia and thrombosis. Am. J. Hematol. 1994;45:252–257. doi: 10.1002/ajh.2830450312. [DOI] [PubMed] [Google Scholar]
- Temple L, Kawabata TT, Munson AE, White KL., Jr Comparison of ELISA and plaque-forming cell assays for measuring the humoral immune response to SRBC in rats and mice treated with benzo[a]pyrene or cyclophosphamide. Fundam. Appl. Toxicol. 1993;21:412–419. doi: 10.1006/faat.1993.1116. [DOI] [PubMed] [Google Scholar]
- Veeramani S, Wang SY, Dahle C, Blackwell S, Jacobus L, Knutson T, Button A, Link BK, Weiner GJ. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood. 2011;118:3347–3349. doi: 10.1182/blood-2011-05-351411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vij M, Srikrishna S, Cardozo L. Interstitial cystitis: diagnosis and management. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012;161:1–7. doi: 10.1016/j.ejogrb.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: "l'union fait la force". Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- White KL, Jr, Sanders VM, Barnes DW, Shopp GM, Jr, Munson AE. Immunotoxicological investigations in the mouse: general approach and methods. Drug Chem. Toxicol. 1985;8:299–331. doi: 10.3109/01480548509041062. [DOI] [PubMed] [Google Scholar]
- White KL, Musgrove DL, Brown RD. The sheep erythrocyte T-dependent antibody response (TDAR) Methods Mol. Biol. 2010;598:173–184. doi: 10.1007/978-1-60761-401-2_12. [DOI] [PubMed] [Google Scholar]
- Wilcoxon F. Individual Comparisons by Ranking Methods. Biometrics Bull. 1945;1:80–83. [Google Scholar]
- Wilson KV. A Distribution-Free Test of Analysis of Variance Hypotheses. Psychol. Bull. 1956;53:96–101. doi: 10.1037/h0047975. [DOI] [PubMed] [Google Scholar]
- Wu J, Guan TJ, Zheng S, Grosjean F, Liu W, Xiong H, Gordon R, Vlassara H, Striker GE, Zheng F. Inhibition of inflammation by pentosan polysulfate impedes the development and progression of severe diabetic nephropathy in aging C57B6 mice. Lab. Invest. 2011;91:1459–1471. doi: 10.1038/labinvest.2011.93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.