SUMMARY
Functional imaging with genetically-encoded calcium and cAMP reporters was used to examine the signal integration underlying learning in Drosophila. Dopamine and octopamine modulated intracellular cAMP in spatially-distinct patterns in mushroom body neurons. Pairing of neuronal depolarization with subsequent dopamine application revealed a synergistic increase in cAMP in the mushroom body lobes, which was dependent on the rutabaga adenylyl cyclase. This synergy was restricted to the axons of mushroom body neurons, and occurred only following forward pairing with time intervals similar to those required for behavioral conditioning. In contrast, forward pairing of neuronal depolarization and octopamine produced a sub-additive effect on cAMP. Finally, elevating intracellular cAMP facilitated calcium transients in mushroom body neurons, suggesting that cAMP elevation is sufficient to induce presynaptic plasticity. These data suggest that rutabaga functions as a coincidence detector in an intact neuronal circuit, with dopamine and octopamine bidirectionally influencing the generation of cAMP.
INTRODUCTION
Olfactory classical conditioning is a well-studied form of associative learning in the fruit fly Drosophila melanogaster. In this paradigm, a fly is trained to associate an odor, the conditioned stimulus (CS), with a reward or punishment, the unconditioned stimulus (US). Genetic screens in Drosophila have uncovered many genes that are required for olfactory classical conditioning (Davis, 1996, 2005). Several of these function in the 3’–5’-cyclic adenosine monophosphate (cAMP) signaling pathway, including rutabaga (rut), which encodes an adenylyl cyclase (AC) related to the mammalian type-I cyclase.
A long-standing hypothesis is that cAMP elevation may “trigger” olfactory memory formation (e.g., Davis, 1993; Davis, 2005; Keene and Waddell, 2007). Neurons in the olfactory pathway are directly activated by odor, resulting in calcium influx through voltage-sensitive calcium channels. They also may receive information about punishment and reward via dopaminergic and octopaminergic interneurons, respectively (Han et al., 1996; Han et al., 1998; Schwaerzel et al., 2003; Schroll et al., 2006). These two input pathways could converge on the rut AC, which is sensitive to both calcium and G protein stimulation (Livingstone et al., 1984; Levin et al., 1992). In Aplysia, pairing calcium with activation of G protein signaling (via serotonin) generates synergistic increases in cAMP in membrane preparations (Abrams et al., 1991; Yovell and Abrams, 1992). A similar effect may occur in Drosophila when calcium is paired with dopamine and/or octopamine. However, there is little direct evidence to support this hypothesis for Drosophila memory formation, and likewise there is little experimental support for synergistic increases in cAMP in a preparation where neural circuitry remains intact.
Several anatomical regions of the Drosophila brain are involved in olfactory associative learning (Davis, 2005; Keene and Waddell, 2007; Liu and Davis, 2006). Functional imaging experiments have revealed memory traces in several areas: the antennal lobe, DPM neuron, APL neuron, and mushroom body α/β and α’/ β’ neurons (Yu et al., 2003, 2005, 2006; Ashraf et al., 2006; Wang et al., 2008; Liu and Davis, 2009). These memory traces were observed as alterations in synaptic transmission and/or calcium influx, and represent direct observations of plasticity that is associated with learning. Memory traces in the DPM neuron and mushroom body neurons are branch specific, occurring only in the vertical lobes (not the medial lobes) – i.e., they appear in one set of axon collaterals independent of the other. In addition, the plasticity associated with appetitive conditioning may be spread across both the antennal lobes and mushroom bodies (Thum et al., 2007).
The mushroom body may be a critical site of CS/US integration through cAMP signaling. Several different enzymes of the cAMP signaling pathway exhibit preferential expression in the mushroom body (Nighorn et al., 1991; Han et al., 1992; Skoulakis et al., 1993). The alpha-lobes-absent mutant, which lacks the vertical α and α’ mushroom body lobes, is deficient in long-term aversive memory (Pascual and Préat, 2001). Most compelling, prior transgenic rescue experiments revealed that wild-type rut function is required only in the adult mushroom body for complete phenotypic rescue in aversive conditioning tests (Zars et al., 2000; McGuire et al., 2003; Mao et al., 2004). Since cAMP signaling is critical for performance in both appetitive and aversive conditioning paradigms (Tempel et al., 1983), the intracellular signaling cascades are likely similar in both types of learning.
Many details about the cellular pathways, temporal steps, and CS/US integration underlying learning are poorly understood. For instance, (i) how do calcium and cAMP levels change upon arrival of the CS or US? (ii) What effect does CS/US coincidence have on cAMP levels? (iii) Is elevation of cAMP sufficient to induce plasticity? Here we have examined these questions using a novel preparation in which functional imaging of calcium and cAMP in intact Drosophila brains was paired with focal application of neurotransmitters, an approach akin to those used effectively to dissect cellular memory in Aplysia (e.g., Martin et al., 1997; Kandel, 2001). This hybrid approach, incorporating a complete intact neuronal circuit with the specificity of focal neurotransmitter application, bridges the gap between in vivo methods and more reduced preparations.
RESULTS
Effects of dopamine and octopamine on calcium and cAMP in mushroom body neurons
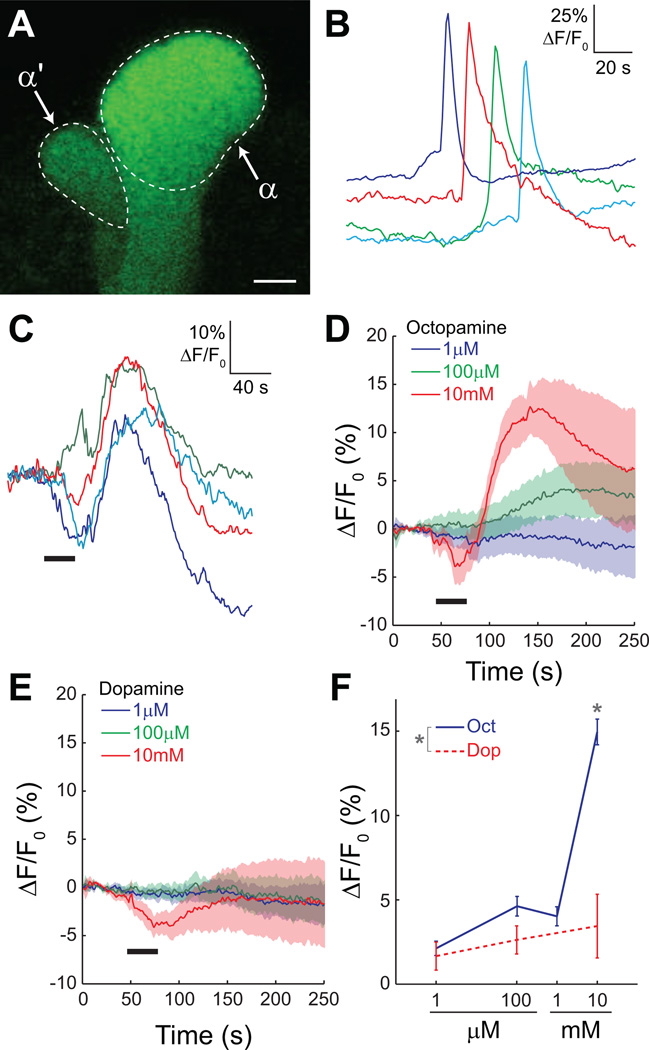
To determine the intracellular signaling cascades activated by the neurotransmitters dopamine and octopamine, we first tested whether mushroom body neurons in dissected brains respond to these transmitters with a change in intracellular calcium. We used the GAL4-UAS system to express a genetically-encoded calcium indicator, G-CaMP 1.6 (Reiff et al., 2005), in the adult Drosophila mushroom bodies using the 238Y-GAL4 driver (Yang et al., 1995). This GAL4 driver expresses in all three class of mushroom body neurons – α/β, α’/β’, and γ The tip of the mushroom body α lobe was imaged, which contains bundled axons of intrinsic mushroom body α/β neurons (Figure 1A). We verified in initial experiments that the isolated brains were viable by depolarizing the neurons with a bath-applied high-K+ solution (saline containing 100 mM K+). Upon depolarization, we observed robust calcium transients in the mushroom body α lobe (Figure 1B). Brains were viable in vitro for ≥ 4 hours, but we restricted recordings to within 1.5 hours of the dissection.
Figure 1.
Dopamine and octopamine have little detectable effect on calcium at low concentrations.
(A) Confocal image showing the tips of the α and α’ lobes in the brain of a 238Y-GAL4> UAS-G-CaMP fly. The image was collected across 256×256 pixels, the same as used for time series imaging. Scale bar = 5 µm.
(B) Examples of calcium responses in the α lobe of four different brains to depolarization with 100 mM K+. The traces are diagonally offset for visibility.
(C) Examples of α lobe responses to 10 mM octopamine. There was a robust increase in calcium in the α lobe following stimulation. Horizontal black bar = stimulus application.
(D) Mean responses of the lobe to three concentrations of octopamine. The shaded area bracketing the mean response (solid line) indicates the S.E.M. Horizontal black bar = stimulus application.
(E) Mean responses of the α lobe to three concentrations of dopamine. There were no consistent or robust responses to dopamine at any of the concentrations tested. Horizontal black bar = stimulus application.
(F) Dose-response curves for α lobe calcium responses to dopamine and octopamine (n = 10). Error bars indicate S.E.M.
The effects of dopamine and octopamine on intracellular calcium were tested by bath-applying each in concentrations ranging from 1 µM to 10 mM. We observed large calcium transients in the α lobe following application of 10 mM octopamine (15.0 ± 2.0% ΔF/F0) (Figure 1 C,D,F). However, at lower concentrations we observed only small responses (Figure 1 D,F). Dopamine did not evoke consistent responses at any concentration that was tested (Figure 1 E,F). Octopamine produced significantly larger responses than dopamine at 10 mM (Fisher’s LSD following ANOVA, p < 0.001). Since depolarization and subsequent firing of action potentials generate large influxes of calcium, it follows that dopamine and octopamine do not significantly depolarize mushroom body neurons at low to moderate concentrations. Similar results were obtained for the α’ lobe (not shown).
Since dopamine and octopamine receptors are coupled to cAMP signaling in addition to calcium (Han et al., 1996, 1998), we turned to cAMP imaging using the genetically-encoded optical reporter epac1-camps (Nikolaev et al., 2004; Shafer et al., 2008). Epac1-camps is a fluorescence resonance energy transfer (FRET)-based reporter comprised of a cAMP binding domain with cyan and yellow fluorescent proteins (CFP and YFP). When the CFP is excited, the inverse FRET ratio (CFP:YFP emission) is proportional to the intracellular cAMP concentration. Epac1-camps has been previously used as a cAMP reporter in Drosophila (Shafer et al., 2008). In initial experiments, we confirmed the responses of the reporter by using forskolin to elevate cAMP via direct stimulation of adenylyl cyclases. Brains were dissected from flies expressing epac1-camps in the mushroom body neurons using the 238Y-GAL4 driver (Figure 2A). Upon stimulation with forskolin, we observed an increase in the inverse FRET ratio (plotted as ΔR/R0) (Figure 2 B,C). When the reporter was excited with a 514-nm laser line, which excites primarily the YFP, there was no decrease in YFP fluorescence. The increases in cAMP following forskolin application were concentration-dependent (Figure 2 B,D). Although epac1-camps been reported to respond to both cAMP and cGMP in the Drosophila neuromuscular junction (Shakiryanova and Levitan, 2008), we found no evidence of cGMP responses in the mushroom body (Supplemental Figure 2). Having verified that we could reliably detect cAMP increases, we turned to measuring the changes in cAMP induced by application of dopamine and octopamine.
Figure 2.
Forskolin, dopamine, and octopamine increase cAMP in mushroom body neurons in a rutabaga-independent manner. Error bars in all panels indicate S.E.M.
(A) Confocal image showing CFP and YFP emission from the mushroom body α and α’ lobes in the brain of a 238Y-GAL4>UAS-epac1-camps fly. The image was collected across 256×256 pixels, the same as used for time series imaging. Scale bar = 10 µm.
(B) Responses of the α lobe to three concentrations of forskolin. The data are plotted in terms of the change in inverse FRET ratio (ΔR/R0).
(C) Pseudocolor images showing the time course of a representative response to forskolin (100 µM).
(D) Dose-response curve for mushroom body α lobe cAMP responses to forskolin in 238Y-GAL4>UAS-epac1-camps flies (n = 7).
(E) Dose-response curves for mushroom body α lobe cAMP responses to bathapplied dopamine and octopamine in 238Y-GAL4>UAS-epac1-camps flies (n = 5). Responses were fit with a four parameter logistic curve. Cyclic AMP responses were observed at lower concentrations of dopamine and octopamine than that required to evoke detectable changes in intracellular calcium (Figure 1). The average maximal responses to dopamine and octopamine (11.9 ± 1.3% and 11.5 ± 1.9% ΔR/R0, respectively) were similar to the response to 10 µM forskolin (11.2 ± 1.2% ΔR/R0).
(F) Dose-response curves for dopamine from rut1 mutants and heterozygous control flies (n = 13). Responses were recorded in the α lobe of brains from 238Y-GAL4>UAS-epac1-camps rut1 or control flies (rut1 heterozygotes).
(G) Dose-response curves for octopamine from rut1 mutants and heterozygous control flies (n = 13). Data are from the same set of flies in panel F.
Responses of the mushroom body α lobe to bath-applied dopamine and octopamine were tested in brains isolated from 238Y-GAL4>UAS-epac1-camps flies. Both dopamine and octopamine produced concentration-dependent increases in cAMP in the lobe (Figure 2E). The octopamine responses were significantly larger at 10 µM and 100 µM (Fisher’s LSD following ANOVA, respectively: p = 0.0085 and 0.0070). The magnitude of the cAMP changes evoked by 10 mM dopamine were not significantly different than those generated by 10 µM forskolin (mean ± S.E.M.: 11.9 ± 1.3% and 11.2 ± 1.2% ΔR/R0, respectively; t test, p = 0.72) (Figure 2 D,E).
Since approximately 50% of initial learning performance is dependent upon the rutabaga AC, it follows that rut-generated cAMP underlies this fraction of initial performance (Han et al., 1992). Therefore, we tested whether the cAMP responses to dopamine and octopamine in the mushroom body were dependent on rut. We focused on the mushroom body, as prior experiments showed that rut expression in mushroom body neurons is sufficient to support performance in aversive conditioning paradigms (Zars et al., 2000; McGuire et al., 2003; Mao et al., 2004). Dose-response curves were collected for dopamine and octopamine in rut1 and control flies (heterozygous rut1/rut+ flies, which perform at wild-type level in initial learning tests; Han et al., 1992; Supplemental Figure 3) (Figure 2 F,G). Expression of epac1-camps was driven in the mushroom bodies by 238Y-GAL4, and responses were recorded from the lobe. There was no significant difference between the responses of the control and mutant flies for either dopamine (repeated-measures ANOVA, p = 0.59, n = 13; Figure 2F) or octopamine (p = 0.29, n = 13; Figure 2G). This suggests that the increases in cAMP that are evoked by bath application of dopamine and octopamine are mediated by a different adenylyl cyclase.
Dopamine and octopamine elevate cAMP in different spatial patterns
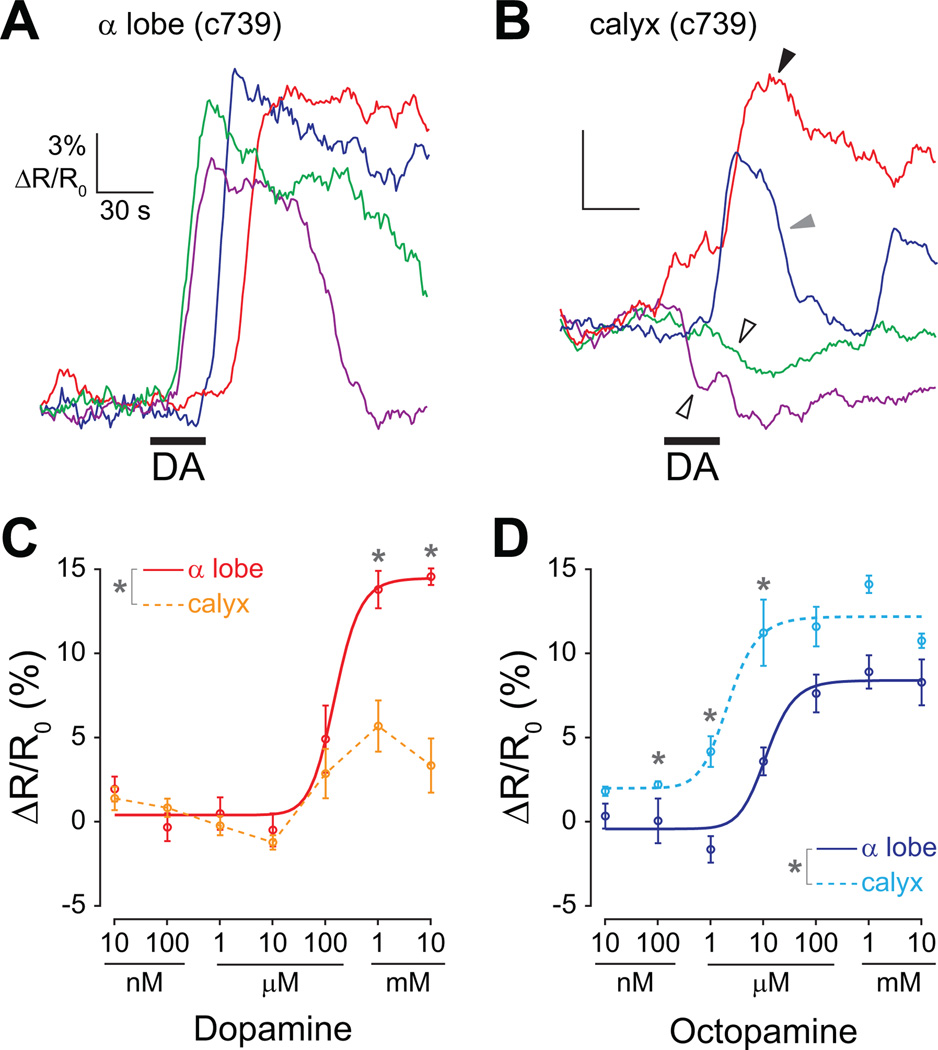
We compared the cAMP responses in the dendrites (calyx) and axons (α lobe) of mushroom body α/β neurons using the α/β neuron-specific c739-GAL4 driver (Figure 3). The responses in the α lobe were similar to those recorded in the lobe with the 238Y-GAL4 driver – we observed consistent concentrationdependent increases in cAMP with both dopamine and octopamine (Figure 3 A,C,D). However, the responses to dopamine in the calyx were remarkably different than those recorded in the α lobe (Figure 3 A–C). We observed heterogeneity in both the sign and magnitude among calyx responses to dopamine. Dopamine increased cAMP in the calyx in some recordings, and decreased it in others (Figure 3B). This heterogeneity resulted in a non-sigmoid, relatively flat dose-response curve (Figure 3C). The dopamine responses in the lobe were significantly larger at 1 mM and 10 mM (Fisher’s LSD following ANOVA, respectively: p = 0.0065 and <0.001). The calyx was highly sensitive to octopamine, with responses observed to concentrations as low as 1 µM and saturation occurring at 10 µM (Figure 3D). There were significantly larger responses to octopamine in the calyx at 100 nM, 1 µM, and 10 µM (Fisher’s LSD following ANOVA, respectively: p = 0.043, 0.0027, and 0.020). Since the responses in the calyx differed significantly from those in the α lobe (especially to dopamine), cAMP diffusion must be spatially-restricted within mushroom body α/β neurons. The difference in dopamine effects between the calyx and lobes further suggests that dopamine may play a different role in these two areas.
Figure 3.
Dopamine has different effects on cAMP in the dendrites (calyx) and axons (α lobe) of mushroom body α/β neurons.
(A) Examples of cAMP responses to 1 mM dopamine in the lobe of brains isolated from c739-GAL4>UAS-epac1-camps flies. Dopamine reliably increased cAMP in recordings. Horizontal black bar = stimulus application.
(B) Examples of cAMP responses to 100 µM dopamine in the calyx of brains isolated from c739-GAL4>UAS-epac1-camps flies. Since the epac1-camps reporter was expressed in mushroom body neurons and not projection neurons, these recordings represent the changes in cAMP in the dendrites of mushroom body α/β neurons. The calyx responses were heterogeneous; in some cases, dopamine elevated cAMP (black arrowhead), and in others dopamine decreased cAMP (unfilled arrowheads). In some brains there was an increase, followed by an abrupt decrease and then another increase (gray arrowhead).
(C) Dose-response curves for dopamine recorded in the α lobe and calyx (n = 5). In the lobe only increases in cAMP were observed, resulting in a sigmoid dose-response curve. However, in the calyx, the heterogeneity of the responses resulted in a non-sigmoid dose-response relationship (data from the calyx are consequently not fitted with a curve).
(D) Dose-response curves for octopamine recorded in the α lobe and calyx (n = 5). In both the α lobe and calyx, consistent increases in cAMP were observed, resulting in a sigmoid dose-response curve. The calyx and α lobe recordings were taken from the same two sets of brains as the calyx and α lobe recordings in panel C (respectively).
The mushroom body α’/β’ and γ neurons were also tested for cAMP responsiveness to dopamine and octopamine by driving epac1-camps with c305a-GAL4 (Krashes et al., 2007) and 1471-GAL4 (Isabel et al., 2004), respectively. We observed concentration-dependent increases in cAMP in these sets of mushroom body neurons (Figure 4 A,B). However, these neurons were less sensitive. Their thresholds were 10–100 µM, and we did not observe an asymptote within the range of concentrations tested. There was no significant difference between the responses to dopamine and octopamine in either the α’ lobe (repeated-measures ANOVA, p = 0.38, n = 5) or the γ lobe (p = 0.95, n = 5).
Figure 4.
Dopamine and octopamine elevate cAMP in multiple anatomical loci across the olfactory system. Four parameter logistic curves were fitted to the responses. Error bars in all panels indicate S.E.M., n = 5 each.
(A) Axons of γ lobe neurons, recorded in the mushroom body γ lobe of brains from 1471-GAL4>UAS-epac1-camps flies.
(B) Axons of α’/β’ neurons, recorded in the mushroom body α’ lobe of brains from c305a-GAL4>UAS-epac1-camps flies.
(C) Neurites of the DPM neuron, recorded in the mushroom body α lobe of brains from c316-GAL4>UAS-epac1-camps flies.
(D) Dendrites of projections neurons (PN), recorded in the antennal lobes of brains from GH146-GAL4>UAS-epac1-camps flies.
(E) Axons of projection neurons, recorded in the mushroom body calyx in brains from GH146-GAL4>UAS-epac1-camps flies.
The DPM neuron, a mushroom body extrinsic neuron that innervates the mushroom body lobes, was tested by imaging its processes innervating the α lobe using the c316-GAL4 driver (Waddell et al., 2000). The responses to octopamine were significantly larger than the dopamine responses at 1 mM (p = 0.034 following ANOVA). We observed responses in the DPM neuron only at the two highest concentrations tested (1 mM and 10 mM) (Figure 4C).
Finally, we tested the projection neurons of the antennal lobe, which relay olfactory information from the antennal lobes to the mushroom body. The GH146-GAL4 driver (Stocker et al., 1997) was used to express epac1-camps in the antennal lobe projection neurons. First we imaged the dendrites of the projection neurons in the antennal lobes. All visible glomeruli were collectively recorded in one region of interest drawn around the antennal lobe. The projection neuron dendrites exhibited concentration-dependent increases in cAMP to dopamine and octopamine (Figure 4D). The responses to octopamine were significantly larger than to dopamine at 100 nM, 1 µM, 10 µM, 100 µM, and 10 mM (Fisher’s LSD following ANOVA, respectively: p = 0.0081, 0.034, 0.0046, <0.001, and <0.001). Similar, though somewhat smaller, responses were observed in the axons of the projection neurons, imaged at the location of the axon terminals in the mushroom body calyx (Figure 4E). The responses to octopamine were significantly larger than to dopamine at 1 µM, 10 µM, 100 µM, and 1 mM (Fisher’s LSD following ANOVA, respectively: p = 0.017, 0.0084, 0.011, and <0.001). The projection neurons exhibited the largest responses of any area imaged in this study (Figure 4).
The rutabaga adenylyl cyclase generates synergistic cAMP elevations
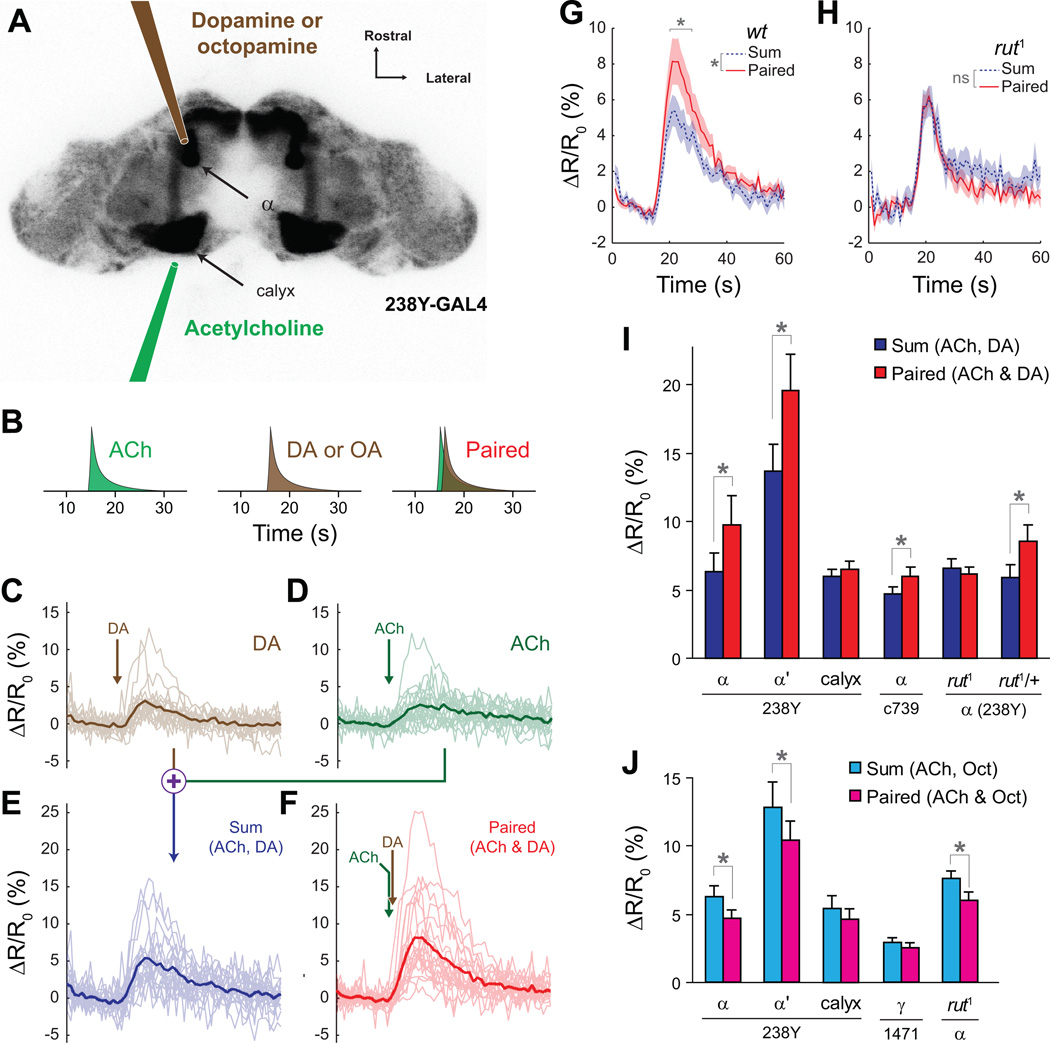
To test whether the rut cyclase functions as a molecular coincidence detector within an intact neural circuit, we studied the dynamics of cAMP changes while focally applying neurotransmitters sequentially to the mushroom body lobes and calyx with micropipettes (Figure 5). Drosophila brains expressing the cAMP reporter in the mushroom bodies (238Y-GAL4>UAS-epac1-camps) were imaged. Acetylcholine (30 mM) was applied to the mushroom body calyx with a glass micropipette (Figure 5A). Mushroom body neurons receive synaptic input from cholinergic synapses of the projection neurons (Gu and O’Dowd, 2006), and acetylcholine thus simulates synaptic input to mushroom body neurons, which encodes the CS during olfactory learning. Dopamine (1 mM) or octopamine (100 µM) was applied to the vertical mushroom body lobes to simulate the US (Figure 5), as previous work has shown that the majority of dopaminergic projections in the mushroom body innervate the lobes (Friggi-Grelin et al., 2003; Mao and Davis, 2009). At the beginning of each experiment, the stimuli were adjusted so that both acetylcholine and dopamine generated a response above threshold but below saturation, as measured in the mushroom body α lobe (Figure 5 C,D) (saturation occurs at 11.9% on average; Figure 2E). A series of pilot experiments revealed that multiple, sequential neurotransmitter stimulations did not produce any priming effect at an interstimulus interval of 3 m (Supplemental Figure 5).
Figure 5.
Pairing of neuronal depolarization with dopamine induced a rutabaga-dependent synergistic elevation of cAMP in mushroom body neurons, while pairing of neuronal depolarization with octopamine resulted in sub-additive 43 increases in cAMP. Panels C–H show cAMP imaging traces recorded from the α lobe of 238Y-GAL4>UAS-epac1-camps brains.
(A) Schematic showing placement of the focal application pipettes. The underlying image is an inverted grayscale confocal image of the brain of a 238Y-GAL4> UAS-epac1-camps fly. The pipettes were placed directly anterior to the α lobe and posterior to the calyx.
(B) Schematic of the stimulus paradigm. Each stimulus was presented three minutes apart.
(C) Recordings from the α lobe of individual brains (thin lines) and the mean response (thick line) during application of dopamine (DA) to the mushroom body vertical lobes. Arrow indicates the stimulus time.
(D) Recordings from the α lobe during application of acetylcholine (ACh) to the calyx.
(E) The sum of the responses to application of DA and ACh were calculated for each brain.
(F) Recordings in which the application of DA and ACh were paired. ACh was applied 1 s before DA. The data from panels C–F are from the same set of brains.
(G) Comparison of the responses from the sum (panel E) and paired (panel F) conditions. The cAMP response to paired application of ACh and DA was synergistic. The shaded area bracketing the mean response (solid line) represents the S.E.M. The horizontal gray bracket denotes an 8 s window in which pairwise tests revealed significantly larger (p < 0.01) paired responses (Fisher’s LSD following significant difference with ANOVA).
(H) Comparison of the responses from the sum and paired conditions in rut1 mutant flies. The response to paired application of ACh and DA was not synergistic in rut1 mutants.
(I) Sum and paired response magnitudes from dopamine/acetylcholine experiments for different driver lines and regions imaged. Error bars indicate S.E.M.; *p<0.05.
(J) Sum and paired response magnitudes from octopamine/acetylcholine experiments for different driver lines and regions imaged. Error bars indicate S.E.M.; *p<0.05.
To test for synergy, responses were recorded for each brain to dopamine and acetylcholine in separate trials (Figure 5 B–D), and the sum of the two individual trials was calculated (Figure 5E). Following washout, we paired the stimuli, applying acetylcholine to depolarize the mushroom body neurons, followed 1 s later by dopamine applied to the lobes (Figure 5B). This paired response was compared to the sum of the individual trials. If there was an additive effect, the sum of responses in the individual trials would equal that of the paired trial. However, if there was a synergistic effect, the response in the paired trial would be greater than the sum of those in the individual trials.
Using 238Y-GAL4>UAS-epac1-camps flies, we found that pairing of neuronal depolarization with dopamine application resulted in a larger increase in cAMP in the α lobe than the sum of both stimuli applied independently; i.e., the response was synergistic (Figure 5 E,F). Both the overall response profiles (repeated-measures ANOVA, p = 0.0082, n = 11) and the response magnitudes (t test, p = 0.041) differed significantly between the sum and paired responses (Figure 5 G,I). This effect was also observed in the α’ lobe (t test; p = 0.0025, n = 11; Figure 5I). To ensure that the synergistic activation was not an artifact induced by either the 238Y-GAL4 or UAS-epac1-camps insertions, we repeated the experiments using the c739-GAL4 driver and a different epac1-camps insertion on the 3rd chromosome. Synergistic activation in the lobe was observed using the c739 driver as well (t test, p = 0.035, n = 12; Figure 5I). The synergistic effect was absent in rut1 mutant flies – neither the overall response profiles (repeated-measures ANOVA, p = 0.15, n = 9) nor the response magnitudes (t test, p = 0.53) differed significantly between the sum and paired responses in rut1 flies (Figure 5 H,I). Heterozygous control flies exhibited the synergistic response, demonstrating that the loss of synergy in the rut1 mutants is specifically due to the absence of the cyclase (t test, p = 0.027, n = 9; Figure 5I). Finally, we tested whether application of dopamine to the calyx would have the same effect. Focal application of both acetylcholine and dopamine to the calyx did not yield any synergy in the cAMP responses of the calyx (t test, p = 0.36, n = 12; Figure 5I). These data collectively demonstrate that neuronal depolarization followed by application of dopamine to the mushroom body lobes generates a rut-dependent, synergistic increase in cAMP in the mushroom body lobes.
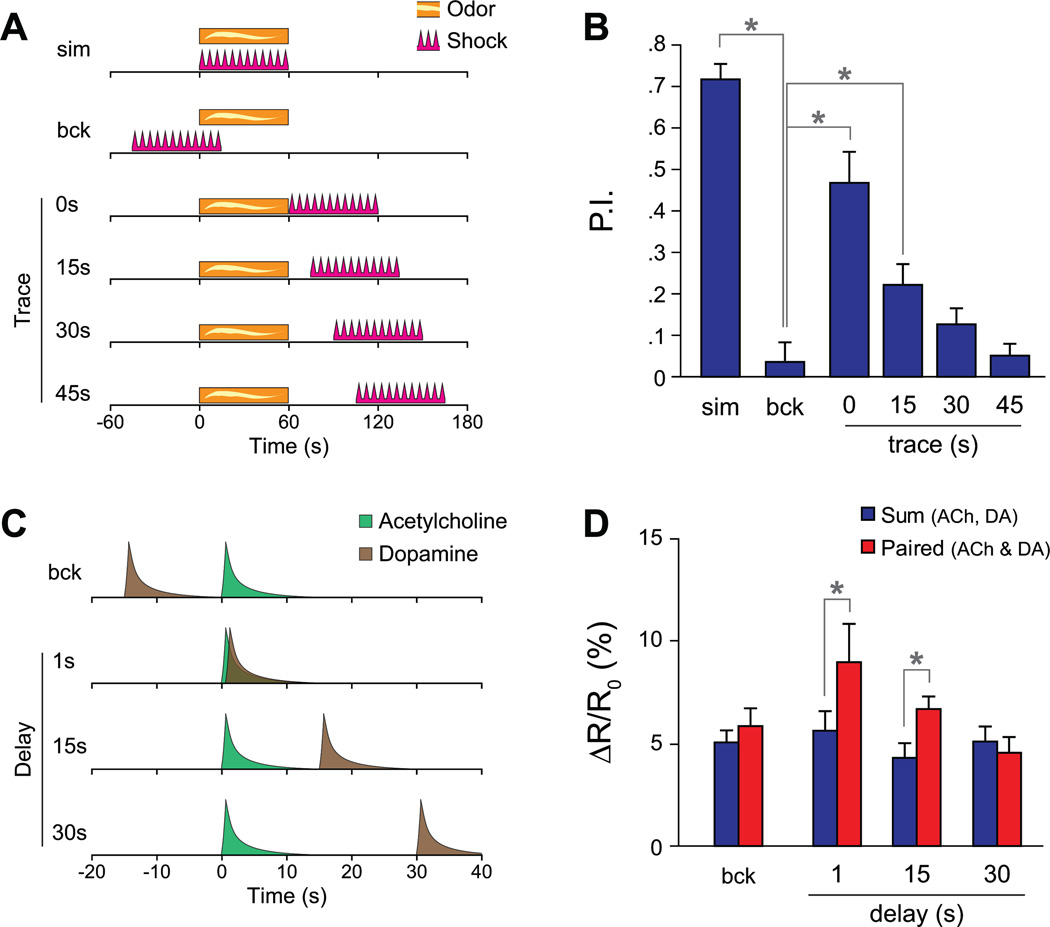
To determine whether the synergistic effect exhibits similar temporal requirements as behavioral conditioning, we varied the delay between the acetylcholine and dopamine application in a series of experiments, and compared these results to behavioral trace conditioning (Figure 6). If the US is delayed 0 – 45 s following the CS, behavioral memory decays with greater time intervals, and backward pairing produces no memory (Tully and Quinn, 1985; Figure 6 A,B). There was a significant difference in performance between the backward conditioned flies and the groups in which the CS and US were applied simultaneously (Fisher’s LSD following ANOVA, p < 0.001, n = 6), as well as between the backward conditioned group and groups trace conditioned with 0 s (p < 0.001) or 15 s (p = .0122) CS-US delays. In imaging experiments, we tested the effect of varying the delay between application of acetylcholine and dopamine (Figure 6 C,D). Backward pairing (dopamine followed 15s later by acetylcholine) produced no significant synergy (t test, p = 0.14, n = 13). Forward pairing with a 1 s delay produced significant synergy (p = 0.022, n = 7). Forward pairing with a 15 s delay also produced a significant synergy (p = 0.011, n = 11), but with a 30 s delay the synergy was insignificant (p = 0.24, n = 9). Therefore, the temporal pairing requirements for synergy on cAMP levels are similar to the CS-US pairing window for performance in behavioral conditioning.
Figure 6.
Synergistic increases in cAMP following dopamine/acetylcholine application exhibit similar temporal pairing requirements as behavioral trace conditioning.
(A) Schematic of the behavioral trace conditioning stimulus paradigm. The CS (odor) and US (shock) were presented for 1 m simultaneously (sim), in a backwards order (bck), or with a forward delay (trace conditioning).
(B) Performance of flies in a trace conditioning experiment (n = 6) with varying delays between the CS (odor) and US (shock). P.I. = performance index; *p<0.05 (Fisher’s LSD following significant differences with ANOVA).
(C) Schematic of the acetylcholine/dopamine stimulus paradigm in the imaging experiments.
(D) Sum and paired response magnitudes when applying different time delays between the acetylcholine and dopamine applications. *p<0.05 (t test)
We additionally tested whether pairing neuronal depolarization with octopamine would produce the same synergistic effect. In contrast to our findings with dopamine, there was no synergistic increase in cAMP in the mushroom body or α’ lobes when acetylcholine was paired with octopamine Figure 5J). In fact, this pairing resulted in significantly less cAMP than the sum of each stimulus individually in both α and α’ lobes (t tests, respectively: p = 0.0026 and 0.03, n = 12). The sub-additive effect of pairing acetylcholine and octopamine was present in rut1 mutant flies (p = 0.03, n = 9; Figure 5J), suggesting that it is not mediated by the rutabaga adenylyl cyclase. There was no significant difference in the sum vs. paired responses when octopamine and acetylcholine were applied to the calyx (p = 0.29, n = 12). Anatomical studies have detected projections of putative octopaminergic neurons to the mushroom body γ lobe (Sinakevitch and Strausfeld, 2006; Busch et al., 2009). Therefore, we tested whether octopamine could function synergistically with neuronal depolarization in the γ lobe. However, we found no effect of pairing acetylcholine and octopamine in this lobe region (p = 0.17, n = 8; Figure 5J). Therefore, pairing acetylcholine and octopamine produces a sub-additive effect in the α and α’ lobes, and a purely additive effect in the calyx or γ lobe.
Cyclic-AMP facilitates mushroom body calcium influx
Normal cAMP signaling is necessary specifically in the mushroom bodies for aversive olfactory learning (Zars et al., 2000; McGuire et al., 2003; Mao et al., 2004). However, it is unknown whether elevating cAMP alone is sufficient for neuronal plasticity that may underlie learning, or whether control of cAMP level is simply necessary for plasticity to occur.
A series of experiments were conducted to test whether elevating cAMP is sufficient to induce changes in the responsiveness of mushroom body neurons using calcium imaging. We searched for any change in the responsivity (either depression or facilitation) of mushroom body neurons to the acetylcholine stimulus. Brains were isolated from 238Y-GAL4>UAS-G-CaMP flies, and the calyx and α/α’ lobes were imaged simultaneously while acetylcholine was focally applied to the calyx. The calcium transients in the calyx likely represent calcium influx through nicotinic acetylcholine receptors in the calyx (Gu and O'Dowd, 2006) with some possible contribution from back-propagating action potentials from the lobes. Calcium transients in the lobes represent calcium influx through voltage-sensitive calcium channels in the axons and presynaptic terminals. Thus, this preparation allowed us to image both the activation of receptors in the calyx and a correlate of the action potential frequency in the lobes. After a second acetylcholine stimulus, forskolin was bath-applied for 2 minutes (during which a third recording was made) and then washed out (Figure 7A). Forskolin at 10 µM was found to produce an equivalent increase in cAMP to that of a saturating concentration of dopamine (Figure 7A, Figure 2 D,E). Thus, the amount of cAMP generated by 10 µM forskolin is within the range of cAMP generated by dopamine receptor activation in the mushroom body neurons.
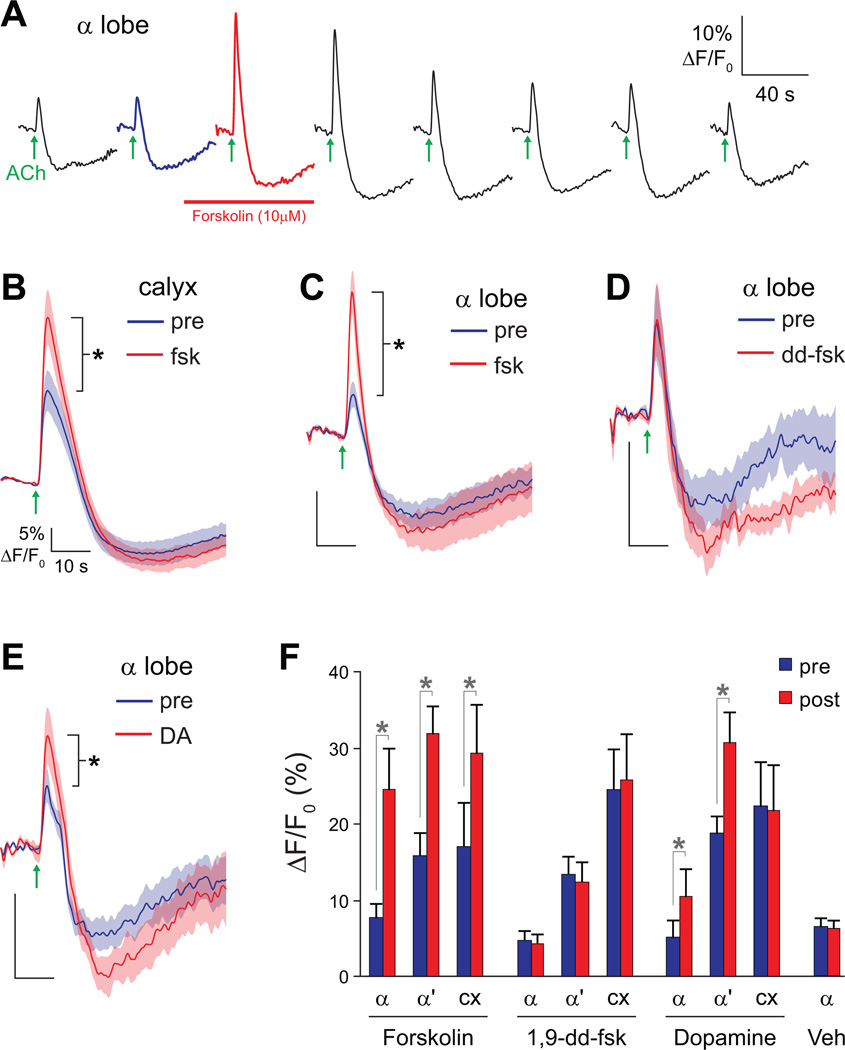
Figure 7.
Elevation of cAMP facilitates responses in mushroom body neurons. Data are from 238Y-GAL4>UAS-G-CaMP flies. Scale bars in panels B–E indicate 5% ΔF/F0 and 10 s. Error bars indicate S.E.M., *p<0.05.
(A) The experimental protocol. Acetylcholine was focally applied to the mushroom body calyx and calcium responses recorded in the mushroom body α and α’ lobes. Recordings were taken every 3 m. Following the second recording, 10 µM forskolin was applied for 2 m. The third recording was taken in the presence of forskolin, which was subsequently washed out.
(B) Acetylcholine-evoked calcium responses in the mushroom body calyx before and after application of 10 µM forskolin.
(C) Acetylcholine-evoked calcium responses in the mushroom body α lobe before and after application of 10 µM forskolin.
(D) Acetylcholine-evoked calcium responses in the mushroom body α lobe before and after application of 10 µM 1,9-dideoxyforskolin (dd-fsk).
(E) Acetylcholine-evoked calcium responses in the mushroom body α lobe before and after application of 10 mM dopamine.
(F) Magnitude of acetylcholine-evoked calcium responses in the α lobe, α’ lobe, and calyx (cx) (recorded simultaneously in each brain), before (pre) and after (post) application of forskolin (10 µM in 0.01% DMSO; n = 13), 1,9-dideoxyforskolin (10 µM in 0.01% DMSO; n = 11), vehicle control (0.01% DMSO; α lobe; n = 7), and dopamine (10 mM in saline; n = 13). *p < 0.05 (t tests).
In the presence of forskolin, the amplitude of the calcium transients in the mushroom body increased significantly in the α lobe, α’ lobe, and calyx (t tests, respectively: p = 0.002, <0.001, <0.001, n = 13; Figure 7 A–C,F). This was not due to the effect of forskolin on voltage-gated potassium channels (Hoshi et al., 1988), since 1,9-dideoxyforskolin (dd-fsk) failed to produce the increase in calcium transients in the α lobe, α’ lobe, or calyx (respectively: p = 0.57, 0.21, 0.40, n = 11; Figure 7 D,F). Dd-fsk has the same nonspecific effects of forskolin but does not elevate cAMP (Hoshi et al., 1988). Likewise, a vehicle control (0.01% DMSO) had no effect (α lobe; p = 0.84, n = 7; Figure 7F). These data collectively demonstrate that elevating cAMP facilitates the calcium responses of mushroom body neurons to stimulation by acetylcholine.
Calcium transients were facilitated across the mushroom body dendrites and axons following cAMP elevation. However, the source of this facilitation is unclear. It could be a neuron-wide change affecting the excitability of both the dendrites and axons. Alternatively, it could be due solely to an increase in excitability of the dendrites, as any widespread increase in dendritic excitability would likely propagate into the axons as a facilitation of action potential frequency and consequently an increase in calcium influx in both the dendrites and axons. These two possibilities can be distinguished. If the plasticity occurred solely in mushroom body dendrites, then it would be impossible to observe facilitation in the mushroom body axons in absence of facilitation in the calyx. Therefore, we examined the data for all of the brains individually, looking for cases in which there was no change in the calyx but facilitation in the axons. In all of the brains, the calcium transients recorded in the α lobe were facilitated. However, in three brains there was a clear increase in calcium responses in the lobes with no change in the calyx (Supplemental Figure 8). Thus, plasticity can occur independently in the mushroom body α lobe, although in most brains elevating cAMP generates facilitation in both the lobe and calyx.
Since dopamine elevates cAMP and acts synergistically with acetylcholine-evoked neuronal depolarization, does it also facilitate responses of mushroom body neurons? We found that a 1-minute application of dopamine significantly facilitated the responses of the α and α’ lobe, while there was no effect in the calyx (t tests, respectively: p = 0.047, 0.0028, 0.57, n = 13; Figure 7 E-F). Therefore, in addition to directly elevating cAMP with forskolin, application of dopamine can facilitate the responses of mushroom body neurons – at least in the axons. This pattern is reminiscent of the distribution of dopaminergic innervation in the mushroom body (Friggi-Grelin et al., 2003; Mao and Davis, 2009), the distribution of dopaminergic receptors (Han et al., 1996; Kim et al., 2007), the pattern of cAMP elevation in mushroom body neurons stimulated by bath-applied dopamine (dopamine consistently elevates cAMP only in the mushroom body lobes), and the requirement of dopamine application to the lobes for synergistic increases in cAMP. Thus, this effect may be due to activation of dopamine receptors on mushroom body axons and subsequent elevation of cAMP specifically in the axons.
DISCUSSION
We have presented data suggesting that rut mediates a synergistic increase in cAMP when dopamine and neuronal depolarization (via acetylcholine stimulation) are paired, an effect that exhibits similar temporal pairing requirements as behavioral conditioning. This provides strong evidence that the rut-encoded AC is a molecular coincidence detector, a long-hypothesized yet never adequately tested idea. The Drosophila rut AC is similar to the mammalian type-I AC (AC1) in that it is sensitive to stimulation by both Gα s and Ca2+/CaM (Livingstone et al., 1984; Levin et al., 1992). When expressed in HEK cells, mammalian AC1 is only stimulated by Gs-coupled receptors if this stimulation is paired with calcium elevation, in which case a synergistic elevation of cAMP is observed (Wayman et al., 1994). Similarly, the only rut-dependent effect we observed was the synergistic increase in cAMP following pairing of dopamine and neuronal depolarization.
We observed cAMP responses to either acetylcholine or dopamine applied in isolation in both wild type and rut1 flies. This suggests that there are additional adenylyl cyclases in the mushroom bodies that respond to unpaired dopamine and acetylcholine but do not generate synergistic increases in cAMP upon coincident depolarization. Several identified and putative ACs could underlie these responses in rut1 mutants (DAC39E, DAC78C, DAC76E, CG32158, CG32301, CG32305). It is unlikely that the responses to acetylcholine-induced depolarization in rut1 mutants are due to calcium-induced adenylyl cyclase activation, as previous studies did not detect any calcium sensitive cyclase activity in rut1 mutants (Livingstone et al., 1984; Livingstone, 1985). There are several possible explanations for why we observed such increases in cAMP in rut1 mutants. Remaining adenylyl cyclases could be directly activated by depolarization (Cooper et al., 1998) or via muscarinic acetylcholine receptors that positively couple cAMP (Dittman et al., 1994). Alternatively, the DPM neurons could be downstream of the mushroom body neurons and provide feedback on the mushroom bodies. The DPM neurons are believed to release a peptide that stimulates cAMP (Waddell et al., 2000). This alternative model emphasizes the need to evaluate responses within the context of a neural circuit, as allowed by the preparation used for our studies.
Synergistic increases in cAMP were observed when acetylcholine was paired with dopamine, but the opposite effect occurred when acetylcholine was paired with octopamine. Various Drosophila tyramine/octopamine receptors can simulate or inhibit the production of cAMP (Evans and Maqueira, 2005), which may explain the inhibition of cAMP generation when octopamine was paired with acetylcholine. This inhibitory effect does not require rut, and is therefore likely mediated by other cyclases. There are several implications of this finding in terms of the role of octopamine in conditioning. Previous data have suggested that dopamine and octopamine may relay the aversive and appetitive US, respectively (Schwaerzel et al., 2003). If this model is correct, then our data suggest that an appetitive stimulus could suppress the responses of mushroom body neurons to a subsequent conditioned stimulus. However, dopamine plays a role in both appetitive and aversive learning (Kim et al., 2007). This opens the alternate possibility that dopamine could relay both appetitive and aversive unconditioned stimuli, with octopamine playing a different role in MB physiology.
Data from our preparation suggest that elevating cAMP is sufficient to induce plasticity in the mushroom bodies, facilitating the calcium responses of mushroom body neurons to stimulation by acetylcholine. Therefore, cAMP appears to be both necessary and sufficient for neuronal plasticity in the mushroom body. There are several mechanisms by which cAMP could facilitate responses of mushroom body neurons. Cyclic-AMP could elevate membrane potential by activating cyclic nucleotide-gated channels, which are expressed in the Drosophila antennal lobes and mushroom body neurons (among other areas) (Miyazu et al., 2000). Cyclic-AMP has been shown to have direct effects on a K+-selective ion channel (e.g., Delgado et al., 1991). In addition, cAMP could affect neuronal excitability via activation of protein kinase A (PKA). Likely targets of PKA phosphorylation include Na+ and K+ channels (Gordon et al., 1990; Brüggemann et al., 1993; Zhou et al., 2002), modulation of which can influence neuronal excitability. In cricket mushroom body neurons, Na+-activated K+ channels are modulated by dopamine and octopamine, as well as cAMP/PKA and cGMP/PKG pathways (Aoki et al., 2008). Notably, presynaptic facilitation in Aplysia sensory neurons relies on modulation of potassium channels by cAMP/PKA (Siegelbaum et al., 1982).
Plasticity in the Drosophila mushroom body shares some features with the plasticity that underlies the siphon withdrawal reflex in Aplysia. In addition to having a large presynaptic component, with cAMP increases being both necessary and sufficient, this type of plasticity involves increased influx of calcium into the presynaptic terminal (Klein and Kandel, 1978). In both Drosophila and Aplysia, the plasticity appears to be heterosynaptic, requiring input from neurons releasing serotonin in Aplysia and dopamine (and/or octopamine) in Drosophila. In Aplysia, facilitation of sensory neuron-motor neuron synapses exhibits similar temporal requirements as behavioral conditioning (Carew et al., 1983; Hawkins et al., 1983). Likewise, we found that the synergistic generation of cAMP has similar temporal requirements as differential behavioral conditioning in flies. Thus, the synergistic increases in cAMP could underlie the plasticity that drives some of the behavioral modification following learning in Drosophila. However, there must be at least one other pathway for plasticity in Drosophila, as performance is reduced, but not eliminated, in rut mutants.
Different sets of mushroom body neurons appear to have different temporal roles in learning and memory, with synaptic transmission from α/β neurons being required during memory retrieval (Dubnau et al., 2001; McGuire et al., 2001) and synaptic transmission from α’/β’ neurons and DPM neurons being required during learning and early memory consolidation (Keene et al., 2006; Krashes et al., 2007). That begs the question: which neurons are responsible for registering CS/US coincidence and initially triggering memory formation? One possibility is that CS/US coincidence is initially registered in the α’/β’ neurons and then the memory is sequentially transferred to the α/β neurons during consolidation. Alternatively, CS/US coincidence could be registered in parallel across both sets of neurons. Our data suggest that initial learning could be triggered in parallel across both the α/β and α’/β’ neurons. We observed synergistic increases in cAMP in both the α and α’ lobes, and increases in cAMP facilitated the responses of axons in both areas as well. This makes sense given that rut is expressed at high levels in both the α/β and α’/β’ lobes (Han et al., 1992), suggesting that the molecular machinery for coincidence detection is present in both. It seems likely that the initial coincidence will occur in different sets of α/β and α’/β’ neurons, depending on how any specific learned odor is represented by subsets of these neurons from the intrinsic wiring with the antennal lobe (Akalal et al., 2006).
EXPERIMENTAL PROCEDURES
Functional imaging
Flies were cultured according to standard methods. Calcium and cAMP were monitored via functional imaging using the geneticallyencoded reporters G-CaMP1.6 (Reiff et al., 2005) or epac1-camps (Nikolaev et al., 2004; Shafer et al., 2008), respectively. The reporters were expressed in specific neuronal populations with the GAL4-UAS system. Mushroom body α/β neurons were visualized with c739- and 238Y-GAL4 drivers, which were chosen for their robust expression in neurons that are critical for olfactory memory (e.g., Zars et al., 2000; Dubnau et al., 2001; McGuire et al., 2001, 2003; Yu et al., 2006). For functional imaging, brains were dissected, maintained in a saline solution (1 ml/min continuous bath perfusion), and imaged with confocal microscopy. G-CaMP and epac1-camps were imaged with appropriate laser lines and emission filters. Responses were plotted as the baseline-normalized change in G-CaMP fluorescence (ΔF/F0) or change in epac1-camps inverse FRET ratio (ΔR/R0) within a circumscribed region of interest.
Bath application experiments
Dopamine and octopamine (dissolved in saline) were applied and washed out by switching the source of the bath perfusion solution for 30 s. A high-K+ solution (100 mM K+ in saline) was used to depolarize neurons in preliminary experiments.
Focal application experiments
Neurotransmitters (dopamine, octopamine, or acetylcholine) were dissolved in saline with 1 µM Texas Red dextran (to allow optical monitoring of the stimulus duration and relative concentration). The solutions were applied focally to the mushroom body lobes or calyx via pressure ejection from a glass micropipette (5–10 µM tip diameter). Dopamine and octopamine were applied to the lobes or calyx, while acetylcholine was applied exclusively to the calyx. The concentration of stimulus solutions reaching each target structure was adjusted by changing the pressure that was applied to the pipettes and/or the position of the pipette tip.
Behavioral Assays
Olfactory learning was tested using a classical conditioning paradigm in which flies were trained and tested in a T-maze (Tully and Quinn, 1985). The CS (odor) and US (electric shock) were applied either simultaneously or with varying time intervals (trace conditioning). The odors 3-octanol and benzaldehyde were used as the CS+/CS- pair. Memory was tested behaviorally three minutes after training, and a performance index (P.I.) was calculated. A P.I. of 0 indicates performance at chance level (50:50 distribution in the T-maze), while a P.I. of 1 indicates that all flies made the correct choice in the T-maze).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Paul Taghert for UAS-epac1-camps flies and Dr. Dierk Reiff for UAS-G-CaMP 1.6 flies. Jennifer Pletting provided assistance with the behavioral experiments, and Dr. Irina Larina provided helpful discussions and assistance with initial imaging experiments. This work was supported by NIH NS052351, the R.P. Doherty-Welch Chair in Science at the Baylor College of Medicine, and a training fellowship from the Biomedical Discovery Training Program of the W. M. Keck Center for Interdisciplinary Bioscience Training of the Gulf Coast Consortia (NIH T90 DA022885 and R90 DA023418).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional details can be found in the Supplemental Experimental Procedures.
Contributor Information
Seth M. Tomchik, Email: stomchik@scripps.edu.
Ronald L. Davis, Email: rdavis@scripps.edu.
References
- Abrams TW, Karl KA, Kandel ER. Biochemical studies of stimulus convergence during classical conditioning in Aplysia: Dual regulation of adenylate cyclase by Ca2+/calmodulin and transmitter. J Neurosci. 1991;11:1655–2665. doi: 10.1523/JNEUROSCI.11-09-02655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akalal DB, Wilson CF, Zong L, Tanaka NK, Ito K, Davis RL. Roles for Drosophila mushroom body neurons in olfactory learning and memory. Learn Mem. 2006;13:659–668. doi: 10.1101/lm.221206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K, Kosakai K, Yoshino M. Monoaminergic modulation of the Na+-activated K+ channel in Kenyon cells isolated from the mushroom body of the cricket (Gryllus bimaculatus) brain. J Neurophysiol. 2008;100:1211–1222. doi: 10.1152/jn.90459.2008. [DOI] [PubMed] [Google Scholar]
- Ashraf SI, McLoon AL, Sclarsic SM, Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. doi: 10.1016/j.cell.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Brüggemann A, Pardo LA, Stühmer W, Pongs O. Ether-à-go-go encodes a voltage-gated channel permeable to K+ and Ca2+ and modulated by cAMP. Nature. 1993;365:445–448. doi: 10.1038/365445a0. [DOI] [PubMed] [Google Scholar]
- Busch S, Selcho M, Ito K, Tanimoto H. A map of octopaminergic neurons in the Drosophila brain. J Comp Neurol. 2009;513:643–667. doi: 10.1002/cne.21966. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Hawkins RD, Kandel ER. Differential classical conditioning of a defensive withdrawal reflex in Aplysia californica. Science. 1983;219:397–400. doi: 10.1126/science.6681571. [DOI] [PubMed] [Google Scholar]
- Cooper DMF, Schell MJ, Thorn P, Irvine RF. Regulation of adenylyl cyclase by membrane potential. J Biol Chem. 1998;273:27703–27707. doi: 10.1074/jbc.273.42.27703. [DOI] [PubMed] [Google Scholar]
- Davis RL. Mushroom bodies and Drosophila learning. Neuron. 1993;11:1–14. doi: 10.1016/0896-6273(93)90266-t. [DOI] [PubMed] [Google Scholar]
- Davis RL. Physiology and biochemistry of Drosophila learning mutants. Physiol Rev. 1996;76:299–317. doi: 10.1152/physrev.1996.76.2.299. [DOI] [PubMed] [Google Scholar]
- Davis RL. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- Davis RL, Cherry J, Dauwalder B, Han PL, Skoulakis E. The cyclic AMP system and Drosophila learning. Mol Cell Biochem. 1995;149–150:271–288. doi: 10.1007/978-1-4615-2015-3_31. [DOI] [PubMed] [Google Scholar]
- Delgado R, Hidalgo P, Diaz F, Latorre R, Labarca P. A cyclic AMP-activated K+ channel in Drosophila larval muscle is persistently activated in dunce. PNAS. 1991;15:557–560. doi: 10.1073/pnas.88.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman AH, Weber JP, Hinds TR, Choi EJ, Migeon JC, Nathanson NM, Storm DR. A novel mechanism for coupling of m4 muscarinic acetylcholine receptors to calmodulin-sensitive adenylyl cyclases: crossover from G protein-coupled inhibition to stimulation. Biochemistry. 1994;33:943–951. doi: 10.1021/bi00170a013. [DOI] [PubMed] [Google Scholar]
- Dubnau J, Grady L, Kitamoto T, Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411:476–480. doi: 10.1038/35078077. [DOI] [PubMed] [Google Scholar]
- Evans PD, Maqueira B. Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert Neurosci. 2005;5:111–118. doi: 10.1007/s10158-005-0001-z. [DOI] [PubMed] [Google Scholar]
- Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Gordon D, Moskowitz H, Zlotkin E. Sodium channel polypeptides in central nervous systems of various insects identified with site directed antibodies. Biochim Biophys Acta. 1990;1026:80–86. doi: 10.1016/0005-2736(90)90335-l. [DOI] [PubMed] [Google Scholar]
- Gu H, O'Dowd DK. Cholinergic synaptic transmission in adult Drosophila Kenyon cells in situ. J Neurosci. 2006;26:265–272. doi: 10.1523/JNEUROSCI.4109-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han PL, Levin LR, Reed RR, Davis RL. Preferential expression of the Drosophila rutabaga gene in mushroom bodies, neural centers for learning in insects. Neuron. 1992;9:619–627. doi: 10.1016/0896-6273(92)90026-a. [DOI] [PubMed] [Google Scholar]
- Han KA, Millar NS, Grotewiel MS, Davis RL. DAMB, a novel dopamine receptor expressed specifically in Drosophila mushroom bodies. Neuron. 1996;16:1127–1135. doi: 10.1016/s0896-6273(00)80139-7. [DOI] [PubMed] [Google Scholar]
- Han KA, Millar NS, Davis RL. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J Neurosci. 1998;18:3650–3658. doi: 10.1523/JNEUROSCI.18-10-03650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RD, Abrams TW, Carew TJ, Kandel ER. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983;219:400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Garber SS, Aldrich RW. Effect of forskolin on voltage-gated K+ channels is independent of adenylate cyclase activation. Science. 1988;240:1652–1655. doi: 10.1126/science.2454506. [DOI] [PubMed] [Google Scholar]
- Isabel G, Pascual A, Preat T. Exclusive consolidated memory phases in Drosophila. Science. 2004;304:1024–1027. doi: 10.1126/science.1094932. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep. 2001;21:565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- Keene AC, Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nat Rev Neurosci. 2007;8:341–354. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- Keene AC, Krashes MJ, Leung B, Bernard JA, Waddell S. Drosophila dorsal paired medial neurons provide a general mechanism for memory consolidation. Curr Biol. 2006;16:1524–1530. doi: 10.1016/j.cub.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Lee HG, Han KA. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci. 2007;27:7640–7647. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Kandel ER. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. PNAS. 1978;75:3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Davis RL. Insect olfactory memory in time and space. Curr Opin Neurobiol. 2006;16:679–685. doi: 10.1016/j.conb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Liu X, Davis RL. The GABAergic anterior paired lateral neuron suppresses and is suppressed by olfactory learning. Nat Neurosci. 2009;12:53–59. doi: 10.1038/nn.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Sziber PP, Quinn WG. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984;37:205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Roman G, Zong L, Davis RL. Pharmacogenetic rescue in time and space of the rutabaga memory impairment by using Gene-Switch. PNAS. 2004;101:198–203. doi: 10.1073/pnas.0306128101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu H, Yaping E, Rose JC, Chen M, Bailey CH, Kandel ER. Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293:1330–1333. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Miyazu M, Tanimura T, Sokabe M. Molecular cloning and characterization of a putative cyclic nucleotide-gated channel from Drosophila melanogaster. Insect Mol Biol. 2000;9:283–292. doi: 10.1046/j.1365-2583.2000.00186.x. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fiber synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- Nighorn A, Healy MJ, Davis RL. The cyclic AMP phosphodiesterase encoded by the Drosophila dunce gene is concentrated in the mushroom body neuropil. Neuron. 1991;6:455–467. doi: 10.1016/0896-6273(91)90253-v. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bünemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- Pascual A, Préat T. Localization of long-term memory within the Drosophila mushroom body. Science. 2001;294:1115–1117. doi: 10.1126/science.1064200. [DOI] [PubMed] [Google Scholar]
- Reiff DF, Ihring A, Guerrero G, Isacoff EY, Joesch M, Nakai J, Borst A. In vivo performance of genetically encoded indicators of neural activity in flies. J Neurosci. 2005;25:4766–4778. doi: 10.1523/JNEUROSCI.4900-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroll C, Riemensperger T, Bucher D, Ehmer J, Völler T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, Fiala A. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron. 2008;58:223–237. doi: 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiryanova D, Levitan ES. Prolonged presynaptic posttetanic cyclic GMP signaling in Drosophila motoneurons. PNAS. 2008;105:13610–13613. doi: 10.1073/pnas.0802131105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum SA, Camardo JS, Kandel ER. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982;299:413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Sinakevitch I, Strausfeld NJ. Comparison of octopamine-like immunoreactivity in the brains of the fruit fly and blow fly. J Comp Neurol. 2006;494:460–475. doi: 10.1002/cne.20799. [DOI] [PubMed] [Google Scholar]
- Skoulakis EM, Kalderon D, Davis RL. Preferential expression in mushroom bodies of the catalytic subunit of protein kinase A and its role in learning and memory. Neuron. 1993;11:197–208. doi: 10.1016/0896-6273(93)90178-t. [DOI] [PubMed] [Google Scholar]
- Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward learning in normal and mutant Drosophila. PNAS. 1983;80:1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum AS, Jenett A, Ito K, Heisenberg M, Tanimoto H. Multiple memory traces for olfactory reward learning in Drosophila. J Neurosci. 2007;27:11132–11138. doi: 10.1523/JNEUROSCI.2712-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Waddell S, Armstrong JD, Kitamoto T, Kaiser K, Quinn WG. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell. 2000;103:805–813. doi: 10.1016/s0092-8674(00)00183-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mamiya A, Chiang AS, Zhong Y. Imaging of an early memory trace in the Drosophila mushroom body. J Neurosci. 2008;28:4368–4376. doi: 10.1523/JNEUROSCI.2958-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Wu Z, Kindsvogel W, Prichard L, Storm DR. Synergistic activation of the type I adenylyl cyclase by Ca2+ and Gs-coupled receptors in vivo. J Biol Chem. 1994;269:25400–25405. [PubMed] [Google Scholar]
- Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- Yang MY, Armstrong JD, Vilinsky I, Strausfeld NJ, Kaiser K. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron. 1995;15:45–54. doi: 10.1016/0896-6273(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Yovell Y, Abrams TW. Temporal asymmetry in activation of Aplysia adenylyl cyclase by calcium and transmitter may explain temporal requirements of conditioning. PNAS. 1992;89:6526–6530. doi: 10.1073/pnas.89.14.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Baird GS, Tsien RY, Davis RL. Detection of calcium transients in Drosophila mushroom body neurons with camgaroo reporters. J Neurosci. 2003;23:64–72. doi: 10.1523/JNEUROSCI.23-01-00064.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Ponomarev A, Davis RL. Altered representation of the spatial code for odors after olfactory classical conditioning; memory trace formation by synaptic recruitment. Neuron. 2004;42:437–449. doi: 10.1016/s0896-6273(04)00217-x. [DOI] [PubMed] [Google Scholar]
- Yu D, Keene AC, Srivatsan A, Waddell S, Davis RL. Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell. 2005;123:945–957. doi: 10.1016/j.cell.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Yu D, Akalal DB, Davis RL. Drosophila alpha/beta mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron. 2006;52:845–855. doi: 10.1016/j.neuron.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zars T, Fischer M, Schulz R, Heisenberg M. Localization of a short-term memory in Drosophila. Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang J, Wen H, Kucherovsky O, Levitan IB. Modulation of Drosophila slowpoke calcium-dependent potassium channel activity by bound protein kinase A catalytic subunit. J Neurosci. 2002;22:3855–3863. doi: 10.1523/JNEUROSCI.22-10-03855.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.