Abstract
During an outbreak of methanol poisonings in the Czech Republic in 2012, we were able to study methanol and formate elimination half-lives during intermittent hemodialysis (IHD) and continuous veno-venous hemodialysis/hemodiafiltration (CVVHD/HDF) and the relative impact of dialysate and blood flow rates on elimination. Data were obtained from 11 IHD and 13 CVVHD/HDF patients. Serum methanol and formate concentrations were measured by gas chromatography and an enzymatic method. The groups were relatively comparable, but the CVVHD/HDF group was significantly more acidotic (mean pH 6.9 vs. 7.1 IHD). The mean elimination half-life of methanol was 3.7 and formate 1.6 h with IHD, versus 8.1 and 3.6 h, respectively, with CVVHD/HDF (both significant). The 54% greater reduction in methanol and 56% reduction in formate elimination half-life during IHD resulted from the higher blood and dialysate flow rates. Increased blood and dialysate flow on the CVVHD/HDF also increased elimination significantly. Thus, IHD is superior to CVVHD/HDF for more rapid methanol and formate elimination, and if CVVHD/HDF is the only treatment available then elimination is greater with greater blood and dialysate flow rates.
Keywords: continuous veno-venous hemodialysis, elimination half-life, formate kinetics, intermittent hemodialysis, methanol kinetics, methanol poisoning
Methanol poisoning is a medical emergency where rapid elimination of the toxin and its metabolite is crucial for recovery. This is because the accumulation of the toxic metabolite formic acid/formate is cytotoxic through the inhibition of mitochondrial respiration.1, 2, 3, 4 In addition, the accumulation of formic acid results in a metabolic acidosis, visual impairment, and damage of the basal ganglia, especially when its concentration rises to 9–11 mmol/l.5, 6, 7, 8
The role of hemodialysis in methanol poisoning is well established.9, 10 Hemodialysis eliminates both methanol and formate, and helps to correct metabolic acidosis. Both intermittent and continuous modalities of hemodialysis are commonly used in the treatment of poisonings; however, comparative studies are scarce for methanol kinetics and nonexistent for formate kinetics.
Given the fact that ∼80% of all dialysis sessions in 2006 were performed in the developed world,11 whereas the majority of methanol poisoning outbreaks occur in underdeveloped countries where resources are scarce; a thorough evaluation of the efficacy and limitations of the various modalities of treatment should be carried out. In addition, challenges regarding the availability of antidotes in some countries (for example, the general lack of fomepizole in the developing world, and cultural limitations regarding the medicinal use of ethanol in some Islamic countries) further emphasize the importance of extracorporeal elimination techniques in the treatment of patients with methanol toxicity.
In addition to the availability of different dialysis equipments, the choice of modality may be governed by the patient's clinical condition, especially with regard to the circulatory status. The risk of performing a dialysis session with a high dialysate and blood flow rate in a patient with a very low blood pressure has to be weighed against the benefits of the increased elimination rate of the toxic substances and correction of the acidosis. We therefore also wanted to study the relative impact of the blood and dialysate flow on the elimination of both methanol and formate to see whether this could add to the recommendations for the treatment.
The above questions were addressed in a prospective observational study on methanol and formate elimination in 24 methanol-poisoned patients treated with antidotes and two different methods of enhanced elimination in order to compare the elimination half-lives of methanol and formate. All patients were treated during the recent outbreak of mass methanol poisoning in the Czech Republic in 2012, where 121 cases of poisoning were admitted and a total of 41 patients died (of whom 21 died in hospital). The epidemiological description of the outbreak is presented elsewhere.12
RESULTS
The clinical features, laboratory data, and patient outcomes are presented in Table 1. Among the 24 patients, 11 were treated with intermittent hemodialysis (IHD), whereas 13 were treated with a continuous modality (eight with continuous veno-venous hemodialysis (CVVHD) and five with continuous veno-venous hemodiafiltration (CVVHDF)). In two patients (patients nos. 8 and 11, Table 1), ethanol infusion was started before the initial blood sample was drawn, explaining why they were acidotic in spite of their serum ethanol concentration.
Table 1. Laboratory and clinical data of 11 patients treated with IHD and 13 patients treated with CVVHD/HDF on admission.
| Sex/age (years) | pH | Base deficit (mmol/l) | pCO2 (kPa) | HCO3− (mmol/l) | S-Methanol (mmol/l) | S-Formate (mmol/l) | Lactate (mmol/l) | S-Ethanol (mmol/l) | Osmolal gap (mOsm per kg H2O) | Anion gap (mmol/l) | Antidote given | Folate substitution | Clinical features | Mean arterial pressure (mm Hg), pulse rate (min) | Glasgow coma scale | Sequelae | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M30 | 6.71 | 38 | ND | 4.3 | 87 | 20 | 11.4 | 0.0 | 128 | 54.8 | Fo | FA | VD.D.C | 79, 102 | 3 | VS |
| 2 | M36 | 7.19 | 20 | 2.9 | 8.6 | 24 | 17 | 2.8 | 0.0 | 42 | 28.1 | E | FLA | VD.D.GI | 117, 100 | 15 | — |
| 3 | F52 | 7.20 | 13 | 4.6 | 13.7 | 166 | 15 | 1.5 | 0.0 | 173 | 25.3 | E | FA | GI | 100, 75 | 15 | — |
| 4 | M58 | 7.17 | 21 | 3.6 | 6.8 | 13 | 16 | ND | 0.0 | 28 | 28.8 | E | FLA | VD. HA | 114, 80 | 15 | — |
| 5 | M79 | 7.32 | 11 | 3.5 | 13.4 | 10 | 5 | 4.4 | 0.0 | 18 | 19.2 | E | No | VD.GI | 107, 80 | 15 | Dieda |
| 6 | F43 | 6.93 | 27 | ND | 7.6 | 56 | 17 | 8.0 | 0.0 | ND | ND | Fo | FA | VD.GI.D.CP.C | 84, 100 | 3 | Died |
| 7 | M36 | 6.98 | 27 | 1.5 | 2.5 | 52 | 4 | 4.0 | 0.0 | 132 | 41.9 | Fo | FLA | VD.GI.CP.HA | 127, 99 | 12 | — |
| 8 | F58 | 7.14 | 22 | 2.5 | 6.0 | 38 | 11 | 2.5 | 36b | 52 | 34.6 | E | No | GI.D.F.IE | 127, 85 | 13 | — |
| 9 | F62 | 7.17 | 18 | 3.5 | 9.3 | 30 | 16 | 2.0 | 0.0 | 30 | 30.5 | E | No | VD.GI.F.D | 113, 110 | 15 | — |
| 10 | F58 | 7.11 | 21 | 3.4 | 7.8 | 105 | 18 | 2.1 | 3.0 | 91 | 23.6 | Fo | No | VD.GI | 117, 70 | 15 | — |
| 11 | F62 | 7.08 | 24 | 2.7 | 5.7 | 55 | 17 | 0.9 | 11b | 74 | 18.4 | E | No | F | 120, 75 | 14 | — |
| Mean | 52 | 7.09 | 22.0 | 3.13 | 7.8 | 58 | 14.2 | 4.0 | 4.5 | 77 | 30.5 | ||||||
| LCL95% | 42 | 6.98 | 17.1 | 2.47 | 5.5 | 26.7 | 10.6 | 1.6 | −2.8 | 39.1 | 22.6 | ||||||
| UCL95% | 62 | 7.20 | 26.9 | 3.80 | 10.1 | 88.9 | 17.7 | 6.3 | 11.9 | 114.5 | 38.4 | ||||||
| 12 | M62 | 6.65 | 29 | 4.3 | 3.2 | 42 | 16 | 12.4 | 0.0 | 108 | 45.1 | E, Fo | FA | VD.D.CP.HA.C | 57, 78 | 3 | Died |
| 13 | M65 | 7.00 | 19 | 5.2 | 10.2 | 24 | 21 | 7.3 | 0.0 | 64 | 17.2 | E | FA | VD.D.CP | 140, 90 | 11 | VS.CS |
| 14 | M60 | 6.65 | 32 | 7.8 | 5 | 76 | 12 | 9.3 | 0.0 | 104 | 33.3 | E, Fo | No | D.CP.RA.C | 70, 89 | 3 | Died |
| 15 | M38 | 6.79 | 35 | ND | 4.4 | 149 | 25 | 8.1 | 0.0 | 183 | 46.8 | E | No | VD.GI.D.CP.C | 96, 102 | 3 | Died |
| 16 | M60 | 7.02 | 26 | 1.3 | 2.5 | 22 | 13 | 3.5 | 0.0 | 41 | 32.2 | F | FA | VD.GI.D.CP | 126, 105 | 15 | — |
| 17 | M33 | 6.72 | 35 | 3.2 | 2.9 | 29 | 15 | 16.3 | 0.0 | 34 | 48.5 | E | FLA | VD.GI.C | 73, 110 | 5 | VS.CS |
| 18 | M61 | 7.28 | 16 | 2.6 | 8.9 | 71 | 12 | 2.4 | 7.2 | 73 | 34.1 | E | FA | IE | 119, 92 | 15 | — |
| 19 | M37 | 7.02 | 25 | 2.0 | 6.8 | 228 | 22 | 1.2 | ND | 148 | 35 | E | FA | VD.D.GI.F | 97, 110 | 8 | — |
| 20 | M48 | 6.82 | 28 | 2.8 | 3.2 | 62 | 20 | 7.6 | 0.0 | 90 | 36.3 | E | FA | D.CP.C | 70, 105 | 3 | VS.CS |
| 21 | M69 | 7.21 | 14 | 4.4 | 12.8 | 45 | 17 | 3.6 | 0.0 | 73 | 29.9 | E | FA | VD.D.GI.C | 81, 80 | 6 | VS |
| 22 | M58 | 6.67 | 28 | 3.8 | 3.8 | 61 | 14 | 12.8 | 0.0 | 78 | 32.8 | E | FA | GI.D.C | 70, 115 | 3 | — |
| 23 | F66 | 7.06 | 25 | 1.5 | 3.2 | 22 | 17 | ND | 1.7 | 52 | 36.3 | E, Fo | FA | VD.D.GI | 118, 88 | 15 | — |
| 24 | F35 | 7.08 | 19 | 4.0 | 8.6 | 33 | 15 | 8.3 | 0.0 | 53 | 27.2 | E | FA | GI.D.C | 100, 110 | 3 | — |
| Mean | 53.2 | 6.92 | 25.5 | 3.6 | 5.8 | 66 | 16.8 | 7.7 | 0.74 | 85 | 35.0 | ||||||
| LCL95% | 45.3 | 6.80 | 21.4 | 2.4 | 3.8 | 30.5 | 14.4 | 4.8 | −0.59 | 58.9 | 29.9 | ||||||
| UCL95% | 61.2 | 7.05 | 29.6 | 4.7 | 7.8 | 102.4 | 19.3 | 10.6 | 2.07 | 110.5 | 40.1 | ||||||
| P | 0.85 | 0.04 | 0.24 | 0.47 | 0.17 | 0.70 | 0.18 | 0.04 | 0.28 | 0.69 | 0.28 |
Abbreviations: C, coma; CP, chest pain; CS, cerebral sequelae; CVVHD/HDF, continuous veno-venous hemodialysis/hemodiafiltration; D, dyspnea; E, ethanol; F, fatigue; Fo, fomepizole; FA, folic acid; FLA, folinic acid; GI, gastrointestinal symptoms; HA, headache; IE, inebriation; IHD, intermittent hemodialysis; LCL, the lower limit of a confidence interval at the 95% level; ND, not determined; RA, respiratory arrest; UCL, the upper limit of a confidence interval at the 95% level; VD, visual disturbances; VS, visual sequelae.
The patient died in the hospital from infectious complications 1 month after the methanol poisoning.
Bolus of ethyl alcohol was administered before blood sampling.Bold values indicate statistically significant P-values, P<0.05 (5%).
The two groups of patients treated with different modes of enhanced elimination were comparable by age, time to diagnosis/treatment, and number of patients. The mean time to diagnosis and treatment after the toxic alcohol consumption was 37 h in the CVVHD/HDF group and 48 h in the IHD group. All collected data were normally distributed with two exceptions: (a) serum ethanol in both groups of patients, and (b) serum methanol in the CVVHD/HDF group. There were no statistical differences between the two groups in admission data with respect to serum methanol, formate, pCO2, HCO3−, base deficit, anion gap, and osmolal gap (all P>0.05). The patients treated with CVVHD/HDF were more acidotic than those treated with IHD (mean pH 6.9±0.1 vs. 7.1±0.1, respectively), with higher lactate levels (both P=0.04).
Data on formate and methanol elimination in 11 patients treated with IHD and 13 patients treated with CVVHD/HDF are presented in Tables 2 and 3, respectively.
Table 2. Formate and methanol kinetics in 11 patients treated on IHD.
| Patient | Methanol t ½, h | R2 methanol | Formate t ½, h | R2 formate | Observation time during HD, h | Blood FR, ml/min | Dialysate FR, ml/min | Dialyzer type | Dialyzer surface area, m2 | Anticoagulation | Dialysate temperature | Number of data points | Rebound after HD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.4 | 0.95 | 2.4 | 0.81 | 11.0 | 250 | 500 | Fresenius 4008S | 1.5 | Hep | 37.0 | 4 | No |
| 2 | 2.0 | 0.80 | 1.6 | 0.85 | 8.0 | 250 | 500 | Fresenius 4008S | 1.6 | Hep | 37.0 | 3 | No |
| 3 | 3.1 | 0.94 | 1.9 | 0.90 | 8.0 | 220 | 500 | Fresenius 4008S | 1.6 | Hep | 36.5 | 3 | No |
| 4 | 3.2 | 0.84 | 1.3 | 0.99 | 8.0 | 250 | 500 | Fresenius 4008S | 1.6 | Hep | 36.5 | 4 | No |
| 5 | 8.0 | 0.87 | 2.3 | 1.00 | 1.9 | 200 | 500 | Fresenius 4008S | 1.5 | Hep | 36.5 | 3 | No |
| 6 | 2.0 | 0.92 | 0.7 | 1.00 | 4.0 | 250 | 500 | Fresenius 4008S | 1.6 | Hep | 37.0 | 3 | No |
| 7 | 4.2 | 1.00 | 1.4 | 1.00 | 12.0 | 180 | 500 | Fresenius 4008S | 1.5 | Hep | 36.5 | 3 | No |
| 8 | 2.1 | 0.84 | 1.2 | 1.00 | 8.0 | 250 | 500 | Fresenius 4008S | 1.6 | Hep | 36.5 | 3 | No |
| 9 | 1.8 | 1.00 | 1.0 | 1.00 | 8.0 | 250 | 500 | Fresenius 4008S | 1.6 | Hep | 36.5 | 3 | No |
| 10 | 4.8 | 0.97 | 2.3 | 0.99 | 10.0 | 180 | 500 | Fresenius 4008S | 1.6 | Hep | 36.5 | 3 | No |
| 11 | 6.8 | 0.89 | 1.7 | 1.00 | 13.0 | 220 | 500 | Fresenius 4008S | 1.5 | Hep | 36.5 | 3 | No |
| Mean | 3.7 | 0.91 | 1.6 | 0.96 | 9.9 | ||||||||
| LCL95% | 2.3 | 1.2 | 7.3 | ||||||||||
| UCL95% | 5.1 | 2.0 | 12.5 |
Abbreviations: FR, flow rate; HD, hemodialysis; Hep, heparin; IHD, intermittent hemodialysis; LCL, the lower limit of a confidence interval at the 95% level; UCL, the upper limit of a confidence interval at the 95% level. Polysulfone dialyzer membranes have been used in all cases.
Table 3. Formate and methanol kinetics in the patients treated on CVVHD12, 13, 14, 15, 16, 17, 18, 19 and CVVHDF20, 21, 22, 23, 24.
| Patient | Methanol t ½, h | R2 methanol | Formate t ½, h | R2 formate | Observation time during CVVHD/HDF, h | Blood FR, ml/min | Dialysate FR, ml/h | Substitution fluid FR, ml/h | Dialyzer type | Dialyzer surface area, m2 | Anticoagulation | Dialysate temperature | Number of data points | Complications | Number of circuits | Frequency of circuit blotting | Ultrafiltration, ml/h |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 11.7 | 0.99 | 5.9 | 0.92 | 34.0 | 100 | 2000 | - | Aquarius | 1.2 | Citrate | 36.5 | 8 | 0 | 1 | 0 | - |
| 13 | 9.8 | 0.99 | 4.5 | 1.00 | 32.0 | 130 | 2400 | - | Multifiltrate Fresenius | 1.8 | Citrate | 38.0 | 4 | 0 | 1 | 0 | - |
| 14 | 7.7 | 0.94 | 5.9 | 0.88 | 32.0 | 130 | 2500 | - | Multifiltrate Fresenius | 1.8 | Citrate | 36.5 | 6 | 0 | 1 | 0 | - |
| 15 | 6.8 | 0.99 | 4.1 | 1.00 | 32.5 | 100 | 2500 | - | Aquarius | 1.9 | Citrate | 36.5 | 6 | 0 | 1 | 0 | - |
| 16 | 9.0 | 0.99 | 2.4 | 0.97 | 26.5 | 250 | 2500 | - | Multifiltrate Fresenius | 1.8 | Hep | 39.0 | 5 | 0 | 1 | 0 | - |
| 17 | 5.6 | 0.95 | 2.1 | 0.96 | 26.0 | 150 | 3000 | - | Multifiltrate Fresenius | 1.8 | Citrate | 36.5 | 6 | 0 | 1 | 0 | - |
| 18 | 5.8 | 0.99 | 2.4 | 1.00 | 28.5 | 200 | 4800 | - | Multifiltrate Fresenius | 1.8 | Citrate | 38.0 | 4 | 0 | 1 | 0 | - |
| 19 | 5.3 | 0.96 | 1.9 | 1.00 | 31.5 | 200 | 4800 | - | Multifiltrate Fresenius | 1.8 | Citrate | 38.0 | 4 | 0 | 1 | 0 | - |
| 20 | 10.6 | 0.99 | 4.0 | 0.97 | 34.5 | 120 | 1000 | 1200 | Aquarius | 1.2 | Hep | 36.5 | 7 | BL | 1 | 0 | 100 |
| 21 | 8.4 | 0.95 | 6.2 | 0.98 | 29.5 | 200 | 1000 | 1200 | Multifiltrate Fresenius | 1.4 | Hep | 38.0 | 7 | 0 | 1 | 0 | 100 (24 h), 50 (5.5 h) |
| 22 | 10.0 | 0.99 | 3.2 | 0.83 | 36.0 | 150 | 1800 | 600 | Multifiltrate Fresenius | 1.8 | Citrate | 35.0 | 9 | 0 | 1 | 0 | 100 |
| 23 | 6.3 | 0.98 | 2.1 | 1.00 | 35.5 | 150 | 1800 | 600 | Aquarius | 1.9 | Hep | 36.5 | 8 | 0 | 1 | 1 | 150 |
| 24 | 8.1 | 0.98 | 1.9 | 0.85 | 37.5 | 150 | 2000 | 600 | Multifiltrate Fresenius | 1.8 | Citrate | 35.0 | 8 | 0 | 1 | 0 | 150 |
| Mean | 8.1 | 0.98 | 3.6 | 0.95 | 32.0 | 155 | 2470 | 1.75 | |||||||||
| LCL95% | 6.9 | 2.6 | 29.8 | 128 | 1750 | 1.65 | |||||||||||
| UCL95% | 9.3 | 4.6 | 34.2 | 183 | 3180 | 1.84 |
Abbreviations: BL, bleeding during HD; CI 95%, 95% confidence interval; CVVHD/HDF, continuous veno-venous hemodialysis/hemodiafiltration; FR, flow rate; LCL, the lower limit of a confidence interval at the 95% level; UCL, the upper limit of a confidence interval at the 95% level. Polysulfone dialyzer membranes have been used in all cases.
The mean methanol elimination half-life in our study was 3.7±1.4 h for IHD and 8.1±1.2 h for CVVHD/HDF. The mean formate elimination half-life was 1.6±0.4 h during IHD and 3.6±1.0 h during CVVHD/HDF. The elimination half-lives of both formate and methanol on IHD were significantly shorter than on CVVHD/HDF (both P<0.001).
Regardless of the mode of dialysis, the elimination half-lives of methanol (Figure 1) and formate (Figure 2) were shorter when the blood flow rates were higher (both P<0.001).
Figure 1.
Elimination half-life of methanol versus blood flow rate (y=−0.04x+13.9, R2=0.52).
Figure 2.
Elimination half-life of formate versus blood flow rate (y=−0.02x+6.5, R2=0.44).
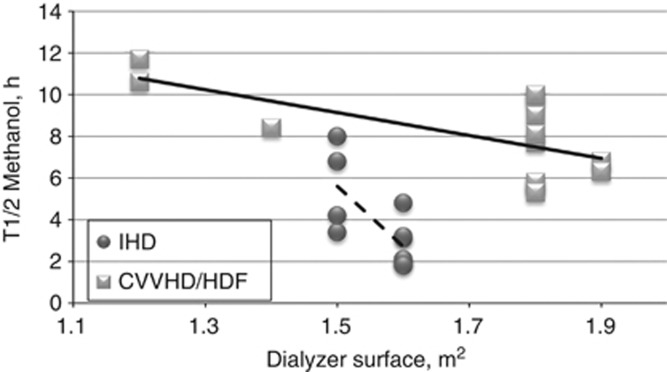
In the CVVHD/HDF group, the elimination half-lives were shorter when the dialysate flow rate was higher (P=0.015, Figure 3) and when the dialyzer membrane surface was larger (both P<0.05). In IHD, only one dialysate flow rate and only two sizes of membrane were used (Table 2), yet a significant correlation was found between the dialyzer membrane surface and the elimination half-life of methanol (P=0.015, Figure 4).
Figure 3.
Elimination half-life of methanol versus dialysate flow rate on CVVHD/HDF (y=−0.0011x+10.91, R2=0.12).
Figure 4.
Elimination half-life of methanol versus dialyzer membrane surface (y=−5.49x+17.37, R2=0.44).
No significant correlation was present between the elimination half-lives of methanol and formate and the predialysis serum concentrations of methanol, formate, ethanol, bicarbonates, and lactate (all P>0.05). However, a significant correlation was present between the elimination half-life of formate and arterial blood pH; the longer half-life of formate was present in more acidotic patients (P=0.038).
No significant correlations of methanol and formate elimination half-lives were found with the folate substitution (treatment with either folic or folinic acid, or without folates) and with the specific antidote (ethanol or fomepizole) administration (all P>0.05). As regards the outcome, the patients with visual and CNS sequelae of methanol poisoning had significantly longer elimination half-lives of formate than those without sequelae (P=0.005). The differences in mortality (P=0.36) and morbidity (survivals with sequelae, P=0.19) between IHD and CVVHD/HDF groups were not significant.
The results of the multivariate regression analysis of variables influencing the elimination half-lives of formate and methanol based on all 24 cases are shown in Tables 4 and 5. Five independent variables included in the model explained 82.8% of the formate elimination half-life variation. The most significant factor was the blood flow rate. The dialysate flow rate (P=0.013) was based on the CVVHD/HDF cases only; however, it was still contributing significantly to the model. The ‘dialyzer membrane surface' was grouped together as interchangeable with ‘dialysate temperature', explaining why ‘other dialyzer properties' is given as the term in the model, in spite of the linear correlation between both the methanol and the formate elimination half-lives. The variable ‘Clinical features' consists of Glasgow coma scale, mean arterial pressure, and pulse rate. The variable ‘metabolic acidosis' includes pH, pCO2, HCO3−, base deficit, and the anion gap (AG). The two latter variables thus reflect the severity of the poisoning.
Table 4. The multivariate regression analysis of formate elimination half-life (R 2=0.828).
|
95% Confidence interval |
|||||||
|---|---|---|---|---|---|---|---|
| Parameter | Regression coefficient | Standard error | t-stat. | P-value | LB | UB | Partial Eta squared |
| Intercept | −39.96 | 8.305 | −4.811 | <0.001 | −57.41 | −22.51 | 56.3% |
| Blood flow rate | −0.022 | 0.004 | −5.467 | <0.001 | −0.03 | −0.014 | 62.4% |
| Dialysate flow rate | −0.0004 | 0.0001 | −2.759 | 0.013 | −0.001 | 0.0001 | 29.7% |
| Other dialyzer properties | 1.328 | 0.234 | 5.678 | <0.001 | 0.836 | 1.819 | 64.2% |
| Clinical features | −0.026 | 0.008 | −3.388 | 0.003 | −0.042 | −0.010 | 38.9% |
| Metabolic acidosis | 0.331 | 0.111 | 2.989 | 0.008 | 0.098 | 0.563 | 33.2% |
Abbreviations: LB, lower bound of a confidence interval at the 95% level of confidence; UB, upper bound of a confidence interval at the 95% level of confidence.
‘Other dialyzer properties' include dialyzer membrane surface and dialysate temperature. ‘Clinical features' include Glasgow coma scale (GCS), mean arterial pressure (MAP), and pulse rate. ‘Metabolic acidosis' includes pH, pCO2, HCO3−, base deficit (BD), and the anion gap (AG).
Table 5. The multivariate regression analysis of methanol elimination half-life (R 2=0.815).
|
95% Confidence interval |
|||||||
|---|---|---|---|---|---|---|---|
| Parameter | Regression coefficient | Standard error | t-stat. | P-value | LB | UB | Partial Eta squared |
| Intercept | 15.761 | 3.325 | 4.740 | <0.001 | 8.802 | 22.72 | 54.2% |
| Dialyzer properties | 4.218 | 0.889 | 4.746 | <0.001 | 2.358 | 6.079 | 54.2% |
| Blood flow rate | −0.018 | 0.008 | −2.219 | 0.039 | −0.035 | −0.001 | 20.6% |
| Dialyzer surface | −5.95 | 1.660 | −3.584 | 0.002 | −9.425 | −2.475 | 40.3% |
| Metabolic acidosis | −0.092 | 0.032 | −2.863 | 0.010 | −0.160 | −0.025 | 30.1% |
Abbreviations: BD, base deficit; LB, lower bound of a confidence interval at the 95% level of confidence; UB, upper bound of a confidence interval at the 95% level of confidence.
‘Metabolic acidosis' includes pH, pCO2, HCO3−, BD, and the anion gap (AG).
For the methanol elimination half-life, the model consisting of four variables explained 81.5% of the variation (Table 5). In this model, the dialysate flow rate and partially the blood flow rate were grouped together within the discrete factor ‘Dialyzer properties' characterizing the mode of hemodialysis, whereas attempting to eliminate this factor led to the devaluation of the model. Thus, this factor represented synthetically the difference in technical parameters of two modes of hemodialysis. The model for methanol was less sensitive to the factors characterizing the clinical state of the patient and more sensitive to the technical parameters of hemodialysis as compared with the model for formate. The ‘metabolic acidosis' was the factor grouping together pH, HCO3−, BD, and the anion gap, reflecting the severity of metabolic acidosis, and thus describing the severity of poisoning in this model.
DISCUSSION
Methanol poisoning is one of the few conditions in clinical toxicology where dialysis has an essential role.10, 13 Both methanol and formate are small molecules (molecular weights 32 and 46 g/mol, respectively) with a volume of distribution 0.6–0.7 l/kg for methanol14 and 0.5 l/kg for formate,9 and no protein binding.5 Extensive toxicokinetic data exist on the capacity of IHD to remove methanol,5, 15, 16, 17, 18, 19, 20 although the evidence is scarce for formate removal.9, 21, 22 There are no studies evaluating both the effects on methanol and formate kinetics with continuous modes of elimination (CVVHD/HDF) and only one that compared the efficacy of continuous and intermittent modes of dialysis.22 In that particular study, there were only three patients, and no formate kinetics was determined.
The elimination of methanol without antidote therapy is of zero order with a rate of 85 mg/l/h—that is i.e., about half of that of ethanol.23 If methanol metabolism is blocked by ethanol or fomepizole, methanol elimination is very slow (elimination half-lives of about 50 h in both cases), predominantly by pulmonary (and some renal) excretion.24, 25 The kinetics of methanol depends upon metabolism, and thus the rate kinetics may vary not only depending on the amount of toxic spirits ingested but also on the time from ingestion to treatment, concomitant ingestion of different amounts of ethanol in other alcoholic beverages and foods, capability of respiratory and renal systems, as well as body weight, muscle/adipose tissue ratio, possible folate, and protein deficiency in chronic alcohol abusers, and other factors.
Both half-lives found during extracorporeal elimination in our study are markedly shorter than the reported endogenous elimination half-life of methanol (mean 43.1–52 h) during treatment with antidotes only.4, 26 Our results further confirm the superiority of IHD over the CVVHD/HDF in methanol elimination from serum, supporting findings from a previous study.27 Nevertheless, the circulatory status is a concern when choosing dialysis modality in conditions where fluid removal is necessary; this is why the continuous modalities are often preferred for hypotensive patients with acute kidney injury. In methanol poisoning, however, fluid removal is usually not necessary; furthermore, the cases with the mean arterial blood pressure lower than 70 mmHg are rare (only one patient in our study), and therefore IHD is not expected to be riskier than CVVHD/HDF in most cases. In fact, IHD would benefit these patients even more because of enhanced clearance.
One complication due to bleeding and one case of circuit clotting were reported in 13 cases of treatment with CVVHD/HDF. No complications were reported in patients treated with IHD. No rebound was present post intermittent hemodialysis. The rebound effect is present when the toxic agent continues to be transferred into the arterial circulation from peripheral body compartments, which is not the case with methanol and formate. The other possibility of methanol rebound is in the cases of delayed absorption of methanol from a full stomach. In our study, the mean time to diagnosis was 48 h in the IHD group, and thus the methanol was completely absorbed before the onset of dialysis. The possibility of formate rebound exists if incomplete ADH blocking is present (due to insufficient serum ethanol levels) and continuation of the metabolic pathway of methanol elimination in addition to the elimination through hemodialysis. In all our cases, ADH was completely blocked either by ethanol or by fomepizole, which was proved by first-order kinetics of methanol elimination.
In contrast to the study by Kan et al.,27 the mean methanol elimination half-life in our study was only 54% shorter during IHD as compared with CVVHD/HDF. The smaller difference between the two modes of dialysis in our study can be explained by the higher dialysate flow in the CVVHD/HDF group. The dialysate flow rate in two patients on CVVHDF in the cited study was 1.0 l/h only, compared with a mean of ∼2.5 l/h in our study.
The results for formate elimination during IHD substantially differed from the endogenous elimination half-life of formate reported from 2.3,4 3.4,21, 22, 28 to 5–6 h,21, 22, 28 and in two case reports 20 (ref. 29) and 77 h.30 It was also 65% shorter than in a previous study by Kerns et al.22 during dialysis. This study, however, possesses some weaknesses: only six patients were dialyzed, and two of them had less than three data points to calculate the half-life of formate (of whom one had an extremely short half-life before initiation of dialysis: 79 min). Further, there was variability in the blood flow in two patients (out of five) during dialysis, which means that half-life cannot be calculated. Our results better correspond with those of Hantson et al.21, who calculated the mean formate elimination half-life during hemodialysis in 18 patients to be 1.81±0.78 h, which was statistically different from the values observed without dialysis. Furthermore, the formate elimination half-life before or in the absence of dialysis calculated in the cited study (6.1±3.3 h) was substantially longer than in the former studies based on fewer cases.
The mean elimination half-life of formate on IHD in our study was 56% shorter as compared with CVVHD/HDF (1.6 vs. 3.6 h, P<0.001) and significantly shorter than the endogenous half-lives calculated in the studies of Kerns et al.22 and Hantson et al.21 Our results suggest that the shorter mean elimination half-life of formate and methanol results from the higher rates of blood flow and dialysate flow. The correlations between the blood flow and the elimination half-lives of methanol and formate were linear (Figures 1 and 2), with elimination half-lives decreasing with increasing blood flow (P<0.001). Blood flow rate was the most significant factor in the multivariate model for formate and one of the most significant factors in the model for methanol. In CVVHD/HDF, the elimination half-lives were shorter when the dialysate flow rate was higher and the dialyzer membrane surface was larger. The elimination half-life of formate in two patients treated with the highest dialysate flow rate possible on CVVHD (4.8 l/h) was significantly shorter (mean 2.2 h) as compared with the mean for this group (3.6±1.0 h; P<0.05). On the basis of these findings, the blood flow and the dialysate flow when using continuous dialysis should be increased as much as possible, taking the patienťs condition into account. Depending on the local dialysis equipment (citrate or heparin anticoagulation), this should be performed under a close evaluation of the acid–base and electrolyte status of the patient.
We did not find any correlation between administration of folates and formate elimination half-lives, supporting the findings by Sanaei-Zadeh et al.31 The difference in formate elimination half-lives between the patients with and without folic or folinic acid administration was not statistically significant. In addition, hemodialysis enhances the formate elimination to such an extent that the effect of the slower pathway of tetrahydrofolate-mediated formate conversion is less apparent. However, further studies are necessary to evaluate this assumption.
Recommendations on the length of dialysis in methanol poisoning often focus on serum methanol, if available. Analyses of the toxic compound formic acid are less available,32 and therefore surrogate markers such as the degree of metabolic acidosis (base deficit) and the anion gap are used.2 In severe methanol poisoning, it is the removal of toxic formate by dialysis and the correction of metabolic acidosis that is crucial, and the lowering of serum methanol is the secondary task if ADH is blocked adequately by ethanol or fomepizole. These facts may explain the clinical experience that ∼8 h of IHD are usually sufficient even in severe methanol poisoning, provided that the metabolic acidosis is corrected, ADH is blocked, and formate is eliminated (normalized anion gap).
In mass methanol outbreaks with a large number of patients, if the dialysis capacities are limited, we recommend a minimum of 8 h of IHD and 18 h of CVVHD/HDF before discontinuation of the dialysis, if the methanol concentration or the osmolal gap cannot be measured. This would theoretically eliminate all formate (5 × T½=8 and 18 h, respectively), as well as a sufficient amount (80%) of the methanol. The continued antidote treatment for at least 12–24 h after the discontinuation of the dialysis, as well as having the possibility of early discovery of any signs of potential rebound acidosis, makes this a clinically and scientifically sound approach.
A similar toxicokinetic approach toward methanol elimination (5 × T½ to remove 100%) would, based on our data, give an estimated need for 18 h of IHD and 40 h of CVVHD/HDF. Assuming that 3 × T½ (12.5% of original serum methanol is remaining) is sufficient (all patients should have antidote treatment for at least 12–24 h post dialysis), it would still require 11 h of IHD and 24 h of CVVHD/HDF, respectively. The problem with this way of guiding the length of dialysis is that one is not addressing the elimination of the (real) toxic agent.
Our study supports the superiority of IHD over CVVHD/HDF in terms of the rate of elimination of both methanol and formate, the latter being especially important in the late-presenting patients. On the basis of the apparent increased elimination with the higher blood and dialysate flow, we recommend optimizing dialysis by increasing the blood flow rate on IHD and the blood and dialysate flow rate on CVVHD/HDF as much as possible, given the limitations of the apparatus and the patient condition. Further studies with more cases are necessary to demonstrate whether the mode of hemodialysis also affects the mortality and long-term visual/CNS sequelae in methanol-poisoned patients; however, our data suggest at least a correlation between the rate of formate elimination and health sequelae of methanol poisoning: the patients with health sequelae had significantly longer elimination half-lives of formate than those without sequelae (P=0.005).
Strength and limitations
The limitations of this study can be attributed to certain confounders: possible variations in the time, amount, and patterns of toxic liquor intake, individual differences in the methanol and formate metabolism, and the available modalities for treatment (other than the modality of enhanced elimination) can add to the limitations. However, the two groups of patients treated with different modes of enhanced elimination were comparable by age, latency period, and size; most of the collected data exhibited normal distribution; for all practical means, there were no statistical differences in admission laboratory data and statistical deviations between both groups. There were limited numbers of data points for most calculations among the IHD patients (predominantly three data points); however, the observation time was sufficiently long, and the correlation on the semilogarithmic plot was good.
This study was not designed as a randomized controlled trial because the choice of the method of enhanced elimination in each case was based on clinical practice at individual sites, as appropriate, and conditioned by different factors, giving the possibility of inherent bias. Nevertheless, this study was not conceived as a study looking at defining the best modality in terms of outcome, rather describing the differences in methanol and formate elimination half-lives only, which should not be biased by the above selection of the groups.
MATERIALS AND METHODS
Patients and treatment
The study design was a prospective, observational—uncontrolled with respect to the hemodialysis modality selection, types of antidotes, and other treatments administered—study. Patients were eligible if they were not pregnant, >18 years of age, and met the following criteria: confirmed methanol poisoning (serum methanol >6.3 mmol/l), documented circumstances of methanol ingestion and time to diagnosis, and sufficient laboratory data, including serum methanol and formate level on admission and at the initiation of hemodialysis.
Twenty-four methanol-poisoned patients were treated in 10 hospitals between September and December 2012. The patients were treated with alkalization, ethanol or fomepizole, or a combination of both antidotes (for example, start of therapy with fomepizole followed by ethanol administration). Folates (folic or folinic acid) were given to 17/24 patients according to the standard protocols.
Patient nos 1–11 were treated with IHD, and patient nos 12–24 were treated with continuous methods of enhanced elimination (nos 12–19 with CVVHD and nos 20–24 with CVVHDF). Most of the patients treated with IHD (9/11 patients) were admitted to the intensive care units of internal medicine departments, and the patients treated with CVVHD/HDF were predominantly admitted to the departments of anesthesiology and resuscitation (12/13 patients).
Patients underwent hemodialysis if they exhibited any of the following: serum methanol higher than 15.8 mmol/l, acidemia, or visual toxicity. The choice of the method of enhanced elimination in each case was based on several factors, such as the hemodynamic stability of the patient on admission (all the patients treated with IHD had mean arterial pressure >70 mm Hg, 4/13 patients treated with CVVHD had mean arterial pressure ⩽70 mm Hg), and severity of clinical symptoms of poisoning (8/13 patients treated with CVVHD were comatose on admission (GCS <8), whereas only 2/11 treated with IHD. Another significant factor was the type of dialyzing equipment available in a medical facility: Some smaller hospitals had the dialyzing equipment in the anesthesiology departments only, making only the continuous mode of hemodialysis available. The larger hospitals usually had IHD equipment available.
As for the antidotes, ethanol was given to seven patients treated with IHD and nine patients were treated with CVVHD/HDF, whereas fomepizole (alone or followed by ethanol administration) was administered to four patients in each group. Six patients treated with IHD and 11 patients treated with CVVHD/HDF received folates. IHD was initiated 2 h after admission to the hospital (range 1–2 h). It was performed for a median duration of 8 h. CVVHD/HDF was started 3.5 h after admission to the hospital (range 0.5–12 h) and lasted for a median duration of 44 h.
Laboratory investigations
Venous blood for methanol and formate analysis was obtained on admission, at the start of hemodialysis, every 2–4 h during the dialysis session, and at the end of dialysis. The blood samples for the osmolality measurements were not necessarily taken at the same time as the samples taken for the methanol and formate analysis in this study, and correlation studies between osmolality and methanol could not be performed. Nonetheless, the first available osmolality was presented. The stated parameters of CVVHD/HDF were maintained during the observation time. In most cases, the duration of CVVHD/HDF was longer than the observation time; however, it was not practical for the study purpose and ethical issues to continue blood sampling. Blood samples were spun, serum was separated, and it was frozen until analyses. Methanol was measured by a gas chromatographic method with flame ionization detection and a direct injection with internal standard (Gas Chromatograph Chrom 5, Laboratory Instruments Prague, Czech Republic), limit of detection 1.9 mmol/l, and day-to-day coefficient of variation 2.5–5.4%. Calibrators and controls were made by dilution of methanol p.a. (Penta, Czech Republic).
Formate was measured enzymatically on a Hitachi analyzer (Hitachi 912, Hitachi Science Systems, Tokyo, Japan) using formate dehydrogenase (Roche, Meylan, France) and NAD (Roche), according to a previously published method.32, 33, 34, 35 Pure sodium formate (Sigma-Aldrich, St Louis, MO) was used to prepare a standard of 1.1 mmol/l phosphate buffer and two control sera. Day-to-day coefficient of variation was 5.6%, and the upper reference limit was 4 mmol/l.
Serum ethanol was analyzed by gas chromatography with flame ionization detection and a direct injection with internal standard (Gas Chromatograph Chrom 5, Laboratory Instruments). The limit of detection was 0.9 mmol/l, and the day-to-day coefficient of variation was 3.8–7.1%. Ethanol standards were purchased (Erba Lachema, Brno, Czech Republic). Osmolality was measured by freezing point depression method on a Fiske one-ten osmometer. The reference range for the osmolal gap was −9–19 mOsm per kg H2O. The osmolal contribution from ethanol (two patients) was subtracted from the measured osmolality.
Calculations and data analysis
Elimination half-life was calculated from the relationship of methanol and formate versus time: linear regression analysis determined the elimination constant (Ke), from the slope of the natural logarithm of methanol or formate versus time.36 The ‘first-order' intradialytic elimination half-life was then calculated from the relationship t1/2=0.693/Ke.
The AG and the osmolal gap were calculated by using the following formulae:
AG=(Na++K+) − (HCO3−+Cl−), and OG=MO − (1.86 × Na++glucose+urea)/0.93, where MO represents the measured osmolality.37
Data are expressed as means with confidence interval (significance level α=0.05). The elimination half-lives were compared using the unpaired t-test. Pearson correlation analysis was performed for characterization of logarithmic dependence concentration of methanol and formate on time, respectively.
Median, modus, arithmetic mean, and confidence intervals were applied for the calculation of average values of different groups of patients. The normality of data was verified using tests based on sample skewness and kurtosis; the normality of one sample was based on the Kolmogorov–Smirnov test. We used the independent t-tests for comparison of independent parameters between two groups of patients, and the multivariate regression models (forward, backward, and stepwise methods) for the determination of optimal sets of explanatory variables for the methanol and formate elimination half-lives. We have used also the exploratory factor analysis on Pearson correlations among the monitored input variables as an auxiliary tool of explanatory regression models. Statistical documentation was performed in Excel (Microsoft, Redmond, WA), and the formal calculations were produced in QC Expert software 3.1 (Trilobyte, Pardubice, Czech Republic) and in IBM SPSS ver. 17.0 and Statistica SF ver. 10.0 (both licensed to First Faculty of Medicine of the Charles University in Prague).
Ethics
The study was approved by the General University Hospital Ethics Committee in Prague, Czech Republic.
Acknowledgments
This study was supported by the Project of the Charles University in Prague P25/1LF/2, the Project 36/13/NAP ‘Prospective study of long-term health effects of acute methanol intoxications' (GUH)—Institutional support, Ministry of Health, Czech Republic, and the Project for development of research organizations 00023001 (IKEM)—Institutional support, Ministry of Health, Czech Republic, and the Project OP Prague Competitiveness, ‘Material and technical base for the research of diagnostics and therapy of civilization and oncological diseases and their significant risk factors in the General University Hospital in Prague‘, CZ.2.16/3.1.00/24012, co-financed by European Regional Development Fund. We thank Professor Robert Hoffman, New York City Poison Control Center, and Professor Dag Jacobsen, Department of Acute Medicine, Oslo University Hospital, for the critical review of the manuscript.
All the authors declared no competing interests.
References
- Liesivuori J, Savolainen H. Methanol and formic-acid toxicity - biochemical-mechanisms. Pharmacol Toxicol. 1991;69:157–163. doi: 10.1111/j.1600-0773.1991.tb01290.x. [DOI] [PubMed] [Google Scholar]
- Hovda KE, Hunderi OH, Rudberg N, et al. Anion and osmolal gaps in the diagnosis of methanol poisoning: clinical study in 28 patients. Intensive Care Med. 2004;30:1842–1846. doi: 10.1007/s00134-004-2373-7. [DOI] [PubMed] [Google Scholar]
- McMartin KE, Ambre JJ, Tephly TR. Methanol poisoning in human-subjects - role for formic-acid accumulation in the metabolic-acidosis. Am J Med. 1980;68:414–418. doi: 10.1016/0002-9343(80)90113-8. [DOI] [PubMed] [Google Scholar]
- Hovda KE, Andersson KS, Urdal P, et al. Methanol and formate kinetics during treatment with fomepizole. Clin Toxicol. 2005;43:221–227. [PubMed] [Google Scholar]
- Jacobsen D, McMartin KE. Methanol and ethylene glycol poisonings. Mechanism of toxicity, clinical course, diagnosis and treatment. Med Toxicol. 1986;1:309–334. doi: 10.1007/BF03259846. [DOI] [PubMed] [Google Scholar]
- Sejersted OM, Jacobsen D, Ovrebo S, et al. Formate concentrations in plasma from patients poisoned with methanol. Acta Med Scand. 1983;213:105–110. doi: 10.1111/j.0954-6820.1983.tb03699.x. [DOI] [PubMed] [Google Scholar]
- Osterloh JD, Pond SM, Grady S, et al. Serum formate concentrations in methanol intoxication as a criterion for hemodialysis. Ann Intern Med. 1986;104:200–203. doi: 10.7326/0003-4819-104-2-200. [DOI] [PubMed] [Google Scholar]
- Salek T, Humpolicek P, Ponizil P.Metabolic disorders due to methanol poisoning Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2013. doi: 10.5507/bp.2013.5074 [DOI] [PubMed]
- Jacobsen D, Ovrebo S, Sejersted OM. Toxicokinetics of formate during hemodialysis. Acta Med Scand. 1983;214:409–412. doi: 10.1111/j.0954-6820.1983.tb08616.x. [DOI] [PubMed] [Google Scholar]
- Barceloux DG, Bond GR, Krenzelok EP, et al. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol-Clin Toxicol. 2002;40:415–446. doi: 10.1081/clt-120006745. [DOI] [PubMed] [Google Scholar]
- Aviles-Gomez R, Luquin-Arellano VH, Garcia-Garcia G, et al. Is renal replacement therapy for all possible in developing countries. Ethn Dis. 2006;16:70–72. [PubMed] [Google Scholar]
- Zakharov S, Pelclova D, Navratil T.Methanol outbreak in the Czech Republic 2012: epidemiology, clinical features and outcomes. In preparation2013
- Jacobsen D, Jansen H, Wiik-Larsen E, et al. Studies on methanol poisoning. Acta Med Scand. 1982;212:5–10. doi: 10.1111/j.0954-6820.1982.tb03160.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen D, McMartin KE. Antidotes for methanol and ethylene glycol poisoning. J Toxicol Clin Toxicol. 1997;35:127–143s. doi: 10.3109/15563659709001182. [DOI] [PubMed] [Google Scholar]
- Garella S. Extracorporeal techniques in the treatment of exogenous intoxications. Kidney Int. 1988;33:735–754. doi: 10.1038/ki.1988.60. [DOI] [PubMed] [Google Scholar]
- Chow MT, Di Silvestro VA, Yung CY, et al. Treatment of acute methanol intoxication with hemodialysis using an ethanol-enriched, bicarbonate-based dialysate. Am J Kidney Dis. 1997;30:568–570. doi: 10.1016/s0272-6386(97)90318-8. [DOI] [PubMed] [Google Scholar]
- Dorval M, Pichette V, Cardinal J, et al. The use of an ethanol- and phosphate-enriched dialysate to maintain stable serum ethanol levels during haemodialysis for methanol intoxication. Nephrol Dial Transplant. 1999;14:1774–1777. doi: 10.1093/ndt/14.7.1774. [DOI] [PubMed] [Google Scholar]
- Chebrolu SB, Hariman A, Eggert CH, et al. Phosphorus-enriched hemodialysis for the treatment of patients with severe methanol intoxication. Int J Artif Organs. 2005;28:270–274. doi: 10.1177/039139880502800313. [DOI] [PubMed] [Google Scholar]
- Peces R, Fernandez R, Peces C, et al. Effectiveness of pre-emptive hemodialysis with high-flux membranes for the treatment of life-threatening alcohol poisoning. Nefrologia. 2008;28:413–418. [PubMed] [Google Scholar]
- Bayliss G. Dialysis in the poisoned patient. Hemodial Int. 2010;14:158–167. doi: 10.1111/j.1542-4758.2009.00427.x. [DOI] [PubMed] [Google Scholar]
- Hantson P, Haufroid V, Wallemacq P. Formate kinetics in methanol poisoning. Hum Exp Toxicol. 2005;24:55–59. doi: 10.1191/0960327105ht503oa. [DOI] [PubMed] [Google Scholar]
- Kerns W, 2nd, Tomaszewski C, McMartin K, et al. Formate kinetics in methanol poisoning. J Toxicol, Clin Toxicol. 2002;40:137–143. doi: 10.1081/clt-120004401. [DOI] [PubMed] [Google Scholar]
- Jacobsen D, Webb R, Collins TD, et al. Methanol and formate kinetics in late diagnosed methanol intoxication. Med Toxicol Adverse Drug Exp. 1988;3:418–423. doi: 10.1007/BF03259893. [DOI] [PubMed] [Google Scholar]
- Brent J, McMartin K, Phillips S, et al. Fomepizole for the treatment of methanol poisoning. N Engl J Med. 2001;344:424–429. doi: 10.1056/NEJM200102083440605. [DOI] [PubMed] [Google Scholar]
- Jacobsen D, Ovrebo S, Arnesen E, et al. Pulmonary excretion of methanol in man. Scand J Clin Lab Invest. 1983;43:377–379. doi: 10.1080/00365518309168275. [DOI] [PubMed] [Google Scholar]
- Palatnick W, Redman LW, Sitar DS, et al. Methanol half-life during ethanol administration: implications for management of methanol poisoning. Ann Emerg Med. 1995;26:202–207. doi: 10.1016/s0196-0644(95)70152-4. [DOI] [PubMed] [Google Scholar]
- Kan G, Jenkins I, Rangan G, et al. Continuous haemodiafiltration compared with intermittent haemodialysis in the treatment of methanol poisoning. Nephrol Dial Transplant. 2003;18:2665–2667. doi: 10.1093/ndt/gfg432. [DOI] [PubMed] [Google Scholar]
- Moore DF, Bentley AM, Dawling S, et al. Folinic acid and enhanced renal elimination in formic acid intoxication. J Toxicol Clin Toxicol. 1994;32:199–204. doi: 10.3109/15563659409000451. [DOI] [PubMed] [Google Scholar]
- Shahangian S, Robinson VL, Jennison TA. Formate concentrations in a case of methanol ingestion. Clin Chem. 1984;30:1413–1414. [PubMed] [Google Scholar]
- Hovda KE, Mundal H, Urdal P, et al. Extremely slow formate elimination in severe methanol poisoning: a fatal case report. Clin Toxicol. 2007;45:516–521. doi: 10.1080/15563650701354150. [DOI] [PubMed] [Google Scholar]
- Sanaei-Zadeh H, Zamani N, Shadnia S. Outcomes of visual disturbances after methanol poisoning. Clin Toxicol. 2011;49:102–107. doi: 10.3109/15563650.2011.556642. [DOI] [PubMed] [Google Scholar]
- Hovda KE, Urdal P, Jacobsen D. Increased serum formate in the diagnosis of methanol poisoning. J Anal Toxicol. 2005;29:586–588. doi: 10.1093/jat/29.6.586. [DOI] [PubMed] [Google Scholar]
- Schaller KH, Triebig GT.Formate determination with formate dehydrogenaseIn: Bergmeyer HU (ed) Methods of Enzymatic Analysis Verlag Chemie: Weinheim; 1984668–672. [Google Scholar]
- Blomme B, Lheureux P, Gerlo E, et al. Cobas Mira S endpoint enzymatic assay for plasma formate. J Anal Toxicol. 2001;25:77–80. doi: 10.1093/jat/25.2.77. [DOI] [PubMed] [Google Scholar]
- Urdal P. Enzymic assay for oxalate in unprocessed urine, as adapted for a centrifugal analyzer. Clin Chem. 1984;30:911–913. [PubMed] [Google Scholar]
- Winter ME.Basic Clinical Pharmacokinetics2nd ednApplied Therapeutics: Vancouver; 1988 [Google Scholar]
- Aabakken L, Johansen KS, Rydningen EB, et al. Osmolal and anion gaps in patients admitted to an emergency medical department. Hum Exp Toxicol. 1994;13:131–134. doi: 10.1177/096032719401300212. [DOI] [PubMed] [Google Scholar]