Abstract
Aims—To develop a protocol that is applicable to single strand conformation polymorphism (SSCP), direct sequencing and loss of heterozygosity analysis of DNA.
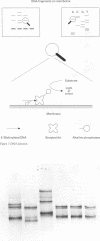
Methods—The protocol is based on the detection of biotinylated DNA by a Streptavidin-alkaline phosphatase conjugate. Biotinylation of DNA was achieved by using 5′-end biotinylated primers for PCR. After polyacrylamide gel electrophoresis, the DNA fragments were transferred to a nylon membrane by contact blotting. Depending on the alkaline phosphatase substrate, DNA was visualised either colorimetrically or by chemiluminescence.
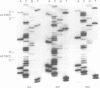
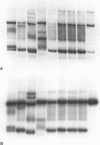
Results—The method was verified by the identification and characterisation of p53 mutations by SSCP analysis and direct DNA sequencing, as well as the assessment of DNA loss in human lung carcinomas by microsatellite polymorphism allelotyping.
Conclusions—The protocol is simple, does not require specialised equipment and would be particularly useful for laboratories with experience in Streptavidin-biotin methodology.
Keywords: SSCP
Keywords: DNA sequencing
Full text
PDF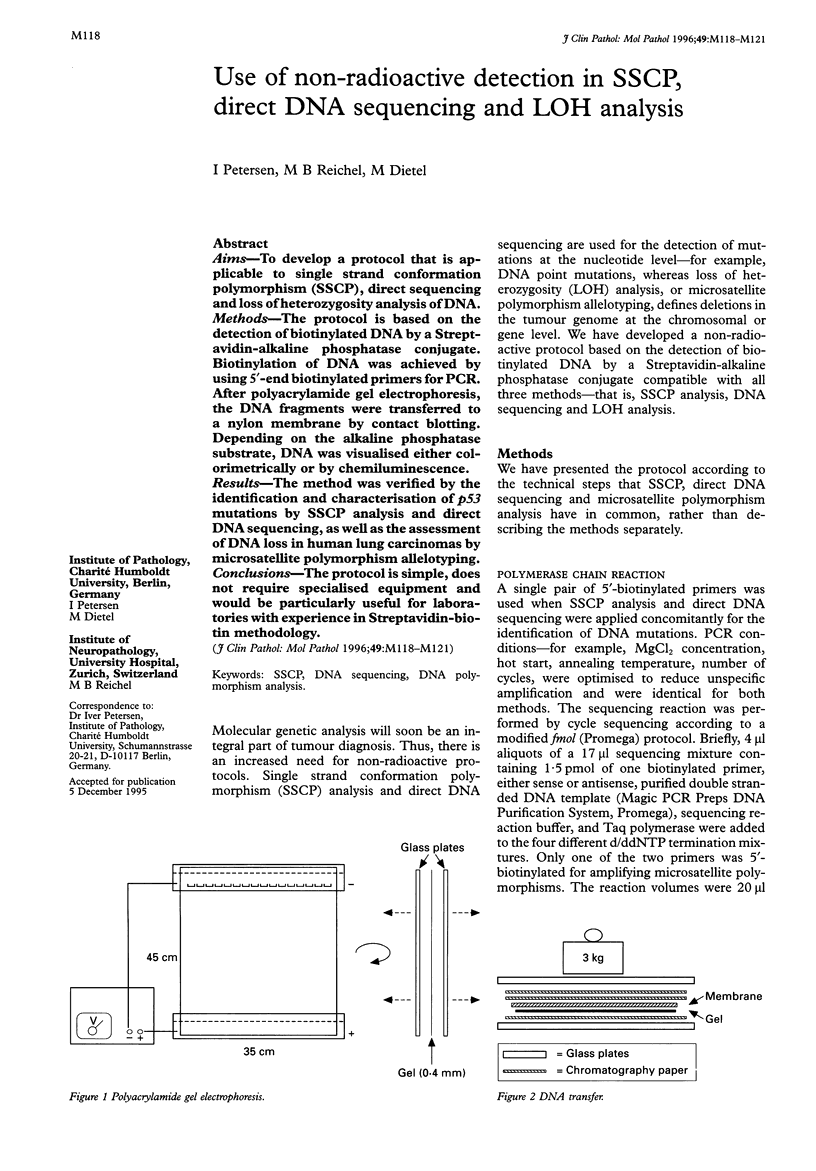
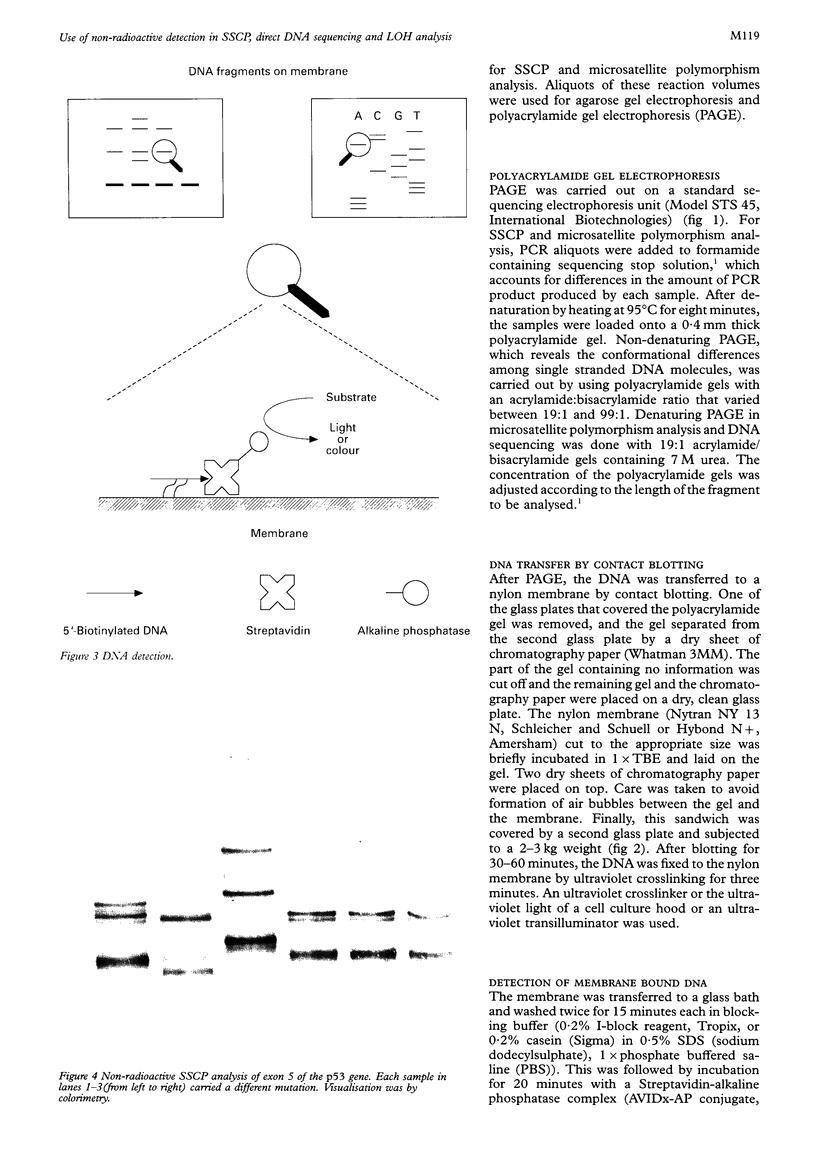
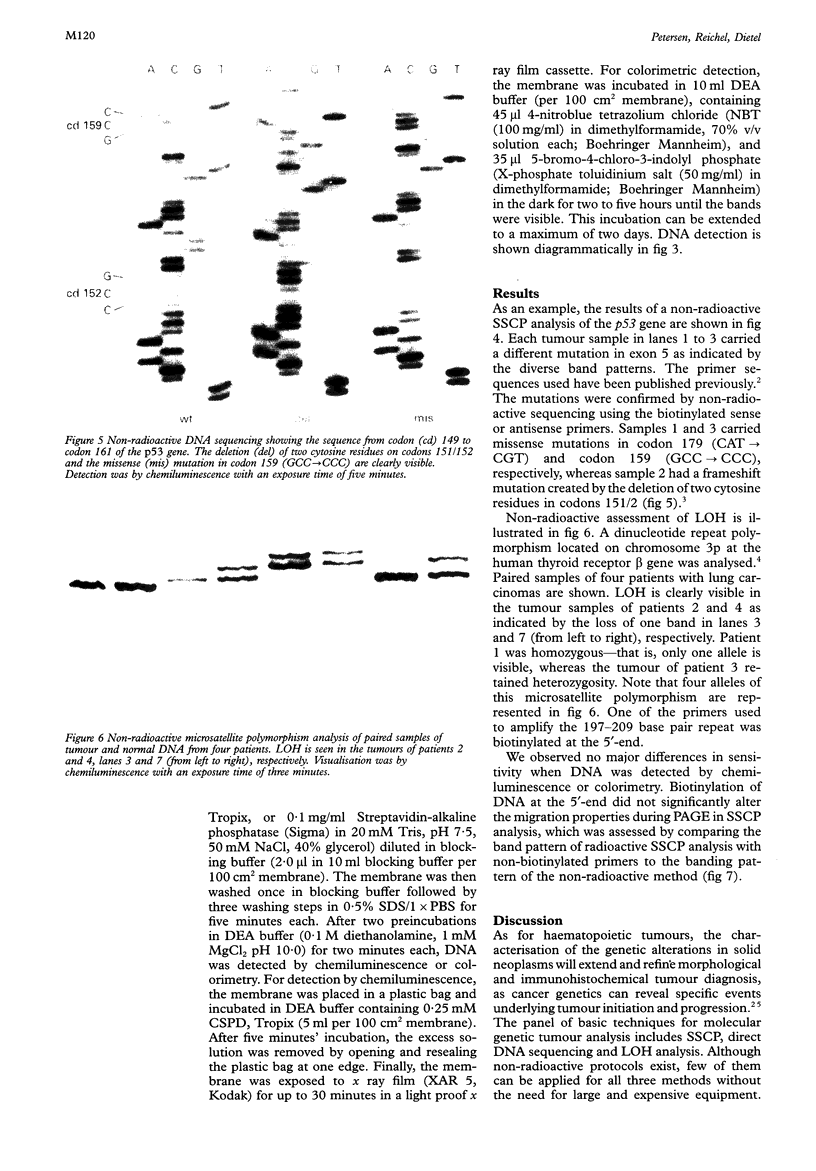
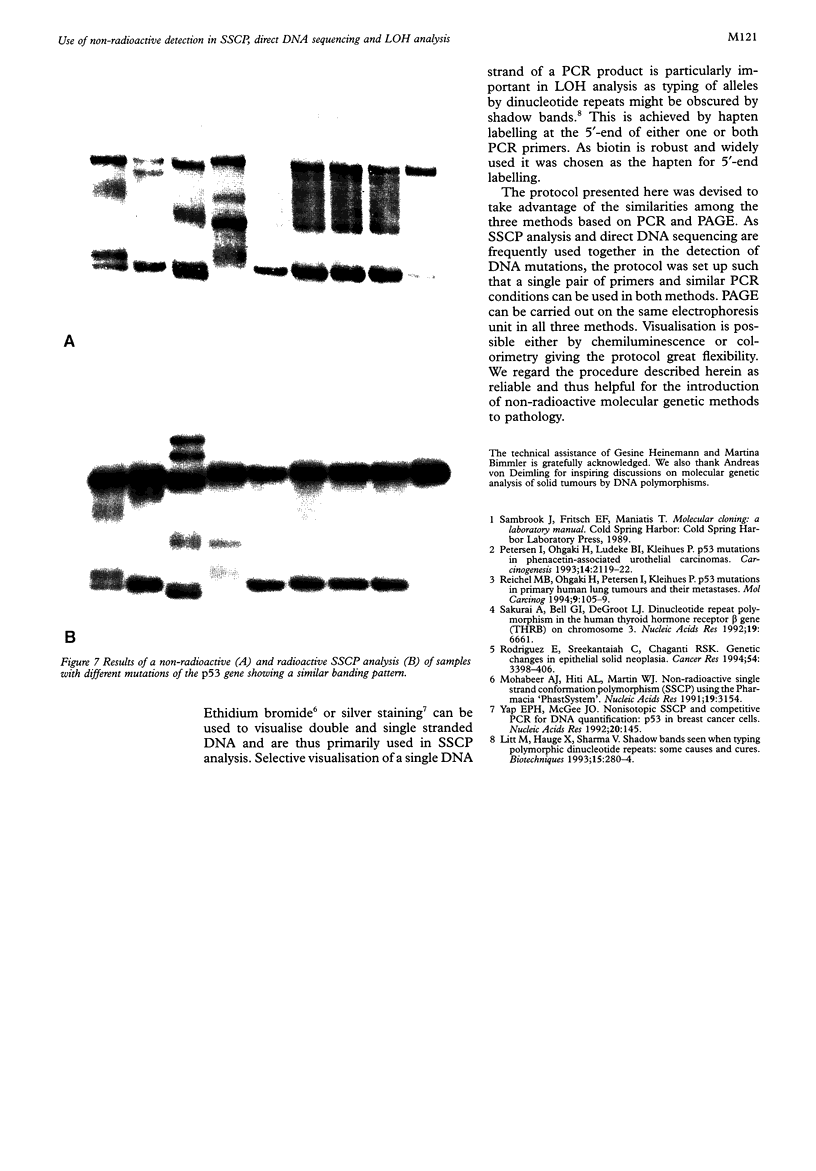
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Litt M., Hauge X., Sharma V. Shadow bands seen when typing polymorphic dinucleotide repeats: some causes and cures. Biotechniques. 1993 Aug;15(2):280–284. [PubMed] [Google Scholar]
- Mohabeer A. J., Hiti A. L., Martin W. J. Non-radioactive single strand conformation polymorphism (SSCP) using the Pharmacia 'PhastSystem'. Nucleic Acids Res. 1991 Jun 11;19(11):3154–3154. doi: 10.1093/nar/19.11.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen I., Ohgaki H., Ludeke B. I., Kleihues P. p53 mutations in phenacetin-associated human urothelial carcinomas. Carcinogenesis. 1993 Oct;14(10):2119–2122. doi: 10.1093/carcin/14.10.2119. [DOI] [PubMed] [Google Scholar]
- Reichel M. B., Ohgaki H., Petersen I., Kleihues P. p53 mutations in primary human lung tumors and their metastases. Mol Carcinog. 1994 Feb;9(2):105–109. doi: 10.1002/mc.2940090208. [DOI] [PubMed] [Google Scholar]
- Rodriguez E., Sreekantaiah C., Chaganti R. S. Genetic changes in epithelial solid neoplasia. Cancer Res. 1994 Jul 1;54(13):3398–3406. [PubMed] [Google Scholar]
- Sakurai A., Bell G. I., DeGroot L. J. Dinucleotide repeat polymorphism in the human thyroid hormone receptor beta gene (THRB) on chromosome 3. Nucleic Acids Res. 1991 Dec 11;19(23):6661–6661. doi: 10.1093/nar/19.23.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap E. P., McGee J. O. Nonisotopic SSCP and competitive PCR for DNA quantification: p53 in breast cancer cells. Nucleic Acids Res. 1992 Jan 11;20(1):145–145. doi: 10.1093/nar/20.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]